Abstract
HIV infects activated CD4+ T cells and induces their depletion. Progressive HIV infection leading to AIDS is fueled by chronic immune hyperactivation, mediated by inflammatory cytokines like TNFα. This has been related to intestinal epithelial damage and microbial LPS translocation into the circulation. Using 11-color flow cytometry, cell sorting, and cell culture, we investigated the numbers and TNFα production of fully defined circulating dendritic cell and monocyte populations during HIV-1 infection. In 15 viremic, untreated patients, compared with 8 treated, virologically suppressed patients or to 13 healthy blood donors, circulating CD141 (BDCA-3)+ and CD1c (BDCA-1)+ dendritic cell counts were reduced. Conversely, CD14+CD16++ monocyte counts were increased, particularly those expressing M-DC8, while classical CD14++CD16−M-DC8− monocyte numbers were unchanged. Blood mononuclear cells from viremic patients produced more TNFα in response to LPS than those from virologically suppressed patients. M-DC8+ monocytes were mostly responsible for this overproduction. Moreover, M-DC8+ monocytes differentiated in vitro from classical monocytes using M-CSF and GM-CSF, which is increased in viremic patient's plasma. This M-DC8+ monocyte population, which is involved in the pathogenesis of chronic inflammatory diseases like Crohn disease, might thus be considered as a major actor in the immune hyperactivation fueling HIV infection progression.
Introduction
HIV-1 infection induces the depletion of CD4+ T lymphocytes in blood and lymphoid organs, particularly in the gut-associated lymphoid tissue.1-3 The absence of immune activation during the chronic phase of the infection distinguishes nonprogressive from progressive infections in patients as well as in nonhuman primate models of HIV infection.4-6 Systemic immune activation is correlated to the increased translocation of gut luminal microbial products such as the Gram-negative bacterial lipopolysaccharide (LPS).7
LPS stimulates the production of proinflammatory cytokines, particularly TNFα. In HIV-1–infected patients, TNFα serum levels increase in correlation with disease progression and drop to normal levels after treatment only in patients with good virologic and immunologic responses.3,8 By activating the NF-κB pathway, TNFα induces viral replication in HIV-infected CD4+ T lymphocytes.3,9 In chronic inflammatory bowel diseases, TNFα affects mucosal integrity, leading to microbial product systemic translocation.10 Granulocyte/macrophage colony-stimulating factor (GM-CSF) and LPS also induce HIV replication in infected myeloid cells.11,12 GM-CSF and TNFα are produced by monocytes and dendritic cells (DCs) after LPS stimulation.
During chronic HIV infection, circulating plasmacytoid and myeloid DC (pDC and mDC) numbers are reduced.13-15 Myeloid DCs were mostly studied in HIV-infected patients using CD11c as a marker. Now, they are further subdivided into BDCA-1+ and BDCA-3+ mDC subsets, the latter recently shown as being the human homolog to the murine CD8α mDC subset.16-19 Circulating classical CD14++CD16− monocyte numbers are normal during HIV infection, whereas CD14+CD16++ monocyte numbers were found to be higher in HIV-infected patients with advanced disease than in healthy donors.20,21 CD14+CD16++ monocyte numbers are high in acute and chronic, viremic SIV infection.22 Interestingly, these cells with low CD14 and strong CD16 expression infiltrate the brains from AIDS patients with HIV-related encephalitis where important amounts of TNFα are detected.23 In addition to CD14+CD16++ monocytes, called nonclassical monocytes in a recent novel unified nomenclature,24 and the classical, CD14++CD16− monocytes, a third population of CD14++CD16+ monocytes, named intermediate, can be distinguished by sensitive multicolor flow cytometry.24,25 Among nonclassical CD14+CD16++ monocytes, a subpopulation expressing M-DC8 (slan, 6-sulfo LacNAc, glycosylation variant of P-selectin glycoprotein ligand-1 [PSGL-1])26 is found in abundance in inflamed tissues of patients with chronic inflammatory diseases such as Crohn disease,27 rheumatoid arthritis, or psoriasis,28,29 but was never found impaired in HIV infection.
In this study, we evaluated the subversion by HIV infection of all these myeloid cell populations, and assessed which myeloid cells might be most involved in TNFα production and in the vicious circle of immune activation found during progressive HIV infection. In viremic HIV-infected patients, our observations point to an accumulation of highly activated M-DC8+ monocytes, which have a predominant role in the TNFα overproduction in response to LPS, and thus might be major actors in this hyperactivation.
Methods
Patient samples
Peripheral blood was collected on heparin from 23 patients with chronic HIV-1 infection, included in the prospective cohorts PREVAC (Clinical Investigation Center of the Cochin Hospital, Paris) and Agence Nationale de Recherche sur le Sida et les Hepatites Virales (ANRS) PRIMO (Table 1). ANRS PRIMO was approved by the Comité de Protection des Personnes (CPP) Paris-Cochin on July 2, 1996 (updated May 23, 2006; no. 1157/18-6-96). PREVAC was approved by the “Ile-de-France 4” Ethics Committee (Paris, France), on June 25, 2010 (no. 2010-13NICB). All patients gave informed consent before inclusion in accordance with the Declaration of Helsinki. Patients were aged 20-64 years (median: 39 years). Eight were treated by combined antiretroviral therapy (cART) and were virologically suppressed, as they had undetectable viral loads (below 50 copies/mL). Fifteen were cART-naive, and viremic: their viral loads (VLs) ranged from 1.63 to 4.98 log10 HIV RNA copies/mL (median: 4.25 log copies of HIV RNA/mL) and their CD4+ T-cell counts from 279 to 803 cells/μL (median: 544 cells/μL). For comparison, peripheral blood from 16 uninfected individuals was collected on heparin at the Etablissement Français du Sang (Saint-Vincent de Paul Hospital, Paris, France) within an ethics convention with Inserm. All experiments were carried out with PBMC freshly purified on a Ficoll density gradient. Plasma (diluted 1:1 with NaCl) were recovered from the top layer of the Ficoll gradient and frozen.
Blood samples, clinical data from patients
| Patient . | HIV infection . | Sex . | Age, y . | Y after infection . | Treatment (duration) . | CD4+ T cells/mL . | VL log10 RNA copies/mL . |
|---|---|---|---|---|---|---|---|
| 1 | Yes | M | 42 | 2 | teno, emtri, efa (2 y) | ND* | 1.00 |
| 2 | Yes | M | 37 | 2 | tdf, emtri, ataza/rito (2 y) | 517 | 1.00 |
| 3 | Yes | M | 20 | 2 | tdf, emtri, ralteg (6 mo) | 523 | 1.00 |
| 4 | Yes | M | 47 | 2 | teno, emtri, efa (1 y) | 526 | 1.00 |
| 5 | Yes | M | 50 | 2 | teno, efa (2 y) | 548 | 1.00 |
| 7 | Yes | M | 50 | 1.5 | tdf, emtri, efa (17 mo) | 668 | 1.00 |
| 6 | Yes | M | 35 | 1.5 | tdf, emtri, ataza/rito (17 mo) | 693 | 1.00 |
| 8 | Yes | M | 43 | 1.5 | tdf, emtri, darun/rito (15 mo) | 793 | 1.00 |
| Treated, n/median | 0F/8M | 42.5 | 2 | 548 | 1.0 | ||
| 9 | Yes | M | 47 | 10 | / | 494 | 1.63 |
| 10 | Yes | F | 33 | 6 | / | 564 | 2.98 |
| 11 | Yes | F | 29 | 2 | / | 354 | 3.67 |
| 12 | Yes | F | 32 | 4 | / | 279 | 3.79 |
| 13 | Yes | M | 49 | 4 | / | 596 | 4.17 |
| 14 | Yes | F | 38 | 9 | / | 544 | 4.12 |
| 15 | Yes | F | 55 | 5 | / | 328 | 4.25 |
| 16 | Yes | M | 41 | 5 | / | 709 | 4.25 |
| 17 | Yes | M | 39 | 1.5 | / | 300 | 4.27 |
| 18 | Yes | M | 39 | 2 | / | 583 | 4.48 |
| 19 | Yes | M | 47 | 21 | / | 434 | 4.53 |
| 20 | Yes | F | 64 | 9 | / | 477 | 4.56 |
| 21 | Yes | F | 32 | 3 | / | 579 | 4.58 |
| 22 | Yes | M | 33 | 2 | / | 755 | 4.6 |
| 23 | Yes | M | 39 | 2 | / | 569 | 4.98 |
| Untreated, n/median | 7F/8M | 39 | 4.5 | 544 | 4.25 |
| Patient . | HIV infection . | Sex . | Age, y . | Y after infection . | Treatment (duration) . | CD4+ T cells/mL . | VL log10 RNA copies/mL . |
|---|---|---|---|---|---|---|---|
| 1 | Yes | M | 42 | 2 | teno, emtri, efa (2 y) | ND* | 1.00 |
| 2 | Yes | M | 37 | 2 | tdf, emtri, ataza/rito (2 y) | 517 | 1.00 |
| 3 | Yes | M | 20 | 2 | tdf, emtri, ralteg (6 mo) | 523 | 1.00 |
| 4 | Yes | M | 47 | 2 | teno, emtri, efa (1 y) | 526 | 1.00 |
| 5 | Yes | M | 50 | 2 | teno, efa (2 y) | 548 | 1.00 |
| 7 | Yes | M | 50 | 1.5 | tdf, emtri, efa (17 mo) | 668 | 1.00 |
| 6 | Yes | M | 35 | 1.5 | tdf, emtri, ataza/rito (17 mo) | 693 | 1.00 |
| 8 | Yes | M | 43 | 1.5 | tdf, emtri, darun/rito (15 mo) | 793 | 1.00 |
| Treated, n/median | 0F/8M | 42.5 | 2 | 548 | 1.0 | ||
| 9 | Yes | M | 47 | 10 | / | 494 | 1.63 |
| 10 | Yes | F | 33 | 6 | / | 564 | 2.98 |
| 11 | Yes | F | 29 | 2 | / | 354 | 3.67 |
| 12 | Yes | F | 32 | 4 | / | 279 | 3.79 |
| 13 | Yes | M | 49 | 4 | / | 596 | 4.17 |
| 14 | Yes | F | 38 | 9 | / | 544 | 4.12 |
| 15 | Yes | F | 55 | 5 | / | 328 | 4.25 |
| 16 | Yes | M | 41 | 5 | / | 709 | 4.25 |
| 17 | Yes | M | 39 | 1.5 | / | 300 | 4.27 |
| 18 | Yes | M | 39 | 2 | / | 583 | 4.48 |
| 19 | Yes | M | 47 | 21 | / | 434 | 4.53 |
| 20 | Yes | F | 64 | 9 | / | 477 | 4.56 |
| 21 | Yes | F | 32 | 3 | / | 579 | 4.58 |
| 22 | Yes | M | 33 | 2 | / | 755 | 4.6 |
| 23 | Yes | M | 39 | 2 | / | 569 | 4.98 |
| Untreated, n/median | 7F/8M | 39 | 4.5 | 544 | 4.25 |
VL indicates viral load (HIV-1 RNA copies/mL plasma); ND, not done; M, male; F, female; teno, tenofovir; emtri, emtricitabine; efa, efavirenz; ataza, atazanavir; rito, ritonavir; tdf, tenofovir DF; ralteg, raltegravir; darun, darunavir; and /, untreated.
CD4 count was not obtained in the sample studied here but was obtained 3 months before: 325 cells/mL.
Eleven-color flow cytometric analyses and intracellular TNFα detection
The following monoclonal antibodies were used in this study: for 11-color membrane flow cytometric analyses: M-DC8-FITC (clone DD-1, dilution factor: 1/20), CD141(BDCA-3)–allophycocyanin (clone AD5-14H12, 1/150) and CD303(BDCA-2)–PE (clone AC144, 1/10) from Miltenyi Biotec; CD1c(BDCA-1)–Pacific Blue (clone L161, 1/400; Biolegend); CD14-QDot655 (clone TüK4, 1/100; Invitrogen), CD19-ECD (clone J3-119, 1/10; Beckman Coulter), CD11c–Alexa Fluor 700 (clone 3.9, 1/10; eBioscience); HLA-DR-PerCP (clone G46-6, 1/10), CD16-allophycocyanin-H7 (clone 3G8, 1/40) and CD45-Amcyan (clone 2D1, 1/25) from BD Biosciences. For intracellular cytokine expression analyses and for FACS sorting of monocyte subsets: CD141(BDCA-3)–PE (clone AC144, 1/10; Miltenyi Biotec); HLA-DR-ECD (clone Immu-357, 1/10; Beckman Coulter); CD19-allophycocyanin-H7 (clone SJ25C1, 1/15), CD14-PE-Cy7 (clone M5E2, 1/30), and TNFα–Alexa Fluor 700 (clone MAB11, 1/20) from BD Biosciences. After 4-day culture of FACS-sorted classical CD14++CD16−M-DC8− monocytes: CD1a-PE (clone HI149, 1/10; BD Biosciences) was also used. In all experiments, the Live/Dead blue Dye (Invitrogen) was used to exclude dead cells.
For 11-color membrane and 9-color intracellular FACS analyses, freshly purified PBMCs (2 × 106 cells/tube) were used, and in the latter experiments, PBMCs were stimulated for 7 hours at 37°C, with 5% CO2, in polypropylene tubes in complete RPMI 1640 supplemented with 10% FCS with or without lipopolysaccharide at 100 ng/mL (LPS; Sigma-Aldrich). Brefeldin A (BFA; 10 μg/mL; Sigma-Aldrich) was added for the last 4 hours. This protocol was established after preliminary kinetic experiments to find the minimal time of incubation and BFA treatment for optimal TNFα expression and minimal cell toxicity. PBMCs were washed and incubated for 30 minutes at +4°C with Live/Dead blue dye in PBS. Five-percent heat-inactivated human AB serum (serum AB; Abcys) was added for an extra 15 minutes at +4°C. Next, cells were labeled for 30 minutes at +4°C with antibodies diluted in PBS with 2% FCS and 2mM EDTA. For intracellular FACS analyses, cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences) following the manufacturer's instructions and incubated with the anti-TNFα monoclonal antibody (45 minutes, +4°C). Cells were then washed, fixed with 0.5% paraformaldehyde, and events acquired using a FACS LSRII (BD Biosciences). All analyses were carried out with the FACSDiva (BD Biosciences) software. The median number of analyzed events for the CD141(BDCA-3)+ dendritic cell population was 188, the minimum was 17, and the highest was 5927. Other DC and monocyte subsets were more numerous. The absolute number of cells per blood microliter was calculated by multiplying the hemocytometer complete blood count (performed independently on whole blood) of mononuclear cells (monocytes + lymphocytes) to the percentage of cells among CD45hi events.
Flow cytometric cell sorting
Fresh PBMCs were incubated for 15 minutes at +4°C with 5% serum AB in PBS and labeled with the following antibodies before FACS sorting using a FACSAriaIII (BD Biosciences) set for high-purity sorting. For the 4 HLA-DR+CD11c+ monocyte subset sorting, cells were labeled with the following antibodies: M-DC8-FITC, HLA-DR-PerCP, CD14-PE-Cy7, CD16-allophycocyanin-H7, and CD11c–Alexa Fluor 700. Purification was obtained to at least 98%. For depletion of CD11c+M-DC8+ monocytes from PBMCs, M-DC8-FITC was used alone to leave sorted cells untouched.
In vitro monocyte differentiation
Freshly FACS-sorted classical HLA-DR+CD11c+CD14++CD16−M-DC8− monocytes were cultured at 5 × 105 cells/mL for 4 days in RPMI 1640 supplemented with 10% FCS and cultured at 37°C with 5% CO2 in the presence or not of GM-CSF (50 ng/mL; AbCys) and M-CSF (10 ng/mL; AbCys) in flat-bottom 96-well plates. When indicated, IL-4 (200 IU/mL; AbCys) or IL-10 (10 ng/mL; R&D Systems) were added. Cells were then thoroughly recovered with ice-cold PBS containing 2mM EDTA without leaving any remaining adherent cell in the wells before either LPS stimulation for intracellular TNFα expression assessment or direct FACS staining as described under “Eleven-color flow cytometric analyses and intracellular TNFα detection” using the following antibodies: M-DC8–FITC, CD11c–Alexa Fluor 700, HLA-DR-PerCP, CD14-PE-Cy7, CD16-allophycocyanin-H7, and CD1a-PE.
Cytokine concentration measurement
Total PBMCs (5 × 105 cells in 500 μL) were cultured in RPMI 1640 supplemented with 10% FCS at 37°C with 5% CO2 in the presence or not of LPS for 18 hours. For M-DC8 depletion experiments, 1 × 105 total PBMCs or M-DC8–depleted PBMCs were cultured in 200 μL. Supernatants were collected after centrifugation and stored at − 80°C until use. For the quantification of plasma sCD14 and TNFα and GM-CSF in supernatants, cytometric bead arrays (BD Biosciences) were used following the manufacturer's instructions (flow cytometric beads were analyzed with a LSRII flow cytometer; BD Biosciences). Concentrations were determined using the FCAP Array software (BD Biosciences). Plasma GM-CSF and TNFα were quantified by ELISA after 1:1 dilution in NaCl (R&D Quantikine and HS ELISA, respectively).
Statistical analysis
Results are given as medians. The Mann-Whitney test was used to compare controls and patient groups or cellular subsets. Correlations were evaluated with the Spearman test. Differences were defined as statistically significant when P < .05. All these nonparametric tests were performed using the GraphPad Prism 5 software.
Results
Depletion of dendritic cells and increased numbers of monocytes expressing CD16 in the blood from viremic, HIV-infected patients
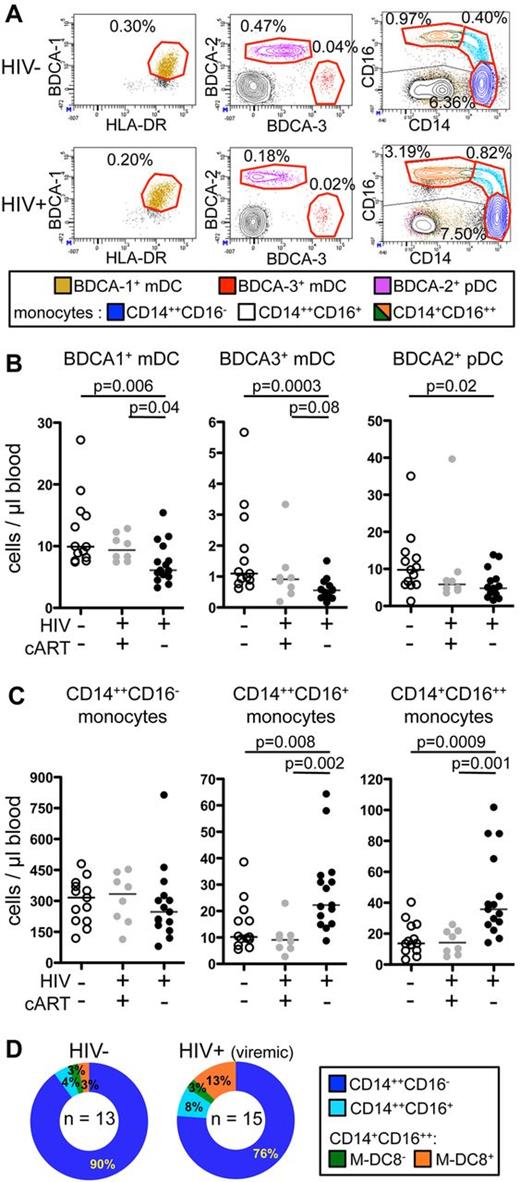
To study all DC and monocyte subsets simultaneously, we carried out 11-color flow cytometric analyses. PBMCs from 13 healthy donors (or “controls”), 8 HIV-infected patients treated by cART (and therefore aviremic, named “virologically suppressed patients”), and 15 HIV-infected untreated patients, who all had detectable viral loads (therefore named “viremic patients”), were studied (Table 1). The gating strategy used to separate the various cellular subsets is shown for a representative healthy donor (populations circled in red, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In these analyses, CD45hiHLA-DR+CD19− cells were subdivided into 3 DC subsets [CD303(BDCA-2)+ pDC, CD141(BDCA-3)+, and CD1c(BDCA-1)+ mDC], and 3 major monocyte subsets (classical CD14++CD16−, intermediate CD14++CD16+, and nonclassical CD14+CD16++ monocytes). The latter subset was further subdivided based on the expression of M-DC8. Dot plots defining circulating DC and monocyte subsets from representative HIV-infected and uninfected individuals are shown (Figures 1A and 2A).
Strong decrease in CD141 (BDCA-3)+ mDC counts and increase in CD16+ and particularly CD14+CD16++ monocyte counts in the circulation of viremic patients compared with controls and virologically suppressed patients. (A) Dot plots of 11-color FACS analysis (described in supplemental Figure 1) of PBMCs from (top panels) a representative healthy donor and (bottom panels) a viremic patient. From left to right, BDCA-1+ mDCs (beige), BDCA-3+ mDCs (red), and BDCA-2+ pDCs (purple); CD14++CD16− classical monocytes (blue), CD14++CD16+ intermediate monocytes (cyan), and CD11c+CD14+CD16++ nonclassical monocytes (green and orange) are shown. The percentage of each cellular subset among CD45+ PBMCs is indicated in each dot plot. (B-C) Absolute counts in the blood from healthy donors (HIV−, cART−, n = 13), virologically suppressed patients (HIV+, cART+, n = 8), and viremic patients (HIV+, cART−, n = 15) of (B) mDC subsets and pDCs and (C) monocyte subsets are shown (P values were calculated using the Mann-Whitney test; bars indicate medians). (D) Ring graphical representation of the mean proportion of monocyte subsets among total monocytes in the healthy donors (HIV−; n = 13) and viremic patients (n = 15).
Strong decrease in CD141 (BDCA-3)+ mDC counts and increase in CD16+ and particularly CD14+CD16++ monocyte counts in the circulation of viremic patients compared with controls and virologically suppressed patients. (A) Dot plots of 11-color FACS analysis (described in supplemental Figure 1) of PBMCs from (top panels) a representative healthy donor and (bottom panels) a viremic patient. From left to right, BDCA-1+ mDCs (beige), BDCA-3+ mDCs (red), and BDCA-2+ pDCs (purple); CD14++CD16− classical monocytes (blue), CD14++CD16+ intermediate monocytes (cyan), and CD11c+CD14+CD16++ nonclassical monocytes (green and orange) are shown. The percentage of each cellular subset among CD45+ PBMCs is indicated in each dot plot. (B-C) Absolute counts in the blood from healthy donors (HIV−, cART−, n = 13), virologically suppressed patients (HIV+, cART+, n = 8), and viremic patients (HIV+, cART−, n = 15) of (B) mDC subsets and pDCs and (C) monocyte subsets are shown (P values were calculated using the Mann-Whitney test; bars indicate medians). (D) Ring graphical representation of the mean proportion of monocyte subsets among total monocytes in the healthy donors (HIV−; n = 13) and viremic patients (n = 15).
Strong increase of M-DC8+ CD11c+CD14+CD16++ monocyte counts in the circulation of viremic patients compared with controls and virologically suppressed patients. (A) Dot plots defining the M-DC8− (green) and M-DC8+ (orange) CD11c+CD14+CD16++ monocyte subsets (gating strategy described in supplemental Figure 1) in the blood from (top panel) a representative uninfected donor and (bottom panel) a viremic patient. The percentage of each CD14+CD16++ monocyte subset among CD45+ PBMCs is indicated in each dot plot. (B) Absolute counts of CD14+CD16++ monocyte subsets in the blood of healthy donors (HIV−, cART−, n = 13), virologically suppressed patients (HIV+, cART+, n = 8), and viremic patients (HIV+, cART−, n = 15) are shown. Filled black circles indicate viremic patients with M-DC8+ monocyte counts below 50 cells/μL blood; and filled red circles indicate those with M-DC8+ monocyte counts above 50 cells/μL blood. (C) Soluble CD14 (sCD14) concentrations in the plasma from viremic patients with low (black circles) or high (red circles) M-DC8+ monocyte blood count (P values are calculated using the Mann-Whitney test; bars indicate medians). (D) Absolute count correlations of total CD14+CD16++ monocytes with either M-DC8− (green) or M-DC8+ (orange) subsets (Spearman test).
Strong increase of M-DC8+ CD11c+CD14+CD16++ monocyte counts in the circulation of viremic patients compared with controls and virologically suppressed patients. (A) Dot plots defining the M-DC8− (green) and M-DC8+ (orange) CD11c+CD14+CD16++ monocyte subsets (gating strategy described in supplemental Figure 1) in the blood from (top panel) a representative uninfected donor and (bottom panel) a viremic patient. The percentage of each CD14+CD16++ monocyte subset among CD45+ PBMCs is indicated in each dot plot. (B) Absolute counts of CD14+CD16++ monocyte subsets in the blood of healthy donors (HIV−, cART−, n = 13), virologically suppressed patients (HIV+, cART+, n = 8), and viremic patients (HIV+, cART−, n = 15) are shown. Filled black circles indicate viremic patients with M-DC8+ monocyte counts below 50 cells/μL blood; and filled red circles indicate those with M-DC8+ monocyte counts above 50 cells/μL blood. (C) Soluble CD14 (sCD14) concentrations in the plasma from viremic patients with low (black circles) or high (red circles) M-DC8+ monocyte blood count (P values are calculated using the Mann-Whitney test; bars indicate medians). (D) Absolute count correlations of total CD14+CD16++ monocytes with either M-DC8− (green) or M-DC8+ (orange) subsets (Spearman test).
The absolute numbers (Figure 1B) and proportions (supplemental Figure 2A) of circulating BDCA-3+ mDCs were reduced in viremic patients, compared with healthy donors (medians: 0.56 ± 0.33 vs 1.1 ± 1.46 cells/μL; 0.02% ± 0.01% vs 0.06% ± 0.04% among CD45+ PBMCs). The absolute numbers (Figure 1B) and proportions (supplemental Figure 2A) of circulating BDCA-1+ mDCs, and of pDCs, labeled by BDCA-2–specific antibodies, were also reduced in viremic patients compared with controls (BDCA-1+ mDC: 6.1 ± 3.3 vs 9.9 ± 5.8 cells/μL; 0.22% ± 0.18% vs 0.51% ± 0.17%, and BDCA-2+ pDC: 4.8 ± 3.9 vs 9.8 ± 8.4 cells/μL; 0.18% ± 0.16% vs 0.47% ± 0.23%, respectively). The numbers and proportions of all DC subsets in the virologically suppressed patients were not statistically different from those of the controls.
Conversely to DC subsets, monocyte subsets were not lower in viremic patients than in virologically suppressed patients or in controls. Classical CD14++CD16− monocyte numbers were statistically unchanged, but the numbers (Figure 1C) and percentages among CD45+ PBMCs (supplemental Figure 2B) of both CD16+ subsets were higher in viremic patients compared with healthy donors. The monocytes with the highest CD16 expression were the most increased (nonclassical CD14+CD16++ monocytes: 35.7 ± 27.3 vs 13.7 ± 10.7 cells/μL blood; 1.23% ± 1.46% vs 0.70% ± 0.54%; and intermediate CD14++CD16+ monocytes: 22.3 ± 15.7 vs 10.2 ± 9.4 cells/μL blood; 0.97% ± 0.50% vs 0.49% ± 0.47%). Virologically suppressed HIV-infected patients had similar counts of all monocyte subsets compared with controls. In addition, the proportion of CD16+ monocytes among total monocytes from viremic patients compared with uninfected controls was increased (Figure 1D). Thus, nonclassical CD14+CD16++ monocytes represented 16% of total monocytes in viremic patients and only 6% in controls (mean 2.7-fold increase), while intermediate CD14++CD16+ monocytes represented 8% of total monocytes in viremic patients and only 4% in controls (mean 2-fold increase). Thus, mDC numbers were reduced, while CD16+ monocyte numbers were increased in viremic compared with virologically suppressed patients.
The M-DC8+ subset mostly accounted for the high numbers of blood CD14+CD16++ monocytes in viremic patients
Nonclassical CD14+CD16++ monocytes can be further subdivided according to M-DC8 and CD11c expression (Figure 2A, supplemental Figure 1). The numbers and percentages of M-DC8+ monocytes (Figure 2B, supplemental Figure 2C) were strongly increased in viremic patients compared with healthy donors and to virologically suppressed patients (23.6 ± 26.1 vs 8.4 ± 6.7 vs 9.4 ± 6.3 cells/μL blood; 0.83% ± 1.35% vs 0.41% ± 0.36% vs 0.45% ± 0.27%). Among viremic patients, 4 had more than 50 M-DC8+ monocytes/μL blood. To assess for a potential role of circulating LPS, we measured soluble CD14 (sCD14) concentrations in the plasma, which reflect LPS translocation more reliably than LPS concentrations themselves (which vary with prandial status). The same 4 patients who had more than 50 M-DC8+ monocytes/μL blood had significantly higher levels of plasma sCD14 than the other viremic patients (red dots, Figure 2B-C). In viremic patients, the proportion of M-DC8+ cells was increased among total CD14+CD16++ monocytes compared with healthy individuals (75% vs 55%, supplemental Figure 2D). As the numbers (Figure 2B) and percentages (supplemental Figure 2C) of M-DC8− CD14+CD16++ monocytes were not significantly different between patients and controls, M-DC8+ monocytes accounted for the increased numbers of CD14+CD16++ monocytes in viremic patients (Spearman r = 0.97, P < .0001; Figure 2D).
M-DC8+ monocytes were responsible for a large part of the LPS-induced TNFα-overproduction in viremic patients
To assess the role of the different myeloid cell populations in TNFα production, freshly purified PBMCs from 8 healthy donors and 7 viremic patients were stimulated with LPS for 18 hours (Figure 3A). TNFα production in response to LPS was strongly increased in the PBMCs from viremic patients compared with healthy donors, particularly for patients (red circles) who had the highest M-DC8+ CD14+CD16++ circulating monocyte counts as observed in Figure 2B. To determine the contribution of M-DC8+ monocytes to the total TNFα production by LPS-stimulated PBMCs, M-DC8–expressing cells were depleted by FACS sorting from the PBMCs of 6 healthy donors and 5 viremic patients before LPS stimulation (Figure 3B). M-DC8 depletion induced a mean 5.7-fold drop in LPS-induced TNFα production in PBMCs from all of the viremic patients (median values: 332 pg/mL vs 40 pg/mL for total PBMCs vs M-DC8–depleted PBMCs, respectively, P = .0625, ie, nonsignificant, but the Wilcoxon test is only a ranking test), but not from healthy donors. Furthermore, TNFα production by M-DC8–depleted PBMCs from HIV-infected patients was comparable with that observed for healthy donor PBMCs. In addition, after LPS stimulation, FACS-sorted M-DC8+ CD14+CD16++ monocytes from viremic patients produced 3.6 times more TNFα than those from healthy donors (Figure 3C), and were also the strongest TNFα-producing monocyte subset (Figure 3D). As controls, unstimulated FACS-sorted M-DC8+CD14+CD16++ monocytes from 1 healthy donor and 1 viremic patient did not produce significant amounts of TNFα (supplemental Figure 3A). After LPS stimulation, other cytokines were also tested by CBA-Flex assays in the supernatants of different FACS-sorted cells. IL-6 was also produced by MDC8+ and MDC8− nonclassical monocytes, more than by mDCs, but less than by intermediate or classical monocytes. Conversely, IFNα was produced by none of these populations (data not shown).
M-DC8+ monocytes from viremic patients produce greater amounts of TNFα than those from healthy donors and are responsible for a large part of the TNFα overproduction after LPS stimulation. (A-D) After 18-hour stimulation with or without LPS, TNFα concentrations were measured in culture supernatants from (A) total PBMCs (1 × 106 cells/mL; 8 healthy donors and 7 viremic patients), (B) total vs [M-DC8+ cell]–depleted PBMC (5 × 105 cells/mL; 6 healthy donors and 5 viremic patients), (C) FACS-sorted M-DC8+ CD11c+CD14+CD16++ monocytes from 4 healthy donors and 4 viremic patients (2.5 × 105 cells/mL), and (D) monocyte subsets from 1 healthy donor (5 × 105 cells/mL; representative of 3 independent experiments). (E) Plasma TNFα concentrations from 16 healthy donors (HIV−, cART−, open circles), 8 virologically suppressed patients (HIV+, cART+, gray circles) and 15 viremic patients (HIV+, cART−, filled circles). Patients with M-DC8+ monocyte counts > 50/μL as defined in Figure 2B are shown (red circles). (F-H) PBMCs were cultured for 7 hours with or without LPS. (F) Dot plot of 9-color intracellular TNFα FACS analysis from (left panels) 1 representative healthy donor and (right panels) 1 representative viremic patient showing TNFα production in different monocyte (top panels), DC, and lymphocyte populations (bottom panels) after stimulation of PBMCs with LPS. Percentages of TNFα-positive cells among the parent population and MFI of the TNFα-positive population (in brackets) are indicated. (G-H) After LPS stimulation, comparison of 6 healthy donors and 8 viremic patients for (G) the percentages of TNFα-positive cells among different monocyte subsets, and (I) of the MFI of the TNFα-positive populations in the same experiments as panel G. P values calculated using the Mann-Whitney test; bars indicate medians. ND indicates not determined; and Unst, unstimulated.
M-DC8+ monocytes from viremic patients produce greater amounts of TNFα than those from healthy donors and are responsible for a large part of the TNFα overproduction after LPS stimulation. (A-D) After 18-hour stimulation with or without LPS, TNFα concentrations were measured in culture supernatants from (A) total PBMCs (1 × 106 cells/mL; 8 healthy donors and 7 viremic patients), (B) total vs [M-DC8+ cell]–depleted PBMC (5 × 105 cells/mL; 6 healthy donors and 5 viremic patients), (C) FACS-sorted M-DC8+ CD11c+CD14+CD16++ monocytes from 4 healthy donors and 4 viremic patients (2.5 × 105 cells/mL), and (D) monocyte subsets from 1 healthy donor (5 × 105 cells/mL; representative of 3 independent experiments). (E) Plasma TNFα concentrations from 16 healthy donors (HIV−, cART−, open circles), 8 virologically suppressed patients (HIV+, cART+, gray circles) and 15 viremic patients (HIV+, cART−, filled circles). Patients with M-DC8+ monocyte counts > 50/μL as defined in Figure 2B are shown (red circles). (F-H) PBMCs were cultured for 7 hours with or without LPS. (F) Dot plot of 9-color intracellular TNFα FACS analysis from (left panels) 1 representative healthy donor and (right panels) 1 representative viremic patient showing TNFα production in different monocyte (top panels), DC, and lymphocyte populations (bottom panels) after stimulation of PBMCs with LPS. Percentages of TNFα-positive cells among the parent population and MFI of the TNFα-positive population (in brackets) are indicated. (G-H) After LPS stimulation, comparison of 6 healthy donors and 8 viremic patients for (G) the percentages of TNFα-positive cells among different monocyte subsets, and (I) of the MFI of the TNFα-positive populations in the same experiments as panel G. P values calculated using the Mann-Whitney test; bars indicate medians. ND indicates not determined; and Unst, unstimulated.
To highlight the relevance of these results obtained in vitro, it must be noted that in vivo, TNFα concentrations were significantly increased in plasma from viremic patients, and particularly those with high M-DC8+ monocyte counts (red circles), compared with either healthy donors or virologically suppressed patients (Figure 3E).
To assess the respective production of TNFα by monocyte and also DC subsets, and from a greater number of donors and viremic patients, TNFα intracellular FACS analyses were carried out using freshly purified PBMCs (Figure 3F-H). Of note, monocytes down-regulated CD16 expression after culture and could therefore not be defined on the basis of CD16 expression. The gating strategy used for these analyses is shown in supplemental Figure 3B. We checked that TNFα detection in the various cellular subsets was not linked to aberrant compensation settings that could lead to unspecific TNFα detection in these subsets (supplemental Figure 3C). Results obtained for different cell subsets from representative individuals are shown (Figure 3F). High proportions of monocytes produced TNFα in response to LPS. While the median percentage of TNFα-positive CD14++ and CD14+M-DC8− monocyte subsets was only moderately increased in viremic patients after LPS stimulation (P = .04 and P = .02, respectively), the percentage of TNFα-positive M-DC8+ monocytes was strongly increased in viremic patients (P = .003; Figure 3G). Furthermore, the median percentage of TNFα-positive M-DC8+ monocytes was much higher than that of both CD14++ and CD14+M-DC8− monocytes from viremic patients (86.7% vs 42.7%, P = .002 and vs 31.2%, P = .0002, respectively). M-DC8+ monocytes from viremic patients not only had a greater percentage of TNFα-positive cells but showed also a much greater mean fluorescence intensity (MFI) of the TNFα-positive population compared with both CD14++ and CD14+M-DC8− monocytes and to M-DC8+ monocytes from controls (Figure 3H). Similar data, with weaker differences between monocyte populations, were obtained using a TLR7/8 agonist, R848 (supplemental Figure 3D). A small proportion of the 2 mDC subsets produced moderate levels of TNFα, whether the individuals were viremic or uninfected, while B and non-B lymphocytes (mostly T cells and NK cells) did not produce any TNFα in response to LPS (Figure 3F). Thus, in vivo, viremic patients had higher plasma TNFα than virologically suppressed patients or controls, and in vitro, their PBMCs, and particularly their M-DC8+ monocytes, produced more TNFα.
CD16+M-DC8+ cell differentiation in vitro from classical CD14++CD16−M-DC8− monocytes in the presence of GM-CSF and M-CSF
To assess whether high M-DC8+ monocyte counts in the blood from viremic patients might originate from another myeloid population, we performed Spearman correlation tests. We found an inverse correlation of their counts with CD14++CD16− classical monocyte counts, and not with the counts of the other monocyte populations (r = −0.61, P = .016; Figure 4A). This led us to raise the hypothesis that M-DC8+ monocytes might differentiate from classical CD14++CD16− monocytes. FACS-sorted CD14++CD16−M-DC8− monocytes from 2 viremic patients and 3 healthy donors were cultured in the presence of GM-CSF and M-CSF, known to induce the differentiation of monocytes into macrophages30 (Figure 4B). After 4 days of culture, regardless of HIV infection, CD16 and M-DC8 expression were acquired, with 9.7% to 39.4% of the cells expressing M-DC8 for the 5 individuals tested (Figure 4B). Addition of IL-4, which in conjunction with GM-CSF leads to monocyte-derived DCs,31 induced expression of the CD1a antigen (Figure 4C middle panel). The addition of either IL-4 or the anti-inflammatory cytokine IL-10 prevented the differentiation into M-DC8–expressing cells (Figure 4C middle and right panels). The increase of M-DC8+ monocyte numbers in viremic patients might be linked to the strong immune activation that occurs during HIV-1 infection. Indeed, we found, as previously published, increased GM-CSF concentrations in the plasma from viremic patients (n = 15) compared with both healthy donors (n = 16) and virologically suppressed patients (n = 8, Figure 4D). We also observed a stronger capacity of both total PBMC (Figure 4E) and FACS-sorted CD14++CD16− classical monocytes (Figure 4F) from viremic patients (n = 3) to produce GM-CSF compared with cells from healthy donors (n = 4). Thus, in vitro stimulation experiments as well as plasma levels in these viremic, chronically HIV-infected patients suggest that the proinflammatory cytokine environment, including GM-CSF, may contribute to the increased proportions and counts of proinflammatory M-DC8+ monocytes.
HIV-1 infection is associated with a greater differentiation of classical CD14++CD16−M-DC8− monocytes into CD16+M-DC8+ cells in vitro. (A) Correlation of the absolute counts of CD14++CD16− classical monocytes with those of nonclassical M-DC8+ or M-DC8− CD14+CD16++ monocytes, and intermediate CD14++CD16+ monocytes for the 15 viremic patients. (B) FACS-sorted classical CD14++CD16− monocytes from a healthy donor were analyzed for CD16 and M-DC8 expression before or after culture for 4 days with GM-CSF and M-CSF. (Bottom panel) Percentages of M-DC8+ cells obtained from 3 healthy (open circles) and 2 viremic patients (filled circles). (C) M-DC8 and CD1a expression after 4-day culture of FACS-sorted CD14++CD16− classical monocytes from 1 viremic patient (representative of 3 independent experiments) cultured as in panel B the presence or not of IL-4 or IL-10 are shown. (D-F) GM-CSF concentrations were measured (D) in the plasma from 16 healthy donors (HIV−, cART−), 8 virologically suppressed patients (HIV+, cART+), or 15 viremic patients (HIV+, cART−), or (E-F) in culture supernatants from 18-hour LPS-stimulated or unstimulated (E) PBMCs (8 healthy donors [open circles] and 7 viremic patients [filled circles]), and (F) from FACS-sorted CD14++CD16− classical (black) or M-DC8+ CD14+CD16++ (gray) monocytes (4 healthy donors [open circles] and 3 viremic patients [filled circles]). When indicated, P values were calculated using the Mann-Whitney test; bars indicate medians.
HIV-1 infection is associated with a greater differentiation of classical CD14++CD16−M-DC8− monocytes into CD16+M-DC8+ cells in vitro. (A) Correlation of the absolute counts of CD14++CD16− classical monocytes with those of nonclassical M-DC8+ or M-DC8− CD14+CD16++ monocytes, and intermediate CD14++CD16+ monocytes for the 15 viremic patients. (B) FACS-sorted classical CD14++CD16− monocytes from a healthy donor were analyzed for CD16 and M-DC8 expression before or after culture for 4 days with GM-CSF and M-CSF. (Bottom panel) Percentages of M-DC8+ cells obtained from 3 healthy (open circles) and 2 viremic patients (filled circles). (C) M-DC8 and CD1a expression after 4-day culture of FACS-sorted CD14++CD16− classical monocytes from 1 viremic patient (representative of 3 independent experiments) cultured as in panel B the presence or not of IL-4 or IL-10 are shown. (D-F) GM-CSF concentrations were measured (D) in the plasma from 16 healthy donors (HIV−, cART−), 8 virologically suppressed patients (HIV+, cART+), or 15 viremic patients (HIV+, cART−), or (E-F) in culture supernatants from 18-hour LPS-stimulated or unstimulated (E) PBMCs (8 healthy donors [open circles] and 7 viremic patients [filled circles]), and (F) from FACS-sorted CD14++CD16− classical (black) or M-DC8+ CD14+CD16++ (gray) monocytes (4 healthy donors [open circles] and 3 viremic patients [filled circles]). When indicated, P values were calculated using the Mann-Whitney test; bars indicate medians.
Discussion
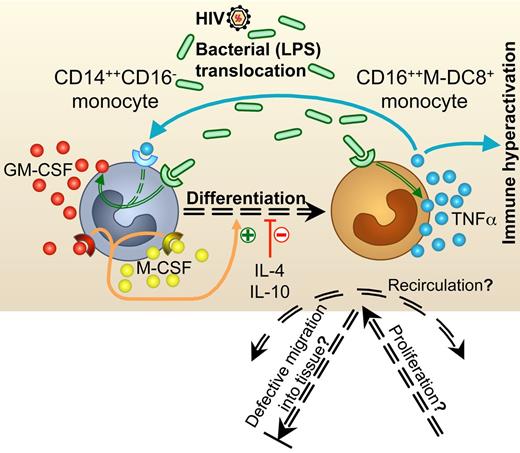
These results point to M-DC8+ CD14+CD16++ monocytes as a specific myeloid cell population that is expanded during chronic HIV infection with active viral replication. Here, using an 11-color flow cytometric strategy, we found high CD16+ monocyte counts in chronically infected patients, who were asymptomatic but viremic, as previously shown in patients with AIDS or AIDS-related dementia20,21,23,32 and in macaques with acute or chronic, viremic SIV infection.22 Furthermore, we pointed to the M-DC8+ subset, which plays a role in several inflammatory diseases,27,29 but had never been found impaired during HIV infection, as the main cell population responsible for this elevation. We found normal counts of these cells in patients whose HIV viral loads were controlled by cART, indicating restoration by treatment, which needs to be confirmed in prospective studies. The untreated patients in this study were all viremic, whereas the cART-treated patients were all virologically suppressed. We strongly feel that viremia, with its consequences on gut dysfunction, rather than the lack of treatment, favors the MDC8+ monocyte number increase, but this remains to be further checked in discordant patients.
One hypothesis (Figure 5) to explain this increase in circulating M-DC8+ monocyte counts would be a defective migration into tissues. This seems unlikely because the proportion of CD16+ monocytes, and especially M-DC8+ monocytes, is also higher in spleens from HIV-infected patients than in uninfected controls (Degrelle, Dutertre et al, manuscript in preparation). M-DC8+ CD14+CD16++ monocytes might home into the spleen and not into other organs, but these cells do infiltrate inflamed tissues in chronic inflammatory diseases,27,29 and CD16+ monocytes (with undocumented M-DC8 expression) infiltrate the brains from patients with AIDS-related dementia.23 A second hypothesis would be a high migration rate and recirculation from one organ to another because of chemokine attraction. The CD16+ monocytes, which infiltrate the brains from AIDS patients, express CX3CR1, and high levels of CX3CL1 are detected in situ in these brains.33-35 In vitro, CD16+ monocytes are indeed attracted by CX3CL1. In addition, high levels of CX3CL1 are induced in vitro in astrocytes after TNFα stimulation. Other cytokines like CCL2 can play a role in transmigration across the blood-brain barrier.36 Further studies are needed to assess chemokine receptor expression levels at the surface of CD14+CD16++M-DC8+ monocytes in HIV-infected patients. A third hypothesis would be a specific proliferation or resistance to apoptosis37 of CD14+CD16++M-DC8+ monocytes during HIV infection. A fourth hypothesis would be a greater differentiation of M-DC8+ cells from another myeloid subset, most probably CD14++CD16− monocytes. Accordingly, we showed here that in the presence of GM-CSF and M-CSF, FACS-sorted primary classical CD14++CD16− monocytes acquired CD16 and M-DC8 expression. This was not the case in the presence of IL-10 or IL-4, in accordance with other authors.38 Indeed, these 2 cytokines rather favor an M2 or DC-like polarization of monocytes in vitro, whereas LPS, TNFα, and GM-CSF cooperate to induce a proinflammatory M1 polarization that is associated with a strong TNFα production by polarized cells.39 Furthermore, activation of the NF-κB pathway, which is mediated by both LPS or TNFα, induces GM-CSF gene expression,12,40 while M-CSF, which is found at high concentrations in healthy human blood,41 is also synergistically induced by GM-CSF and TNFα.42 This differentiation may really have happened in vivo in the viremic patients because of the inverse correlation between classical CD14++CD16− and CD14+CD16++M-DC8+ monocyte counts and the high plasma levels of TNFα and GM-CSF of these patients.
Potential mechanisms underlying the strong increase in CD16++M-DC8+ monocytes that might be a therapeutic target to fight TNFα-mediated chronic inflammation during HIV infection. Chronic immune activation drives the progression of HIV infection and is thought to be one of the prediction parameters of disease outcome. As in Crohn disease, systemic LPS translocation and TNFα overproduction seem to play a major role in this activation. However, the cellular origins of this TNFα overproduction have remained elusive. We demonstrate here that in the blood from viremic patients, CD16++M-DC8+ monocytes are found in higher numbers than in virologically suppressed patients, and that they account for a large part of the TNFα overproduction in response to LPS. The potential mechanisms underlying this increase in proinflammatory CD16++M-DC8+ monocytes may be a defective migration into tissues, a high level of recirculation between organs, a specific proliferation or resistance to apoptosis, or a greater differentiation from another myeloid cell subset. In line with this hypothesis, we show that CD16++M-DC8+ monocytes can arise in vitro from CD14++CD16− classical monocytes in the presence of GM-CSF and M-CSF, and high GM-CSF and TNFα levels, as well as high levels of sCD14, an indicator of LPS translocation, are indeed found in the plasma from viremic patients with high numbers of M-DC8+ monocytes.
Potential mechanisms underlying the strong increase in CD16++M-DC8+ monocytes that might be a therapeutic target to fight TNFα-mediated chronic inflammation during HIV infection. Chronic immune activation drives the progression of HIV infection and is thought to be one of the prediction parameters of disease outcome. As in Crohn disease, systemic LPS translocation and TNFα overproduction seem to play a major role in this activation. However, the cellular origins of this TNFα overproduction have remained elusive. We demonstrate here that in the blood from viremic patients, CD16++M-DC8+ monocytes are found in higher numbers than in virologically suppressed patients, and that they account for a large part of the TNFα overproduction in response to LPS. The potential mechanisms underlying this increase in proinflammatory CD16++M-DC8+ monocytes may be a defective migration into tissues, a high level of recirculation between organs, a specific proliferation or resistance to apoptosis, or a greater differentiation from another myeloid cell subset. In line with this hypothesis, we show that CD16++M-DC8+ monocytes can arise in vitro from CD14++CD16− classical monocytes in the presence of GM-CSF and M-CSF, and high GM-CSF and TNFα levels, as well as high levels of sCD14, an indicator of LPS translocation, are indeed found in the plasma from viremic patients with high numbers of M-DC8+ monocytes.
In this study, we also characterized a major functional consequence of the increase in proinflammatory M-DC8+ monocytes, showing that despite their low numbers, they were responsible for a large part of the overproduction of TNFα in vitro in response to LPS of PBMCs from viremic patients. CD8+ T cells from viremic HIV-infected patients are widely known to produce IFNγ and TNFα in response to stimulation by HIV peptides or other T-cell stimuli.43 Therefore, they probably participate, with NK cells, in TNFα production in vivo, but not directly in response to LPS (as confirmed in our experiments in vitro). TNFα production by myeloid cells may occur when there is bacterial product translocation and then synergize with TNFα production from CD8+ T cells, or act in different compartments.
This is likely to happen in vivo, as plasma TNFα levels were increased in these viremic patients compared with controls, consistently with other studies.3,8,44,45 In AIDS-related dementia, high TNFα levels are also found in the spinal fluid, opening the way for HIV-1 invasion of CD16+ monocytes from blood to brain,46 and cognitive dysfunction correlates with high plasma levels of LPS, sCD14, and TNFRII.32,47 Nonclassical CD14++CD16− monocytes can produce high levels of TNFα in response to LPS.48 In Crohn disease, M-DC8+ cells are found in abundance in inflamed mucosal tissues,27 and they produce large amounts of TNFα, which is a central actor of the intestinal epithelial cell destruction leading to LPS translocation.10 Like in Crohn disease, TNFα-producing M-DC8+ cells in the mucosa from HIV-infected patients may have a major role in the maintenance of chronic immune activation leading to the strong mucosal CD4+ T-lymphocyte depletion.7 Indeed, we found that sCD14 levels, which correlate with microbial translocation and were claimed to be a more reliable measure than LPS levels themselves,7 were significantly higher in patients with very high M-DC8+ cell numbers than in the other viremic patients.
In former studies during HIV infection, mDCs were usually defined as Lin(CD3/CD19/CD14/CD56)−HLA-DR+CD11c+. This includes both BDCA-1+ and BDCA-3+ subsets. Our 11-color flow cytometric strategy made it possible to precisely define mDC subsets by avoiding contamination or exclusion of cells of interest. Indeed, we observed that both subsets expressed lineage markers, BDCA-1+ mDC expressing CD14 and subsets of the 2 mDC subpopulations expressing CD56, particularly BDCA-3+ mDC (data not shown). Thus, we observed lower counts of circulating BDCA-1+ and even more significantly, of BDCA-3+ mDC counts in viremic patients than in controls. A reduction in BDCA-3+ mDC counts has been reported once in HIV-infected patients as data not shown.49 Moreover, these counts were normal in virologically controlled patients, as already found for CD11c mDCs. Longitudinal studies will be needed to really prove whether cART restores BDCA3+ mDC counts. As expected, pDC counts were low in the blood from viremic patients.14,50,51
In summary, during chronic HIV infection with viremia, the 2 types of mDCs and pDCs are depleted in the blood. Concomitantly, we evidence here for the first time that TNFα-producing M-DC8+ monocytes are expanded in the blood from these patients and may have a major role in the maintenance of chronic immune activation leading to AIDS through their overproduction of TNFα in response to LPS.7 This makes HIV infection a particular case of inflammatory disease. In Crohn disease, anti-TNFα antibodies are used successfully to ablate intestinal inflammation, and anti–IL-12p40 are currently under trial. Similar approaches might be useful against the intestinal inflammation which fuels chronic immune activation during HIV infection. However, these antibodies induce a systemic immune suppression, which leads to increased susceptibility to mycobacteria, and this may be even more harmful during HIV infection. Rather than a global cytokine inhibition, targeting the cells that entertain a vicious immune activation cycle during HIV infection would be more specific. Therefore, our findings open the way to new therapeutic avenues using anti–M-DC8 monoclonal antibodies, which by specifically depleting M-DC8+ monocyte/macrophages, could break the vicious circle of chronic immune activation. This treatment would help patients to reach a nonactivated status similar to that of long-term nonprogressor or elite patients, who control HIV replication without antiretroviral treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors greatly acknowledge the Cochin Immunobiology Facility for maintaining the flow cytometers used for analyses and sorting. The authors thank other members of the Antigen Presentation by Dendritic Cell team, Frédéric Geissmann, Rémi Cheynier, Van Truster, and the ANRS AC31 members for discussions, and Marc Dalod and Cédric Auffray for critical reading of the manuscript.
This work was supported by Inserm, Centre National de la Recherche Scientifique, Université Paris Descartes, Université Paris-Diderot, ANRS (fellowship to C-.A.D. and funding), Sidaction (fellowship and funding to A.-S.L.), and Inserm Direction de l'hospitalisation et de l'organisation des soins, Ministère de la Santé et des Sports.
Authorship
Contribution: C.-A.D. and A.H. designed the experiments and wrote the manuscript; C.-A.D., S.A., A.D., J.-P.J., L.V., M.G., S.D., V.F., and A.-S.L. conducted the experiments; C.-A.D. conducted the data analyses; M.-M.T. and Y.R. supervised experiments and edited the manuscript; N.D., C.D., L.M., C.G., P.L., and O.L. procured samples and clinical data and recruited subjects for the human clinical studies; A.H. supervised the project; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Hosmalin, Immunohematology De-partment, Institut Cochin, Bâtiment Gustave Roussy, 27 rue du Faubourg Saint Jacques, 75014 Paris, France; e-mail: anne.hosmalin@inserm.fr.
References
Author notes
S.A. and A.D. contributed equally to this work.


![Figure 3. M-DC8+ monocytes from viremic patients produce greater amounts of TNFα than those from healthy donors and are responsible for a large part of the TNFα overproduction after LPS stimulation. (A-D) After 18-hour stimulation with or without LPS, TNFα concentrations were measured in culture supernatants from (A) total PBMCs (1 × 106 cells/mL; 8 healthy donors and 7 viremic patients), (B) total vs [M-DC8+ cell]–depleted PBMC (5 × 105 cells/mL; 6 healthy donors and 5 viremic patients), (C) FACS-sorted M-DC8+ CD11c+CD14+CD16++ monocytes from 4 healthy donors and 4 viremic patients (2.5 × 105 cells/mL), and (D) monocyte subsets from 1 healthy donor (5 × 105 cells/mL; representative of 3 independent experiments). (E) Plasma TNFα concentrations from 16 healthy donors (HIV−, cART−, open circles), 8 virologically suppressed patients (HIV+, cART+, gray circles) and 15 viremic patients (HIV+, cART−, filled circles). Patients with M-DC8+ monocyte counts > 50/μL as defined in Figure 2B are shown (red circles). (F-H) PBMCs were cultured for 7 hours with or without LPS. (F) Dot plot of 9-color intracellular TNFα FACS analysis from (left panels) 1 representative healthy donor and (right panels) 1 representative viremic patient showing TNFα production in different monocyte (top panels), DC, and lymphocyte populations (bottom panels) after stimulation of PBMCs with LPS. Percentages of TNFα-positive cells among the parent population and MFI of the TNFα-positive population (in brackets) are indicated. (G-H) After LPS stimulation, comparison of 6 healthy donors and 8 viremic patients for (G) the percentages of TNFα-positive cells among different monocyte subsets, and (I) of the MFI of the TNFα-positive populations in the same experiments as panel G. P values calculated using the Mann-Whitney test; bars indicate medians. ND indicates not determined; and Unst, unstimulated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/11/10.1182_blood-2012-03-418681/4/m_zh89991295780003.jpeg?Expires=1767701525&Signature=FkjV7e1Mkat1vbciTY7d7C1Rhwp2Hzj78Z1riR6Nmkej7ovKDsOdDkJ9oE6kGW9daCeRLjAb3oCNv9Tqri651gUtMs5PG7NJRzKYCnXAsXtqmwGcSAjO8gHkS7lRQ7Mm4kbLaMBIIE8OfyvUZTP8aHPlo6oilMpCi9GAC5yor1QnQ0vgB7yqXR~rmPSq-0YteFzgsqzKgwv37DzKa1R5bUAvksA3knd4MhBfQ0u2BbqCwtVBo9dlps~39-dfL4q39pF-RDD39HqbwH8jJ2Rk8wPPg4f7MjmJ6zE1MJ3rFRkZsBwLLwed9QrhvfEItkddjMvlY0LlIX166w-rrypUkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. HIV-1 infection is associated with a greater differentiation of classical CD14++CD16−M-DC8− monocytes into CD16+M-DC8+ cells in vitro. (A) Correlation of the absolute counts of CD14++CD16− classical monocytes with those of nonclassical M-DC8+ or M-DC8− CD14+CD16++ monocytes, and intermediate CD14++CD16+ monocytes for the 15 viremic patients. (B) FACS-sorted classical CD14++CD16− monocytes from a healthy donor were analyzed for CD16 and M-DC8 expression before or after culture for 4 days with GM-CSF and M-CSF. (Bottom panel) Percentages of M-DC8+ cells obtained from 3 healthy (open circles) and 2 viremic patients (filled circles). (C) M-DC8 and CD1a expression after 4-day culture of FACS-sorted CD14++CD16− classical monocytes from 1 viremic patient (representative of 3 independent experiments) cultured as in panel B the presence or not of IL-4 or IL-10 are shown. (D-F) GM-CSF concentrations were measured (D) in the plasma from 16 healthy donors (HIV−, cART−), 8 virologically suppressed patients (HIV+, cART+), or 15 viremic patients (HIV+, cART−), or (E-F) in culture supernatants from 18-hour LPS-stimulated or unstimulated (E) PBMCs (8 healthy donors [open circles] and 7 viremic patients [filled circles]), and (F) from FACS-sorted CD14++CD16− classical (black) or M-DC8+ CD14+CD16++ (gray) monocytes (4 healthy donors [open circles] and 3 viremic patients [filled circles]). When indicated, P values were calculated using the Mann-Whitney test; bars indicate medians.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/11/10.1182_blood-2012-03-418681/4/m_zh89991295780004.jpeg?Expires=1767701525&Signature=NoYN3dY2jWUpHC458WbnCUismJ9yq1FDhgSBBnusDHVEUTrWvbQC3Sa2QRybJREhrUSEc2-GISn1VgiJADuQTFKQTpnu~CoWoIvCCrhKqe--tS4w1MiayFr66X0pMHnCku092OVX5h0es7pEDJ3jS-nHH8PUgE9l2rpMi0PVnb2cp4YfseXWQO~YVHwQHKMwvPhIXwurq4MZPcz8ktO6GC5gaytdZyq9A5qpGq8c69B61ux2qYxjCxSJAo8vM9DOksXnioivwgh7fpDPa-aSuC4k6~9VaE9uWpudCChj1GfdG7roAOpQ6Yu6iPrOjTmq3J245zYqf0nb5ytu99riMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal