Abstract
Human peripheral Vγ9Vδ2 T cells are activated by phosphorylated metabolites (phosphoagonists [PAg]) of the mammalian mevalonate or the microbial desoxyxylulose-phosphate pathways accumulated by infected or metabolically distressed cells. The underlying mechanisms are unknown. We show that treatment of nonsusceptible target cells with antibody 20.1 against CD277, a member of the extended B7 superfamily related to butyrophilin, mimics PAg-induced Vγ9Vδ2 T-cell activation and that the Vγ9Vδ2 T-cell receptor is implicated in this effect. Vγ9Vδ2 T-cell activation can be abrogated by exposing susceptible cells (tumor and mycobacteria-infected cells, or aminobisphosphonate-treated cells with up-regulated PAg levels) to antibody 103.2 against CD277. CD277 knockdown and domain-shuffling approaches confirm the key implication of the CD277 isoform BTN3A1 in PAg sensing by Vγ9Vδ2 T cells. Fluorescence recovery after photobleaching (FRAP) experiments support a causal link between intracellular PAg accumulation, decreased BTN3A1 membrane mobility, and ensuing Vγ9Vδ2 T-cell activation. This study demonstrates a novel role played by B7-like molecules in human γδ T-cell antigenic activation and paves the way for new strategies to improve the efficiency of immunotherapies using Vγ9Vδ2 T cells.
Introduction
γδ T cells are key players in the immune surveillance of cellular distress, thanks to their ability to recognize conserved determinants up-regulated after inflammation, infection, or cell transformation.1,2 Although γδ T-cell receptors (TCRs) contribute to detection of danger-associated determinants, ligands for these receptors have been identified in a few cases only.3 Thus, the antigenic specificity of γδ T cells and their fine activation modalities in response to cell stress remain largely unknown.
One of the best studied γδ T-cell subsets in humans expresses Vγ9Vδ2 TCR and predominates in blood, composing several percent of the whole peripheral lymphoid pool in most adults. Vγ9Vδ2 T cells are activated by nonpeptidic phosphorylated isoprenoid pathway metabolites,4-6 hereafter referred to as phosphoagonists (PAg). Natural Vγ9Vδ2-stimulating PAg include isopentenyl pyrophosphate (IPP),7 a metabolite of the mevalonate pathway found in mammalian cells and the desoxyxylulose phosphate pathway shared by many microorganisms, and hydroxy-methyl-butyl-pyrophosphate,8 an intermediate of the latter pathway. PAg detection by γδ T cells underlies their broad reactivity toward infected and transformed cells. Indeed, tumor cell recognition by Vγ9Vδ2 T cells is linked to enhanced production of the weak agonist IPP, resulting from increased cell metabolism and cholesterol biosynthesis. Accordingly, pharmacologic inhibitors of the mevalonate pathway that up-regulate (eg, aminobisphosphonates, NBP) or down-regulate (eg, statins) IPP production, respectively, increase or decrease antitumor Vγ9Vδ2 T-cell responses.9,10 Moreover, because of the high Vγ9Vδ2 T cell-stimulating activity of the microbial agonist hydroxy-methyl-butyl-pyrophosphate, Vγ9Vδ2 T-cell responses are elicited by infected cells producing even traces of this PAg.8
Although PAg-induced activation is restricted to Vγ9Vδ2 T cells and can be conferred by Vγ9Vδ2 TCR gene transfer,11,12 attempts to detect cognate interactions between PAg and Vγ9Vδ2 TCR have failed so far.13 So how Vγ9Vδ2 T cells sense PAg remains an enigma. PAg rapidly induce Ca2+ signaling and activation of Vγ9Vδ2 T-cell clones, but this requires cell-to-cell contact, suggesting the implication of additional target cell surface receptors in this phenomenon.11,14 PAg elicit Vγ9Vδ2 T-cell responses against basically all human cells, irrespective of their tissue origin, but do not induce recognition of any murine target cells. Therefore, the putative target cell receptors involved in PAg-mediated T-cell activation are expected to be broadly expressed by human, but not murine, cells.
Activation of antigen-stimulated T cells is tuned by interactions involving T cell-derived CD28-related receptors and target cell-derived B7-related counter-receptors,15 which family includes members, such as Skint and butyrophilin (BTN) receptors. The mandatory role played by Skint-1 in the intrathymic positive selection and functional maturation of the murine intraepidermal Vγ5Vδ1 T-cell subset raises questions about the general role played by Skint1-related receptors in the selection and activation of γδ T cells in mice and other species.16-19 Although there are 10 intact skint1 paralogs in the mouse, the only human skint1 ortholog is a pseudogene. The human proteins with the greatest similarity to the extracellular domain of murine Skint-1 are the BTNs. BTNs are type I membrane proteins with 2 extracellular Ig domains frequently linked to an intracellular B30.2 (PRY/SPRY) domain, and encompass 3 gene subfamilies in humans: btn1, btn2, and btn3. Some BTN receptors exert nonimmunologic functions (eg, milk fat globule formation for BTN1A1). Others, in line with their homology with several B7-related coinhibitory receptors, can up- or down-modulate TCR-induced T-cell activation and contribute to dampening of inflammatory responses.17,20,21 This can result from either direct engagement of BTN receptors expressed on responding T cells by target cell ligands, or interactions involving target cell BTN molecules and responding T cell-derived ligands.22-25
In this study, we provide evidence that ubiquitous human CD277/BTN3A plays a key role in PAg-induced activation of Vγ9Vδ2 T cells in both tumor and infectious contexts and that CD277-dependent activation is conferred by Vγ9Vδ2 TCR. Our results suggest that intracellular PAg accumulation is associated with membrane reorganization of CD277 molecules, which in turn leads to Vγ9Vδ2 T-cell activation, and bring new insights into the mechanisms linking metabolic and infectious distress and human γδ T-cell activation. We also describe agonist and blocking CD277-specific antibodies that could be used for the immunotherapeutic modulation of Vγ9Vδ2 T-cell responses toward tumor or infected cells.
Methods
Monoclonal antibodies and reagents
The following monoclonal antibodies were from Beckman Coulter: phycoerythrin–cyanin 5.1–anti-TCRγδ (#IMMU510) and anti-CD69 (#TP1.55.3), FITC–anti-Vδ2 (#IMMU389), phycoerythrin–cyanin 7– and purified anti-CD3ϵ (#UCHT1). The following monoclonal antibodies were from BD Biosciences: mouse IgG1, allophycocyanin-TCRγδ (#B1), allophycocyanin-H7 (analog of cyanin 7)–anti-CD3 (#SK7), FITC- or phycoerythrin–cyanin 5.1–CD107a (#H4A3), anti-CD107b (#H4B4). Fluorescein isothiocyanate– or phycoerythrin–anti–IFN-γ (#B27) and phycoerythrin- or allophycocyanin–anti–TNF-α (MAb11) were used for intracellular stainings. Purified anti-CD3 (#OKT3) was kindly provided by H. Vié (Inserm U892, Nantes, France). Alexa-647–labeled goat anti–mouse IgG and anti-IgG1 were from Invitrogen. Mouse anti-CD277 mAbs (clones 7.2, 20.1, and 103.2) were generated, produced, and purified as described.26 Synthetic bromohydrin pyrophosphate (BrHPP) was kindly provided by Innate Pharma. Pamidronate was obtained from Mayne Pharma. Monensin A, phorbol-12-myristate-13-acetate (PMA), ionomycin, leucoagglutinin, mevastatin, and sec-butylamine were purchased from Sigma-Aldrich. Guinea pig myelin basic protein was kindly provided by R. Dörries (Institut für Medizinische Mikrobiologie und Hygiene, Mannheim, Germany). Live/Dead, Lipofectamine, and hygromycin were from Invitrogen. Recombinant human IL-2 (rhIL-2) was from Chiron Therapeutics. Green fluorescent protein strain of Mycobacterium bovis bacillus Calmette-Guérin (GFP-BCG) was provided by F. Altare (Inserm U892, Nantes, France).
Cells
PBMCs of human healthy donors were isolated from blood samples obtained from the Etablissement Français du Sang. Human Vγ9Vδ2 (clone GR4, polyclonal lines GUI and AL), Vγ8Vδ3 (clone 73R9), iNKT (polyclonal line MAD11), and αβ CD8+ (HCV-1/A2–restricted clone 13) T cells were cultured in complete RPMI 1640 medium (10% FCS, 2mM l-glutamine, 10 μg/mL streptomycin, 100 IU/mL penicillin, and 300 IU/mL rhIL-2) as described.35 Human polyclonal αβ T cells expressing the Vγ9Vδ2 G115 TCR after retroviral transduction were generated as described.27 Human Jurkat T cells (J.RT3-T3.5, β-chain-deficient variant) expressing either Vγ9Vδ2 (from clone G115) or Vγ8Vδ3 (from clone 73R9) TCR were generated as described.28 58C-CD28+ are αβ TCR− 58C mouse T hybridoma cells transduced for rat/mouse CD28 expression.29,30 53/4-CD28 cells are 53/4 cells (a sister clone of the rat/mouse T-cell hybridoma 35/2), which express a rat αβ TCR recognizing a guinea pig MBP68-88 peptide restricted to RT1BI (rat MHC class II) and are also transduced for rat/mouse CD28 expression.29 The 2Aγδ TCR MOP is a 2A peptide-linked Vγ9Vδ2 TCR cloned into either pczCFG5IEGN- or pczCFG5IH MuLV-based retroviral expression vectors31 and transduced into either 58C-CD28+ or in 53/4-CD28+ T-cell hybridomas. RajiRT1Bl are Raji cells transduced with RT1Bl.30 The following human cell lines were kept in complete RPMI 1640 or DMEM: Epstein-Barr virus+ and 721.221 B-lymphoblastoid cell lines; C91 T2.2 (T-cell leukemia); Daudi and Raji (Burkitt lymphoma); RPMI 8226 (myeloma); THP-1 (acute monocytic leukemia); HeLa (cervix adenocarcinoma); SVK14 (simian virus-40-transformed keratinocytes); HEK293FT (embryonic kidney); Med (breast cancer); MG63, HOS, and U2OS (osteosarcoma), provided by D. Heymann (Inserm U957, Nantes, France); PC3 (prostate cancer); HMEC-1 (endothelial cells), provided by F. Paris (Inserm U892, Nantes, France); M88 and M6 (melanoma) provided by N. Labarrière (Inserm U892, Nantes, France); and meso 45, meso 152 and meso 134 (mesothelioma), provided by M. Grégoire (Inserm U892, Nantes, France).
Functional assays
Target cells were pretreated for the indicated time periods with either CD277-specific mAbs or NBP (pamidronate) at the indicated concentrations. Treated target cells were extensively washed and cocultured together with effector T cells at 37°C in complete RPMI 1640 medium. T cells were also activated by either soluble PAg (BrHPP), at the indicated concentrations, or PMA and ionomycin (used, respectively, at 1μM and 0.5 μg/mL). T cells were activated at 37°C in the presence of 10μM monensin and fluorochrome-labeled CD107a- and CD107b-specific mAbs, used alone or in combination. After 4 hours, T cells were harvested and stained with fluorochrome-labeled TCR-specific mAbs and a viability marker (Live/Dead cell marker, 0.3μM). When indicated, intracellular stainings of IFN-γ and TNF-α were performed within the same samples. Surface expression of CD69 was measured by flow cytometry after activation for either 4 hours (Jurkat) or 7 hours (PBMCs) at 37°C in complete RPMI 1640 medium. Data were collected on FACSCantoII, LSR or FacsCalibur cytometers (BD Biosciences) and analyzed with FlowJo software Version 7 (TreeStar). TNF-α concentration was assessed by a biologic assay based on WEHI164 clone 13 cell viability as described.32 ELISpot for IFN-γ was performed as described.27 When indicated, the cytolytic activity of human γδ T cells was also measured in standard 4-hour 51Cr release assays. Stimulation of 2Aγδ TCR MOP+ 58C-CD28 or 2Aγδ TCR MOP+ 53/4-CD28 murine hybridoma cells was performed similarly as previously described.30,31
Expression and knockdown of BTN3 isoforms in HEK293FT cells
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Microscopy
See supplemental Methods.
Statistical analysis
The statistical significance of differences between populations was assessed with 2-tailed Student t, 2-way ANOVA, or Mann-Whitney tests. P values less than .05 were considered significant.
Results
Broad activation of human Vγ9Vδ2 T cells by anti-CD277 antibodies
While screening mouse mAbs raised against isoforms of the human BTN3 (CD277) subfamily,26 we noticed that 1 mAb (clone 20.1) elicited dramatic expansion and proliferation of γδ T cells in IL-2–supplemented PBMC cultures (Figure 1A; supplemental Figure 1A). Accordingly, soluble 20.1 mAb induced: ex vivo production of IFN-γ and TNF-α by γδ T cells (Figure 1B), rapid CD69 up-regulation and IFN-γ production by most Vδ2+-γδ PBL ex vivo (Figure 1C; supplemental Figure 1B), and degranulation and cytokine responses of Vγ9Vδ2 T-cell clones specifically (Figure 1D; data not shown). In agreement with recent studies,23,25 soluble 20.1 mAb had no stimulatory effect on αβ T cells. Moreover, it had no effect on Vδ2−-γδ PBL (Figure 1C) or T-cell clones (Figure 1D). Early activation events induced in Vγ9Vδ2 T cells by 20.1 mAb were studied by videomicroscopy. Although 20.1 mAb had no effect on the intracellular Ca2+ levels of αβ T cells, it induced a progressive increase of Ca2+ signaling in Vγ9Vδ2 T-cell clones, which became significant as of 10 minutes after mAb addition (Figure 1E). This unusual Ca2+ signaling pattern, in stark contrast with the sharp increase of Ca2+ signals induced after γδ or αβ TCR crosslinking by anti-CD3 mAb, or after antigenic stimulation of αβ T cells, is similar to the delayed and progressive increase of Ca2+ signals previously described for Vγ9Vδ2 T cells stimulated by PAg.33
Broad activation of human Vγ9Vδ2 T cells by anti-CD277 mAbs. (A) Frequency of human γδ T cells in IL-2–supplemented ex vivo PBMCs after a 2 week-incubation in the presence of anti-CD277 mAb (#20.1; 10 μg/mL). The values for the percentage of γδ T cells within PBLs are indicated. No stim. indicates no stimulation. (B) Intracellular stainings of IFN-γ and TNF-α in ex vivo human PBL-γδ T cells after incubation for 5 hours with anti-CD277 mAb (#20.1; 10 μg/mL). Numbers inside or adjacent to outlined areas indicate the values for the percentage of IFN-γ+ (top row) or TNF-α+ (bottom row) cells within γδ− and γδ+ PBL subsets. (C) Expression of CD69 on ex vivo human PBL subsets after incubation for 7 hours with either anti-CD277 mAb (#20.1; 10 μg/mL) or soluble PAg (BrHPP; 3μM). MFI indicates geometric mean of fluorescence intensity. Data in graph are mean ± SD (n = 3 healthy donors). *P < .05 (Student t test). **P < .005 (Student t test). (D) Stainings of CD107a/b (left), IFN-γ (middle), and TNF-α (right) of Vγ9Vδ2 (clone GR4), Vγ8Vδ3 (clone 73R9), CD8+ αβ (clone 13), and invariant NK (iNKT, line MAD11) human T cells after treatment with anti-CD277 mAb (#20.1; 10 μg/mL, 5 hours). PMA/Iono. indicates nonspecific activation induced by PMA and ionomycin. (A-B,D) Data are representative of at least 3 experiments. (E) Intracellular Ca2+ levels were measured by videomicroscopy within clusters of Fura-2 AM-loaded polyclonal human Vγ9Vδ2 (γδ) T-cell lines after addition (t = 0 minutes) of anti-CD277 mAb (#20.1; 10 μg/mL) and compared with CD8+ αβ (αβ) or isolated γδ T cells. IgG1 indicates isotype control. Values correspond to the mean of emissions (340/380 nm ratio) measured among all T cells present in the field. γδ, n = 70; αβ, n = 30; isolated γδ, n = 30; and IgG1, n = 30.
Broad activation of human Vγ9Vδ2 T cells by anti-CD277 mAbs. (A) Frequency of human γδ T cells in IL-2–supplemented ex vivo PBMCs after a 2 week-incubation in the presence of anti-CD277 mAb (#20.1; 10 μg/mL). The values for the percentage of γδ T cells within PBLs are indicated. No stim. indicates no stimulation. (B) Intracellular stainings of IFN-γ and TNF-α in ex vivo human PBL-γδ T cells after incubation for 5 hours with anti-CD277 mAb (#20.1; 10 μg/mL). Numbers inside or adjacent to outlined areas indicate the values for the percentage of IFN-γ+ (top row) or TNF-α+ (bottom row) cells within γδ− and γδ+ PBL subsets. (C) Expression of CD69 on ex vivo human PBL subsets after incubation for 7 hours with either anti-CD277 mAb (#20.1; 10 μg/mL) or soluble PAg (BrHPP; 3μM). MFI indicates geometric mean of fluorescence intensity. Data in graph are mean ± SD (n = 3 healthy donors). *P < .05 (Student t test). **P < .005 (Student t test). (D) Stainings of CD107a/b (left), IFN-γ (middle), and TNF-α (right) of Vγ9Vδ2 (clone GR4), Vγ8Vδ3 (clone 73R9), CD8+ αβ (clone 13), and invariant NK (iNKT, line MAD11) human T cells after treatment with anti-CD277 mAb (#20.1; 10 μg/mL, 5 hours). PMA/Iono. indicates nonspecific activation induced by PMA and ionomycin. (A-B,D) Data are representative of at least 3 experiments. (E) Intracellular Ca2+ levels were measured by videomicroscopy within clusters of Fura-2 AM-loaded polyclonal human Vγ9Vδ2 (γδ) T-cell lines after addition (t = 0 minutes) of anti-CD277 mAb (#20.1; 10 μg/mL) and compared with CD8+ αβ (αβ) or isolated γδ T cells. IgG1 indicates isotype control. Values correspond to the mean of emissions (340/380 nm ratio) measured among all T cells present in the field. γδ, n = 70; αβ, n = 30; isolated γδ, n = 30; and IgG1, n = 30.
Agonist anti-CD277 antibodies sensitize CD277+ target cells to Vγ9Vδ2 T-cell recognition
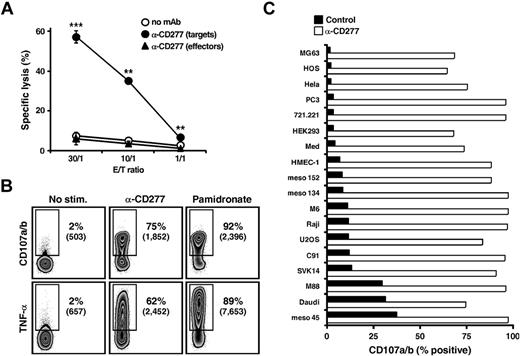
When immobilized onto plastic, anti-CD277 mAbs can induce costimulatory signals in TCR-stimulated αβ T cells, through direct engagement of CD277 molecules.23,25 They can also modulate T-cell costimulatory or coinhibitory signals through interactions with target cell-expressed CD277.24,34 Thus, although anti-CD277 mAb readily induced Vγ9Vδ2 T-cell activation in the absence of third-party cells, this could be the result of either a “direct” or “cis” effect of the Ab (CD277-induced signaling in responder Vγ9Vδ2 T cells) or an “indirect” or “trans” effect (enhanced reactivity of Vγ9Vδ2 T cells toward anti-CD277–coated T cells). Two observations argue against a direct effect of the 20.1 mAb: mAb immobilized on FcRI+ murine P815 cells fails to activate Vγ9Vδ2 T cells (supplemental Figure 2) and mAb-treated Vγ9Vδ2 T cells maintained in the absence of cell-to-cell contacts do not become activated (Figure 1E). Evidence for an indirect effect was obtained by comparing the effect of 20.1 mAb pretreatment of either effector or target cells in cytotoxicity assays. Pretreatment of the Vγ9Vδ2-resistant Raji lymphoma target cells with the 20.1 mAb (and other anti-CD277 mAbs, such as the clone 7.2) enhanced tumor cell killing by Vγ9Vδ2 T cells, whereas pretreatment of effector Vγ9Vδ2 T-cell lines had no such effect (Figure 2A; supplemental Figure 3A). Importantly, the lack of effect of effector cell pretreatment with 20.1 mAb on target lysis remained compatible with the ability of this mAb to readily activate effector cells alone (Figure 1). Indeed, because target, but not effector, cells were 51Cr-labeled, this did not allow assessment of effector cell fratricide, degranulation, or cytokines responses, which are presumably induced on effector cell preincubation. Raji target cell preincubation with 20.1 mAb also induced Vγ9Vδ2 T-cell degranulation and TNF-α cytokine response to levels reached after target cell pretreatment with NBP (Figure 2B). In line with the lack of effect of 20.1 mAb on αβ T-cell proliferation or cytokine responses, this mAb did not enhance the cytolytic response of human CD8+ αβ cytotoxic T-cell clones toward Ag-loaded target cells pretreated with anti-CD277 mAb (data not shown). Taken together, these results indicate that anti-CD277 mAbs act indirectly (ie, through sensitization of human CD277+ target cells to Vγ9Vδ2 T-cell recognition). Importantly, enhancement of Vγ9Vδ2 T-cell responses by 20.1 mAb also occurs for a wide array of CD277+ human cell lines, irrespective of their tissue origin and basal susceptibility to Vγ9Vδ2 T-cell recognition (Figure 2C; supplemental Figure 3B). This effect was not the result of mAb-redirected killing or mAb-dependent cell cytotoxicity, as it did not depend on CD16, CD32, or CD64 Fc-receptors expression on target or effector cells (supplemental Figure 3C).
Anti-CD277 mAb induces strong Vγ9Vδ2 T cell cytolytic and cytokine responses. (A) Cytolytic activity of human Vγ9Vδ2 T cells (clone GR4) against Raji Burkitt lymphoma cells. Raji (targets) or Vγ9Vδ2 T (effectors) cells were treated for 2 hours with anti-CD277 mAb (#20.1; 10 μg/mL), washed, and cocultured at the indicated γδ T cell-to-target (E:T) ratios. Data are mean ± SD of triplicate measurements. **P < .005. ***P < .0005. (B) Stainings of CD107a/b (top row; surface) and IFN-γ (bottom row; intracellular) of Vγ9Vδ2 T cells (clone GR4) after coculture with Raji cells, pretreated for 2 hours with either anti-CD277 mAb (#20.1; 10 μg/mL) or NBP (pamidronate; 250μM). Numbers adjacent to outlined areas indicate percent and geometric mean of fluorescence intensity (numbers in brackets) of CD107a/b+ and TNF-α+ T cells. (C) Expression of CD107a/b on Vγ9Vδ2 T cells (clone GR4) after coculture with human tumor/transformed cell lines (n = 18), pretreated for 2 hours with anti-CD277 mAb (#20.1; 10 μg/mL). The values for the percentage of CD107a/b+ Vγ9Vδ2 T cells are represented in the graph. Control indicates no antibody. Data are representative of at least 3 independent experiments.
Anti-CD277 mAb induces strong Vγ9Vδ2 T cell cytolytic and cytokine responses. (A) Cytolytic activity of human Vγ9Vδ2 T cells (clone GR4) against Raji Burkitt lymphoma cells. Raji (targets) or Vγ9Vδ2 T (effectors) cells were treated for 2 hours with anti-CD277 mAb (#20.1; 10 μg/mL), washed, and cocultured at the indicated γδ T cell-to-target (E:T) ratios. Data are mean ± SD of triplicate measurements. **P < .005. ***P < .0005. (B) Stainings of CD107a/b (top row; surface) and IFN-γ (bottom row; intracellular) of Vγ9Vδ2 T cells (clone GR4) after coculture with Raji cells, pretreated for 2 hours with either anti-CD277 mAb (#20.1; 10 μg/mL) or NBP (pamidronate; 250μM). Numbers adjacent to outlined areas indicate percent and geometric mean of fluorescence intensity (numbers in brackets) of CD107a/b+ and TNF-α+ T cells. (C) Expression of CD107a/b on Vγ9Vδ2 T cells (clone GR4) after coculture with human tumor/transformed cell lines (n = 18), pretreated for 2 hours with anti-CD277 mAb (#20.1; 10 μg/mL). The values for the percentage of CD107a/b+ Vγ9Vδ2 T cells are represented in the graph. Control indicates no antibody. Data are representative of at least 3 independent experiments.
Implication of the Vγ9Vδ2 TCR in anti-CD277 mAb-induced activation
The broad stimulatory effects of anti-CD277 mAbs are limited to Vγ9Vδ2 T cells, which suggests involvement of the Vγ9Vδ2 TCR. 20.1 mAb-treatment induced co-recruitment of target cell-derived CD277 and effector cell-derived CD3 molecules at the target/γδ T cell interface, as shown by a confocal microscopy study (Figure 3A). To assess the Vγ9Vδ2 TCR dependency of this activation more directly, we studied the responses of human and mouse T-cell lines transduced with human γδ TCRs. Unlike their nontransduced or Vγ8Vδ3+ counterparts,28 Vγ9Vδ2+ human Jurkat T lymphoma cells were readily activated by 20.1 mAb (Figure 3B). Similarly, Vγ9Vδ2 TCR gene transfer into nontransformed human CD4+ αβ T cells27 conferred T-cell responsiveness to 20.1 mAb- and NBP-pretreated human target cells (Figure 3C). We also tested the reactivity of murine T-cell hybridomas transduced with a human Vγ9Vδ2 TCR derived from a PAg-reactive γδ T-cell clone. Human Raji cells treated with 20.1 mAb, but not cells treated with control IgG, triggered IL-2 responses of both Vγ9Vδ2 TCR murine transductants (Figure 3D-E). Control αβ TCR-expressing cells did not respond to Raji cells, whether mAb treated or not (not shown). We conclude that Vγ9Vδ2 TCR expression is necessary for recognition of anti-CD277 mAb-treated target cells. Moreover, recognition of 20.1 mAb-treated cells by murine Vγ9Vδ2 transductants, which are CD277−, formally rules out a direct effect of 20.1 mAb on Vγ9Vδ2 T-cell activation.
Vγ9Vδ2 TCR implication in anti-CD277 mAb-induced activation. (A) Confocal microscopy. CD3ε (red) and CD277 (green) distribution in conjugates of Vγ9Vδ2 T cells (line AL) and HEK293 cells expressing EmGFP tagged-CD277 molecules. HEK293 cells were pretreated for 2 hours with anti-CD277 mAb (20.1; 10 μg/mL) and washed before coculture with Vγ9Vδ2 T cells. Composite pictures showing overlays of both colors and bright-field pictures. Confocal images of representative γδ T-cell–HEK293 conjugates are shown. Bars represent 10 μm. (−) indicates untreated cells. One experiment representative of 3 is shown. (B) Surface expression of CD69 on human Jurkat T-cell γδ TCR transductants (human Vγ8Vδ3 or Vγ9Vδ2 TCRs) after incubation for 4 hours with either anti-CD3 (OKT3; 10 μg/mL) or anti-CD277 (20.1; 10 μg/mL) mAbs. WT indicates wild-type Jurkat cells with no TCR expression. The values for the MFI of stained cells are represented in the graph. **P < .005 (Student t test). ***P < .0005 (Student t test). Data are representative of 3 independent experiments. (C) IFN-γ response in PBL-derived human αβ T cells expressing a human Vγ9Vδ2 TCR after incubation with NBP (pamidronate)- or anti-CD277 (20.1; 10 μg/mL) mAb-treated Daudi cells. Production of IFN-γ was determined via ELIspot analysis. Data are mean ± SEM. **P < .005 (2-way ANOVA). ***P < .0005 (2-way ANOVA). Comparable results were obtained in 3 independent experiments. (D-E) IL-2 release by murine T-cell hybridomas, transduced for the expression of a human Vγ9Vδ2 TCR, after coculture with human Raji cells in the presence of anti-CD277 mAb (20.1). (D) IL-2 release by mouse γδTCR58C-CD28+ hydridoma cells. IgG indicates isotype control. Data are representative of 4 independent experiments. *P < .05 (paired Student t test and Mann-Whitney test). (E) IL-2 release by γδTCR53/4-CD28+ (αβ TCR− variant) rat/mouse T-cell hybridoma in the presence of Raji cells pretreated with grading doses of anti-CD277 mAb (20.1). Data are mean ± SEM and are representative of 3 independent experiments. *P < .05 (paired Student t test). **P < .005 (paired Student t test). IL-2 production in both panels is presented relative to those of cells cocultured with Raji cells in the absence of antibody with (100%) or without 3mM sec-butylamine (0%).
Vγ9Vδ2 TCR implication in anti-CD277 mAb-induced activation. (A) Confocal microscopy. CD3ε (red) and CD277 (green) distribution in conjugates of Vγ9Vδ2 T cells (line AL) and HEK293 cells expressing EmGFP tagged-CD277 molecules. HEK293 cells were pretreated for 2 hours with anti-CD277 mAb (20.1; 10 μg/mL) and washed before coculture with Vγ9Vδ2 T cells. Composite pictures showing overlays of both colors and bright-field pictures. Confocal images of representative γδ T-cell–HEK293 conjugates are shown. Bars represent 10 μm. (−) indicates untreated cells. One experiment representative of 3 is shown. (B) Surface expression of CD69 on human Jurkat T-cell γδ TCR transductants (human Vγ8Vδ3 or Vγ9Vδ2 TCRs) after incubation for 4 hours with either anti-CD3 (OKT3; 10 μg/mL) or anti-CD277 (20.1; 10 μg/mL) mAbs. WT indicates wild-type Jurkat cells with no TCR expression. The values for the MFI of stained cells are represented in the graph. **P < .005 (Student t test). ***P < .0005 (Student t test). Data are representative of 3 independent experiments. (C) IFN-γ response in PBL-derived human αβ T cells expressing a human Vγ9Vδ2 TCR after incubation with NBP (pamidronate)- or anti-CD277 (20.1; 10 μg/mL) mAb-treated Daudi cells. Production of IFN-γ was determined via ELIspot analysis. Data are mean ± SEM. **P < .005 (2-way ANOVA). ***P < .0005 (2-way ANOVA). Comparable results were obtained in 3 independent experiments. (D-E) IL-2 release by murine T-cell hybridomas, transduced for the expression of a human Vγ9Vδ2 TCR, after coculture with human Raji cells in the presence of anti-CD277 mAb (20.1). (D) IL-2 release by mouse γδTCR58C-CD28+ hydridoma cells. IgG indicates isotype control. Data are representative of 4 independent experiments. *P < .05 (paired Student t test and Mann-Whitney test). (E) IL-2 release by γδTCR53/4-CD28+ (αβ TCR− variant) rat/mouse T-cell hybridoma in the presence of Raji cells pretreated with grading doses of anti-CD277 mAb (20.1). Data are mean ± SEM and are representative of 3 independent experiments. *P < .05 (paired Student t test). **P < .005 (paired Student t test). IL-2 production in both panels is presented relative to those of cells cocultured with Raji cells in the absence of antibody with (100%) or without 3mM sec-butylamine (0%).
Although PAg-reactive Vγ9Vδ2 T cells express TCR with extensive junctional diversity, they share several highly conserved junctional motifs, such as small hydrophobic residues at position δ97,35 which are key for PAg responsiveness.36 Mutation of these residues affected responses of Jurkat Vγ9Vδ2 TCR transfectants to both NBP- and anti-CD277–treated cells, which suggests close similarities between both activation processes (supplemental Figure 4A; data not shown). This observation led us to assess by mass spectrometric analysis the levels of 2 major Vγ9Vδ2-stimulating PAg (IPP and its adenosine triphosphate isopentenol [ApppI] derivative)37 in cells treated with NBP or 20.1 mAb. High levels of both IPP and ApppI were detected in Raji and B-lymphoblastoid cell lines target cells treated with NBP, but not in cells treated with 20.1 mAb, although both treatments led to strong Vγ9Vδ2 T-cell activation (supplemental Figure 4B-D). Therefore, although anti-CD277 mAbs and PAg stimulate Vγ9Vδ2 T cells, up-regulation of production and/or accumulation of PAg in target cells by anti-CD277 mAb seems unlikely. This assumption is also supported by the lack of any inhibitory effect of statins, which block endogenous PAg production, on Vγ9Vδ2 T-cell recognition of anti-CD277–treated target cells (not shown).
Abrogation of Vγ9Vδ2 T-cell responses by blocking anti-CD277 mAbs and CD277 expression knockdown
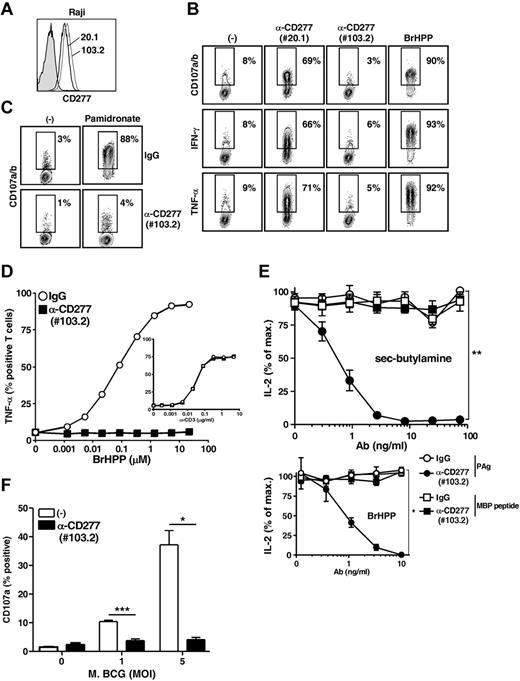
Further insight into the mechanisms underlying CD277-dependent activation of γδ T cells was gained from analysis of additional anti-CD277 mAbs. Among the various clones that were tested, 1 clone (103.2) efficiently stained CD277+ cells, such as Raji cells (Figure 4A), but did not sensitize them to Vγ9Vδ2 T-cell recognition (Figure 4B middle right panels). Indeed, this mAb strongly inhibited Vγ9Vδ2 T-cell degranulation (Figure 4C), cytokine (Figure 4D left panel) and Ca2+ responses (data not shown) induced by NBP- and PAg-treated cells. Importantly, 103.2 mAb did not modulate anti-CD3–induced activation of Vγ9Vδ2 T cells (Figure 4D inset). Moreover, unlike a recently described inhibitory anti-CD277 mAb,23 103.2 mAb did not affect antigenic activation of CD8+ αβ T-cell clones (supplemental Figure 5A). Preincubation studies indicated that, like their stimulating counterparts, blocking CD277-specific mAb acted on target rather than on effector cells (supplemental Figure 5B). The indirect and specific effect of 103.2 mAb on Vγ9Vδ2 T-cell responses was more formally documented using CD277− mouse T-cell hybridomas coexpressing both Vγ9Vδ2 and MBP-specific αβ TCRs. The 103.2 mAb inhibited responses of this transductant to human target cells with PAg accumulation, but not to cells treated with the MBP peptide (Figure 4E). Screening of Vγ9Vδ2 T-cell reactivity against a large array of human tumor cells previously identified several tumor cell lines readily recognized by Vγ9Vδ2 T cells (like Daudi or RPMI 8226 tumor cells). Recognition of these Vγ9Vδ2-susceptible cell lines was strongly inhibited by the blocking mAb 103.2 mAb (supplemental Figure 6). Vγ9Vδ2 T cells have been reported to be activated by mycobacteria-infected dendritic cells or macrophagic cell lines in vitro.38 In this regard, 103.2 mAb abrogated Vγ9Vδ2 T-cell responses induced by THP-1 cells infected by Mycobacterium bovis BCG (Figure 4F).
Abrogation of Vγ9Vδ2 T-cell responses by a blocking anti-CD277 mAb. (A) Surface stainings of Raji cells with either 20.1 and 103.2 anti-CD277 mAbs. Filled histogram represents IgG isotype control. (B) Stainings of CD107a/b (top), IFN-γ (middle), and TNF-α (bottom) of Vγ9Vδ2 T cells (clone GR4) after coculture with Raji cells pretreated for 2 hours with either 20.1 or 103.2 anti-CD277 mAbs (10 μg/mL). Numbers adjacent to outlined areas indicate the percentage of CD107a/b+, IFN-γ+, and TNF-α+ γδ T cells. Positive control indicates PAg (BrHPP, 3μM); and (−), no activation. (C) Staining of CD107a/b on Vγ9Vδ2 T cells (line GUI) after coculture with 721.221 B cells, pretreated, or not, for 2 hours with NBP (pamidronate; 250μM). Cocultures were performed in the presence of either control IgG or 103.2 anti-CD277 Abs (10 μg/mL). Numbers adjacent to outlined areas indicate the percentage of CD107a/b+ γδ T cells. (D) TNF-α production by Vγ9Vδ2 T cells (clone GR4) after activation induced by grading doses of soluble PAg (BrHPP) or anti-CD3 mAb (UCHT1, inset) in the presence of control IgG or anti-CD277 (103.2) Abs (10 μg/mL). (E) Top: Effects of grading doses of 103.2 anti-CD277 mAb on IL-2 release by γδTCR53/4-CD28+ hybridoma cells coexpressing both an MBP-specific αβ TCR (RT1Bl/MHC II) and a human Vγ9Vδ2 TCR. Human Raji cells transduced for RT1Bl expression were cocultured with hybridoma T cells in the presence of Guinea pig myelin basic protein peptide (0.1 μg/mL) or sec-butylamine (1mM). Bottom: Activation induced by soluble PAg (BrHPP, 3μM). IgG indicates isotype control. IL-2 production (ELISA) is presented relative to those of cells activated in the absence of antibody. *P < .05 (paired Student t test). **P < .005 (paired Student t test). (F) Expression of CD107a on Vγ9Vδ2 T cells (polyclonal line AL) after coculture with Mycobacterium bovis BCG-infected THP-1 cells, in the presence or in the absence of 103.2 anti-CD277 mAb (10 μg/mL). The values for the percentage of CD107a+ γδ T cells are indicated on the y-axis. MOI indicates multiplicity of infection; and (−), no antibody. Data are mean ± SD (n = 3 experiments). *P < .05 (Student t test). ***P < .0005 (Student t test).
Abrogation of Vγ9Vδ2 T-cell responses by a blocking anti-CD277 mAb. (A) Surface stainings of Raji cells with either 20.1 and 103.2 anti-CD277 mAbs. Filled histogram represents IgG isotype control. (B) Stainings of CD107a/b (top), IFN-γ (middle), and TNF-α (bottom) of Vγ9Vδ2 T cells (clone GR4) after coculture with Raji cells pretreated for 2 hours with either 20.1 or 103.2 anti-CD277 mAbs (10 μg/mL). Numbers adjacent to outlined areas indicate the percentage of CD107a/b+, IFN-γ+, and TNF-α+ γδ T cells. Positive control indicates PAg (BrHPP, 3μM); and (−), no activation. (C) Staining of CD107a/b on Vγ9Vδ2 T cells (line GUI) after coculture with 721.221 B cells, pretreated, or not, for 2 hours with NBP (pamidronate; 250μM). Cocultures were performed in the presence of either control IgG or 103.2 anti-CD277 Abs (10 μg/mL). Numbers adjacent to outlined areas indicate the percentage of CD107a/b+ γδ T cells. (D) TNF-α production by Vγ9Vδ2 T cells (clone GR4) after activation induced by grading doses of soluble PAg (BrHPP) or anti-CD3 mAb (UCHT1, inset) in the presence of control IgG or anti-CD277 (103.2) Abs (10 μg/mL). (E) Top: Effects of grading doses of 103.2 anti-CD277 mAb on IL-2 release by γδTCR53/4-CD28+ hybridoma cells coexpressing both an MBP-specific αβ TCR (RT1Bl/MHC II) and a human Vγ9Vδ2 TCR. Human Raji cells transduced for RT1Bl expression were cocultured with hybridoma T cells in the presence of Guinea pig myelin basic protein peptide (0.1 μg/mL) or sec-butylamine (1mM). Bottom: Activation induced by soluble PAg (BrHPP, 3μM). IgG indicates isotype control. IL-2 production (ELISA) is presented relative to those of cells activated in the absence of antibody. *P < .05 (paired Student t test). **P < .005 (paired Student t test). (F) Expression of CD107a on Vγ9Vδ2 T cells (polyclonal line AL) after coculture with Mycobacterium bovis BCG-infected THP-1 cells, in the presence or in the absence of 103.2 anti-CD277 mAb (10 μg/mL). The values for the percentage of CD107a+ γδ T cells are indicated on the y-axis. MOI indicates multiplicity of infection; and (−), no antibody. Data are mean ± SD (n = 3 experiments). *P < .05 (Student t test). ***P < .0005 (Student t test).
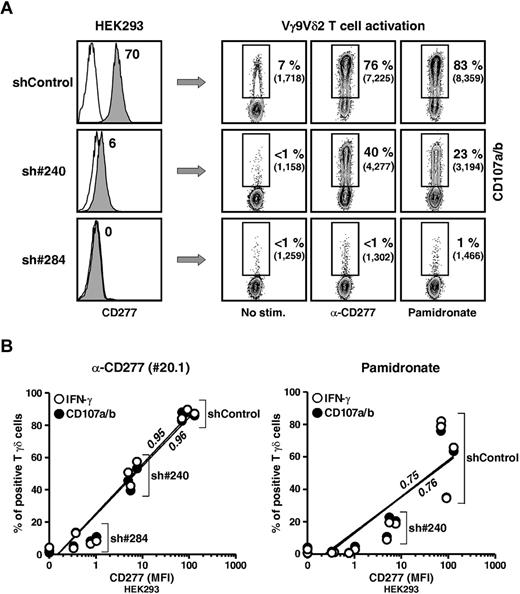
The CD277 requirement for Vγ9Vδ2 T-cell activation was directly addressed by a knockdown approach. The CD277 subfamily is composed of 3 isoforms (BTN3A1, -A2, and -A3) with highly (> 95%) homologous extracellular domains but less conserved intracellular sequences. Two short hairpin RNAs (shRNA) mediating knockdown of all 3 CD277 isoforms (Figure 5A left panel) in HEK293 cells significantly reduced Vγ9Vδ2 T-cell cytolytic and cytokine responses to target cells treated with agonist 20.1 mAb, NBP, or soluble PAg (Figure 5A-B; data not shown). The shRNA most effective for CD277 knockdown (sh#284) provoked the greatest reduction in γδ T-cell responses. In contrast, CD277 knockdown had no effect on antigenic activation of αβ T cells (not shown).
Abrogation of Vγ9Vδ2 T-cell responses by CD277 expression knockdown. (A) Left: Surface expression of CD277 molecules on human HEK293FT clones after transduction with lentivirus delivering irrelevant (shControl) or CD277-specific (sh#240 and sh#284) shRNAs. Open histograms represent control IgG. The value for the intensity of specific CD277 staining (mAb 103.2) is indicated. Right: CD107a/b expression on human Vγ9Vδ2 T cells (line GUI) after coculture with shRNA-transduced HEK293FT clones, pretreated for 2 hours with either anti-CD277 mAb (20.1; 10 μg/mL) or NBP (pamidronate, 100μM). Numbers adjacent to outlined areas indicate the percentage and mean of fluorescence intensity (MFI) of CD107a/b+ T cells. (B) CD107a/b expression and IFN-γ production by Vγ9Vδ2 T cells (line GUI) after incubation with shRNA-transduced HEK293FT clones pretreated for 2 hours with either anti-CD277 mAb (left, 20.1; 10 μg/mL) or NBP (right, pamidronate, 100μM). Data are percentage of CD107a+ (black circles) or IFN-γ+ (white circles) T cells versus the value for the intensity (MFI) of specific CD277 staining (mAb 103.2). sh#284, n = 6; sh#240, n = 3; and shControl, n = 3. Regression lines and calculated r2 values (0.95 and 0.75, for IFN-γ; 0.96 and 0.76 for CD107a/b) are indicated on the graphs.
Abrogation of Vγ9Vδ2 T-cell responses by CD277 expression knockdown. (A) Left: Surface expression of CD277 molecules on human HEK293FT clones after transduction with lentivirus delivering irrelevant (shControl) or CD277-specific (sh#240 and sh#284) shRNAs. Open histograms represent control IgG. The value for the intensity of specific CD277 staining (mAb 103.2) is indicated. Right: CD107a/b expression on human Vγ9Vδ2 T cells (line GUI) after coculture with shRNA-transduced HEK293FT clones, pretreated for 2 hours with either anti-CD277 mAb (20.1; 10 μg/mL) or NBP (pamidronate, 100μM). Numbers adjacent to outlined areas indicate the percentage and mean of fluorescence intensity (MFI) of CD107a/b+ T cells. (B) CD107a/b expression and IFN-γ production by Vγ9Vδ2 T cells (line GUI) after incubation with shRNA-transduced HEK293FT clones pretreated for 2 hours with either anti-CD277 mAb (left, 20.1; 10 μg/mL) or NBP (right, pamidronate, 100μM). Data are percentage of CD107a+ (black circles) or IFN-γ+ (white circles) T cells versus the value for the intensity (MFI) of specific CD277 staining (mAb 103.2). sh#284, n = 6; sh#240, n = 3; and shControl, n = 3. Regression lines and calculated r2 values (0.95 and 0.75, for IFN-γ; 0.96 and 0.76 for CD107a/b) are indicated on the graphs.
Taken together, these results indicate a unique mandatory role played by CD277 in tumor or infectious contexts associated with spontaneous Vγ9Vδ2 T-cell activation and in Vγ9Vδ2 T-cell responses elicited by PAg.
Key contribution of BTN3A1 to PAg-induced activation of Vγ9Vδ2 T cells
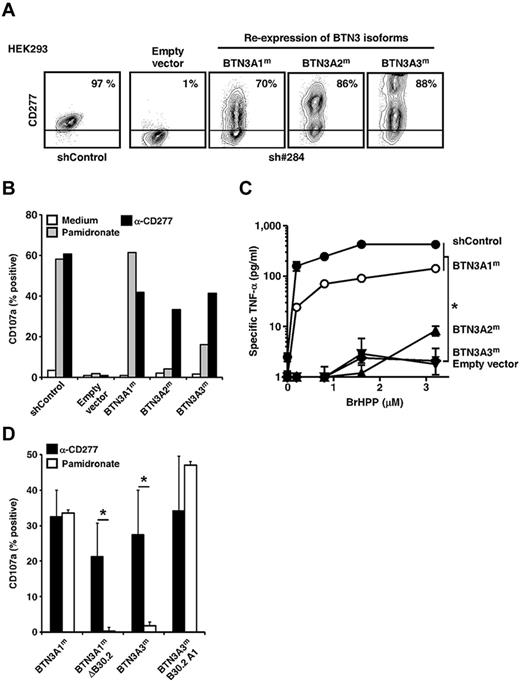
Because CD277-specific mAbs cross-react with all 3 isoforms of CD277,26 they did not allow their role played in Vγ9Vδ2 T-cell activation to be assessed. To address this, we generated cells expressing only one of each of the isoforms, by transfecting CD277-knockdown (sh#284) HEK293 cells with mutated cDNAs designed to confer resistance to knockdown without changing the protein sequence coded (Figure 6A). Selective re-expression of each isoform restored Vγ9Vδ2 T-cell responses induced by the agonist 20.1 mAb (Figure 6B). In contrast, only re-expression of BTN3A1, and to a much lesser extent BTN3A3, restored the ability to activate Vγ9Vδ2 T cells to NBP-treated knockdown cells. Similarly, soluble PAg-induced γδ T-cell responses were restored after BTN3A1, but not BTN3A2 or BTN3A3, re-expression in CD277-knockdown cells (Figure 6C).
Key role played by BTN3A1 in PAg-induced activation of Vγ9Vδ2 T cells. (A) Surface expression of CD277 molecules on shRNA 284 transduced-HEK293FT cells, knocked down for the expression of endogenous CD277 molecules and selectively re-expressing, after transfection, silently mutated CD277 isoforms (BTN3A1m, BTN3A2m, or BTN3A3m). shControl indicates clone 3, random shRNA sequence; sh#284, shRNA-specific for CD277, clone 30; and empty vector, transfection of an empty plasmid. The values for the percentage of CD277+ cells are indicated. Data are representative of more than 3 independent experiments. (B) Expression of CD107a on Vγ9Vδ2 T cells (line GUI) after coculture with HEK293FT cells re-expressing BTN3A1m, BTN3A2m, or BTN3A3m isoforms and pretreated for 2 hours with NBP (pamidronate, 250μM) or anti-CD277 mAb (20.1, 10 μg/mL). (C) TNF-α release from Vγ9Vδ2 T cells (line GUI) induced by grading doses of soluble PAg (BrHPP) in coculture with HEK293FT cells re-expressing BTN3A1, BTN3A2, or BTN3A3 isoforms. Data are mean ± SD and are representative of 3 independent experiments. *P < .05 (paired Student t test). (D) Expression of CD107a on Vγ9Vδ2 T cells (line AL) after coculture with HEK293FT cells expressing full-length BTN3A1, BTN3A3, truncated BTN3A1 proteins lacking the intracellular B30.2 domain (BTN3A1mΔB30.2), or chimeric BTN3A3 proteins assembled with the BTN3A1 intracellular B30.2 domain (BTN3A3mB30.2A1). Target cells were pretreated for 2 hours with anti-CD277 mAb (20.1, 10 μg/mL) or NBP (pamidronate, 250μM). Data are mean ± SD of the percentage of CD107a+ γδ T cells (n = 3). *P < .05 (Student t test).
Key role played by BTN3A1 in PAg-induced activation of Vγ9Vδ2 T cells. (A) Surface expression of CD277 molecules on shRNA 284 transduced-HEK293FT cells, knocked down for the expression of endogenous CD277 molecules and selectively re-expressing, after transfection, silently mutated CD277 isoforms (BTN3A1m, BTN3A2m, or BTN3A3m). shControl indicates clone 3, random shRNA sequence; sh#284, shRNA-specific for CD277, clone 30; and empty vector, transfection of an empty plasmid. The values for the percentage of CD277+ cells are indicated. Data are representative of more than 3 independent experiments. (B) Expression of CD107a on Vγ9Vδ2 T cells (line GUI) after coculture with HEK293FT cells re-expressing BTN3A1m, BTN3A2m, or BTN3A3m isoforms and pretreated for 2 hours with NBP (pamidronate, 250μM) or anti-CD277 mAb (20.1, 10 μg/mL). (C) TNF-α release from Vγ9Vδ2 T cells (line GUI) induced by grading doses of soluble PAg (BrHPP) in coculture with HEK293FT cells re-expressing BTN3A1, BTN3A2, or BTN3A3 isoforms. Data are mean ± SD and are representative of 3 independent experiments. *P < .05 (paired Student t test). (D) Expression of CD107a on Vγ9Vδ2 T cells (line AL) after coculture with HEK293FT cells expressing full-length BTN3A1, BTN3A3, truncated BTN3A1 proteins lacking the intracellular B30.2 domain (BTN3A1mΔB30.2), or chimeric BTN3A3 proteins assembled with the BTN3A1 intracellular B30.2 domain (BTN3A3mB30.2A1). Target cells were pretreated for 2 hours with anti-CD277 mAb (20.1, 10 μg/mL) or NBP (pamidronate, 250μM). Data are mean ± SD of the percentage of CD107a+ γδ T cells (n = 3). *P < .05 (Student t test).
BTN3A2 is devoid of an intracellular B30.2 domain,21 which could be involved in translating intracellular PAg accumulation into a Vγ9Vδ2 activation signal. In agreement with this hypothesis, truncated BTN3A1 molecules lacking a B30.2 intracellular domain failed to restore NBP-induced γδ T-cell responses, although they were still able to activate Vγ9Vδ2 T cells after incubation with agonist 20.1 mAb. Furthermore, we detected strong Vγ9Vδ2 T-cell responses against NBP-treated target cells expressing chimeric CD277 molecules (extracellular domain of BTN3A3 fused to the intracellular B30.2 domain of BTN3A1; Figure 6D). This indicates that expression of the B30.2 intracellular domain of BTN3A1 is required for sensing both endogenous and exogenous PAg by human Vγ9Vδ2 T cells. Weakness of PAg-induced γδ T-cell responses toward transductants expressing BTN3A3, which carries a B30.2 domain highly homologous to that of BTN3A1, may possibly be linked to the presence of a 70-amino acid tail following the B30.2 domain, which could interfere with proper B30.2-dependent CD277 recruitment.
Vγ9Vδ2 T-cell activation correlates with decreased membrane mobility of BTN3A1 induced by anti-CD277 mAb and NBP
All BTN3A isoforms restored Vγ9Vδ2 T-cell responses induced by agonist anti-CD277 mAb, whereas only those carrying the B30.2 domain of BTN3A1 could restore responses toward NBP- or PAg-treated cells. This suggests that NBP and PAg could act upstream of Vγ9Vδ2 T-cell activation by inducing B30.2-dependent changes in the conformation or membrane topology of CD277. These changes might be mimicked by agonist anti-CD277 mAbs. To test this, we generated transfectants expressing BTN3A1 or BTN3A2 fused to either EmGFP or mCherry at their carboxytermini, and studied membrane mobility of the tagged chimeras by fluorescence recovery after photobleaching (FRAP) analysis. Functionality of chimeras was first tested and EmGFP or mCherry BTN3A1 and BTN3A2 chimeras restored Vγ9Vδ2 T-cell responses to transductants pretreated with agonist 20.1 mAb (supplemental Figure 7). In line with results obtained with wild-type BTN3A2 molecules, fluorescent BTN3A2 chimeras failed to restore Vγ9Vδ2 T-cell responses to NBP-treated cells. mCherry-BTN3A1 chimeras triggered more efficient Vγ9Vδ2 T-cell responses after NBP treatment compared with EmGFP-BTN3A1 chimeras. Because EmGFP, unlike mCherry, was fused directly to the carboxy-terminus end of the B30.2 domain without any spacer sequence, the weak functionality of EmGFP-BTN3A1 may be the result of interference with proper B30.2-dependent recruitment of BTN3A1 by PAg.
We next investigated by FRAP analysis whether Vγ9Vδ2 T-cell responses correlated with alterations of CD277 membrane mobility. Approximately 80%-90% fluorescence prebleach levels were recovered within 80 seconds (Tp) after photobleaching of untreated transfectants expressing either BTN3A1 or BTN3A2 fused to EmGFP or mCherry. The 103.2 mAb induced a dramatic aggregation of membrane CD277 molecules within a few minutes after treatment and subsequent CD277 down-modulation (C.H., S. Nedellec, E.S., M.B., unpublished data, January 2012). This was not the case for the agonist 20. mAb. A 20- to 30-minute treatment with this mAb strongly decreased FRAP of BTN3A1 and BTN3A2 chimeras (average recovery reduced to 30%-40% of prebleach levels), indicating decreased membrane mobility (Figure 7A-B). Importantly, NBP treatment decreased mobility of mCherry-BTN3A1 chimeras but had no effect on mCherry-BTN3A2 chimeras (Figure 7C). Furthermore, treatment with mevalonate pathway inhibitors, such as mevastatin, that act upstream of PAg production did not change the mobility of mCherry-BTN3A1 chimeras and actually inhibited the effects of NBPs on this phenomenon. This suggests that NBP-induced changes of BTN3A1 mobility are primarily the result of PAg accumulation, rather than downstream effects linked to the overall blockade of the mevalonate pathway. Therefore, Vγ9Vδ2 T-cell activation tightly correlates with decreased CD277 membrane mobility provoked in a B30.2-dependent way by intracellular PAg accumulation.
Vγ9Vδ2 T-cell activation correlates with decreased mobility of BTN3A1 induced by agonist anti-CD277 mAb and NBP. (A) Left: Confocal images of HEK293 cells expressing EmGFP-BTN3A1 molecules after incubation for 30 minutes with anti-CD277 mAb (#20.1; 10 μg/mL), shown before (Pre-bleach), immediately after (T0-bleach), and 80 seconds (Tp-80 seconds) after photobleaching of regions of interest (indicated rectangular areas). Scale bars represent 6 μm. (−) indicates no treatment. Right: Mean FRAP and fit curves in EmGFP-BTN3A1-expressing HEK293 cells (n = 18), after treatment with anti-CD277 mAb (#20.1; 10 μg/mL). Control indicates no treatment (n = 17). The symbols correspond to the mean ± SEM of FRAP collected every 5 seconds. The curves were fitted by 1-phase exponential equations. The average fluorescence before photobleaching was counted as 100% (dashed line). Immobile fractions are indicated for each condition. (B) FRAP analysis of HEK293 cells expressing EmGFP-BTN3A1 or EmGFP-BTN3A2 molecules after incubation for 30 minutes with anti-CD277 mAbs (#20.1; 10 μg/mL). Data are presented as the value for the percentage of immobile fraction, measured as described under “Microscopy.” Control indicates no treatment. BTN3A1: (−), n = 21; 20.1, n = 13; BTN3A2: (−), n = 12; 20.1, n = 8. Bars represent mean values. (C) FRAP analysis of HEK293 cells expressing mCherry-BTN3A1 or mCherry-BTN3A2 molecules after either incubation for 30 minutes with anti-CD277 mAb (#20.1; 10 μg/mL), incubation overnight with NBP (Pam; pamidronate; 250μM) or statin (mevastatin, 50μM) only, or treatment with statin for 6 hours before incubation overnight with both statin and NBP (Pam + Meva). Data are presented as the value for the percentage of immobile fraction. Control indicates no treatment. BTN3A1: (−), n = 21; 20.1, n = 16; Pam, n = 28; Pam + Meva, n = 18; Meva, n = 8. BTN3A2: (−), n = 12; 20.1, n = 14; Pam, n = 14. Bars represent mean values. (B-C) ***P < .005 (Student t test).
Vγ9Vδ2 T-cell activation correlates with decreased mobility of BTN3A1 induced by agonist anti-CD277 mAb and NBP. (A) Left: Confocal images of HEK293 cells expressing EmGFP-BTN3A1 molecules after incubation for 30 minutes with anti-CD277 mAb (#20.1; 10 μg/mL), shown before (Pre-bleach), immediately after (T0-bleach), and 80 seconds (Tp-80 seconds) after photobleaching of regions of interest (indicated rectangular areas). Scale bars represent 6 μm. (−) indicates no treatment. Right: Mean FRAP and fit curves in EmGFP-BTN3A1-expressing HEK293 cells (n = 18), after treatment with anti-CD277 mAb (#20.1; 10 μg/mL). Control indicates no treatment (n = 17). The symbols correspond to the mean ± SEM of FRAP collected every 5 seconds. The curves were fitted by 1-phase exponential equations. The average fluorescence before photobleaching was counted as 100% (dashed line). Immobile fractions are indicated for each condition. (B) FRAP analysis of HEK293 cells expressing EmGFP-BTN3A1 or EmGFP-BTN3A2 molecules after incubation for 30 minutes with anti-CD277 mAbs (#20.1; 10 μg/mL). Data are presented as the value for the percentage of immobile fraction, measured as described under “Microscopy.” Control indicates no treatment. BTN3A1: (−), n = 21; 20.1, n = 13; BTN3A2: (−), n = 12; 20.1, n = 8. Bars represent mean values. (C) FRAP analysis of HEK293 cells expressing mCherry-BTN3A1 or mCherry-BTN3A2 molecules after either incubation for 30 minutes with anti-CD277 mAb (#20.1; 10 μg/mL), incubation overnight with NBP (Pam; pamidronate; 250μM) or statin (mevastatin, 50μM) only, or treatment with statin for 6 hours before incubation overnight with both statin and NBP (Pam + Meva). Data are presented as the value for the percentage of immobile fraction. Control indicates no treatment. BTN3A1: (−), n = 21; 20.1, n = 16; Pam, n = 28; Pam + Meva, n = 18; Meva, n = 8. BTN3A2: (−), n = 12; 20.1, n = 14; Pam, n = 14. Bars represent mean values. (B-C) ***P < .005 (Student t test).
Discussion
Sensing of microbial and mammalian PAg is an elegant strategy for immune detection of infectious and metabolic distress exploited by a major γδ T-cell subset shared by primates but absent in other species.2,4,5 Although expression of Vγ9Vδ2 TCR has been known for years to be a prerequisite for PAg-induced activation,12,39 how such small nonpeptidic metabolites promote γδ TCR engagement has remained a mystery. Our results identifying a key role for CD277, a recently identified member of the extended B7 receptor family, yield new insights into this mechanism. The key implication of the ubiquitous CD277 molecule in γδ T-cell activation is consistent with the lack of tissue restriction of NBP- and PAg-induced γδ T-cell responses.11 Moreover, the lack of functional CD277 orthologs in rodents goes hand in hand with the absence of PAg-responding γδ T-cell subsets in these species,2 and the lack of Vγ9Vδ2 T-cell reactivity toward NBP- or PAg-treated murine cell lines.5,11
How do the effects of anti-CD277 mAb on Vγ9Vδ2 T-cell activation fit in with the recently reported modulation of αβ T-cell responses by several CD277-specific mAbs? In agreement with previous studies,23,25 soluble 20.1 mAb had neither stimulatory nor costimulatory activity on αβ T cells. Costimulatory activity of mAb 20.1 on TCR-stimulated αβ T cells has been reported with plate-bound mAb only and is the consequence of direct engagement of CD277 expressed by responder T cells.25 In marked contrast, soluble, but not immobilized, mAb 20.1 induced full activation of Vγ9Vδ2 T cells in the absence of any other stimuli (eg, anti-CD3 mAb) and acted primarily, if not exclusively, in an indirect manner (ie, through sensitization of CD277+ target cells to Vγ9Vδ2 T-cell recognition. Yamashiro et al also described inhibition of anti-CD3–stimulated αβ T cells by a high-affinity anti-CD277 mAb able to induce CD277 phosphorylation in T cells.23 This again suggests a direct coinhibitory effect of the mAb. Although we could not assess activity of this particular mAb on Vγ9Vδ2 T-cell responses, its mode of action clearly differs from that of the blocking mAb 103.2 we used. In particular, mAb 103.2 inhibited PAg-induced Vγ9Vδ2 T-cell responses, but not anti-CD3–stimulated Vγ9Vδ2 or αβ T cells, and acted indirectly, by interfering with Vγ9Vδ2 T-cell recognition of NBP-treated or BCG-infected target cells (as indicated by mAb preincubation experiments and analysis of the responses of murine Vγ9Vδ2 TCR transductants).
Results of anti-CD277 mAb treatments and CD277-knockdown approaches both point to a unique role played by CD277 in PAg-induced Vγ9Vδ2 T-cell responses. Moreover, the effects of both agonist and blocking anti-CD277 mAbs on Vγ9Vδ2 TCR transductant responses indicate that the TCR is necessary for this CD277-dependent activation process. How CD277, PAg, and the Vγ9Vδ2 TCR are linked together remains nevertheless unclear. The CD277-dependent recognition of PAg-treated human cells by murine Vγ9Vδ2 TCR transductants argues against engagement of CD277 by a counter-receptor expressed by responder T cells. Indeed, because CD277 is not conserved in the mouse,21 existence of a murine counter-receptor seems unlikely.
Interestingly, whereas Vγ9Vδ2 T-cell activation by agonist anti-CD277 mAbs was restored after re-expression of any of the 3 CD277 isoforms, responses to PAg or NBP were obtained after re-expression of BTN3A1 only. This suggests that PAg are not bona fide γδ T-cell antigens, consistent with previous failures to demonstrate interactions between PAg and Vγ9Vδ2 TCR.13 We propose that PAg act through the intracytoplasmic domain of the BTN3A1 isoform to induce membrane reorganization of CD277 complexes sensed directly or indirectly by Vγ9Vδ2 T cells. Because of the high conservation of the extracellular domain of all 3 CD277 isoforms, such Vγ9Vδ2-stimulatory CD277 complexes could be mimicked by agonist anti-CD277 mAbs, irrespective of the isoform expressed. This model (supplemental Figure 8) is supported by the similar Ca2+ signaling kinetics of Vγ9Vδ2 T cells stimulated by agonist anti-CD277 mAb and PAg as well as by the results of FRAP experiments. Both agonist mAb and NBP induce changes of CD277 membrane mobility that correlate with Vγ9Vδ2 T-cell activation. Here again, NBP induced topologic rearrangement of only BTN3A1, whereas agonist mAb affected the mobility of both BTN3A1 and BTN3A2 isoforms. This establishes a specific and presumably causal link between intracellular PAg accumulation and CD277 reorganization, which occurs independently of Vγ9Vδ2 T-cell activation. PAg-dependent recruitment of CD277 molecules could be achieved through direct interactions between PAg and the intracellular domain of BTN3A1. Alternatively, in line with the role played by some B30.2 domains in protein/protein interactions,40,41 BTN3A1 recruitment could involve additional intracellular or membrane-bound partners. Although PAg are likely to act intracellularly, we cannot rule out an extracellular mode of action (eg, after interaction with the extracellular part of a transmembrane receptor that would in turn induce BTN3A1 reorganization in a B30.2-dependent fashion). This hypothesis would be in line with evidence for the existence of surface receptors able to interact with PAg.42,43
How Vγ9Vδ2 T cells detect PAg-induced changes of CD277 remains to be determined. These changes could be sensed by the Vγ9Vδ2 TCR directly, although we failed to demonstrate cognate interactions between recombinant Vγ9Vδ2 TCR and CD277 (E.J.A., unpublished results, January 2012), or to restore Vγ9Vδ2 T-cell responses toward NBP-treated murine target cells after BTN3A1 transduction (C.H., R.B., M.B., and E.S., unpublished results, January 2011). Alternatively, CD277 might promote recruitment of other receptors recognized by the Vγ9Vδ2 TCR, such as ecto-F1-ATPase, which is a known ligand for Vγ9Vδ2 TCR.44,45
The restricted but mandatory implication of CD277 in the activation of particular human γδ T-cell subsets brings to mind the mandatory role played by Skint-1, another member of the extended B7 receptor family, in the selection of murine intraepidermal γδ T cells.16-18 Although Skint-1 deficiency prevents differentiation of T cells expressing invariant Vγ5Vδ1 TCR, it has no effect on other γδ or αβ T-cell compartments. Whether Skint-1 directly interacts with Vγ5Vδ1 TCR or modulates TCR engagement by yet undefined antigens remains unclear. It is striking that the human B7-related molecules, which are most homologous to SKINT1 in their extracellular regions, are the mouse and human BTNs, including CD277.21 Therefore, the implication of BTN/Skint-related members of the extended B7 receptor family in cell selection and/or peripheral activation could represent a unifying feature shared by seemingly unrelated γδ T-cell subsets from distinct species.
From an applied standpoint, trials using clinical grade 5γ9Vδ2 T-cell agonists (like NBP or synthetic PAg) have yielded promising preclinical and clinical responses in viral infections and several cancers.2,5,46 Such protocols are nevertheless suboptimal because of the progressive exhaustion of Vγ9Vδ2 T-cell responses after repeated treatment with specific agonists and because of the rather weak reactivity of in vitro or in vivo expanded Vγ9Vδ2 T cells toward fresh autologous tumor cells.5 Enhancing antitumor responses of Vγ9Vδ2 T cells with agonist anti-CD277 mAbs may prove a good way to improve their antitumor efficacy. On the other hand, antagonist anti-CD277 mAbs might be exploited to dampen deleterious Vγ9Vδ2 T-cell responses,47 for instance, during Mycobacterium tuberculosis–induced immune restoration syndrome in AIDS patients under therapy.48
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Santolaria and A. Giraud for assistance and discussion; M. C. Devilder for assistance with cytometry; M. C. Gesnel, S. Heijhuurs, Ingrid Müller, and Lisa Starick for technical assistance; J. Desfrançois-Noel and P. Hulin at the Flow Cytometry and Cellular and Tissular Imaging Core Facility of Nantes University (MicroPICell) and IFR26 (Nantes) for expert technical assistance; and N. Minato, X. Saulquin, B. Clémenceau, H. Vié, N. Labarrière, F. Paris, M. Grégoire, F. Altare, D. Heymann, and Innate Pharma (Marseille, France) for sharing cells and reagents.
This work was supported by Inserm, Université de Nantes, Association pour la Recherche contre le Cancer (R10139NN), Institut National du Cancer (V9V2THER), AICR (10-0736), UU (2010-4669), and IZKF Würzburg (01KS9603).
Authorship
Contribution: C.H., Y.G., S. Nedellec, C.-M.P., H.M., J.M., J.L., J.K., S. Netzer, A.L., and R.B. performed experiments; C.H., Y.G., S. Nedellec, J.M., H.M., J.K., E.J.A., J.D.-M., T.H., R.B., D.O., and E.S. analyzed results and made the figures; M.B., R.B., and E.S. designed the research and wrote the manuscript; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuel Scotet, Inserm UMR 892, Centre de Recherche en Cancérologie Nantes-Angers, IRT UN, 8 quai Moncousu, 44007 Nantes cedex, France; e-mail: emmanuel.scotet@inserm.fr; and Marc Bonneville, Inserm UMR892, Centre de Recherche en Cancérologie Nantes-Angers, IRT UN, 8 quai Moncousu, 44007 Nantes cedex, France; e-mail: bonnevil@inserm.fr.
References
Author notes
C.H. and Y.G. contributed equally to this study.
D.O., M.B., and E.S. are co–senior authors.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal