Abstract
Vascular development and angiogenesis initially depend on endothelial tip cell invasion, which is followed by a series of maturation steps, including lumen formation and recruitment of perivascular cells. Notch ligands expressed on the endothelium and their cognate receptors expressed on perivascular cells are involved in blood vessel maturation, though little is known regarding the Notchdependent effectors that facilitate perivascular coverage of nascent vessels. Here, we report that vascular smooth muscle cell (VSMC) recognition of the Notch ligand Jagged1 on endothelial cells leads to expression of integrin αvβ3 on VSMCs. Once expressed, integrin αvβ3 facilitates VSMC adhesion to VWF in the endothelial basement membrane of developing retinal arteries, leading to vessel maturation. Genetic or pharmacologic disruption of Jagged1, Notch, αvβ3, or VWF suppresses VSMC coverage of nascent vessels and arterial maturation during vascular development. Therefore, we define a Notch-mediated interaction between the developing endothelium and VSMCs leading to adhesion of VSMCs to the endothelial basement membrane and arterial maturation.
Introduction
The process of angiogenesis must be highly regulated to produce a patent vascular system with adequate perfusion to support the surrounding tissue. While multiple cell types, secreted proteins, and growth factors coordinately regulate angiogenesis and vessel stabilization, the Notch signaling pathway is unique in that it is involved at multiple stages of angiogenesis, from initial vascular plexus formation and artery/vein patterning, to vascular smooth muscle cell (VSMC) recruitment and vascular remodeling.
Notch signaling, which is characterized by heterotypic cell-cell interactions between Notch-ligand and -receptor expressing cells, represents an evolutionarily conserved mechanism that is known to be important for cell-fate decisions during embryogenesis and, more recently, angiogenesis. Mammals express 4 Notch receptors, Notch1-4, and 5 Notch ligands, Delta-like ligand (Dll) 1, Dll3, Dll4, Jagged1 (Jag1), and Jagged2 (Jag2). Notch1, Dll4, and Jag1 are required for vascular development, and genetic deletion of each of these genes results in embryonic lethality.1–5 The loss of Notch3, whose expression is restricted primarily to VSMCs in the vasculature, is not lethal, but rather demonstrates that Notch3 is required for arterial differentiation and VSMC maturation.6 While Notch3 seems to be the critical receptor for mural cell differentiation, Jag1 is the corresponding ligand that has been shown to be most important for this process.7 Endothelial-specific deletion of Jag1 results in severe mural cell defects, whereas the expression of Jag1 on endothelial cells promotes mural cell differentiation.8–10 While there has been research into the role that Notch signaling plays in vessel patterning and VSMC differentiation, the role of Notch signaling in vascular maturation has not been explored because the mouse models used to date are embryonic lethal.
The recruitment of pericytes and VSMCs to nascent vessels is critical to vessel maturation.11 In the absence of perivascular cell coverage, newly formed vessels are subject to regression and are dependent on growth factor stimulation from the environment for their survival.12 However, once invested with pericytes, vessels are stabilized and resistant to regression. One mechanism by which Notch signaling regulates VSMC recruitment to vessels is by the up-regulation of PDGFRβ,13 however, other Notch-downstream effector genes involved in the recruitment or retention of VSMCs in vessel maturation have not yet been described.
In addition to mural cell recruitment, the deposition of an endothelial basement membrane also regulates vessel maturation. Basement membranes are thin layers (50-100 nm) of specialized extracellular matrix shared by endothelial and epithelial cells which provide structure and support for those cells.14 The endothelial basement membrane is unique in its accumulation of the protein VWF, which is derived from endothelial Weibel-Palade body secretions.15 After the formation of a quiescent vasculature, VWF plays an essential role in hemostasis. The lack of VWF, or dysfunctional VWF, leads to the congenital bleeding disorder VWD.16 In addition, VWF in the endothelium acts in the recruitment, adhesion, and migration of leukocytes.17,18 It has also recently been shown to play a role in vessel patterning.19 Here, we provide evidence that VWF regulates the recruitment of VSMCs to mature arteries.
In this report, we identified a novel Notch-downstream effector, integrin αvβ3, on VSMCs. Furthermore, we demonstrate that Notch-induced up-regulation of αvβ3 enables VSMC interaction with VWF in the endothelial basement membrane. We propose that Notch ligand expression on endothelial cells, in combination with VWF accumulation in the endothelial basement membrane, promote arterial maturation via αvβ3 expression on VSMCs.
Methods
In vivo assays
Mouse experiments were performed under approval of the University of California, San Diego Institutional Animal Care and Use Committee. Balb/c mice (The Jackson Laboratory) were used for DAPT (N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester) treatment, anti-CD61 injection, and the VWF/αSMA time course. DiYF mice were a gift from T. Byzova (The Cleveland Clinic) and VWF KO mice were a gift from D. Wagner (Harvard University). Both DiYF and VWF KO mice were on a C57BL/6 background, and age-matched C57BL/6 mice (The Jackson Laboratory) were used as controls.
For injection of anti-CD61, postnatal day 5 (P5) Balb/c pups received a single intravitreal injection of purified NA/LE hamster anti–mouse CD61 (BD Biosciences; 0.5 μg in 0.5-μL volume) using a 2.5-μL Hamilton syringe fitted with a 33-GA 0.5-inch removable needle (point style 4; Hamilton) into 1 eye. The contralateral eye received a single injection of purified NA/LE hamster IgGκ isotype control. Retinas were harvested on day P8 and processed for IHC.
For injection of DAPT, DAPT (Tocris) was dissolved in 20% DMSO and 80% corn oil (Sigma-Aldrich). Balb/c pups were injected subcutaneously over 3 consecutive days, at 100 mg/kg in a volume of 10 μL/g daily, either from P2-P5 or from P3-P6.
Whole-mount immunohistochemical staining of retinas
Retinas were harvested from postnatal pups aged P1-P28, fixed in 4% paraformaldehyde (PFA) for 10 minutes and dissected in PBS.20 The retinas were permeabilized in ice-cold methanol for 10 minutes then blocked in 20% FBS/20% normal goat serum (NGS; or 20% NDS [normal donkey serum]) + 0.3% Triton X-100 in PBS for 2 hours. Primary Abs were added to Ab diluents (10% FBS/10% NGS or 10%NDS) + 0.3% Triton X-100 in PBS) overnight (O/N) at 4°C. The next day, retinas were washed for 2 hours and secondary Abs were added.
Primary Abs
Blood vessels were labeled with either isolectin B4 directly conjugated to Alexa 647 (Invitrogen) or with rat anti–mouse CD31/PECAM-1 (BD Biosciences). VSMCs were labeled with mouse-αSMA (alpha smooth muscle actin) directly conjugated to either FITC or Cy3 (Sigma-Aldrich). Other primary Abs used: rabbit anti–human VWF (Millipore), rabbit anti-NG2 chondroitin sulfate proteoglycan (Millipore), rabbit anti-Jagged1 (Santa Cruz Biotechnology), goat anti-Jagged1 (Santa Cruz Biotechnology), goat anti-Notch3 (Santa Cruz Biotechnology). Secondary Abs: goat anti–rabbit A568 or A647, goat anti–rat A488, and donkey anti–goat A568 or A647 (Invitrogen). All Abs were used at a 1:200 dilution.
IHC of retinal frozen sections
Retinas were harvested as described, fixed in 4% PFA in PBS O/N at 4°C, incubated in 20% sucrose in PBS for 2 hours, and embedded in OCT (Tissue-Tek). Thick frozen sections (10 μm) were cut, postfixed in acetone, rehydrated in PBS, then blocked with 5% BSA, 2% NGS in PBS for 2 hours. Sections were incubated with primary Abs: rat anti–mouse CD31, 1:200 (BD Biosciences), mouse anti-SMA FITC-conjugated, 1:200 (Sigma-Aldrich), and hamster anti–mouse CD61, 1:10 (BD Biosciences) O/N in 5% BSA, 2% NGS in PBS. After washing, sections were incubated with goat anti–rat A546 and goat anti–hamster A647 (Invitrogen) for 2 hours and washed again.
Confocal fluorescence microscopy
Imaging was performed on a Nikon Spectral C1 confocal microscope (Nikon C1si with EZC1 acquisition software, Nikon Instruments) with Plan Apo 10×/0.45 air, Plan Apo 20×/0.75 air, and Plan Apo 60×/1.40 oil-objective lenses (Nikon). All images were recorded with a sequential acquisition of the fluorescent channels to prevent fluorescence bleed-through. Images were analyzed with MetaMorph software (Molecular Devices) for determination of VSMC coverage and with ImageJ for VSMC-EC coalignment (RG2B colocalization plugin).
Transmission electron microscopy
Samples were immersed in modified Karnovsky fixative (1.5% glutaraldehyde, 3% PFA and 5% sucrose in 0.1M sodium cacodylate buffer, pH 7.4) for at least 8 hours, postfixed in 1% osmium tetroxide in 0.1M cacodylate buffer for 1 hour and stained en bloc in 1% uranyl acetate for 1 hour. Samples were dehydrated in ethanol, embedded in epoxy resin, sectioned at 60 to 70 nm, and picked up on Formvar and carbon-coated copper grids. Grids were stained with uranyl acetate and lead nitrate, viewed using a JEOL 1200EX II transmission electron microscope and photographed using a Gatan digital camera.
Cell culture
HUVECs (Lonza) were cultured in EBM-2 media (Lonza) supplemented with a bullet kit containing endothelial-specific growth factors (Lonza) and 10% FBS (Hyclone). Human aortic VSMCs from a young donor (Invitrogen) were cultured in Media 231 with smooth muscle cell growth supplement (Invitrogen).
Jag-1 stimulation of VSMC in vitro
VSMCs between passages 3-7 were cultured on 6-well tissue culture treated (TCT) dishes coated with 5 μg/mL IgG or Jagged1 for 18 hours as previously described.13
Microarray analysis
VSMCs were cultured on Jag1 or IgG-coated plates as described in “Jag-1 stimulation of VSMC in vitro.” RNA was extracted using the RNA isolation kit (QIAGEN). Reverse-transcription PCR was done with the RNA-to-cDNA kit (Applied Biosystems). cDNA was run on a TaqMan low-density array, human angiogenesis panel (Applied Biosystems), amplified on a 7900 HT Fast Real-Time PCR system (Applied Biosystems) according to the manufacturer's instructions. All microarray data are available at the Gene Expression Omnibus (GEO) database under accession number GSE34024.
RNA extraction, RT-PCR
VSMCs were cultured on Jag1 or IgG-coated plates as described. RNA was harvested with TRIzol (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Individual quantitative RT-PCRs were performed with Absolute qPCR mix (Thermo Scientific) and SYBR Green (Invitrogen) on 20 μg of cDNA on a SmartCycler (Cepheid) according to the manufacturers' instructions. Primers: Hey2 (CAACCCCTTGTCGCCTCTC/CCGTGGATGGCATTCGGAG, PrimerBank ID 6912414a3); Hes1 (CAGGAATGTTTTGACCGAGTCT/AACGTGCTCAGGTTTTTAG- CC), PrimerBank ID 5031691a2); integrin β3 (GTGACCTGAAGGAGAATC- TGC/TCACTCACTGGGAACTCGATG, PrimerBank ID 2443452a3),21 β-actin (GGAGGAGCTGGAAGCAGCC/GCTG TGCTACGTCGCCCTG).22
Tube formation assay
HUVECs at passage 2 were trypsinized and coated onto Cytodex3 microcarrier beads (GE Healthcare) as previously described.23 After O/N culture in EGM-2 (Lonza), HUVEC-coated beads were suspended in fibrinogen (Sigma-Aldrich; 2 mg/mL) with aprotinin (Sigma-Aldrich; 0.15 U/mL) in PBS at a concentration of 500 beads/mL. VSMCs were labeled with DiIC12(3) (1:1000 in culture media for 2 hours; BD Biosciences) and added to bead solution at 1 × 105 cells/mL. Fibrin gels were made in 24-well TCT plates by mixing 0.5 mL of fibrinogen solution with thrombin (final concentration 0.625 U/mL). After gels solidified, 1 mL of EGM2 was added to each well with Ulex europaeus agglutinin I conjugated to FITC (1:200; Vector Laboratories) to visualize endothelial tubes. For IHC, 1-mL gels were made on glass chamber slides. Fibrin gels were fixed with 4% PFA O/N, permeabilized with methanol (4°C) for 15 minutes. Staining proceeded as described for the retinas, with all Ab incubations done O/N.
Endothelial cell-VSMC coalignment quantification
The overlap between endothelial cells and VSMCs was taken as a percentage of the endothelial tubes in each image analyzed. To do this, stacks of confocal fluorescent images were analyzed in ImageJ with the RG2B colocalization plugin. The resultant colocalization images, which show only pixel overlap, were converted to 8-bit images and thresholded. These are the “colocalization” images. Separately, the corresponding RGB confocal images were split into individual color channels and processed to binary. Of these binary images, the channel of interest is that of the endothelial tubes. These are the “EC tubes” images. For analyzing colocalization in the bead assay, the pixels corresponding to the beads themselves were deleted from all images analyzed because we were interested in colocalization along the tubes. Finally, the ImageJ “analyze particles” function was run on all images, with the readout being area fraction. To determine colocalization, the following calculation was used: area fraction(colocalization)/area fraction(EC tubes) = colocalization.
Adhesion assay
Sixteen-well E plates (Roche) were coated with VWF (gift from Z.M.R.) at 10 μg/mL O/N and blocked with 1% heat-denatured BSA (Hyclone). VSMCs in Opti-MEM with 0.25mM MnCl2, 1mM MgCl2, 1mM CaCl2, 20mM HEPES were added to wells, each condition was run in triplicate. Data were collected on the xCelligence system (Roche) according to the manufacturer's instructions. Impedance was measured, resulting in a cell index number that correlated to VSMC adhesion.
Inhibition of Notch, VWF, αvβ3, and β3 in vitro
A 100mM stock solution of DAPT (Tocris) in DMSO was added to VSMC culture media at a final concentration of 25μM, vehicle was DMSO. A VWF blocking Ab specific to the β3 RGD binding site (α-152B, gift from Z.M.R.) was added to the culture media at a final concentration of 30 μg/mL; the αvβ3 blocking Ab LM609 and the integrin β1 blocking Ab P4C10 were each added to the culture media at a final concentration of 20 μg/mL. Vehicle was mouse IgG (BD Biosciences).
siRNA knockdown in VSMCs
VSMCs were transfected with siRNAs against Notch3 (QIAGEN; Notch3-3) or All Stars control siRNA (QIAGEN) using human aortic SMC nucleofector kit (Lonza) in the Amaxa nucleofector system (Lonza) according to manufacturer's instructions. Knockdown was confirmed by Western blot with rabbit anti-Notch3 (Sigma-Aldrich), rabbit anti-Hey2 (ProteinTech Group), mouse anti-HSP60 (Santa Cruz Biotechnology) was used as a loading control.
Statistical analyses
All statistical analyses were performed with Excel (Microsoft). The 2-tailed Student t test was used to calculate statistical significance. A P value < .05 was considered to be significant.
Results
Notch is required for VSMC coverage during postnatal angiogenesis
To understand the role of Notch signaling in the perivascular compartment during postnatal developmental angiogenesis, we examined vascular mural cell coverage in the developing mouse retina following pharmacologic inhibition of Notch signaling activity. The γ-secretase inhibitor DAPT blocks the cleavage and release of the intracellular domain of Notch, which is the critical effector that controls gene expression24 and as such acts as a global Notch inhibitor both in vivo and in vitro.9,24,25 As previously reported,25 DAPT treatment from P2-P5 leads to the formation of an immature vascular plexus in the periphery of the mouse retina (Figure 1A-B). Because we are interested in the effect of Notch inhibition on vessel maturation, we examined VSMC coverage in the arterial region closest to the optic nerve, which is more mature. Not only did we observe a reduction in the arterial length covered by VSMCs (Figure 1 A,B,E), we also found many areas of incomplete VSMC coverage (Figure 1C-D) following DAPT treatment, indicative of an immature artery. The effect of Notch inhibition on mural cell coverage was limited to αSMA+ perivascular cells and did not impact αSMA−/NG2+pericytes (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Notch signaling is required for arterial maturation in vivo. Representative image of P6 retinas, following (A) vehicle (DMSO) or (B) γ-secretase inhibitor (DAPT) treatment to block Notch signaling from P2-P5, immunostained with Abs specific to CD31 (red) and αSMA (green). DAPT treatment results in an immature vascular plexus, represented by enhanced vascular branching (arrows) and reduced αSMA+ perivascular cell coverage of arteries (arrowheads). (C) Vehicle-treated pups show continuous VSMC coverage of arteries, marked by αSMA, unlike (D) DAPT-treated pups where individual VSMCs are clearly visible (arrowhead) and arterial coverage is interrupted (arrows). (E) DAPT treatment prevents arterial maturation, measured as a 40% decrease in arterial VSMC (αSMA+) coverage compared with controls. Values represent means ± SEM. DMSO (n = 17), DAPT (n = 25), where n is the number of arteries analyzed. P = .004. Scale bars: (A-B) 600 μm; (C-D) 25 μm.
Notch signaling is required for arterial maturation in vivo. Representative image of P6 retinas, following (A) vehicle (DMSO) or (B) γ-secretase inhibitor (DAPT) treatment to block Notch signaling from P2-P5, immunostained with Abs specific to CD31 (red) and αSMA (green). DAPT treatment results in an immature vascular plexus, represented by enhanced vascular branching (arrows) and reduced αSMA+ perivascular cell coverage of arteries (arrowheads). (C) Vehicle-treated pups show continuous VSMC coverage of arteries, marked by αSMA, unlike (D) DAPT-treated pups where individual VSMCs are clearly visible (arrowhead) and arterial coverage is interrupted (arrows). (E) DAPT treatment prevents arterial maturation, measured as a 40% decrease in arterial VSMC (αSMA+) coverage compared with controls. Values represent means ± SEM. DMSO (n = 17), DAPT (n = 25), where n is the number of arteries analyzed. P = .004. Scale bars: (A-B) 600 μm; (C-D) 25 μm.
Endothelial Jag1 is required for arterial VSMC coverage and maintenance
Endothelial cell Jag1, a Notch ligand recognized by VSMCs, is predominantly expressed in the arterial endothelium during murine retinal development26 (supplemental Figure 2) and is required for VSMC differentiation during embryogenesis.8 Genetic ablation of Jag1 from the endothelium of mice using a Tie2-Cre mouse line resulted in embryonic lethality with severe VSMC defects.8 To investigate the role of Jag1 in postnatal arterial maturation, we used a genetic murine model that bypasses the critical requirement for Jag1 in early endothelial development (VE-Cadherin-Cre; Jag1 ECKO mice).27 In the retinas of these mice, we observed weak arterial αSMA+ perivascular staining in P7 pups compared with controls, indicating that VSMC recruitment was deficient, leading to less mature arteries during development (Figure 2A-B). Staining with an alternative marker for VSMCs, SM22α, confirmed that it is the VSMC compartment which is affected by loss of endothelial Jag1 (supplemental Figure 3). Pericyte coverage was not affected (supplemental Figure 4). In adults, we observed a loss of VSMCs in the arteries immediately adjacent to the Jag1− endothelium (Figure 2C-H). These findings suggest that Jag1 is an endothelial cell Notch ligand capable of regulating arterial maturation and maintaining arteries in a mature state, without loss of VSMCs.
Endothelial Jag1 maintains arterial VSMC coverage in development and adulthood. (A-B) Representative images of vessels in P7 retinas from mice with (Jag1 lox/lox VECAD-Cre negative, “wild-type”) and without (Jag1 lox/lox VECAD-Cre positive, “JAG1 ECKO”) Jag1 in the endothelium. Retinas were immunostained with Abs to CD31 (red) and αSMA (green). (C-H) Large vessels from young adult mice were immunostained with Abs to CD31 (red), αSMA (green), and TOPRO (blue). Aorta from wild-type (C-D) and Jag1 ECKO (E-F) adult mouse. Arrows (F) indicate loss of smooth muscle cells in the layer immediately below the Jag1-negative endothelium. (G-H) Mesenteric vessel from Jag1 ECKO mouse, arrow (H) notes lack of smooth muscle cells. (I) Scale bars: (A-B) 100 μm; (C-F) 50 μm; and (G) 20 μm.
Endothelial Jag1 maintains arterial VSMC coverage in development and adulthood. (A-B) Representative images of vessels in P7 retinas from mice with (Jag1 lox/lox VECAD-Cre negative, “wild-type”) and without (Jag1 lox/lox VECAD-Cre positive, “JAG1 ECKO”) Jag1 in the endothelium. Retinas were immunostained with Abs to CD31 (red) and αSMA (green). (C-H) Large vessels from young adult mice were immunostained with Abs to CD31 (red), αSMA (green), and TOPRO (blue). Aorta from wild-type (C-D) and Jag1 ECKO (E-F) adult mouse. Arrows (F) indicate loss of smooth muscle cells in the layer immediately below the Jag1-negative endothelium. (G-H) Mesenteric vessel from Jag1 ECKO mouse, arrow (H) notes lack of smooth muscle cells. (I) Scale bars: (A-B) 100 μm; (C-F) 50 μm; and (G) 20 μm.
Interaction with Jag1 induces integrin αvβ3 expression on VSMCs
To identify Jag1-dependent Notch-mediated genes that might contribute to arterial maturation, VSMCs were seeded onto plates coated with or without immobilized recombinant Jag1. Eighteen hours later, we analyzed changes in gene expression using a low-density array featuring a panel of genes associated with human angiogenesis (Applied Biosystems). Cell interaction with Jag1 altered the expression of several angiogenesis-related genes in VSMCs, including PDGFRβ, integrin β3, and the Notch-downstream gene Hey1 (Table 1).
VSMC mRNA fold-change following culture on Jag1
| Down > 3-fold . | Up > 3-fold . |
|---|---|
| Follistatin | Angiopoietin 2 |
| ADAMTS1 | ECGF1 |
| TGFa | Hey1 |
| VEGFC | Integrin beta3 |
| IL12A | |
| PDGFRβ | |
| Platelet factor 4 |
| Down > 3-fold . | Up > 3-fold . |
|---|---|
| Follistatin | Angiopoietin 2 |
| ADAMTS1 | ECGF1 |
| TGFa | Hey1 |
| VEGFC | Integrin beta3 |
| IL12A | |
| PDGFRβ | |
| Platelet factor 4 |
VSMC indicates vascular smooth muscle cell.
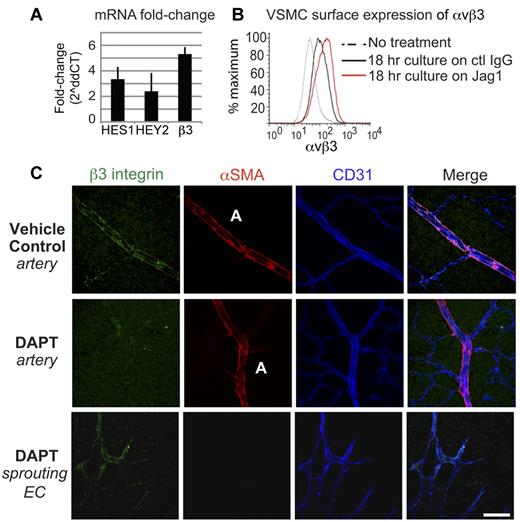
Integrin β3, which associates with αv to form the integrin heterodimer αvβ3, is known to play an important role in vascular cell adhesion, migration, and development.28,29 We confirmed that integrin β3 mRNA expression was increased 5.3-fold by RT-PCR (± 0.7 SEM, n = 6; Figure 3A) and observed a 3-fold increase in αvβ3 surface expression by FACS on VSMCs following interaction with immobilized Jag1 (Figure 3B). We also verified that integrin αvβ3 was expressed on arterial-associated VSMCs during retinal development, and that this expression was dependent on Notch signaling (Figure 3C). Blockade of Notch signaling by DAPT administration selectively prevented the expression of αvβ3 on VSMCs of maturing arteries, but did not impact the expected pattern of β3 expression on angiogenic sprouting tip cells (Figure 3C). These findings reveal that VSMC integrin αvβ3 expression depends, in part, on a Jag1/Notch interaction.
A Jagged1/Notch interaction drives integrin αvβ3 expression in VSMCs. (A) VSMC culture on a Jag1-coated substrate increased mRNA expression of integrin β3 5.3-fold by qPCR (± 0.7; n = 6). Culture on Jag1 also up-regulated canonical Notch-downstream genes HES1 (3.4 ± 1.4, n = 3) and HEY2 (2.4 ± 0.5, n = 4). (B) FACS analysis of VSMCs found that surface expression of integrin αvβ3 increased 3-fold in response to culture on a Jag1-coated substrate. (C) Representative images of retinas from mice treated with vehicle (DMSO) or DAPT to block Notch signaling from P3-P6, and analyzed P7. Retinas were immunostained with Abs against integrin β3 (green) and αSMA (red) to label VSMCs, and CD31 (blue) to label endothelial cells. DAPT treatment inhibited the expression of αvβ3 in arterial-associated VSMCs, but did not inhibit the expression of αvβ3 in the sprouting angiogenic tip cells. Values represent means ± SEM, 2ΔΔCT analysis. Scale bar: 50 μm.
A Jagged1/Notch interaction drives integrin αvβ3 expression in VSMCs. (A) VSMC culture on a Jag1-coated substrate increased mRNA expression of integrin β3 5.3-fold by qPCR (± 0.7; n = 6). Culture on Jag1 also up-regulated canonical Notch-downstream genes HES1 (3.4 ± 1.4, n = 3) and HEY2 (2.4 ± 0.5, n = 4). (B) FACS analysis of VSMCs found that surface expression of integrin αvβ3 increased 3-fold in response to culture on a Jag1-coated substrate. (C) Representative images of retinas from mice treated with vehicle (DMSO) or DAPT to block Notch signaling from P3-P6, and analyzed P7. Retinas were immunostained with Abs against integrin β3 (green) and αSMA (red) to label VSMCs, and CD31 (blue) to label endothelial cells. DAPT treatment inhibited the expression of αvβ3 in arterial-associated VSMCs, but did not inhibit the expression of αvβ3 in the sprouting angiogenic tip cells. Values represent means ± SEM, 2ΔΔCT analysis. Scale bar: 50 μm.
Integrin αvβ3 regulates arterial VSMC coverage in vivo
We next considered whether αvβ3 expression on VSMCs might contribute to arterial VSMC coverage during retinal development. We used pharmacologic and genetic approaches to block β3 expression and function in vivo. First, we characterized retinas from the β3 knockin “DiYF mice,” which express an inactive β3 subunit with 2 critical tyrosines converted to phenylalanines, producing deficient αvβ3 signaling.30,31 Retinas were harvested at P3, P5, and P7 and blood vessels were labeled using an endothelial-specific lectin and αSMA to visualize endothelial cells and VSMCs, respectively (Figure 4A). VSMC coverage was quantified by measuring the arterial αSMA labeling as a percentage of endothelial outgrowth from the optic nerve to the vascular plexus periphery, along each arterial branch. At each time point, we observed significantly less αSMA+ perivascular cell coverage in DiYF compared with wild-type mice (Figure 4B). The reduced mural cell coverage seems specific to VSMCs, as pericyte coverage did not differ between DiYF and wild-type animals (supplemental Figure 5). Arteries in retinas from mice completely deficient in αvβ3 showed a delay in VSMC coverage compared with age-matched controls at P4, yet by P7, we observed normal vascular patterning and maturation (data not shown).
Integrin β3 is required for VSMC coverage in developmental angiogenesis. (A) Representative images of retinas undergoing early postnatal retinal developmental angiogenesis at 3 different time points: P3, P5, and P7. Mice with deficient downstream αvβ3 signaling (DiYF) have less arterial VSMC coverage at each time point than age-matched wild-type (C57BL/6) controls. Retinas were immunostained with isolectin GS-IB4 from Griffonia simplificonia (lectin, red) to label the endothelium and αSMA to label VSMCs (green). (B) VSMC coverage was measured along each artery as a percentage of the outgrowth of the vascular plexus (dotted lines). Arterial coverage is reduced by 30% at p3, 22% at p5, and 14% at P7. (C-D) Intravitreal injection of blocking Abs against integrin β3 (D) reduces arterial αSMA staining compared with control (C). (E) The β3 function-blocking Ab reduced arterial VSMC coverage by 74% (P = .01). Values represent means ± SEM. C57BL/6 (n = 12 [P3], n = 23 [P5], n = 19 [P7]), DiYF (n = 15 [P3], n = 23 [P5], n = 21 [P7]), isotype control-injected, n = 5; anti-β3 injected, n = 6, where n is the number of retinas analyzed. For the DiYF time course, P = 1.4 × 10−4 (P3), P = 7.5 × 10−9 (P5), P = 1.0 × 10−3 (P7). Scale bars: (A) 150 μm (P3), 300 μm (P5), 600 μm (P7); and (C-D) 50 μm.
Integrin β3 is required for VSMC coverage in developmental angiogenesis. (A) Representative images of retinas undergoing early postnatal retinal developmental angiogenesis at 3 different time points: P3, P5, and P7. Mice with deficient downstream αvβ3 signaling (DiYF) have less arterial VSMC coverage at each time point than age-matched wild-type (C57BL/6) controls. Retinas were immunostained with isolectin GS-IB4 from Griffonia simplificonia (lectin, red) to label the endothelium and αSMA to label VSMCs (green). (B) VSMC coverage was measured along each artery as a percentage of the outgrowth of the vascular plexus (dotted lines). Arterial coverage is reduced by 30% at p3, 22% at p5, and 14% at P7. (C-D) Intravitreal injection of blocking Abs against integrin β3 (D) reduces arterial αSMA staining compared with control (C). (E) The β3 function-blocking Ab reduced arterial VSMC coverage by 74% (P = .01). Values represent means ± SEM. C57BL/6 (n = 12 [P3], n = 23 [P5], n = 19 [P7]), DiYF (n = 15 [P3], n = 23 [P5], n = 21 [P7]), isotype control-injected, n = 5; anti-β3 injected, n = 6, where n is the number of retinas analyzed. For the DiYF time course, P = 1.4 × 10−4 (P3), P = 7.5 × 10−9 (P5), P = 1.0 × 10−3 (P7). Scale bars: (A) 150 μm (P3), 300 μm (P5), 600 μm (P7); and (C-D) 50 μm.
To confirm a role for αvβ3 in arterial maturation, a β3 integrin function-blocking Ab was injected intravitreally into wild-type mice at P5 and retinas were examined at P8 for the extent of vessel maturation and VSMC arterial coverage. We chose to initiate treatment at P5 because arterial patterning has been established at this developmental stage, but with minimal arterial VSMC coverage. The β3 function-blocking Ab suppressed angiogenesis in the deep vascular plexus of the retina, consistent with previous reports of the antiangiogenic activity of β3 integrin antagonists28,32 (supplemental Figure 6). After treatment with anti-β3, we observed reduced αSMA+ coverage of the developing arteries of P8 mice relative to control (IgG)–injected mice (Figure 4C-E). Thus, both genetic and pharmacologic approaches indicate that αvβ3 expression on VSMCs is required for the coverage and maturation of newly developing arteries.
VWF regulates arterial VSMC coverage in vivo
During vascular development both endothelial cells28 and VSMCs33 transiently express integrin αvβ3, which is a receptor for VWF. Importantly, VWF is an RGD-containing adhesion protein that is unique to the vascular basement membrane.34,35 Previous studies have revealed that endothelial cells and VSMCs share a common basement membrane in mature arteries.36,37 Because endothelial cells and VSMCs both up-regulate integrin ανβ3, a receptor for their shared basement membrane, during angiogenesis, we considered whether VWF might regulate VSMC-dependent arterial maturation. To test this in vivo, we first characterized the VWF expression pattern during retinal development in mice from P1 to P21. Whole-mount staining revealed that VWF accumulated in the endothelial basement membrane during vessel maturation (supplemental Figure 7). We observed a strong accumulation in major arteries and veins, but only minimal accumulation in the capillary beds. During the first month of development, VWF accumulated along arteries in a pattern that completely coaligned with the perivascular outgrowth of VSMCs (supplemental Figure 7).
To determine the functional role of basement membrane VWF in arterial maturation, we examined retinas from VWF KO mice. Arterial VSMC coverage was quantified by measuring the arterial αSMA labeling as a percentage of endothelial outgrowth, from the optic nerve to the vascular plexus periphery, along each arterial branch. During the early stages of developmental angiogenesis, VWF KO mice showed significantly less VSMC coverage compared with wild-type controls (Figure 5). However, pericyte coverage did not differ between VWF KO and wild-type animals (supplemental Figure 5). Similar to our observations in DiYF mice (Figure 4), early defects in developmental angiogenesis were resolved by 3 weeks of age, suggesting the existence of compensatory/redundancy mechanisms. These finding suggest that VWF regulates an early step in VSMC coverage of developing arteries.
VWF is required for VSMC coverage in developmental angiogenesis. (A) Representative images of retinas undergoing early postnatal retinal developmental angiogenesis at 3 different time points: P3, P5, and P7. Retinas were immunostained with CD31 (red) to label the endothelium and αSMA to label VSMCs (green). (B) VWF KO mice have significantly less VSMC coverage compared with age-matched controls, measured along each artery as a percentage of the outgrowth of the vascular plexus (dotted lines). Arterial coverage is reduced 37% at P3, 21% at P5, and 17% at P7. Values represent means ± SEM. C57BL/6 (n = 12 [P3], n = 23 [P5], n = 19 [P7]), VWF KO (n = 14 [P3], n = 25 [P5], n = 14 [P7]), where n is the number of retinas analyzed. P = 9.3 × 10−4 (P3), P = 1.7 × 10−9 (P5), P = 7.3 × 10−3 (P7). Scale bars: 150 μm (P3), 300 μm (P5), and 600 μm (P7).
VWF is required for VSMC coverage in developmental angiogenesis. (A) Representative images of retinas undergoing early postnatal retinal developmental angiogenesis at 3 different time points: P3, P5, and P7. Retinas were immunostained with CD31 (red) to label the endothelium and αSMA to label VSMCs (green). (B) VWF KO mice have significantly less VSMC coverage compared with age-matched controls, measured along each artery as a percentage of the outgrowth of the vascular plexus (dotted lines). Arterial coverage is reduced 37% at P3, 21% at P5, and 17% at P7. Values represent means ± SEM. C57BL/6 (n = 12 [P3], n = 23 [P5], n = 19 [P7]), VWF KO (n = 14 [P3], n = 25 [P5], n = 14 [P7]), where n is the number of retinas analyzed. P = 9.3 × 10−4 (P3), P = 1.7 × 10−9 (P5), P = 7.3 × 10−3 (P7). Scale bars: 150 μm (P3), 300 μm (P5), and 600 μm (P7).
Notch up-regulates VSMC adhesion to endothelial VWF via αvβ3
To explore the link between Notch signaling in VSMCs and their adhesion to VWF, VSMCs treated with or without Jag1 were allowed to attach to a VWF-coated substrate in vitro. Jag1-stimulated VSMCs, which up-regulate αvβ3 expression (Figure 3A-B), showed enhanced adhesion to VWF compared with control cells not exposed to Jag1 (Figure 6A-B). This increased adhesion could be disrupted by addition of function-blocking Abs specific to either αvβ3 or to the RGD-binding site on VWF, but not the addition of β1 integrin function-blocking Abs (Figure 6A-B). In addition, inhibition of Notch signaling with DAPT suppressed VSMC adhesion to VWF (supplemental Figure 8A). Together, these findings suggest that Jag1 ligation leads to up-regulation of integrin αvβ3, allowing VSMCs to adhere to the VWF-containing vascular basement membrane.
VSMC adhesion to VWF is because of a Notch-dependent up-regulation of integrin αvβ3. (A-B) The ability of VSMCs to adhere to a VWF-coated substrate was quantified following 18-hour culture on control (IgG) or Jag1-coated plates (to stimulate Notch signaling). Blocking Abs specific to αvβ3 (A, LM609) and the RGD-binding site of VWF (B, anti-152B) prevented Notch-dependent adhesion to VWF, whereas a blocking Ab specific to β1 integrins (A, anti-P4C10) did not. (C-K) Representative images of in vitro fibrin bead angiogenesis assays in which HUVECs (green) were cultured on microcarrier beads and suspended in a fibrin gel with VSMCs (red). (C) A fibrin gel was fixed and immunostained with a fluorescein-labeled Ulex lectin specific to endothelial cells and with Abs to VWF showed that the in vitro endothelial tubes produced a VWF-rich basement membrane. The addition of a blocking Ab to VWF (anti-152B) significantly reduced copatterning, as quantified by pixel overlap in ImageJ, between VSMCs and endothelial tubes (F-G) compared with controls (D-E). Blocking Notch signaling specifically in VSMCs by siRNA knockdown (J-K) significantly reduced copatterning compared with transfection with All Stars negative control siRNA (H-I). Arrows point to regions magnified, shown in panels E, G, I, and K. (L) VSMC-endothelial copatterning was reduced 66% when VWF blocking Ab was added to culture media and 69% when Notch3 was transfected into VSMCs. (M) Notch3 receptor knockdown in VSMCs was confirmed by Western blot in comparison to VSMCs transfected with All Stars negative control siRNA, as was the resultant decrease in Notch signaling as measured by decreased Hey2 expression, a canonical Notch downstream transcription factor. Molecular weight (MW) of N3 precursor: 280 kDa; MW of truncated N3: 120 kDa. Values represent means ± SEM. IgG control (n = 14); α-152b (n = 17); control siRNA (n = 23), Notch3 siRNA (n = 23); where n is the number of beads analyzed; P = 2.5 × 10−13 (α-152b); P = 8.4 × 10−13 (N3 siRNA). Scale bars: (D,F,H,J) 325 μm; and (C,E,G,I,K) 150 μm.
VSMC adhesion to VWF is because of a Notch-dependent up-regulation of integrin αvβ3. (A-B) The ability of VSMCs to adhere to a VWF-coated substrate was quantified following 18-hour culture on control (IgG) or Jag1-coated plates (to stimulate Notch signaling). Blocking Abs specific to αvβ3 (A, LM609) and the RGD-binding site of VWF (B, anti-152B) prevented Notch-dependent adhesion to VWF, whereas a blocking Ab specific to β1 integrins (A, anti-P4C10) did not. (C-K) Representative images of in vitro fibrin bead angiogenesis assays in which HUVECs (green) were cultured on microcarrier beads and suspended in a fibrin gel with VSMCs (red). (C) A fibrin gel was fixed and immunostained with a fluorescein-labeled Ulex lectin specific to endothelial cells and with Abs to VWF showed that the in vitro endothelial tubes produced a VWF-rich basement membrane. The addition of a blocking Ab to VWF (anti-152B) significantly reduced copatterning, as quantified by pixel overlap in ImageJ, between VSMCs and endothelial tubes (F-G) compared with controls (D-E). Blocking Notch signaling specifically in VSMCs by siRNA knockdown (J-K) significantly reduced copatterning compared with transfection with All Stars negative control siRNA (H-I). Arrows point to regions magnified, shown in panels E, G, I, and K. (L) VSMC-endothelial copatterning was reduced 66% when VWF blocking Ab was added to culture media and 69% when Notch3 was transfected into VSMCs. (M) Notch3 receptor knockdown in VSMCs was confirmed by Western blot in comparison to VSMCs transfected with All Stars negative control siRNA, as was the resultant decrease in Notch signaling as measured by decreased Hey2 expression, a canonical Notch downstream transcription factor. Molecular weight (MW) of N3 precursor: 280 kDa; MW of truncated N3: 120 kDa. Values represent means ± SEM. IgG control (n = 14); α-152b (n = 17); control siRNA (n = 23), Notch3 siRNA (n = 23); where n is the number of beads analyzed; P = 2.5 × 10−13 (α-152b); P = 8.4 × 10−13 (N3 siRNA). Scale bars: (D,F,H,J) 325 μm; and (C,E,G,I,K) 150 μm.
To examine the functional relevance of VWF in the basement membrane in the context of a VSMC-endothelial cell interaction, we adapted an in vitro model system in which VSMCs associate with outgrowing endothelial VWF-producing tubes, a process which could be readily visualized in real time.23 Microcarrier beads coated with primary HUVECs were suspended in a fibrin gel containing human aortic VSMCs. The endothelial cells form large lumen-containing vascular networks within the fibrin gel. The vascular networks that form deposit VWF into a basement membrane-like structure (Figure 6C). VSMCs migrate toward the outgrowing tubes and associate with them, approximating the relationship between endothelial cells and mural cells observed in vivo. The addition of a VWF function-blocking Ab, specific to the RGD site on VWF (anti-152b),35 to the culture media reduced VSMC interaction with outgrowing endothelial tubes by ∼ 70% compared with control IgG (Figure 6D-G,L). These findings indicate that VWF can serve as a common adhesion protein for both endothelial cells and VSMCs, providing a mechanism for these cell types to engage a common basement membrane and thereby enable specific perivascular coverage during arterial development.
To investigate the impact of Notch signaling on VSMC interaction with VWF in the endothelial basement membrane, we examined the effects of blocking Notch signaling on copatterning during tube formation in vitro. When Notch signaling was inhibited with DAPT, we observed decreased copatterning between VSMCs and endothelial tubes by ∼ 50% relative to control cells (supplemental Figure 8B-F). When Notch signaling was specifically blocked in VSMCs by knockdown of Notch3 gene expression (Figure 6M), we found that VSMCs lacking Notch3 showed significantly less copatterning with endothelial tubes compared with control VSMCs (Figure 6H-L). Knockdown of Notch3 decreased both the mRNA and protein expression of integrin β3 by ∼ 40%, as measured by qPCR and Western blot (supplemental Figure 9). Similar to our observations that VWF was required for VSMC-endothelial interaction (Figure 6D-G), we found that Notch signaling in VSMCs, which up-regulates αvβ3, was also required for this interaction. Taken together, these findings reveal a central role for Notch signaling leading to αvβ3-mediated adhesion between VSMC and VWF in the endothelial basement membrane.
Discussion
Vascular development and angiogenesis depend first on endothelial tip cell invasion, followed by a series of maturation steps, which include recruitment of perivascular cells and the deposition of a mature basement membrane. Notch signaling has been linked to artery/vein patterning,38 endothelial tip and stalk cell formation,25,39 as well as VSMC coverage of newly formed arteries,6,8 leading to the formation of functional vascular plexus. However, it is unclear how Notch signaling facilitates vessel maturation, specifically arterial VSMC coverage. Previous studies have implicated Notch in PDGFRβ expression,13 a receptor critically involved in pericyte function.40 In the current study, we have identified a new role for Notch that appears to regulate the specific recruitment of VSMCs to maturing arteries.
We present several lines of evidence that Notch signaling is critical for VSMC recruitment to arteries because of its regulation of αvβ3, leading to VSMC interaction with VWF in the endothelial basement membrane. First, integrin αvβ3 was among the most up-regulated genes following VSMC interaction with the Notch ligand Jag1 in vitro. Integrins are known to play a critical role in cell adhesion and cell migration.41 Importantly, αvβ3 expression gives a nucleated cell the unique ability to bind to VWF, which is the only tissue-restricted αvβ3 ligand available in the endothelial basement membrane.34 When Notch signaling was inhibited in neonatal pups, we observed discontinuous arterial VSMC coverage of arteries and a VSMC morphology that was consistent with an adhesion or migratory defect. In addition, mice specifically lacking the Notch ligand Jag1 in the endothelium showed a paucity of VSMCs immediately adjacent to the endothelium. Second, mice expressing a defective integrin αvβ3 or those deficient in VWF showed a significant delay in VSMC arterial coverage, suggesting that this receptor-ligand pair plays a critical role in VSMC recruitment and subsequent arterial maturation. However, it is important to note that this phenotype was transient and that by day 21 of development the arteries appeared normal, suggesting a redundant or compensatory mechanism might be involved. This is not entirely surprising because adult mice deficient in VWF16 or αvβ342 do not show a discernible vascular defect; this has been linked in part to compensation.43 Injection of an αvβ3 antagonist into wild-type mice at postnatal day 5 resulted in a disruption of VSMC arterial coverage measured on day 8, corroborating the developmental phenotype observed in the genetically altered mice.
From their restricted location on the arterial endothelium, Notch ligands and receptors ensure the maturation of arterial vessels. This process is specific to arterial development because arterial VWF staining and VSMC coverage were coincident, and this was not observed in developing veins within the retina. This cascade ensures that VSMCs are selectively localized to mature arterial regions, rather than regions that continue to undergo extensive vascular remodeling.
Previous in vivo studies point to a critical role for Notch in the VSMC compartment.6,8,9 Notch3-deficient mice have thinner VSMC coverage and a discontinuous layer of noncohesive VSMCs,6 reminiscent of what we observed in mice with deficiencies in β3 signaling or VWF expression. Furthermore, VSMCs from Notch3−/− mice displayed migratory deficiencies, whereas Notch3 overexpression affected actin cytoskeleton dynamics.6 These observations are consistent with our description that Notch signaling in VSMCs regulates integrin αvβ3, thereby affecting cell adhesion and migration.
It was recently reported that endothelial VWF regulates angiogenesis.19 The work described herein reveals that VWF is acting not only on endothelial cells,19 but is also critical for VSMCs and their ability to interact with the endothelial basement membrane. Our findings raise the possibility that VWF secreted into the basement membrane by endothelial cells could participate in VSMC outside-in signaling. As such, VWF in the endothelial basement membrane may serve a dual purpose, regulating both endothelial network formation as well as VSMC recruitment leading to arterial maturation.
Vessel maturation, or the lack thereof, has relevance to cancer, diabetic retinopathy, restenosis, wound healing, and stroke, among other pathologies. During pathologic angiogenesis associated with tumor growth, blood vessels lack an intact basement membrane and are highly disorganized, leading to increased vascular permeability.44 Interestingly, these vessels show minimal expression of VWF and are relatively devoid of perivascular cell coverage.45
While tumor vessels have reduced perivascular coverage and generally lack VSMCs, other pathologic vessels, such as those formed during wound repair, suffer from the recruitment of too many VSMCs.46 Accumulation of VSMCs in the tunica intima leads to coronary atherosclerosis and restenosis following angioplasty, and Notch signaling likely plays a role in this recruitment.47 In addition, integrin αvβ3 is up-regulated on VSMCs during restenosis, and is considered a therapeutic target to prevent arterial narrowing.48 In addition to osteopontin,48 Notch signaling could be a driving factor in the well-described up-regulation of αvβ3 on VSMCs in restenosis. In this case, inhibitors of Notch signaling as well as αvβ3 antagonists are potential therapeutics worth considering.
In the current study, we have identified new roles for both Notch and VWF in regulating arterial maturation. Our findings suggest that endothelial cells display the ligand Jag1 to increase the expression of integrin αvβ3, a receptor for VWF, on VSMCs. We found that arterial VWF is coincident with VSMC patterning during development and is required for endothelial VSMC coverage in vitro, suggesting that VWF in the endothelial basement membrane is used by αvβ3-expressing VSMCs. We propose a model in which developing arteries use Notch signaling between endothelial cells and local perivascular cells to generate integrin β3-expressing VSMCs that gain the ability to engage VWF within the endothelial basement membrane and potentiate the maturation of newly formed arteries (supplemental Figure 10).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Marilyn Farquhar for the use of the electron microscopy facility, and Dylan Barnes for electron microscopy sample preparation. They also thank Dennis Young for use of the FACS facility and sample analysis, and Denisa Wagner for the kind gift of VWF KO mice.
This work was supported by grants from the National Institutes of Health: PO1 HL057900 (D.A.C), R37 CA050286 (D.A.C.), and T32 CA009523 (L.S.).
National Institutes of Health
Authorship
Contribution: L.S. and D.A.C. designed the project; L.S. carried out the experiments, except the microarray which was carried out by E.A.M. and D.J.S., and the IHC of Jag1 ECKO adult tissue which was carried out and analyzed by J.J.H. and M.L.I.-A.; A.Z., A.M., T.V.B., and Z.M.R. contributed new reagents; E.A.M., D.J.S., and S.M.W. contributed to experimental analysis; and L.S. and D.A.C. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr David A. Cheresh, Moores UCSD Cancer Center, 3855 Health Sciences Dr 0803, La Jolla, CA 92093-0803; e-mail: dcheresh@ucsd.edu.



![Figure 4. Integrin β3 is required for VSMC coverage in developmental angiogenesis. (A) Representative images of retinas undergoing early postnatal retinal developmental angiogenesis at 3 different time points: P3, P5, and P7. Mice with deficient downstream αvβ3 signaling (DiYF) have less arterial VSMC coverage at each time point than age-matched wild-type (C57BL/6) controls. Retinas were immunostained with isolectin GS-IB4 from Griffonia simplificonia (lectin, red) to label the endothelium and αSMA to label VSMCs (green). (B) VSMC coverage was measured along each artery as a percentage of the outgrowth of the vascular plexus (dotted lines). Arterial coverage is reduced by 30% at p3, 22% at p5, and 14% at P7. (C-D) Intravitreal injection of blocking Abs against integrin β3 (D) reduces arterial αSMA staining compared with control (C). (E) The β3 function-blocking Ab reduced arterial VSMC coverage by 74% (P = .01). Values represent means ± SEM. C57BL/6 (n = 12 [P3], n = 23 [P5], n = 19 [P7]), DiYF (n = 15 [P3], n = 23 [P5], n = 21 [P7]), isotype control-injected, n = 5; anti-β3 injected, n = 6, where n is the number of retinas analyzed. For the DiYF time course, P = 1.4 × 10−4 (P3), P = 7.5 × 10−9 (P5), P = 1.0 × 10−3 (P7). Scale bars: (A) 150 μm (P3), 300 μm (P5), 600 μm (P7); and (C-D) 50 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/9/10.1182_blood-2011-04-348706/4/m_zh89991185040004.jpeg?Expires=1765890422&Signature=3~sp~mXltFkozg6szlcSKihMyeN0gdEDJQ4nrDNw1eXL40~sDRBp~XeZoQ6tjGftDDDUpPDOiWaKJWDRAWU1MRi2WWGheB90OYYLyJ2RC2x1CWsDXmn0rqOMqwO8iNmYYMatBYFOJKtMG27hs7Yl4iYWWoM3DfuUjpxsJB7EFCw~uEscs9Gn97XxY3Jhxl-KyCH~llhUGRNqEWkeprAkH8728D6jSZVuSJf7KIjI7xavr1GCcmUq4-Qo86k~Bd2YRS80VJfqs9JgvDShyD6zWF1zLYavLsTbdhsNF5ItY25mMm~G7pB4k5kYQNUPD58o0-s3HWSJKRFxIaRpHSJ~Ag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. VWF is required for VSMC coverage in developmental angiogenesis. (A) Representative images of retinas undergoing early postnatal retinal developmental angiogenesis at 3 different time points: P3, P5, and P7. Retinas were immunostained with CD31 (red) to label the endothelium and αSMA to label VSMCs (green). (B) VWF KO mice have significantly less VSMC coverage compared with age-matched controls, measured along each artery as a percentage of the outgrowth of the vascular plexus (dotted lines). Arterial coverage is reduced 37% at P3, 21% at P5, and 17% at P7. Values represent means ± SEM. C57BL/6 (n = 12 [P3], n = 23 [P5], n = 19 [P7]), VWF KO (n = 14 [P3], n = 25 [P5], n = 14 [P7]), where n is the number of retinas analyzed. P = 9.3 × 10−4 (P3), P = 1.7 × 10−9 (P5), P = 7.3 × 10−3 (P7). Scale bars: 150 μm (P3), 300 μm (P5), and 600 μm (P7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/9/10.1182_blood-2011-04-348706/4/m_zh89991185040005.jpeg?Expires=1765890422&Signature=QM3Mob4VSp0gfk5kFBgMyOuoH8IVyUiN9a36K~sz5OmYV0e3nNIZCTyfhC4A0vPHHRZhqmTwJAccKz7KsWultmL1Ks6wgZRLJEkUGAKgGN1Caz3Q9XhWEVPhIWoWFraToL2LQ3XtvFOd79P6FZVH~qmAEeIbdsALNBJPcqvq1pkSgI19bjjtllt7qZYXnTiIjJ6mejNMBhnCgul~KUKO9lnhWwDT5qxHrhCKHBsfIA4DKrsTOgxAepSj~0GeU1QMrLsOrFOrRRaNOrAiWhKxrE6qngvbGHrGYrqAv9Cz7X0ZBfPkNa6pdQsDVGqxmbsOYMGSHAXqBlnz2pcVyA4yOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
