Abstract
Multipotent, bone marrow–derived stromal cells (BMSCs, also known as mesenchymal stem cells [MSCs]), are culture-expanded, nonhematopoietic cells with immunomodulatory effects currently being investigated as novel cellular therapy to prevent and to treat clinical disease associated with aberrant immune response. Emerging preclinical studies suggest that BMSCs may protect against infectious challenge either by direct effects on the pathogen or through indirect effects on the host. BMSCs may reduce pathogen burden by inhibiting growth through soluble factors or by enhancing immune cell antimicrobial function. In the host, BMSCs may attenuate pro-inflammatory cytokine and chemokine induction, reduce pro-inflammatory cell migration into sites of injury and infection, and induce immunoregulatory soluble and cellular factors to preserve organ function. These preclinical studies provide provocative hints into the direction MSC therapeutics may take in the future. Notably, BMSCs appear to function as a critical fulcrum, providing balance by promoting pathogen clearance during the initial inflammatory response while suppressing inflammation to preserve host integrity and facilitate tissue repair. Such exquisite balance in BMSC function appears intrinsically linked to Toll-like receptor signaling and immune crosstalk.
Introduction
Mesenchymal stromal cells (MSCs) are nonhematopoietic, multipotent progenitor cells that differentiate into bone marrow (BM) stroma as well as adipocytes, chondrocytes, and osteocytes. Initially expanded from BM, MSCs can also be culture-expanded from other sources, including umbilical blood, adipose tissue, and dental pulp. BMSC designation requires that in vitro–expanded cells be plastic-adherent, express surface CD73, CD90, and CD105 but not hematopoietic markers (CD14, CD34, CD45, and HLA-DR), and differentiate into osteoblastic, adipocytic, and chondroblastic lineages in vitro.1 BMSCs produce cytokines, chemokines, and extracellular matrix proteins that support hematopoietic stem cell (HSC) survival and engraftment, influence immune effector cell development, maturation, and function, and inhibit alloreactive T-cell responses2-5 (Figure 1). Given their immunomodulatory properties, BMSCs are being used as cellular agents to treat autoimmune6 and alloimmune7 diseases. Yet a role for BMSCs in host defense is also emerging.8 In this capacity, BMSCs may augment antimicrobial responses, abridge pro-inflammatory and damage responses, and ameliorate injury caused by the host response to pathogen. This review summarizes preclinical studies reporting on the capabilities of BMSCs to augment host defense and preserve host integrity after infectious challenge. Furthermore, the review broadens the appreciation for how nonhematopoietic cells contribute to host defense and offers direction for further investigation into how BMSCs may ultimately evolve into novel antimicrobial cellular therapy for immunocompromised patients.
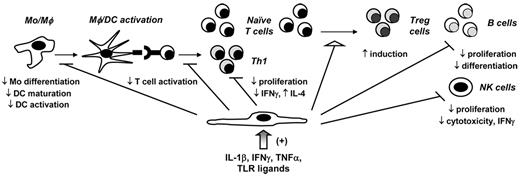
MSCs down-modulate in vitro pro-inflammatory responses. Using in vitro assays, BMSCs have been shown to interact with immune effector cells either through direct contact or through the induction of paracrine immunomodulatory soluble factors, such as galectin-1, HO-1, HLA-G5, hepatocyte growth factor, IL-10, IDO, PGE2, and TGF-β1. Specifically, BMSCs inhibit monocyte (Mo) differentiation into DCs as well as DC activation and maturation; inhibit naive T-cell activation, Th1 proliferation, and cytokine production (TNF-α and IFN-γ); inhibit B-cell proliferation and differentiation into plasma cells; and inhibit NK cell proliferation, cytotoxicity, and IFN-γ production. In contrast, BMSCs induce Treg cells. Soluble factors, including IL-1β, IFN-γ, TNF-α, and TLR ligands, have been shown to provide necessary signals for in vitro MSC activation.
MSCs down-modulate in vitro pro-inflammatory responses. Using in vitro assays, BMSCs have been shown to interact with immune effector cells either through direct contact or through the induction of paracrine immunomodulatory soluble factors, such as galectin-1, HO-1, HLA-G5, hepatocyte growth factor, IL-10, IDO, PGE2, and TGF-β1. Specifically, BMSCs inhibit monocyte (Mo) differentiation into DCs as well as DC activation and maturation; inhibit naive T-cell activation, Th1 proliferation, and cytokine production (TNF-α and IFN-γ); inhibit B-cell proliferation and differentiation into plasma cells; and inhibit NK cell proliferation, cytotoxicity, and IFN-γ production. In contrast, BMSCs induce Treg cells. Soluble factors, including IL-1β, IFN-γ, TNF-α, and TLR ligands, have been shown to provide necessary signals for in vitro MSC activation.
TLRs and their ligands link pathogen- and damage-associated inflammatory pathways and modulate BMSC activation and function
Immunity works in a coordinated and redundant manner to eliminate nonself (“immune surveillance”) and to preserve host integrity by not reacting against self (“immune tolerance”). Both innate and adaptive immune responses produce soluble factors, including cytokines and chemokines that culminate to eliminate pathogen challenge and to regulate immune cell function. These distinct, but not mutually exclusive, arms of the immune system crosstalk at multiple levels through direct contact as well as an elaborate paracrine network of soluble factors.9 Hematopoietic and nonhematopoietic cells produce an inflammatory microenvironment that directly influences innate and adaptive immune cell activation, differentiation, and function. Within hours of exposure to microbes, soluble pathogen-associated molecular patterns (PAMPs), expressed by microbes and danger-associated molecular patterns (DAMPs) associated with tissue injury, are recognized by Toll-like receptors (TLRs) present on innate effector cells.10 TLR ligation triggers phagocytosis and the release of inflammatory mediators, which also serve as secondary signals to initiate humoral and cellular antimicrobial immunity. Consequently, TLR activation provides an immediate host response to microbial invasion as well as links innate and adaptive responses.11
TLR ligands can also function as DAMPs, promoting inflammation through macrophage production of reactive oxygen species (ROS) in response to both infectious12 and noninfectious injury.13 Interestingly, TLR1, TLR2, and TLR4 have recently been shown to enhance murine macrophage bactericidal activity by augmenting mitochondrial ROS production, demonstrating a novel pathway for eliminating intracellular pathogens mediated by TLR signaling.14 Whether released from necrotic cells or induced by pro-inflammatory cytokines, DAMPs can co-associate with nuclear (DNA, RNA) and soluble (PAMPs, cytokines) factors and bind to TLR2, TLR4, and RAGE (receptor for advanced glycation end products). In this regard, TLR-DAMP ligation activates pro-inflammatory transcription cascades, further linking pathogen- and sterile-induced inflammatory pathways.15
Given the extent to which pro-inflammatory cascades are activated by PAMPs and DAMPs, regulation is vital to ensure that acute inflammation does not progress to chronic inflammation, fibrosis, or carcinogenesis.16 Pathogen- and sterile-induced inflammatory responses are regulated at multiple levels, including at the level of TLR signaling,17 cytokine activation and signaling,18 and MAPK and NF-κB gene transcription.19 For example, after TLR4-mediated ROS induction,20 antioxidant enzymes, such as heme oxygenase 1 (HO-1), attenuate pro-inflammatory innate and adaptive immune cell function during endotoxemia.21,22 In addition to soluble factors, hematopoietic cell populations, such as myeloid suppressor cells23 and regulatory T cells,24 can also decrease inflammatory responses through their production of indolamine-2,3-dioxygenase (IDO), IL-10, prostaglandin 2 (PGE2), and TGF-β.
TLR-mediated modulation in human BMSC function
TLR activation and signaling have traditionally been associated with host defense to microbes, so it is intriguing to note that human BMSCs express TLRs. Human BMSCs express TLR2, TLR3, and TLR4. TLR2 recognizes the most diverse amount of PAMPs, including bacterial lipoproteins, peptidoglycan, and lipoteichoic acids.25 Like TLR2, TLR4 recognizes bacterial motifs, such as lipopolysaccharide (LPS), as well as fungal elements.26 TLR3 recognizes viral double-stranded RNA and the synthetic TLR3 agonist, polyinosine-polycytidylic acid (poly I:C), and TLR2 ligation induces type I IFN production (IFN-α/β).27
TLR ligation has been reported to influence MSC differentiation, proliferation, migration, and immunomodulation.28,29 After in vitro LPS-TLR4 ligation, human BMSCs produce cytokines, chemokines, and growth factors.30,31 Specifically, TLR4- and cytokine-stimulated human MSCs may protect against oxidative stress through activation of antioxidant and anti-inflammatory pathways.32,33 With respect to effects of TLR ligation on MSC-mediated immunosuppression, Lombardo et al found that TLR3 and TLR4 ligation did not affect the ability of adipose-derived stem cells (ASCs) to inhibit T-cell proliferation, nor did TLR ligation change MSC surface HLA-II and costimulatory CD80/CD86 expression.32 However, using human BMSCs, Liotta et al found that costimulation with TLR3 and TLR4 agonists inhibited MSC-mediated suppression by down-regulating Notch ligand Jagged-1.34 Furthermore, TLR3 and TLR4 activation did not affect MSC induction of IDO and PGE2, implying that TLR ligation could restore host defense against viral infections.34 In contrast to the results of Liotta et al,34 Opitz et al showed that human BMSCs prestimulated with TLR3 and TLR4 agonists had enhanced IDO-mediated T-cell suppression.35 The investigators defined a novel IFN-γ–independent pathway involving an autocrine IFN-β signaling loop and requiring protein kinase R.35
Why the disparate results in MSC-mediated immune modulation following TLR ligation? Differences in study techniques, purity of MSC populations, and magnitude of pro-inflammatory stimuli may be possible explanations. Alternatively, it is possible that TLR ligation offers a novel way to modify human BMSCs to function as either pro-inflammatory (“MSC1”) or immunosuppressive (“MSC2”) cells, as suggested by studies from Waterman et al.36 Using LPS (TLR4) and poly I:C (TLR3) priming, these investigators showed that MSCs produce different cytokine profiles, reflecting activation of distinct TLR signaling cascades. Specifically, MSC1 (TLR4-primed) cells produced IL-6, IL-8, and TGF-β1/3, whereas MSC2 (TLR3-primed) cells produced PGE2 and IDO but had decreased TGF-β1/3 induction. Furthermore, when cocultured with T cells, LPS-primed MSCs augmented T-cell activation, whereas poly I:C-primed MSCs suppressed T-cell activation.36
In addition to effects of TLR ligation on MSC function, in vitro culture conditions have been reported to up-regulate TLR expression on human MSCs. Cho et al showed that hypoxic conditions increase TLR2 mRNA expression on human ASCs.37 Hypoxia also induces human BMSCs to produce vascular endothelial growth factor, fibroblast growth factor 2, insulin-like growth factor 1, and hepatocyte growth factor production,31 which may ultimately influence MSC repair and migratory capacity. In vitro culture conditions using IL-1β (25 ng/mL), IFN-γ (103 U/mL), TNF-α (50 ng/mL), and IFN-α (3 × 103 U/mL), composed to mimic inflammatory conditions, can also up-regulate TLR expression on BMSCs and enhance their gene expression of TGF-β and protein secretion of IL-6 and IL-23.38 BMSCs stimulated with these inflammatory conditions in combination with TLR ligation had reduced ability to suppress T-cell proliferation38 and had enhanced recruitment of immune inflammatory cells,39 suggesting that BMSCs may function more like pro-inflammatory cells in the context of TLR ligation. In contrast, human ASCs have not been found to up-regulate TLR expression in response to TLR ligands (LPS and poly I:C) alone or in combination with IFN-γ.32 However, further testing is required before concluding that stromal-cell source associates with distinct TLR responses.
Cytokine-mediated modulation in human BMSC function
Host response to infectious challenge results in induction of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IFN-γ, which have direct antimicrobial effects as well as immunomodulatory effects on innate and adaptive immune cell activation and function.40 Similar to immune effector cells, human BMSCs require activation by pro-inflammatory cytokines to mediate their in vitro anti-inflammatory effects. For example, IL-1β1 produced by CD14+ cells activates human BMSCs to suppress in vitro T-cell activation and proliferation.41 However, cytokine-mediated activation of MSC function does not necessarily result in MSC response that is unilaterally immunosuppressive. For example, Chan et al have shown that levels of endogenous IFN-γ change human BMSC function from antigen-presenting cells to allo-inhibitory cells.42 In addition, Krampera et al have shown that exogenous IFN-γ–stimulated BMSCs inhibit NK cells and T cells via IDO production.43 Finally, other pro-inflammatory cytokines, such as TNF-α and IFN-γ, can modify human BMSCs to produce PGE2, TGF-β1, and HO-1, which can induce CD4+CD25+FoxP3+ T-regulatory (Treg) cells from naive T cells in vitro.44,45 However, Mougiakakos et al have recently shown that human BMSCs prestimulated with an alloreactive in vitro microenvironment had decreased production in HO-1 and IL-10 and subsequent attenuation in HO-1–mediated Treg induction.46
Collectively, results from these in vitro studies highlight key areas of investigation for defining how human BMSCs might respond to infectious challenge. First, pro-inflammatory cytokines and TLR ligands inherent to infectious challenge can modify in vitro BMSC activation and function. Second, BMSC activation and response may be augmented through the ability of MSCs to up-regulate cytokine and TLR receptor expression in response to a pro-inflammatory microenvironment. Third, BMSC immune function can change, suggesting that plasticity in function may allow MSCs to respond to a changing in vivo microenvironment. That is, levels of pro-inflammatory soluble factors (cytokines, chemokines, oxygen radicals, and hypoxia) in the microenvironment probably fluctuate based on levels of pathogen burden, anti-inflammatory soluble factors, and longevity of response (ie, soluble factor half-life). In this regard, BMSCs may function to provide a form of “immune surveillance,” characterized by the ability to sense a changing microenvironment to which MSCs respond as either pro-inflammatory or anti-inflammatory cells in the context of infection.
Defining how the in vivo microenvironment affects BMSC activation and function
BMSCs serve as significant modulators of both innate and adaptive immune responses in the context of inflammation and injury.47 Constitutive and inducible cytokines, chemokines, and growth factors within the microenvironment probably compose an immunomodulatory milieu that initiates potent BMSC suppression of inflammation.48 That is, in vivo MSC activation may be mediated by soluble factors within the microenvironment that induce transcription factors within MSCs, similar to how transcription factor activation differentiates naive CD4+ T cells into distinct CD4+ subsets.49 Likewise, cytokines and TLR ligands might modify MSC function using intracellular signaling pathways that dictate monocytes to function as either pro-inflammatory or anti-inflammatory cells.50,51 Nonetheless, how pathogen itself and how host inflammatory and immunomodulatory soluble factors produced in response to pathogen influence in vivo human BMSC activation and what in vivo effects activated MSCs have on host immune function currently remain undefined (Figure 2).
Signals mediating in vivo BMSC activation in the context of infectious challenge and their subsequent effects on MSC-mediated immunomodulation remain largely undefined. Infectious challenge is associated with induction in pathogen- and sterile-induced soluble factors that may influence BMSC activation and subsequent immunomodulation. As infectious burden increases, so too do associated inflammatory and damage burdens at the site of infection. By nature of their multipotency, BMSCs may possess plasticity in their immunomodulatory effects to respond to the changing microenvironment at the site of infectious challenge. Specifically, the inflammatory and damage milieu may instruct or “license” BMSC activation and function specific to the microenvironment in which BMSCs reside or to which they migrate as a consequence of infection. Further investigation is needed to define these signals within the in vivo microenvironment as well as their effects on MSC activation in addition to resultant immunomodulatory effects of MSCs on host immunity.
Signals mediating in vivo BMSC activation in the context of infectious challenge and their subsequent effects on MSC-mediated immunomodulation remain largely undefined. Infectious challenge is associated with induction in pathogen- and sterile-induced soluble factors that may influence BMSC activation and subsequent immunomodulation. As infectious burden increases, so too do associated inflammatory and damage burdens at the site of infection. By nature of their multipotency, BMSCs may possess plasticity in their immunomodulatory effects to respond to the changing microenvironment at the site of infectious challenge. Specifically, the inflammatory and damage milieu may instruct or “license” BMSC activation and function specific to the microenvironment in which BMSCs reside or to which they migrate as a consequence of infection. Further investigation is needed to define these signals within the in vivo microenvironment as well as their effects on MSC activation in addition to resultant immunomodulatory effects of MSCs on host immunity.
In vivo MSC-mediated immunomodulation: lessons from the mouse
Animal models have provided insight into how the pro-inflammatory microenvironment might modify in vivo BMSC immunomodulation. Ren et al observed that IFN-γ, in combination with other IL-6, IL-1α, and TNF-α in the microenvironment, induced murine BMSCs to produce chemokines (CXCL9, MIG and CXCL10, IP-10).52 These chemokines approximated alloreactive T cells to MSCs for activated MSCs to suppress T-cell alloreactivity via NO production. Polchert et al showed that the microenvironment into which BMSCs were infused determined how well MSCs prevented GVHD.53 Specifically, coinfusion of BMSCs on the day of BM transplantation (day 0) did not prevent GVHD, whereas BMSC infusions given at 2 or 20 days after transplantation significantly improved overall survival. These outcome differences suggested that the early pro-inflammatory microenvironment into which MSCs were infused activated MSC-mediated immunomodulation. To interrogate this effect further, the investigators pretreated MSCs with increasing concentrations of IFN-γ and showed that GVHD prevention correlated with dose-dependent in vitro IFN-γ priming. Despite the limitations inherent to these models as well as the immunomodulatory differences between murine and human BMSCs, their results show that MSC immunomodulation is a dynamic process, reflecting an active exchange or crosstalk between MSCs and their microenvironment.54
Emerging roles for BMSCs in host defense
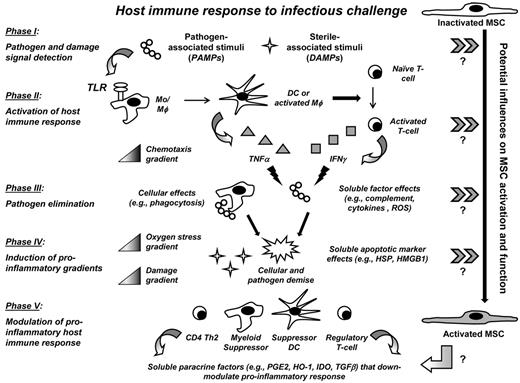
BMSCs are activated by pro-inflammatory stimuli, which also seemingly modify MSC function. An accumulating literature further suggests that MSCs could potentially be involved at multiple levels of host defense, assuming roles in hematopoiesis and mobilizing immune effector cells, in direct elimination of pathogen, and in modulation of pro-inflammatory immune responses so as to minimize inflammatory-induced tissue damage. Yet defined activating signal(s) and level(s) of the immune response at which BMSCs function during infection will require further investigation (Figure 3). This section will review the potential ways in which BMSCs may be activated and/or mediate host defense after infectious challenge.
The host immune response to infectious challenge affords multiple potential signals for BMSC activation. TLRs recognize PAMPs and DAMPs within the microenvironment. The microenvironment milieu is the composite of resident and immigrating hematopoietic and nonhematopoietic cells responding to pathogen and the associated constitutive and inducible soluble factors that these cells produce. During infectious challenge, TLR ligands activate immune effector cells, such as DCs, monocytes, and macrophages (Mo/Mφ), and T cells to produce pro-inflammatory cytokines and chemokines. These soluble factors in turn activate additional host defense responses, including recruitment of immune effector cells (chemotaxis gradient). Pathogen is eliminated directly through immune effector cells themselves (eg, phagocytosis) and by the antimicrobial soluble factors they produce (eg, TNF-α and IFN-γ). Resultant pathogen cell necrosis and tissue toxicity release additional DAMPs, which accumulate and form pro-inflammatory damage and oxygen stress gradients. To preserve host integrity, regulatory hematopoietic cells, also activated by PAMPs and DAMPs, function to counter inflammation through the production of anti-inflammatory and antioxidant paracrine soluble factors. BMSCs possess TLRs, which could potentially recognize pathogen and danger signals and activate BMSCs to function as nonhematopoietic immunomodulatory cells during infection. Block arrows (≫) indicate potential influences within each phase of host response to infection that may influence BMSC activation and function. Further investigation is needed to confirm these putative signals.
The host immune response to infectious challenge affords multiple potential signals for BMSC activation. TLRs recognize PAMPs and DAMPs within the microenvironment. The microenvironment milieu is the composite of resident and immigrating hematopoietic and nonhematopoietic cells responding to pathogen and the associated constitutive and inducible soluble factors that these cells produce. During infectious challenge, TLR ligands activate immune effector cells, such as DCs, monocytes, and macrophages (Mo/Mφ), and T cells to produce pro-inflammatory cytokines and chemokines. These soluble factors in turn activate additional host defense responses, including recruitment of immune effector cells (chemotaxis gradient). Pathogen is eliminated directly through immune effector cells themselves (eg, phagocytosis) and by the antimicrobial soluble factors they produce (eg, TNF-α and IFN-γ). Resultant pathogen cell necrosis and tissue toxicity release additional DAMPs, which accumulate and form pro-inflammatory damage and oxygen stress gradients. To preserve host integrity, regulatory hematopoietic cells, also activated by PAMPs and DAMPs, function to counter inflammation through the production of anti-inflammatory and antioxidant paracrine soluble factors. BMSCs possess TLRs, which could potentially recognize pathogen and danger signals and activate BMSCs to function as nonhematopoietic immunomodulatory cells during infection. Block arrows (≫) indicate potential influences within each phase of host response to infection that may influence BMSC activation and function. Further investigation is needed to confirm these putative signals.
TLR-mediated effects on hematopoiesis and immune effector cell mobilization
Hematopoietic immune effector cells arise from common myeloid and lymphoid progenitor cells. Interestingly, TLR ligation stimulates HSC differentiation of common myeloid progenitors into innate immune effector cells, bypassing the need for growth factors and suggesting an “emergency response” of the BM to infection.55 Therefore, maintaining the HSC pool is essential to mounting an effective antimicrobial response. The endosteal niche is composed of the most primitive and quiescent HSCs, whereas the perivascular niche contains active or self-renewing HSCs.56 The endosteal niche contains osteoblasts that supply HSC maintenance and quiescent factors and actively engage dormant HSCs, literally fastening them to the endosteal niche. Given their ability to differentiate into osteoblasts and their inherent location within the marrow, Nes+ BMSCs have been identified as critical constituents of the HSC niche, active in both HSC maintenance and BM homing.57 Furthermore, Nes+ BMSCs have been shown to induce monocyte emigration from the BM into the circulation in response to TLR ligands. In this regard, Shi et al demonstrated that murine Nes+ BMSCs respond to TLR4 ligand (LPS) by up-regulating monocyte chemotactic protein-1 (MCP1) expression, inducing CCR2-dependent migration of monocytes to sinusoidal endothelium within the BM and ultimately into the circulation.58 In addition to Nes+ BMSCs, CXC chemokines ligand (CXCL)12-abundant reticular cells also produced MCP1 after LPS administration. The investigators further showed that select depletion of MCP1 in Nes+ BMSCs decreased monocyte emigration and increased susceptibility to in vivo infectious challenge with Listeria monocytogenes. Taken together, in vivo murine models suggest that TLR ligation may underlie a holistic BM response to infection, in which both hematopoietic and nonhematopoietic cells respond to systemic infectious challenge.
Antimicrobial and immunomodulatory effects are linked in preserving host integrity
Mechanisms for direct pathogen elimination include phagocytosis and subsequent oxidative (eg, generation of ROS via NADPH-oxidase complex in combination with superoxide dismutase and myeloperoxidase) and nonoxidative killing (eg, release of neutrophil contents of specific and gelatinase granules); complement activation and formation of terminal membrane attack complex; and release of microbiocidal soluble factors (eg, IFN-γ and TNF-α) by immune effector cells.40 Given their ability to release paracrine factors as well as migrate to sites of injury and inflammation, BMSCs may indeed “be at the right place, at the right time” to mediate and to modulate anti-infective host immune responses. As evidence, the in vivo cellular counterpart to in vitro expanded BMSCs has recently been shown to assume a perivascular position and to compose a subset of pericytes, which are strategically placed on the front line to recognize pathogen and to integrate into early inflammatory events.59 Through their expression of selectin, integrin, and chemokine receptors, BMSCs within the infectious microenvironment may even directly engage pathogen, holding pathogen hostage to impede pathogen growth by competing for nutrient resources or by making the microenvironment less “immuno-hospitable” for pathogen survival. Whether MSCs actually perform these tasks remains to be determined. Yet MSCs have recently been shown to impede in vitro pathogen growth,60,61 to reduce microbial burden after infectious challenge,61-63 and to prolong host survival in the context of sepsis.62-64
The tryptophan catabolic enzyme, IDO, is a key mediator of immune tolerance. Pro-inflammatory cytokines, such as IFN-γ, and Treg cell signals, such as CTLA-4, induce IDO expression in dendritic cells (DCs), resulting in their transformation into tolerogenic DCs that can inhibit T-cell expansion as well as induce Tregs.65 Along with these immunomodulatory effects, IDO also possesses antimicrobial effects, probably through its ability to down-modulate the inflammatory response to infection.66 Knowing these effects of IDO and having previously shown that human BMSCs produce IDO in response to IFN-γ priming,67 Meisel et al sought to investigate further the role of IDO on pathogen growth.67 Specifically, human BMSCs stimulated with pro-inflammatory cytokines for 72 hours were cocultured for 16 hours with various microbes, including bacterium (Staphylococcus aureus, Enterococcus faecium, Escherichia coli, and Staphylococcus epidermidis), parasite (Toxoplasma gondii), or virus (Cytomegalovirus), and pathogen growth was measured either photometrically or by colony counts.60 Investigators demonstrated that IFN-γ–primed human BMSCs decreased in vitro microbial growth, an effect further enhanced by TNF-α costimulation. To show that the observed antimicrobial effect was IDO-dependent, the IDO-specific inhibitor, 1-MT, added to cocultures containing both human BMSCs and microbes reversed microbe growth inhibition. Compared with human BMSCs, BMSCs derived from different mouse strains did not express functional IDO but did express significant iNOS activity. However, murine BMSCs did not inhibit bacteria growth. Based on these results, the investigators concluded that inducible IDO mediates both antimicrobial and immunomodulatory properties unique to human BMSCs.
Animal models of pathogen challenge have also demonstrated that BMSCs decrease in vivo infectious burden and prolong host survival. Using a murine model of cecal ligation and puncture (CLP), Nemeth et al showed that MSC-associated prolongation in survival and improvement in organ (kidney, liver, and pancreas) function coincided with reduced bacterial burden, as measured by blood colony-forming units (CFUs) from mice given MSCs.63 The investigators further showed that murine BMSCs mediated their effects by enhancing IL-10 production from macrophages, which, in turn, decreased neutrophil migration into the pro-inflammatory microenvironment and reduced myeloperoxidase expression in kidney and liver. Specifically, pro-inflammatory cytokines inherent to the septic microenvironment induced MSC production in COX2 and PGE2. Subsequently, PGE2 modulated macrophage function to decrease TNF-α and IL-6 production and to increase IL-10. Collectively, these data show that BMSCs decrease migration of additional immune cells responding to infection, which may further induce tissue injury by their release of pro-inflammatory cytokines and free radicals.
Mei et al also used the murine CLP model to define the effects of murine BMSCs on lung injury in the context of sepsis.62 These investigators showed that BMSC therapy decreased systemic inflammation and multiorgan dysfunction, resulting in reduced mortality after CLP. Specifically, BMSCs attenuated lung injury, as measured by vascular permeability (protein and albumin concentrations measured in bronchoalveolar lavage [BAL]) fluid, total BAL cellularity, and histopathology. In addition, the numbers of apoptotic cells and levels of pro-inflammatory cytokines and chemokines in BAL fluid were reduced in MSC-treated, CLP-challenged animals versus untreated, CLP-challenged animals. MSC-treated animals also had enhanced clearance of E coli and S aureus as measured by CFU content in the spleen and peritoneal fluid. BMSCs demonstrated, albeit limited, in vitro phagocytosis of fluorescence-labeled E coli and S aureus compared with macrophages. However, CD11b+ cells purified from the spleen and peritoneal fluid of MSC-treated, CLP-challenged animals showed enhanced phagocytosis of E coli and S aureus versus purified CD11b+ cells from CLP-challenged only animals. These data show that BMSCs suppress systemic inflammation by preserving organ function and may even enhance antimicrobial immune effector cell function during infectious challenge. However, whether BMSCs can effectively phagocytose bacteria themselves requires further investigation given the limited results observed from this study.
In a murine model of colitis and sepsis, human ASCs were shown to protect against dextran-induced colitis by decreasing pro-inflammatory cytokines (IL-1β, IL-6, IL-12, IFN-γ, and TNF-α) and chemokines (eg, RANTES, regulated on activation normal T cell expressed and secreted, and MIP-2, macrophage inflammatory protein 2), by producing IL-10 themselves and by inducing IL-10 production from macrophages.64 Gonzalez-Rey et al further demonstrated that human ASCs induced IL-10–producing Treg cells in colitic mice, and Treg induction correlated with reduced infiltration of inflammatory immune cells in the colonic mucosa and preservation in colonic histology.64 Human ASCs also prolonged survival in mice after LPS- and CLP-induced endotoxemia, correlating with reduced CFUs in peritoneal lavage, blood, spleen, and liver. Finally, the investigators used both syngeneic and allogeneic murine ASCs to show that the beneficial effect of ASCs on colitis was not xenogeneically restricted. Together, these results show that human MSCs can also mediate in vivo antimicrobial effects and induce Treg cells in modulating the systemic and local (ie, colonic) microenvironment in the context of infectious challenge.
Lastly, Krasnodembskaya et al have shown that human BMSCs inhibited in vitro bacterial (E coli, P aeruginosa, and S aureus) growth through secretion of human cathelicidin antimicrobial peptide, hCAP (LL-37).61 These investigators further demonstrated that monoclonal blocking antibody against LL-37 reversed bacterial growth inhibition (CFU assessment). Protective in vivo effects of MSC-induced LL-37 were also demonstrated in a murine model of E coli pneumonia in which BAL counts and bacteria growth were lower in MSC-treated versus untreated animals challenged with E coli.
These preclinical studies suggest that BMSCs can mediate potentially favorable effects in the context of infectious challenge (summarized in Table 1). Whether measured improvement in survival is a reflection of BMSC immunomodulatory or antimicrobial effects remains unclear. Yet the intrigue in further defining these contributions lies in the unique potential for BMSCs to mediate both types of effects during infectious challenge. That is, plasticity in immunomodulatory function may underlie the ability of BMSCs to respond initially to augment antimicrobial immunity and decrease infectious burden, and then shift function to preserve host integrity by attenuating systemic inflammation and augmenting tissue repair (Figure 4).
Putative roles for BMSCs during infection
| Phase 1: Pathogen and damage signal detection |
| BMSCs |
| TLR-mediated modulation of activation and function28-32,34-36,54 |
| Recruitment to site of infection through chemotaxis gradients52,54,71 |
| Phase 2: Activation of host immune response |
| BMSC effects on HSCs |
| Maintenance of quiescent HSC pool56,57 |
| BM emigration of activated HSCs57 |
| BMSC effects on immune effector cells |
| Mobilization and emigration from BM58 |
| Thymic development to augment immune effector cell response79-82 |
| Phase 3: Pathogen elimination |
| BMSC effects on pathogen |
| Production of microbiocidal soluble factors3,60,61 |
| Containment of infectious pathogen within microenvironment (eg, pathogen phagocytosis)62 |
| Enhance microbiocidal function of immune effector cells62,83 |
| Phase 4: Induction of pro-inflammatory gradients |
| BMSC production of immunomodulatory soluble factors |
| Antioxidant soluble factors (HO-1)45,46 |
| Anti-inflammatory soluble factors (galectin-1, IDO, IL-10, HGF, HLA-G5, PGE2, TGFβ, TSG-6)2-4,47,52-54,67,72,84 |
| Phase 5: Modulation of pro-inflammatory host immune response |
| BMSC-mediated effects on host immune response and on immune-induced damage to the host |
| Effects on immune effector cell activation, differentiation, function, or migration47,52-54,73,75,84 |
| Augment wound healing, minimize tissue cytotoxicity, or enhance revascularization62-64,71,72 |
| Modulation of inflammation and organ dysfunction after in vivo infectious challenge61-64 |
| Phase 1: Pathogen and damage signal detection |
| BMSCs |
| TLR-mediated modulation of activation and function28-32,34-36,54 |
| Recruitment to site of infection through chemotaxis gradients52,54,71 |
| Phase 2: Activation of host immune response |
| BMSC effects on HSCs |
| Maintenance of quiescent HSC pool56,57 |
| BM emigration of activated HSCs57 |
| BMSC effects on immune effector cells |
| Mobilization and emigration from BM58 |
| Thymic development to augment immune effector cell response79-82 |
| Phase 3: Pathogen elimination |
| BMSC effects on pathogen |
| Production of microbiocidal soluble factors3,60,61 |
| Containment of infectious pathogen within microenvironment (eg, pathogen phagocytosis)62 |
| Enhance microbiocidal function of immune effector cells62,83 |
| Phase 4: Induction of pro-inflammatory gradients |
| BMSC production of immunomodulatory soluble factors |
| Antioxidant soluble factors (HO-1)45,46 |
| Anti-inflammatory soluble factors (galectin-1, IDO, IL-10, HGF, HLA-G5, PGE2, TGFβ, TSG-6)2-4,47,52-54,67,72,84 |
| Phase 5: Modulation of pro-inflammatory host immune response |
| BMSC-mediated effects on host immune response and on immune-induced damage to the host |
| Effects on immune effector cell activation, differentiation, function, or migration47,52-54,73,75,84 |
| Augment wound healing, minimize tissue cytotoxicity, or enhance revascularization62-64,71,72 |
| Modulation of inflammation and organ dysfunction after in vivo infectious challenge61-64 |
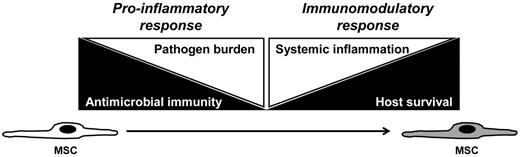
Proposed plasticity in BMSC response and function during infectious challenge. To decrease infectious burden (left white triangle), the host requires intact immunity (left black triangle). That is, level of pathogen burden in the host inversely correlates with host immune competency. BMSCs may act as pro-inflammatory agents in the initial stages of the host response to infectious challenge, thereby decreasing pathogen burden by augmenting antimicrobial immune responses. However, high-level, persistent systemic inflammation (right white triangle) ultimately results in decreased host survival (right black triangle). Therefore, BMSCs may assume an immunomodulatory function to dampen the pro-inflammatory response associated with host immune response to pathogen. In this regard, BMSCs might maintain immune homeostasis after infectious challenge, by preserving host integrity through mediating initial pro-inflammatory, antimicrobial effects and then shifting function to attenuate inflammation and to augment tissue repair.
Proposed plasticity in BMSC response and function during infectious challenge. To decrease infectious burden (left white triangle), the host requires intact immunity (left black triangle). That is, level of pathogen burden in the host inversely correlates with host immune competency. BMSCs may act as pro-inflammatory agents in the initial stages of the host response to infectious challenge, thereby decreasing pathogen burden by augmenting antimicrobial immune responses. However, high-level, persistent systemic inflammation (right white triangle) ultimately results in decreased host survival (right black triangle). Therefore, BMSCs may assume an immunomodulatory function to dampen the pro-inflammatory response associated with host immune response to pathogen. In this regard, BMSCs might maintain immune homeostasis after infectious challenge, by preserving host integrity through mediating initial pro-inflammatory, antimicrobial effects and then shifting function to attenuate inflammation and to augment tissue repair.
MSC-induced endothelial repair: accelerated resolution of microbial-induced inflammation
Sepsis-related inflammatory mediators, such as LPS, TNF-α, and IL-1β, can directly activate microvascular endothelial cells to promote the additional release of pro-inflammatory cytokines and increase expression of adhesion molecules (E-selectin, ICAM-1, VCAM-1).68 The septic milieu containing LPS and IFN-γ can preferentially stimulate endothelial production of superoxide over beneficial nitric oxide. Resultant superoxide and loss of nitric oxide increase vascular permeability, further promoting pro-inflammatory and pro-coagulation changes in endothelium.69,70 ROS produced by recruited neutrophils augment this effect, and the resultant endothelial barrier failure paves the way for organ dysfunction in sepsis.70
MSCs are recruited to vascular endothelium via the up-regulation of adhesion molecules, with increased binding mediated in part by increased levels of TNF-α and IL-1β.71 Binding with ICAM and VCAM potentiates MSC immunosuppressive properties,72 thereby placing these potentially highly immunomodulatory cells at the dysfunctional endothelial barrier, along with recruited macrophages and neutrophils. Macrophage interface with MSCs may lead to alternative activation, with macrophage reduced production of pro-inflammatory IL-12 and increased production of IL-10.75 Alternatively, activated macrophages have the capacity to produce pro-angiogenic, pro-reparative factors, such as vascular endothelial growth factor, hepatocyte growth factor, angiopoietin-1, and matrix. Such polarization in macrophage function can occur in self-limiting inflammatory processes ushering regenerative and reparative processes.74 MSCs can also produce vascular endothelial growth factor and angiopoietin-1, thereby accelerating endothelial proliferation and inhibition of apoptosis and ultimately stabilizing vascular function. Finally, MSCs have been reported to recruit endothelial progenitors to site of tissue injury, further augmenting a reparative effect on the endothelium.75 In summary, direct and paracrine effects of MSCs may combine to reduce the endothelial dysfunction associated with sepsis and its inflammatory state by rapidly facilitating pro-regenerative effects. This accelerated adoption of a pro-regenerative state by the endothelium has the potential to limit the deleterious effects of sepsis, fortifying organ endothelium and limiting associated organ dysfunction.
Considerations for the use of BMSCs as novel antimicrobial therapy
Preclinical studies have shown that BMSCs have important roles in regulating repair and immune homeostasis during the inflammatory response, with evidence that, as a consequence of the microenvironment, BMSCs may have dual roles as pro- and anti-inflammatory mediators. These immunomodulatory roles and other emerging roles in hematopoiesis and antimicrobial response poise BMSCs for further investigation as novel cellular antimicrobial therapy to reduce infection-related morbidity and mortality, particularly in immunocompromised patients, for whom BMSC therapy may have multiple benefits. For example, clinical applications for BMSCs in allogeneic HSC transplantation (HSCT) include enhancing hematopoietic engraftment, modulating GVHD, and augmenting host defense in the context of immunosuppressive therapy and delayed immune reconstitution. Indications for BMSC therapy with respect to engraftment and GVHD have been extensively reviewed.54,76,77 With respect to enhancing host antimicrobial immunity, BMSC therapy could potentially decrease adverse effects of GVHD on donor hematopoiesis78 and on target lymphoid organs, such as the thymus,79 wherein MSCs assume vital roles in thymic epithelial progenitor cell proliferation and differentiation.80,81 As evidence, labeled murine BMSCs have recently been shown to home to the thymus of haploidentical transplant recipients, decrease thymocyte apoptosis, and enhance peripheral T-cell recovery.82 Preclinical MSC-mediated restoration in both hematopoiesis and lymphoid organ architecture and function could translate into faster immune recovery and mobilization of immune effector cells in response to clinical infection.
BMSC therapy might also directly augment immune effector cell function against pathogen. To this end, TLR3-primed human BMSCs have recently been shown to prolong in vitro neutrophil survival and function.83 MSCs seem resistant to immune effector cell lysis, particularly by NK cells and cytotoxic T lymphocytes.84 Therefore, BMSCs should then remain functionally viable cells during host immune response to infection. However, whether BMSCs suppress immune effector cell function in the clinical setting of infection remains an important unanswered question. Clinical experience using BMSCs for GVHD treatment or prophylaxis does not suggest any increased risk of infectious complications in allogeneic HSCT patients. However, these patients also receive prophylactic antimicrobials, and these studies generally did not report on infectious burden at the time of MSC treatment.7,85 In addition, human BMSCs do not seem to interfere with in vitro EBV- and CMV-specific cytotoxic T lymphocyte proliferation, IFN-γ production, and viral killing,86 as peripheral blood mononuclear cells from patients who received BMSCs for acute GVHD had persistent CMV-specific T cells and retention of IFN-γ response to CMV post-MSC infusion.86
Yet attention must be paid to the direct susceptibility of BMSCs to infectious pathogens. Fetal BMSCs have been shown to be in vitro targets for Kaposi sarcoma virus,87 which may explain hematopoietic deficiencies and BM failures in these patients given the role of MSCs in hematopoiesis as previously reviewed. BMSCs have also been shown to harbor and transmit parvovirus B19 to hematopoietic cells in vitro.88 Limited clinical experience with parvovirus B19-harboring BMSCs did not result in clinical viremia or viral transmission.88 BMSC susceptibility to parvovirus 19, varicella zoster virus, and human herpesvirus-6 has also been observed.89 Notably, these clinical studies did not specify clinical response to MSC therapy in relationship to viral history or load. That is, reduced biologic efficacy in the context of viral inactivation of a cellular therapeutic agent like BMSCs remains unstudied.
Sepsis-induced acute lung injury may be an initial clinical setting to test the use of BMSCs as novel antimicrobial therapy. The high vulnerability for managing respiratory function during acute lung inflammation not only balances the risk-benefit ratio for BMSC treatment in the presence of sepsis but also provides clinical read-outs of intended benefit (eg, decreases in BAL cell counts and ventilation settings as well as clearance of pathogen from sterile sites). An inherent difficulty remains in separating the anti-inflammatory from the antimicrobial effects of BMSCs in the clinical setting. Notwithstanding, clinical protocols are currently under review by the FDA and under consideration at society workshops.90 In addition, the field of BMSC therapeutics requires sound clinical trial development, including defined hypotheses and clinical endpoints. Further clinical investigation should emphasize optimizing dosing and other pharmacokinetic and pharmacodynamic properties of BMSCs and defining their in vivo mechanism of action. In light of the emerging hypothesis that BMSCs contribute to microbial defense, inclusion of biomarkers to monitor pathogen burden may also be warranted. Building on an improved understanding for the dynamic crosstalk between BMSCs and immune effector cells in the context of infectious challenge should not only provide a stronger and more effective rationale for clinical design but also positively influence further investigation into this emerging, novel therapeutic potential for BMSC therapy.
Acknowledgments
J.J.A. was supported by the Hematopoietic Stem Cell Core Facility of the Case Comprehensive Cancer Center (P30 CA 43703), the National Center for Stem Cell and Regenerative Medicine at Case Western Reserve University, and the National Institutes of Health/National Institute of Allergy and Infectious Diseases (AI57801). A.M.B. was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (AI089556).
National Institutes of Health
Authorship
Contribution: J.J.A. wrote the manuscript and designed the figures; and A.M.B. and R.J.D. cowrote and provided critical revisions to the manuscript.
Conflict-of-interest disclosure: R.J.D. is a paid employee and share option holder of Athersys Inc. The remaining authors declare no competing financial interests.
Correspondence: Jeffery J. Auletta, Rainbow Babies and Children's Hospital, 11100 Euclid Ave, Mailstop 6054, Cleveland, OH 44106; e-mail: jeffery.auletta@case.edu.