Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by lung endothelial dysfunction and vascular remodeling. Recently, bone marrow progenitor cells have been localized to PAH lungs, raising the question of their role in disease progression. Independently, serotonin (5-HT) and its receptors have been identified as contributors to the PAH pathogenesis. We hypothesized that 1 of these receptors, 5-HT2B, is involved in bone marrow stem cell mobilization that participates in the development of PAH and pulmonary vascular remodeling. A first study revealed expression of 5-HT2B receptors by circulating c-kit+ precursor cells, whereas mice lacking 5-HT2B receptors showed alterations in platelets and monocyte-macrophage numbers, and in myeloid lineages of bone marrow. Strikingly, mice with restricted expression of 5-HT2B receptors in bone marrow cells developed hypoxia or monocrotaline-induced increase in pulmonary pressure and vascular remodeling, whereas restricted elimination of 5-HT2B receptors on bone marrow cells confers a complete resistance. Moreover, ex vivo culture of human CD34+ or mice c-kit+ progenitor cells in the presence of a 5-HT2B receptor antagonist resulted in altered myeloid differentiation potential. Thus, we demonstrate that activation of 5-HT2B receptors on bone marrow lineage progenitors is critical for the development of PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a rare but fatal disease, often of unknown origin, characterized by progressive increase in pulmonary vascular resistance and remodeling associated with vasoconstriction.1 PAH is histologically characterized by a neomuscularization of small pulmonary arteries with intimal thickening, medial hypertrophy, adventitial proliferation, and abnormal extracellular matrix deposition. The progression of vascular remodeling results in vascular lumen narrowing, increased pulmonary artery resistance, hypoxia, and right heart hypertrophy, although the molecular pathways initiating this remodeling are not clearly established.
On the one hand, stem cells, resident or not, may give rise to a significant proportion of differentiating/proliferating smooth muscle cells (SMCs) that contribute to intimal hyperplasia in lung vascular remodeling.2 Moreover, genetic ablation of the transmembrane tyrosine kinase receptor for stem cell factor/c-kit pathway results in a marked reduction in intimal hyperplasia in animal models of vascular injury; conversely, wild-type (WT) bone marrow (BM) reconstitution in c-kit mutant mice led to intimal hyperplasia comparable with WT animals.3 Pharmacologic antagonism of the c-kit pathway with STI-571 (imatinib mesylate; Gleevec) also results in a marked reduction in hyperplasia.3 Mobilization of c-kit expressing cells from BM to blood circulation is a physiologic response to hypoxia. Increasing evidence supports the idea that these progenitor cells of BM origin may also contribute to vascular wall remodeling that is characteristic of PAH.4-8 However, it is unclear, whether this entry of progenitors represents a protective or a worsening process in the development of PAH.9 Other observations have also identified an association between PAH and BM-related hematologic disorders10 : in proliferative disorders of the hematopoietic stem cells such as myeloproliferative cancers, there is an unexplained high incidence of PAH. PAH is now a recognized complication of BM transplantation for leukemia,11 chronic myeloproliferative disorders,12 or in the treatment of malignant infantile osteopetrosis.13
On the other hand, serotonin (5-hydroxytryptamine [5-HT]) is associated with the pathogenesis of PAH.14 Therapeutic drugs with PAH as a side effect, such as the amphetamine derivative and anorexigen dexfenfluramine, are potent 5-HT releasers acting at 5-HT transporter (SERT) and/or agonists at 5-HT receptors (5-HTRs).15 An over-expression of 5-HT2BRs is observed in PAH.16 Blockade of 5-HT2BRs using independent approaches, either genetic (5-HT2BR knock-out mice; 5-HT2B−/−) or pharmacologic (5-HT2B antagonist RS-12744) inactivation, completely prevented the development of hypoxia-induced pulmonary hypertension in mice.16 By using the monocrotaline (MCT)–induced pulmonary hypertension rat model, recent studies confirmed that other 5-HT2B antagonists (terguride, PRX-08066, or C-122) significantly reduced pulmonary pressure, arterial wall thickening, and lumen occlusion but maintained cardiac function.17-19 Independently, 5-HT was shown to stimulate human BM stromal cells and synergize with other pleiotropic growth factors that promote hematopoietic stem and progenitor cells.20 The 5-HT action on hematopoiesis or BM microenvironment at pathophysiologic conditions warrant further investigation. 5-HT is a potent vasoconstrictor of pulmonary arteries but also stimulates pulmonary SMC proliferation. It may thus affect various processes associated with pulmonary vascular remodeling, but its exact contribution remains unclear.
The prognosis of PAH remains unsatisfying,21 although the number of therapeutic options has increased over the past years, and several novel therapeutic targets are under active investigation.1 Currently, the available vasodilator therapies for PAH, although helpful for improving exercise tolerance and quality of life, are only moderately effective in improving survival. These therapies are targeted to ameliorate the physiologic consequences of the remodeled pulmonary arterial vasculature and probably do not directly alter the underlying defects in the pulmonary vascular remodeling. Here, we investigated a possible contribution of 5-HT signaling pathways to BM-derived progenitor cells in animal models of PAH. We found that the expression of 5-HT2BRs restricted to BM cells is necessary and sufficient for pulmonary hypertension to develop via an action at the hematopoietic stem cell differentiation.
Methods
Reagents
RS-127445, 2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine, STI-571, 4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]-phenyl]-benzamide and all other chemicals were reagent grade, purchased from Sigma-Aldrich and Tocris. The radioactive compounds [1,2-3H]-5-HT binoxalate (specific activity 1.11 TBq/mmol), [methyl,1′,2′-3H]-thymidine (specific activity 4.44 TBq/mmol), and (6)-[125I]1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride ([125I]DOI, specific activity 81.4 TBq/mmol) were purchased from NEN Perkin Elmer. Monoclonal rat anti–c-kit antibody and the polyclonal rabbit anti–5-HT2BR were from AbCam.
Animals
The mice strains used in all experiments, including 5-HT2BRs deficient mice (5-HT2B−/−) are in a 129S2/SvPAS background.22 Adult (7-9 weeks old) 5-HT2B−/−, and WT control mice (originally obtained at Charles River Laboratories) were all derived from heterozygote crosses bred at our animal facilities. All mice were maintained according to the EC directive 86/609/CEE, and housed in groups of 3 to 5 of the same genetic background after weaning.
BM transplantation
As previously described, 6 males and 6 females 8-week-old WT and 5-HT2B−/− mice were subjected to 9.5 gray lethal total body irradiation.23 The day after, mice were reconstituted by direct intravenous injection with 2.5 × 106 cells of freshly isolated BM from femurs and tibias of age and sex-matched WT or 5-HT2B−/− mice. All lethally irradiated and transplanted mice survived, revealing the efficiency of BM reconstitution. After 4 weeks of recovery, transplanted mice were then exposed to hypoxia as a validated pulmonary hypertension inducer in mice.
Pulmonary hypertension induction
As previously observed, after BM transplantation, mice were more sensitive to pulmonary hypertension inducers.24 Control and test mice were thus exposed to progressive hypoxia (20%-10% O2) for 3 weeks. Control, normoxic mice were kept in the same 12/12 light-dark cycle. The vehicle or drug was delivered by miniosmotic pump (Alzet) at the beginning of the hypoxic (or normoxic) treatment.
Cardiovascular evaluations
As previously described, mice were anesthetized with intraperitoneal ketamine hydrochloride (60 mg/kg) and xylazine (8 mg/kg).16 The arterial pulmonary pressure was estimated by assessing the cardiac right ventricular systolic pressure (RVSP), measured by insertion into the heart right ventricle of a 26-gauge needle connected to a pressure transducer. The pulmonary artery was cannulated through an incision in the right ventricle and perfused with Earle balanced salt solution (37°C, 20 cm H2O pressure). The heart and lungs were removed and the airways were distended with 10% formaldehyde solution and fixed for 3 days. Measurements of the 5-HT2BR expression was performed using 5-HT2R–specific iodinated radioligand (125I-DOI) sensitive to RS 127445, as previously described.22
Cell preparations, FACS analysis, and sorting
Murine BM cells were recovered and suspended in culture medium, as described.25 Briefly, BM cells, flushed from the femur and tibia, were resuspended in Hanks buffer before being passed through a 70 μm strainer. Cells were then incubated with appropriately labeled antibodies (CD11b, Gr1, CD31, and c-kit antibodies; BD Biosciences, eBioscience) and acquisition was carried out on a FACS CantoII. Murine c-kit+ cells (> 95% purity) were next sorted electronically using a FACSVantageTM cell sorter (BD Biosciences). Peripheral blood was collected from mouse tails and cells were counted on a MS9-5 Hematology Counter (Melet Schloesing Laboratories).
Cell cultures
Murine hematopoietic progenitors were quantified in MethoCult M3334 (StemCell Technologies) supplemented with IL-3, IL-6, SCF and in MethoCult GF H4434 (complete with growth factors), respectively. Cells were plated in a final volume of 1 mL at a concentration of 2-5 × 104 total BM cells/culture dish (Falcon 1008) for murine and 5000 CD34+ cells/mL for human progenitors. Colonies were scored on day 7 and day 14. All cultures were incubated at 37°C in a humidified chamber < 5% CO2. For methylcellulose cultures, human CD34+ cells were cultured in BFU-E/CFU-E medium with EPO, IL-3, SCF (StemCell Technologies, 4434), and murine total or c-kit+ cells were cultured in CFU-GEMM/CFU-GM, and CFU-E/BFU-E medium with IL-3, IL-6, SCF, and EPO (StemCell Technologies, 03434).
Statistics
The reported data represent the mean of individual values ± standard error of the mean (SEM; n = number of individuals at the end of treatments as indicated in the text). For simple comparisons, unpaired t test was used. Significance was set at P < .05. For the groups, statistical comparisons were made by 1-way ANOVA. When statistical significance was attained (P < .05), difference between groups was established using the Bonferroni multiple comparison test.
Results
Alterations of blood composition in 5-HT2BR mutant mice
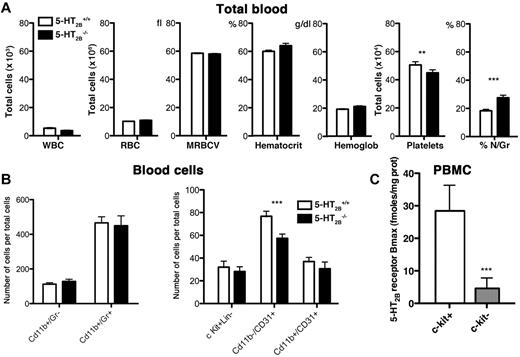
Starting from the previous observations that 5-HT may act on hematopoiesis,20 we first investigated the blood composition of WT and 5-HT2B−/− mice. Interestingly, we found a significant decrease in platelet number and increase in circulating granulocyte/macrophage population in 5-HT2B−/− compared with WT mice, but not in other parameters, white blood cells, red blood cells, mean red blood cell volume, hematocrit, or hemoglobin content (Figure 1A). FACS was performed using antibodies to identify different populations, Mac-1 (CD11b) for monocytes/macrophages, Gr-1 (Gr+) for granulocytes, PECAM (CD31) for endothelial cells/platelets/macrophages, and determine the blood cell composition of WT and 5-HT2B−/− mice. Completing our previous results, we found a significant reduction in CD11b−/CD31+ population that labeled immature endothelial progenitor cells in 5-HT2B−/− mice (Figure 1B). Interestingly, by FACS of peripheral blood mononuclear cells, we also identified expression of 5-HT2BRs in c-kit+ (CD117+) cells. Importantly, c-kit negative cells were negative for 5-HT2BR expression (Figure 1C). Together, these observations support that 5-HT2BRs may affect the differentiation of hematopoietic and BM precursor cells.
Alterations of blood composition in 5-HT2BR mutant mice. (A) In total blood, the number of white blood cells (WBCs), red blood cells (RBCs), the mean red blood cell volume (MRBCV), the hematocrit, and the hemoglobin content were not different among genotypes, whereas the number of platelets was significantly reduced and the percentage of neutrophil/granulocyte (% N/Gr) significantly increased in the blood of 5-HT2B−/− mice. Values are means ± SEM (n = 20, P < .05). Any statistical difference by unpaired t test versus control is indicated by ***P < .001 and **P < .01. (B) Flow cytometric analysis of blood cells using cell-surface markers allows identification of different populations. Mac-1 (CD11b) for monocytes/macrophages, Gr-1 (Gr+) for Granulocytes, PECAM (CD31) for endothelial cells/platelets/macrophages antibodies were used to identify lin+ cells or late multipotent progenitors. In blood, although the number of c-Kit+Lin− cells was unaffected, the 5-HT2B−/− mice had reduced number of CD11b−/CD31+, immature endothelial progenitors. Values are means ± SEM (n = 10, P < .05). Any statistical difference by unpaired t test versus control is indicated by ***P < .001. (C) By flow cytometric purification of peripheral blood mononuclear cells, we first identified expression of 5-HT2BRs in c-kit+ cells but not in c-kit− cells (28.4 ± 7.9 fmoles/mg protein vs 4.6 ± 3.2, P < .05, n = 14). Any statistical difference by unpaired t test versus control is indicated by ***P < .001.
Alterations of blood composition in 5-HT2BR mutant mice. (A) In total blood, the number of white blood cells (WBCs), red blood cells (RBCs), the mean red blood cell volume (MRBCV), the hematocrit, and the hemoglobin content were not different among genotypes, whereas the number of platelets was significantly reduced and the percentage of neutrophil/granulocyte (% N/Gr) significantly increased in the blood of 5-HT2B−/− mice. Values are means ± SEM (n = 20, P < .05). Any statistical difference by unpaired t test versus control is indicated by ***P < .001 and **P < .01. (B) Flow cytometric analysis of blood cells using cell-surface markers allows identification of different populations. Mac-1 (CD11b) for monocytes/macrophages, Gr-1 (Gr+) for Granulocytes, PECAM (CD31) for endothelial cells/platelets/macrophages antibodies were used to identify lin+ cells or late multipotent progenitors. In blood, although the number of c-Kit+Lin− cells was unaffected, the 5-HT2B−/− mice had reduced number of CD11b−/CD31+, immature endothelial progenitors. Values are means ± SEM (n = 10, P < .05). Any statistical difference by unpaired t test versus control is indicated by ***P < .001. (C) By flow cytometric purification of peripheral blood mononuclear cells, we first identified expression of 5-HT2BRs in c-kit+ cells but not in c-kit− cells (28.4 ± 7.9 fmoles/mg protein vs 4.6 ± 3.2, P < .05, n = 14). Any statistical difference by unpaired t test versus control is indicated by ***P < .001.
Alterations of BM composition in 5-HT2BR mutant mice
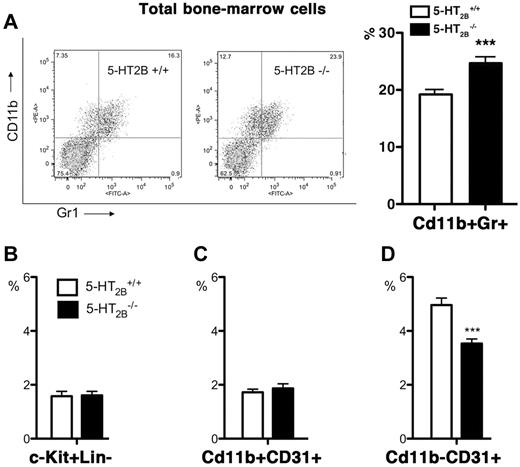
From these blood defects in 5-HT2B−/− mice, we could expect associated alterations in BM cell composition. Using FACS analysis, we determined the BM composition of WT and 5-HT2B−/− mice (Figure 2). In accordance with our blood results, we found in 5-HT2BR mutant BM a significant increase in Cd11b+/Gr+ that represents granulocyte precursors (Figure 2A). Interestingly, this was associated with a significant reduction in Cd11b−/Cd31+ population that labeled immature endothelial progenitor cells in 5-HT2B−/− mice (Figure 2D). Together, these observations support that the lack of 5-HT2BRs alters the differentiation of myeloid precursor cells; these precursors may be required for endothelial progenitor cells that have been proposed to participate to the development of pulmonary hypertension and pulmonary vascular remodeling.26
Alterations of BM composition in 5-HT2BR mutant mice. (A) In BM of 5-HT2B−/− mice, the number of CD11b+/Gr+ cells, corresponding to committed precursor cells of the monocyte/granulocyte lineage, was significantly increased as shown by the distribution of cells by FACS (left, representative experiment) and quantification in percent of total cells (right, n = 3). No modifications of the c-kit+Lin− cells, multipotent stem cells (B) or Cd11b+CD31+ cells (C) was observed in BM of 5-HT2B−/− mice. However, BM of 5-HT2B−/− mice present a significant reduction of CD11b−/CD31+, immature committed endothelial/SMC precursor cells (D). Values are means ± SEM, n = 3 independent determinations. Any statistical difference by unpaired t test versus control is indicated by ***P < .001.
Alterations of BM composition in 5-HT2BR mutant mice. (A) In BM of 5-HT2B−/− mice, the number of CD11b+/Gr+ cells, corresponding to committed precursor cells of the monocyte/granulocyte lineage, was significantly increased as shown by the distribution of cells by FACS (left, representative experiment) and quantification in percent of total cells (right, n = 3). No modifications of the c-kit+Lin− cells, multipotent stem cells (B) or Cd11b+CD31+ cells (C) was observed in BM of 5-HT2B−/− mice. However, BM of 5-HT2B−/− mice present a significant reduction of CD11b−/CD31+, immature committed endothelial/SMC precursor cells (D). Values are means ± SEM, n = 3 independent determinations. Any statistical difference by unpaired t test versus control is indicated by ***P < .001.
Mice with restricted ablation of 5-HT2BRs to BM are resistant to pulmonary hypertension
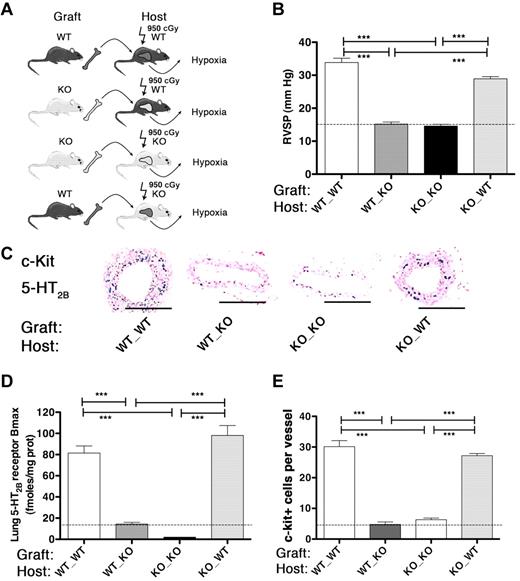
We investigated whether 5-HT2BRs interact with BM-derived stem cells in the development of pulmonary hypertension. For this, we generated mice with restricted deletion or rescue of 5-HT2BRs in BM cells using lethal total body irradiation to eliminate all stem cells combined to BM transplantation (Figure 3A; see “BM transplantation,” and supplemental Methods; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Strikingly, after this procedure, we found that only mice transplanted with WT BM (ie, expressing 5-HT2BRs) responded to chronic hypoxia in increasing their pulmonary arterial pressure as measured in the heart by RVSP. Irradiated mice transplanted with WT BM presented a significant increase in RVSP, including 5-HT2B−/− host mice after 3 weeks of hypoxia. By contrast, all mice having received BM from 5-HT2B−/− mice, were completely insensitive to hypoxia and never showed any sign of RVSP increase, including WT host mice (Figure 3B). Mice with restricted 5-HT2BR deletion to BM thus behaved as RS-127445–treated WT, or full 5-HT2B−/− mice.16 To exclude putative bias in these results because of the hypoxic model of pulmonary hypertension, we tested the effects of BM transplantation using another animal model of pulmonary hypertension induced by a single MCT injection. Strictly similar results were obtained in MCT injected mice: only mice with 5-HT2BR expressing WT BM developed an increase in RVSP (supplemental Figure 1). These results support a need for 5-HT2BR–dependent processes in BM for the development of pulmonary hypertension.
Mice with restricted ablation of 5-HT2BRs to BM are resistant to pulmonary hypertension. (A) WT mice transplanted with BM cells from WT or 5-HT2B−/− (WT_WT, WT_KO, respectively) and 5-HT2B−/− knock-out (KO) mice transplanted with BM cells from 5-HT2B−/− or WT (KO_KO, KO_WT, respectively) were exposed to progressive hypoxia (20%-10% for 3 weeks). (B) Pulmonary arterial pressure as assessed by RVSP increased in mice bearing WT BM either in WT (WT_WT) or 5-HT2B−/− (KO_WT) background. Conversely, mice with 5-HT2B−/− BM into WT (WT_KO) or 5-HT2B−/− (KO_KO) background did not show any RVSP increase. (C) Immunohistochemistry of c-Kit and 5-HT2BR. Staining was performed with antibodies against 5-HT2BR (pink; rabbit polyclonal AbCam) or against c-Kit (blue; rat monoclonal AbCam). Sections were analyzed under ×200 original magnification using a Zeiss Axiophot microscope with inbuilt camera (Carl Zeiss). A 20×/0.30 air objective was used. Images were acquired using a Zeiss Axiocam camera and Axiovision Version 3.1 software. Scale bars represent 50 μm. (D) Lung 5-HT2BR expression (Bmax) increased in mice bearing WT BM not only in WT (WT_WT) but also in 5-HT2B−/− (KO_WT) background. Conversely, not only mice with 5-HT2B−/− BM into 5-HT2B−/− (KO_KO), but also in WT (WT_KO) background did not show any change in lung 5-HT2BR expression compared with controls WT (18 fmoles/mg prot) or full 5-HT2B−/− (less than 5 fmoles/mg prot). (E) Similarly, the pulmonary perivascular c-kit+ BM derived progenitor cell recruitment induced by hypoxia was higher in mice with WT BM to WT (WT_WT) or 5-HT2B−/− (KO_WT) background as revealed by quantification of the immuno-labeling. Values are means ± SEM (n = 12, P < .05). Lines are normoxic values. Any statistical difference by 1-way ANOVA followed by Bonferroni posthoc test is indicated by ***P < .001.
Mice with restricted ablation of 5-HT2BRs to BM are resistant to pulmonary hypertension. (A) WT mice transplanted with BM cells from WT or 5-HT2B−/− (WT_WT, WT_KO, respectively) and 5-HT2B−/− knock-out (KO) mice transplanted with BM cells from 5-HT2B−/− or WT (KO_KO, KO_WT, respectively) were exposed to progressive hypoxia (20%-10% for 3 weeks). (B) Pulmonary arterial pressure as assessed by RVSP increased in mice bearing WT BM either in WT (WT_WT) or 5-HT2B−/− (KO_WT) background. Conversely, mice with 5-HT2B−/− BM into WT (WT_KO) or 5-HT2B−/− (KO_KO) background did not show any RVSP increase. (C) Immunohistochemistry of c-Kit and 5-HT2BR. Staining was performed with antibodies against 5-HT2BR (pink; rabbit polyclonal AbCam) or against c-Kit (blue; rat monoclonal AbCam). Sections were analyzed under ×200 original magnification using a Zeiss Axiophot microscope with inbuilt camera (Carl Zeiss). A 20×/0.30 air objective was used. Images were acquired using a Zeiss Axiocam camera and Axiovision Version 3.1 software. Scale bars represent 50 μm. (D) Lung 5-HT2BR expression (Bmax) increased in mice bearing WT BM not only in WT (WT_WT) but also in 5-HT2B−/− (KO_WT) background. Conversely, not only mice with 5-HT2B−/− BM into 5-HT2B−/− (KO_KO), but also in WT (WT_KO) background did not show any change in lung 5-HT2BR expression compared with controls WT (18 fmoles/mg prot) or full 5-HT2B−/− (less than 5 fmoles/mg prot). (E) Similarly, the pulmonary perivascular c-kit+ BM derived progenitor cell recruitment induced by hypoxia was higher in mice with WT BM to WT (WT_WT) or 5-HT2B−/− (KO_WT) background as revealed by quantification of the immuno-labeling. Values are means ± SEM (n = 12, P < .05). Lines are normoxic values. Any statistical difference by 1-way ANOVA followed by Bonferroni posthoc test is indicated by ***P < .001.
Lung 5-HT2BR– and c-kit–expressing cells are of BM origin
Because an increase in lung 5-HT2BR expression had been previously documented in PAH,16,17 we tested lung 5-HT2BR expression after irradiation and BM replacement. Noteworthy, irradiated 5-HT2B−/− mice having received WT BM and exposed to hypoxia showed a similar increase in lung 5-HT2BR expression (approximately 7-fold) as irradiated WT host mice having received WT BM (Figure 3C-D). To the opposite, WT mice having received 5-HT2B−/− BM showed no increase in 5-HT2BR expression in the lung after exposure to hypoxia as 5-HT2B−/− mice having received 5-HT2B−/− BM. Strictly similar results were obtained in MCT injected mice: only mice with 5-HT2BR expressing WT BM developed an increase in lung 5-HT2BR overexpression (supplemental Figure 1). These results support that lung cells overexpressing 5-HT2BRs during pulmonary hypertension are originating from BM precursors and not from lung resident cells. Because c-kit has been shown to be expressed in lungs of PAH patients,26,27 we completed these investigations by performing c-kit and 5-HT2BR immunohistochemistry of lung tissues. Interestingly, a partially overlapping increase in both c-kit and 5-HT2BR expression was observed in small arteries of hypoxic WT or 5-HT2B−/− mice transplanted with 5-HT2BR expressing WT BM, but not with 5-HT2B−/− BM (Figure 3C-E). These combinations of BM transplantation into different background confirm that, c-kit and 5-HT2BR expressing cells in the diseased lung are requiring active 5-HT2BRs in BM stem cells. In addition, these results support that the initial trigger of pulmonary hypertension is originating from BM progenitors.
Alterations of BM differentiation after 5-HT2BR inhibition
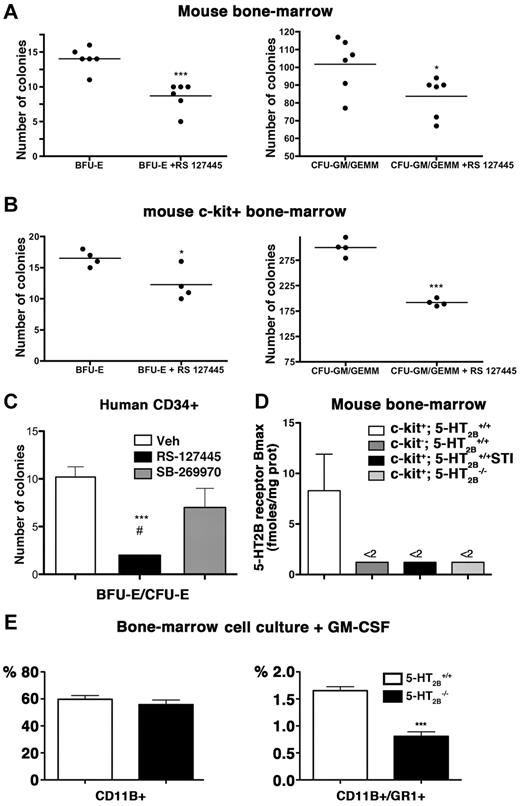
Previously, 5-HT has been shown to enhance the expansion of CD34+ cells to early stem/progenitors (CD34+ cells, colony-forming unit-mixed [CFU-GEMM]) and committed progenitors (burst-forming unit/colony-forming unit-erythroid [BFU/CFU-E].20,28 We have assessed the effect of 5-HT2BR blockade using the selective antagonist, RS-127445, during the differentiation of total BM cell cultures from mice. When mice BM was used in methylcellulose colony-forming assay, we observed a significant reduction in both CFU-GEMM/CFU-GM and CFU-E/BFU-E colonies with the 5-HT2BR antagonist (Figure 4A). Similar results were obtained when analyzing the clonogenic potential of isolated c-kit+ cells from mice BM that showed also a reduction in both CFU-GEMM/CFU-GM and CFU-E/BFU-E colonies with the 5-HT2BR antagonist (Figure 4B). To validate these findings in humans, we then analyzed the clonogenic potential of human blood cord CD34+ cells in the methylcellulose colony-forming assay. The most immature CD34+ cells isolated from cord blood, were expanded for 14 days in methylcellulose, with or without 5-HT2BR or 5-HT7R antagonists. The number of BFU-E/CFU-E colonies arising from CD34+ cells was strongly reduced (5-fold) on exposure to the antagonist RS-127445, whereas no significant difference was observed using the 5-HT7R antagonist, SB-269970 (Figure 4C). By flow cytometric purification of BM cells, we also identified expression of 5-HT2BRs in c-kit+ (CD117 positive) cells. Importantly, c-kit− cells, as c-kit+ cells after chronic STI-571 treatment (5 weeks, see Figure 5) or c-kit+ cells from 5-HT2B−/− mice were negative for 5-HT2BR expression (Figure 4D). Interestingly, in cultures performed in the presence of GM-CSF, we found that the lack of 5-HT2BR reduced the apparition of granulocyte precursors (CD11b+/Gr+) lineage by 50% (Figure 4E). Because GM-CSF controls the differentiation of monocyte/macrophages and all granulocytes,29 this finding strongly supports a role of 5-HT2BRs at precursor stage differentiation toward these lineages. These results confirm the need of the 5-HT2BR action including in human stem cells for early stem/progenitor cells and multilineage committed progenitors differentiation of myeloid and erythroid lineages that may ultimately participate in the pulmonary hypertension pathogenesis.
Alterations of BM differentiation after 5-HT2BR inhibition. (A) In mouse BM cells, methyl cellulose cultures, RS-127445 reduced CFU-GEMM/CFU-GEM/BFU-E/CFU-E (n = 3 independent cultures). Statistical difference by unpaired t test versus control is indicated by ***P < .001, *P < .05 versus Vehicle. (B) Similarly, in c-kit+ BM cells, methyl cellulose cultures, RS-127445 reduced CFU-GEMM/CFU-GEM/BFU-E/CFU-E (n = 3 independent cultures). Statistical difference by unpaired t test versus control is indicated by ***P < .001, *P < .05 versus Vehicle. (C) On methyl cellulose cultures of human blood cord CD34+ cells, the 5-HT2BR antagonist RS-127445 significantly reduced expansion of BFU-E/CFU-E, burst-forming unit/colony-forming unit-erythroid, but not the 5-HT1BR antagonist SB-269970 (n = 4 independent cultures). Any statistical difference by unpaired t test versus control is indicated by ***P < .001 versus Vehicle, #P < .05 versus SB-269970. (D) After flow cytometric purification of BM cells, expression of 5-HT2BRs was detected in c-kit+ (CD117 positive) cells (n = 8), but remained undetectable in c-kit− cells (n = 5), c-kit+ cells after STI-571 (STI) treatment (n = 6) or c-kit+ cells from 5-HT2B−/− mice (n = 5). (E) On ex vivo expansion of total BM cells in the presence of GM-CSF driving the monocyte/macrophages and all granulocytes differentiation, the lack of 5-HT2BRs significantly reduced the number of CD11b+/Gr+ cells (n = 3-5 mice of each genotype). Any statistical difference by unpaired t test versus control is indicated by ***P < .001 versus 5-HT2B+/+ culture.
Alterations of BM differentiation after 5-HT2BR inhibition. (A) In mouse BM cells, methyl cellulose cultures, RS-127445 reduced CFU-GEMM/CFU-GEM/BFU-E/CFU-E (n = 3 independent cultures). Statistical difference by unpaired t test versus control is indicated by ***P < .001, *P < .05 versus Vehicle. (B) Similarly, in c-kit+ BM cells, methyl cellulose cultures, RS-127445 reduced CFU-GEMM/CFU-GEM/BFU-E/CFU-E (n = 3 independent cultures). Statistical difference by unpaired t test versus control is indicated by ***P < .001, *P < .05 versus Vehicle. (C) On methyl cellulose cultures of human blood cord CD34+ cells, the 5-HT2BR antagonist RS-127445 significantly reduced expansion of BFU-E/CFU-E, burst-forming unit/colony-forming unit-erythroid, but not the 5-HT1BR antagonist SB-269970 (n = 4 independent cultures). Any statistical difference by unpaired t test versus control is indicated by ***P < .001 versus Vehicle, #P < .05 versus SB-269970. (D) After flow cytometric purification of BM cells, expression of 5-HT2BRs was detected in c-kit+ (CD117 positive) cells (n = 8), but remained undetectable in c-kit− cells (n = 5), c-kit+ cells after STI-571 (STI) treatment (n = 6) or c-kit+ cells from 5-HT2B−/− mice (n = 5). (E) On ex vivo expansion of total BM cells in the presence of GM-CSF driving the monocyte/macrophages and all granulocytes differentiation, the lack of 5-HT2BRs significantly reduced the number of CD11b+/Gr+ cells (n = 3-5 mice of each genotype). Any statistical difference by unpaired t test versus control is indicated by ***P < .001 versus 5-HT2B+/+ culture.
Blocking tyrosine kinase c-kit activity prevents pulmonary hypertension and 5-HT2BR overexpression
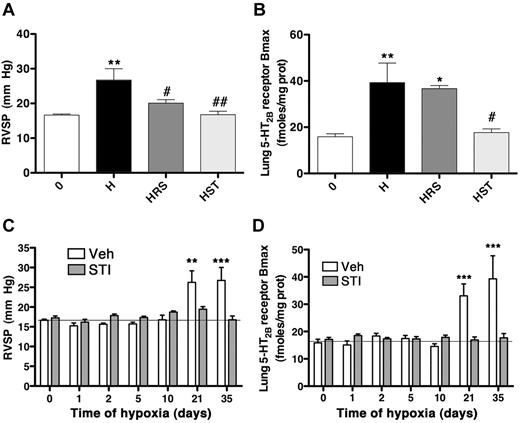
Among compounds that are under investigation for PAH treatment, the tyrosine kinase inhibitor, STI-571 has been shown to be effective.30 We thus compared the 5-HT2B selective antagonist RS-127445 with STI-571 in their ability to prevent pulmonary hypertension in the mouse hypoxic model and found they were both equally effective for tested parameters (Figure 5A, supplemental Figure 2A-D). However, we found that only STI-571 was able to prevent the increase in lung 5-HT2BR expression (Figure 5B). We therefore investigated the kinetic in establishment of hypoxic pulmonary hypertension with that of 5-HT2BR overexpression. Interestingly, we found that STI-571 was able to prevent both the increase in RVSP and the 5-HT2BR overexpression with similar kinetic (Figure 5C-D). These results support an action of both inhibitors at a common pathway that ultimately participate in the pulmonary hypertension pathogenesis.
Blocking c-kit activity prevents pulmonary hypertension and 5-HT2BR overexpression. Groups of 10 WT mice were exposed to hypoxia (10% 02) for 5 weeks (H), and in the presence of either RS-127445 (0.5 mg/kg-HRS), or STI-571 (1 mg/kg-HST) and compared with normoxic mice (0). (A) The significant increase in hypoxia-induced RVSP was totally prevented by RS-127445 or STI-571. (B) Chronic hypoxia increased significantly the maximal number of lung 5-HT2BR specific binding sites (Bmax), which was not significantly prevented by RS-127445, but STI-571 did prevent this increase. (C) Groups of 10 WT mice were exposed to hypoxia (10% 02) for 0 to 35 days in the presence of vehicle (Veh) or STI-571 (STI; 1 mg/kg). The significant increase in hypoxia-induced RVSP observed at 21 and 35 days was totally prevented by STI-571 with similar kinetic. (D) Chronic hypoxia increased significantly the maximal number of lung 5-HT2BR specific binding sites (Bmax) at 21 and 35 days, which was significantly prevented by STI-571 with similar kinetic. Values are means ± SEM (n = 10, P < .05), and are representative of at least 2 independent experiments. Straight lines are normoxic values. Any statistical difference by 1-way ANOVA followed by Bonferroni posthoc test versus normoxic untreated control values is indicated by * and versus chronic hypoxia values by a #; ***P < .001; **P < .01; *P < .05; ##P < .01; #P < .05.
Blocking c-kit activity prevents pulmonary hypertension and 5-HT2BR overexpression. Groups of 10 WT mice were exposed to hypoxia (10% 02) for 5 weeks (H), and in the presence of either RS-127445 (0.5 mg/kg-HRS), or STI-571 (1 mg/kg-HST) and compared with normoxic mice (0). (A) The significant increase in hypoxia-induced RVSP was totally prevented by RS-127445 or STI-571. (B) Chronic hypoxia increased significantly the maximal number of lung 5-HT2BR specific binding sites (Bmax), which was not significantly prevented by RS-127445, but STI-571 did prevent this increase. (C) Groups of 10 WT mice were exposed to hypoxia (10% 02) for 0 to 35 days in the presence of vehicle (Veh) or STI-571 (STI; 1 mg/kg). The significant increase in hypoxia-induced RVSP observed at 21 and 35 days was totally prevented by STI-571 with similar kinetic. (D) Chronic hypoxia increased significantly the maximal number of lung 5-HT2BR specific binding sites (Bmax) at 21 and 35 days, which was significantly prevented by STI-571 with similar kinetic. Values are means ± SEM (n = 10, P < .05), and are representative of at least 2 independent experiments. Straight lines are normoxic values. Any statistical difference by 1-way ANOVA followed by Bonferroni posthoc test versus normoxic untreated control values is indicated by * and versus chronic hypoxia values by a #; ***P < .001; **P < .01; *P < .05; ##P < .01; #P < .05.
Discussion
Both 5-HT and BM-derived stem cells have been shown to participate to some extent in PAH. Our work establishes, for the first time, a causal link between these 2 issues by showing that (1) the initial functions in pulmonary hypertension of 5-HT2BR restricted to BM cells, (2) the contribution to stem cells differentiation/proliferation of 5-HT2BRs, and (3) the critical functions of BM-derived cells for pulmonary hypertension development.
Several independent investigations have described a mobilization of BM-derived cells during pulmonary hypertension.2 Not only cells expressing c-kit are mobilized from BM in the circulation in response to hypoxia, but they are also found in the remodeled lung vessel wall in PAH (Figure 3 and Hayashida et al,7 Davie et al,8 Farha et al,12 Spees et al,24 and Sata et al31 ). Activation of c-kit was reported as necessary for mobilization of reparative BM progenitor cells32 and for the remodeling of blood vessels from these progenitor cells.3,27 Recently, a role of c-kit+ progenitors in hypoxia-induced vascular remodeling26,33 was evidenced: stromal derived factor-1 (SDF-1/CXCL12) and its receptors CXCR4 and CXCR7 have been shown to be critical for homing of hematopoietic c-kit+ progenitor cells in the perivascular niche, including in chronic hypoxia-exposed mice. These mice showed increased lung expression of CXCR4, CXCR7, and CXCL12, associated with significantly increased RVSP, vascular remodeling, and perivascular c-kit+/sca-1+ progenitor cell accumulation.26 In humans, pulmonary arterial lesions are also associated with expression of CXCL12 that may recruit c-kit+ cells.27 As we previously reported,16 5-HT2BRs were found overexpressed (as in mice) on human vascular SMC layer in lungs from PAH patients and at least partially colocalized with SMC α-actin.17 Independently, c-kit was shown to colocalize partially with SMC α-actin.26 Here, we show that c-kit and 5-HT2BR staining are also partially overlapping in hypoxic lung arteries, supporting a common link to SMC lineage. Further work is needed to clarify the exact contribution of these different partners into vascular remodeling during PAH.
STI-571 is a protein-tyrosine kinase inhibitor, which selectively blocks Abl, c-kit, and PDGFR,34 but has no 5-HT2BR agonist or antagonist properties (see supplemental data of Huang et al35 ). Expression of PDGFR was found to be significantly increased in lung tissue from pulmonary arterial hypertension patients and beneficial action of STI-571 has been proposed to act directly at lung PDGFR,36 which is well known to mediate SMC proliferation. However, in experimental vascular injury, expression of PDGFRβ by medial SMCs is up-regulated only several days after vascular injury,3 whereas c-kit+ cells appear within the vessel medial wall by the first day and early intervention with STI-571 can significantly attenuate intima hyperplasia formation. Finally, the STI-571-mediated inhibition of hyperplasia appears to act through the c-kit pathway, because the blocking anti–c-kit receptor monoclonal antibody ACK2 generated similar results.3 In this work, we show that the 5-HT2BR-overexpression in lungs is fully prevented by STI-571 treatment or by lack of 5-HT2BR in BM, both of which can hardly be explained only by a direct inhibition of lung resident cells expressing PDGFR. Our result strongly supports that cells, which give rise to 5-HT2BR expressing cells in lungs, are c-kit+ progenitor cells originating from BM, although contribution of other receptors-tyrosine kinase sensitive to STI-571 cannot be excluded.
Plasma 5-HT is increased in PAH patients, even after lung transplantation,37 which suggests that 5-HT either is an extra pulmonary causative factor in PAH or is associated with such a factor. Deletion of tryptophan hydroxylase 1 (the limiting enzyme in peripheral 5-HT synthesis), SERT, or 5-HT1BRs reduces pulmonary vascular remodeling and hypoxic pulmonary hypertension.38 Locally, 5-HT has been shown to be a potent vasoconstrictor of isolated pulmonary arteries but also to stimulate pulmonary SMC proliferation. It has thus been implicated in various processes associated with pulmonary vascular remodeling. Here, we found 5-HT2BR expression in both c-kit+ BM-derived cells and c-kit+ circulating blood cells. In addition, the results of BM transplantation establish for the first time that 5-HT2BR–expressing cells in hypertensive lungs are exclusively of extra-pulmonary origin probably deriving from c-kit+ BM precursors. These results clearly reveal that the initial requirement for 5-HT2BRs in PAH is extrinsic to the lung and BM-derived, independently of resident lung cells.
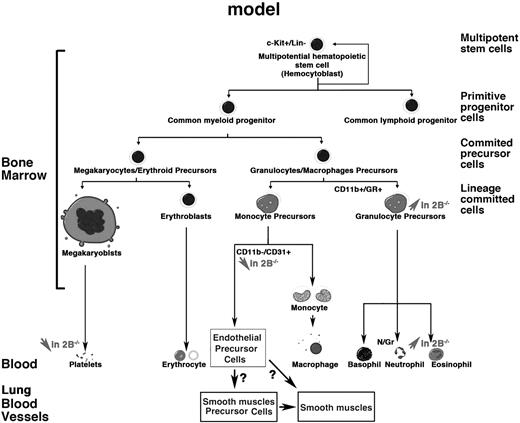
Although probably not instructive in stem cell lineages, 5-HT may rather modulate the balance between different lineages by participating at the proliferation and/or survival of specific lineage via its receptors (Figure 6). Similar to what we found here using 5-HT2BR antagonist RS-127445, a decrease in colony-forming capacity was observed with STI-571 with inhibition of both CFU-GEMM and BFU-E formation attributed to a reduction of cell proliferation and/or an apoptotic effect.39 In addition, we found 5-HT2BR expression in c-kit+ BM cells, but not in these cells after STI-571 treatment. To the opposite, 5-HT significantly enhanced the expansion of CD34+ cells to early stem/progenitors (CFU-GEMM) and committed progenitors (BFU/CFU-E).20 Recently, the absence of 5-HT was shown to reduce erythroid precursors in BM via 5-HT2A and 5-HT2BRs because a 5-HT2 agonist, PNU 22394, produced the same proliferative effect on erythroid precursors as observed with 5-HT.28 At early stages of megakaryocytopoiesis, 5-HT regulates proliferation and survival by antiapoptotic effects on megakaryoblastic cells.40 On megakaryocytic cell line HEL, pretreatment with 5-HT protected subsequent nitric oxide-induced apoptosis.41 We previously showed that 5-HT2BRs were required for proliferation of embryonic and survival of newborn cardiomyocytes via regulation of mitochondrial membrane permeability, caspase activation, and Akt/ERK1/2 pathways.42 Combined with these observations, the present data clearly highlight the importance of 5-HT via 5-HT2BRs at various levels of the myeloid lineages as summarized on the scheme (Figure 6). Our data support their requirement for proliferation/survival after the c-kit–dependent mobilization of precursors from BM.
Working model illustrating the present findings. This work implicates 5-HT2B receptors at different levels of the myeloid lineage. The absence of 5-HT2BRs in mutant mice (2B−/−) leads to a reduction in (↓) platelets and immature endothelial progenitors and lineages but to an increase (↑) in granulocyte precursors and lineages.
Working model illustrating the present findings. This work implicates 5-HT2B receptors at different levels of the myeloid lineage. The absence of 5-HT2BRs in mutant mice (2B−/−) leads to a reduction in (↓) platelets and immature endothelial progenitors and lineages but to an increase (↑) in granulocyte precursors and lineages.
BM progenitor cell mobilization is a physiologic response to hypoxic conditions,4,6 and altered circulating BM-derived precursors have been reported in PAH as contributors to vascular remodeling.7,8,24,31 Patients with myeloproliferative diseases often develop PAH, but this secondary form of PAH has been reported to resolve with treatment of the underlying myeloproliferative process.43 Other data highlight a potential interdependence of dysregulated hematopoiesis and abnormal pulmonary vascular endothelial behavior that may play a key role in the development of PAH.10 It was previously demonstrated that cells from the mononuclear lineage could differentiate into endothelial and SMC-like cells at injured vessels,44-47 and that circulating precursors of this lineage can contribute to hypoxia-induced pulmonary vascular remodeling.6 In this context, a stimulus-dependent generation of proangiogenic and BM-derived early progenitors could be required for physiologic repair response to ongoing pulmonary vascular shear stress and endothelial injury. That this process ultimately contribute to the abnormal proliferation of SMC and endothelial cells leading to progression of pulmonary hypertension was still hypothetical.9 We validate here the interdependence of dysregulated hematopoiesis and abnormal pulmonary vascular remodeling, which play key roles in the development of PAH.
Studies in patients and experimental models have led to a dichotomy of views regarding the role of BM progenitor cells in PAH. On the one hand, a relative deficiency of circulating endothelial precursor cells might contribute to pulmonary vascular pathology, and the transfusion of native autologous or genetically modified endothelial precursor cells could offer a novel cell-based therapy; long-term pharmacologic augmentation of endogenous progenitors represents an additional strategy. On the other hand, dysfunctional, apoptotic-resistant, and proliferative pulmonary vascular cells are implicated in the pathogenesis of PAH,12 and the inclusion of progenitor cells in vascular lesions may have adverse long-term consequences.9 Our work reveals that the absence of 5-HT2BRs generates permanent alterations of blood and BM composition in myeloid lineages and in particular, in endothelial/SMC progenitors. The present finding clearly supports a role of 5-HT2BRs in the maturation of different myeloid progenitors including immature endothelial/SMC precursors required for PAH pathogenesis.
Our work supports the concept that BM-derived cells contributing to pulmonary vascular remodeling may be the link between PAH and BM-related hematologic disorders.4 By demonstrating a causal role for 5-HT2BRs in BM progenitors, which controls precursor cells contributing to pulmonary hypertension vascular remodeling, this work switches the attention on PAH-initiating events from an intrinsic lung issue to a BM originating problem.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank O. Hermine and Anne Roumier for critical reading of the paper, and K. Boutourlinsky and Imane Moutkine for excellent technical assistance.
This work has been supported by funds from the Centre National de la Recherche Scientifique, Inserm, the Université Pierre et Marie Curie, and by grants from the Fondation de France, the Fondation pour la Recherche Médicale, and the European Union (DEVANX). S.L.D. is supported by a Region Ile de France DIM STEM, A.B. by a SFPT, and S.D. by a Lefoulon-DeLalande fellowship.
Authorship
Contribution: J.-M.L., F.C., and L.M. conceived and designed the experiments; P.H., J.C., Z.M., C.C., A.B., S.D., S.L.D., and S.H. performed the experiments; ZM, FC, J.-M.L., M.H., and L.M. analyzed the data: P.H., J.C., Z.M., C.C., S.D., S.L.D., and S.H. contributed reagents, materials, and analysis tools; and Z.M., F.C., J.M.L., M.H., and L.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luc Maroteaux, Inserm UMR-S 839, Institut du Fer à Moulin, 17 rue du Fer a moulin 75005, Paris, France; e-mail: luc.maroteaux@upmc.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal