Abstract
Although scid mice have been widely used for human HSC engraftment studies, the function of HSCs of scid mice has not been characterized. We hypothesized that the DNA repair defect of scid mice results in a stem cell defect that facilitates HSC engraftment. scid BM cells showed severely impaired repopulation potentials in the competitive repopulation assay. To assess the BM hematopoietic niche occupancy ability of scid HSC, WT BM cells were transplanted into scid mice without any conditioning and observed to achieve long-term engraftment. Furthermore, the defects of scid HSCs are independent of their inability to perform lymphopoiesis because a similar defect in hematopoietic niche occupancy was not observed with Rag1−/− recipients. These results demonstrate that scid HSCs are impaired in maintenance within the niche, which may explain the nature of the conducive marrow niche environment of scid mice for xenotransplantation.
Introduction
Engraftment of human HSCs into NOD-scid (ie, nonobese diabetic-severe combined immunodeficiency) mice has become the gold standard in the stem cell research community for the evaluation of human HSC function.1,2 The scid mutation is a spontaneous nonsense mutation in protein kinase, DNA-activated catalytic polypeptide (Prkdc), which is the catalytic subunit of DNA-dependent kinase (DNA PKcs) in the nonhomologous end-joining (NHEJ) pathway.3-5 Mice homozygous for the mutation (Prkdcscid, commonly referred to as scid) are characterized by an absence of functional T cells and B cells because of the inability of V(D)J recombination, although some mice show a “leaky” phenotype.6 The immunodeficiency had been considered responsible for the engraftment of human hematopoietic tissues; however, xenograft mouse models determined on the basis of mice deficient in Rag1 (recombination activating gene 1) or Rag2, which show “nonleaky” SCID phenotype,7,8 are less conducive for xeno-engraftment than scid mice,2,9 and only slight HSC engraftment was observed when large numbers of wild-type (WT) HSCs were transplanted into unconditioned Rag2−/− or WT mice,10,11 suggesting that immunodeficiency itself may not be the primary characteristic permitting engraftment in scid mice.
The authors of previous studies have shown that HSCs deficient in the NHEJ pathway are defective in repopulation and BM niche occupancy.12,13 We hypothesize that HSCs in scid mice have intrinsic defects because of loss of the NHEJ pathway, which facilitate exogenous engraftment. Here, we assess the self-renewal and BM hematopoietic niche occupancy capacities of scid HSC and compare those with Rag1−/− HSC.
Methods
Mice
The C57BL/6, B6.CB17-Prkdcscid/SzJ (scid), Rag1−/− (all C57BL/6 background strains), and the congenic strain B6.SJL-PtprcaPep3b/BoyJ (BoyJ; The Jackson Laboratory) were bred and housed in specific pathogen-free facility. All mouse studies were approved by the institutional animal care and use committee at Case Western Reserve University.
Transplantation assay
Flow cytometry
Flow cytometry was performed on a BD LSRI or LSRII (BD Biosciences), and we analyzed data using BDFACS Diva 6.2 software or FlowJo Version 8.8 software (TreeStar).
Results and discussion
HSC pools in scid mice are maintained at steady-state
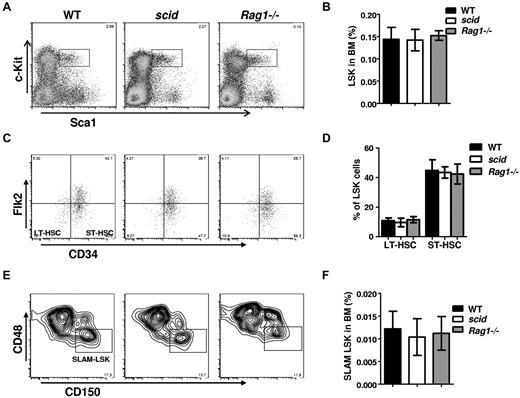
To determine whether scid affects the maintenance of HSC pools, we examined HSC pools in BM by flow cytometry. The frequency of LSK cells in scid or Rag1−/− BM was within the normal range compared with WT BM (Figure 1A-B), as were frequencies of long- and short-term HSCs (Figure 1C-D). SLAM markers were also used to analyze the primitive HSC pool. The frequency of SLAM+LSK was slightly decreased but not significantly altered in scid mice, whereas SLAM+LSK frequency in Rag1−/− BM was compatible with WT BM (Figure 1E-F). BM cellularity in scid and WT mice were compatible (data not shown), indicating that HSC cell numbers in scid mice were compatible with those in WT mice. In addition, steady-state proliferation and apoptosis of HSCs in scid mice did not differ significantly from WT mice (data not shown). These results suggested that scid mutation does not change the size of immunophenotypic HSC pools.
scid mutation does not affect HSC pools. BM cells from scid, Rag1−/−, and WT mice were assayed by multiparameter FACS for proportion of HSC populations. At least 6 age-matched (8-10 weeks old) mice per genotype were compared. Representative FACS profiles of pregated on live, lineage negative cells, Sca1+, c-Kit+ (LSK) are shown in panel A, long-term HSCs (LSK, CD34−, Flk2−) and short-term HSCs (LSK, CD34−, Flk2+) are shown in panel C, and SLAM+ LSK (CD48−, CD150+,LSK) are shown in panel E. Frequencies of HSCs, LT-HSCs, ST-HSCs, and SLAM+LSK in BM from WT, scid, and Rag1−/− mice are analyzed in panels B, D, and F. Error bars indicate SD; significance was determined by a Student 2-tailed t test.
scid mutation does not affect HSC pools. BM cells from scid, Rag1−/−, and WT mice were assayed by multiparameter FACS for proportion of HSC populations. At least 6 age-matched (8-10 weeks old) mice per genotype were compared. Representative FACS profiles of pregated on live, lineage negative cells, Sca1+, c-Kit+ (LSK) are shown in panel A, long-term HSCs (LSK, CD34−, Flk2−) and short-term HSCs (LSK, CD34−, Flk2+) are shown in panel C, and SLAM+ LSK (CD48−, CD150+,LSK) are shown in panel E. Frequencies of HSCs, LT-HSCs, ST-HSCs, and SLAM+LSK in BM from WT, scid, and Rag1−/− mice are analyzed in panels B, D, and F. Error bars indicate SD; significance was determined by a Student 2-tailed t test.
HSCs from scid mice are defective in self-renewal
To examine the long-term repopulation activity of scid HSC in vivo, serial transplantation and competitive repopulation assays were performed. However, because of the high frequency of lymphoma development in primary recipients, we were unable to assess the long-term repopulation activity of scid HSC using serial transplantation (data not shown). scid BM had a significant competitive repopulation disadvantage, yielding < 1% overall chimerism in peripheral blood of recipients when mixed 1:1 with WT BM (Figure 2A top). Because scid BM cannot generate T and B lymphocytes, myeloid (Mac1+) chimerism in the recipient peripheral blood was examined. scid BM was impaired in myeloid generation, contributing only approximately 7% of peripheral blood myeloid chimerism (Figure 2A bottom, B). Consistently, HSCs derived from scid BM in the transplant recipients' BM comprised < 5% of the total HSC population (Figure 2C).
scid HSCs are defective in self-renewal and niche occupancy. (A-C) Competitive repopulation assay of scid and Rag1−/− HSCs. A total of 2 × 106 BM from 8-week-old healthy WT, scid, or Rag1−/− mice (CD45.2) were mixed with age-matched WT competitors (CD45.1) at 1:1 ratio, and transplanted into lethally irradiated WT recipients (CD45.1). Sixteen weeks after transplantation, donor chimerisms of myeloid cells (Mac1+) in the peripheral blood and HSCs (LSK) in the BM were analyzed and quantitated in panels B and C. Donor chimerisms of Mac1+ cells in the peripheral blood are calculated as the CD45.2+Mac1+ portion of the total Mac1+ cells. Donor chimerisms of LSK in the BM are calculated as the CD45.2+LSK portion of the total LSK. Error bars indicate SD; significance was determined by a Student 2-tailed t test. *P < .005. (D-F) Engraftment of WT HSC into unconditioned scid and Rag1−/− mice. WT BM cells (5 × 106; CD45.1) were transplanted into unconditioned WT, scid, and Rag1−/− mice (CD45.2). Sixteen weeks after transplantation, donor chimerisms of multilineages in peripheral blood of recipients were analyzed by FACS, and representative results are shown in panel D. Myeloid chimerisms in the peripheral blood were quantitated in panel E, donor chimerism of myeloid cells is the CD45.1+Mac1+ portion of the total Mac1+ cells. Engraftment of transplanted WT BM HSCs (LSK) in the recipients were analyzed in panel F, donor chimerism of HSC is the CD45.1+LSK portion of the total LSK cells. Error bars indicate SD; the engraftment of WT BM into scid and Rag1−/− mice was compared; significance was determined by a Student 2-tailed t test. *P < .01.
scid HSCs are defective in self-renewal and niche occupancy. (A-C) Competitive repopulation assay of scid and Rag1−/− HSCs. A total of 2 × 106 BM from 8-week-old healthy WT, scid, or Rag1−/− mice (CD45.2) were mixed with age-matched WT competitors (CD45.1) at 1:1 ratio, and transplanted into lethally irradiated WT recipients (CD45.1). Sixteen weeks after transplantation, donor chimerisms of myeloid cells (Mac1+) in the peripheral blood and HSCs (LSK) in the BM were analyzed and quantitated in panels B and C. Donor chimerisms of Mac1+ cells in the peripheral blood are calculated as the CD45.2+Mac1+ portion of the total Mac1+ cells. Donor chimerisms of LSK in the BM are calculated as the CD45.2+LSK portion of the total LSK. Error bars indicate SD; significance was determined by a Student 2-tailed t test. *P < .005. (D-F) Engraftment of WT HSC into unconditioned scid and Rag1−/− mice. WT BM cells (5 × 106; CD45.1) were transplanted into unconditioned WT, scid, and Rag1−/− mice (CD45.2). Sixteen weeks after transplantation, donor chimerisms of multilineages in peripheral blood of recipients were analyzed by FACS, and representative results are shown in panel D. Myeloid chimerisms in the peripheral blood were quantitated in panel E, donor chimerism of myeloid cells is the CD45.1+Mac1+ portion of the total Mac1+ cells. Engraftment of transplanted WT BM HSCs (LSK) in the recipients were analyzed in panel F, donor chimerism of HSC is the CD45.1+LSK portion of the total LSK cells. Error bars indicate SD; the engraftment of WT BM into scid and Rag1−/− mice was compared; significance was determined by a Student 2-tailed t test. *P < .01.
To examine whether the self-renewal defect of scid HSCs occurred because of its V(D)J deficiency, we evaluated Rag1−/− mice in the competitive repopulation assay. Although the overall chimerism derived from Rag1−/− BM in the peripheral blood of recipients was significantly decreased from WT BM because of a lack of lymphocytes production (Figure 2A top), Rag1−/− BM did not exhibit a competitive repopulation disadvantage, instead contributing almost 50% of the myeloid cells in the peripheral blood and approximately 50% of HSC in the BM of the recipients (Figure 2A bottom, B-C). In addition, scid HSCs were not defective in BM homing, as measured by recovery of progenitors 16 hours after transplantation (data not shown). These results demonstrate that scid HSCs are severely impaired in competitive repopulation independently of their immunodeficiency.
HSC from scid mice are defective in BM hematopoietic niche occupancy
scid mice are “permissive” to exogenous HSC transplantation, suggesting that scid HSCs are disadvantaged against WT HSCs in competing for the BM niche to sustain long-term hematopoiesis, which we term a BM hematopoietic niche occupancy defect. To assess the competitiveness of scid HSC in situ, 5 × 106 WT BM cells were transplanted into scid and WT recipients without myeloablative conditioning. As expected, little if any measurable stem cell engraftment occurred in WT recipients. In contrast, transplanted WT BM made a long-term multilineage contribution to hematopoiesis in scid recipients. By 16 weeks after transplantation, 12% to 18% of myeloid cells in blood and 8% to 10% of LSK cells in BM were WT donor derived (Figure 2D-F). These engrafted WT HSCs were transplantable (data not shown). In addition, WT donor–derived lymphoid cells, both T and B cells, were present in the peripheral blood of scid recipients, indicating that the BM microenvironment in scid mice is capable of supporting HSC differentiation and lymphoid development. Thus, there does not appear to be a microenvironment defect in the scid mouse; rather, scid HSCs have an intrinsic defect in their maintenance within the BM niche. Rag1−/− mice, which cannot complete V(D)J, but do not have a defect in NHEJ, have a minimal hematopoietic niche occupancy defect, allowing very slight (< 1%) engraftment of WT HSCs under the conditions used (Figure 2D-F).
These data demonstrate that HSCs in scid mice have intrinsic defects in self-renewal and BM hematopoietic niche occupancy, and importantly, these HSC defects are independent of the immunodefiency. DNA PKcs is essential for NHEJ, and although the NHEJ pathway is involved in both DNA repair and V(D)J recombination, our results indicate that, from the vantage point of hematopoiesis, these functions are distinct. HSCs in Rag1−/− mice and Rag2−/− mice10 retain functional, whereas data presented here clearly show that it is the deficiency in NHEJ DNA repair rather than the inability of V(D)J recombination that results in the HSC defect in scid mice.
Our results suggest that both self-renewal and BM hematopoietic niche occupancy defects of scid HSCs facilitate the engraftment of exogenous HSCs, including human HSCs. This BM hematopoietic niche occupancy defect is distinct from the self-renewal defect of scid HSCs; heterozygous Kit mutant mice exhibit a large HSC self-renewal defect (Y.Q., Y.L., and S.L.G., unpublished data, May 2011)16 but do not allow WT BM engraftment without conditioning (Y.Q., Y.L., and S.L.G., unpublished data, May 2011). This finding is important to the interpretation of hematopoietic xenotransplantation in the scid model system and suggests that scid conditions may not mimic human/human transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center (P30 CA43703) and by National Institute of Health grants R01AG024916 and R01CA063193.
National Institutes of Health
Authorship
Contribution: Y.L. performed research and analyzed data; and Y.Q. and S.L.G. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stanton L. Gerson, University Hospitals Case Medical Center and Case Western Reserve University, 10900 Euclid Ave, Wearn 151, Cleveland, OH 44106; e-mail: slg5@case.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal