Abstract
It is believed that the size of the CD8+ T-cell pool is fixed and that with every new viral challenge, the size of the pre-existing memory-cell population shrinks to make way for the new virus-specific cells. CMV-seropositive individuals have high numbers of CMV-specific resting-effector type CD8+ T cells in their peripheral blood (PB). This prompted us to investigate whether CMV infection limits immunologic space at sites where immune reactions are initiated, such as in the lymph nodes (LNs). LN and paired PB samples were analyzed for CMV-, EBV-, and influenza-specific CD8+ T cells. In marked contrast to blood, LNs contained significantly lower numbers of CX3CR1-expressing effector-type CD8+ T cells, whereas the CMV-specific cells that were found in the LNs resembled polyfunctional memory-type cells. In contrast, EBV- and influenza-specific CD8+ T cells were highly similar between PB and LNs both in number and function. Therefore, it is unlikely that CMV-specific CD8+ T cells in the LNs restrain the immunologic space of other virus-specific cells.
Introduction
The elderly have an increased susceptibility to infections and impaired responses to vaccination, which may be caused by aging of the immune system, also referred to as immunosenescence. This poses a major challenge to public health, and may become even more significant as the percentage of older people increases in the Western population. Various factors, including an impaired innate immune system and reduced T- and B-cell responses, may contribute to immunosenescence.1-3 Longitudinal studies in the aged (> 85 years) have suggested that poor immunologic responses are associated with an “immune risk profile.” Characteristics of this risk profile are an inverted CD4/CD8 ratio and increased numbers of CD27−CD28−CD57+ effector-type CD8+ T cells.4 We and others have shown that the presence of large numbers of these CD27−CD45RA+ and CD57+CD28− CD8+ T cells, which are largely overlapping populations, is associated with latent CMV infection.5-8 Therefore, CMV infection may contribute to immunosenescence.
It is generally believed that the size of the CD8+ T-cell pool is fixed and that with every new viral challenge, the size of the preexisting memory-cell population shrinks to make way for new virus-specific cells, a process referred to as “memory-cell attrition.”9 However, Vezys et al have challenged this notion by demonstrating, in an elegant murine model system of repetitive antigenic challenge, that the CD8+ T-cell compartment is remarkably flexible and has the capacity to expand and accommodate vast numbers of Ag-specific CD8+ T cells.10 This enlargement predominantly occurs within the effector memory subset and, therefore, the major enlargements of the CD8+ T-cell pool take place in the spleen, blood, and solid tissues, but not in the lymph nodes (LNs). Memory CD8+ T cells specific for previously encountered infections were found to be largely preserved. In agreement with these observations in experimental animal models, we have shown in humans that the entrance of CMV-specific CD8+ T cells expanded the Ag-primed CD8+ T-cell compartment rather than competing for “immune-space” with preexisting memory T cells specific for persistent or cleared viruses.11 In this respect, it is interesting that circulating CMV-specific CD8+ T cells in latent virus carriers do not express CCR7,12-14 but do have a high expression level of CX3CR1,15 the chemokine receptor for fractalkine, a cell-bound chemokine expressed on stressed endothelial cells. Therefore, the expression of CX3CR1 provides CMV-specific CD8+ T cells with the ability to migrate to inflamed vascular endothelium,16,17 whereas the absence of CCR7 indicates that these cells are unlikely to home to LNs.
To test this notion directly, we investigated whether CMV infection limits immunologic space at sites where immune reactions are initiated, such as in the LNs. We had the unique opportunity to study LN-derived human T cells. These cells were isolated from LNs gathered from surgical waste material collected during living donor kidney transplantation. We investigated the presence, phenotype, and polyfunctionality of CMV-, EBV-, and influenza-specific and total CD8+ T cells in the LNs and compared this with the peripheral blood (PB) compartment.
Methods
Subjects
We studied absolute numbers of CD4+ and CD8+ T cells, B cells, and natural killer (NK) cells in a large group of patients on the waiting list for renal transplantation (N = 560). In a smaller group of transplantation recipients (n = 21), we studied paired heparinized PBMCs and LN mononuclear cells (LNMCs), which were isolated before or during kidney transplantation, respectively. All of these patients were EBV-seropositive, and the majority were also CMV-seropositive (16 of 21 patients). All patients were treated with quadruple immunosuppression consisting of CD25mAb induction therapy, prednisolone, calcineurin inhibitor, and mycophenolic acid. Except for CD25mAb, immunosuppressive treatment was started after transplantation. At the time the LNs were gathered, the first dose of CD25mAb was already administered. However, we have demonstrated that CD25mAb (basiliximab; Novartis Pharma) was not detectable in LNMCs and that ex vivo CD25 expression on LNMCs could be blocked with CD25mAb (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The medical ethics committee of the Academic Medical Center, Amsterdam, approved this study and all subjects gave written informed consent in accordance with the Declaration of Helsinki.
Isolation of mononuclear cells from PB and LNs
PBMCs were isolated using standard density gradient centrifugation. LNs were collected from kidney transplantation recipients during living donor kidney transplantation. Briefly, LNMCs were isolated from surgical residual material of the recipient that was gathered during implantation of the transplanted kidney. Before anastomosing the arteria and vena renalis, the iliac artery and vein were dissected free. The residual tissue that was removed in this procedure often contains LNs. Directly after extraction, the gathered LNs were chopped into small pieces. A cell suspension was obtained by grinding the material through a flow-through chamber. PBMCs and LNMCs were subsequently cryopreserved until the day of analysis.
Determination of absolute numbers of CD4+ and CD8+ T cells, B cells, and NK cells
Absolute numbers were determined in EDTA whole blood with Multitest 6 color reagents (BD Biosciences) according to the manufacturer's instructions. Analysis was performed on a FACSCanto II (BD Biosciences) using FACSCanto Version 2.2 software for analysis.
Virological analysis
To determine CMV serostatus, anti–CMV IgG was measured in the serum using the AxSYM microparticle enzyme immunoassay (Abbott Laboratories) according to the manufacturer's instructions. Measurements were calibrated relative to a standard serum. EBV serostatus was determined by qualitative measurement of specific IgG against the viral capsid Ag and against the nuclear Ag of EBV using, respectively, the anti–EBV viral capsid Ag IgG ELISA and the anti–EBV nuclear Ag of EBV IgG ELISA (Biotest). All tests were performed following the instructions of the manufacturers.
Tetrameric complexes
HLA-peptide tetramer complexes were obtained from Sanquin Reagents. For CMV, we used 8 different tetramers loaded with pp65- and IE-derived peptides; for EBV, we used 6 different tetramers loaded with BMLF1-, EBNA3A-, and BZLF1-derived peptides; and for influenza (FLU), we used 2 different tetramers loaded with nucleoprotein- and matrix protein–derived peptide (supplemental Table 1).
Immunofluorescence staining and flow cytometry
PBMCs and LNMCs were washed in PBS containing 0.01% NaN3 and 0.5% BSA. Two million PBMCs and LNMCs were incubated with an appropriate concentration of tetrameric complexes for 30 minutes at 4°C and protected from light. Fluorescence-labeled mAbs were added and incubated for 30 minutes at 4°C, protected from light and at concentrations according to the manufacturer's instructions. The following surface Abs were used: CD8 V450, CD3 V500, CCR7 PE-Cy7, CD8 PerCP-Cy5.5, and CD3 PE-Cy7 (BD Biosciences); CD45RA eFluor 605NC (eBioscience); CX3CR1 FITC (BioLegend); CD27 APC-Alexa Fluor 750 and CD4 PE-Cy5.5 (Invitrogen); and CXCR3 PE (R&D Systems).
For intracellular staining, cells were fixed after surface staining with FACS Lysing Solution (BD Biosciences). After permeabilization (FACS Permeabilizing Solution 2; BD Biosciences), cells were stained with anti–granzyme K FITC (Immunotools) and anti–granzyme B PE (Sanquin). The Live/Dead Fixable Red Cell Stain Kit (Invitrogen) was used in every staining to exclude dead cells from the analysis. Cells were measured on an LSR-Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo Version 9.3.3 software (TreeStar).
Detection of polyfunctional T cells
Cytokine release after peptide or phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation was performed as described by Lamoreaux et al.18 PBMCs and LNMCs were thawed and rested overnight in suspension flasks (Greiner) in RPMI supplemented with 10% FCS, penicillin, and streptomycin (culture medium). Two million cells were stimulated with PMA/ionomycin or with the viral peptides in culture medium in the presence of CD107a FITC (eBioscience); αCD28 (15E8; 2 μg/mL), αCD29 (TS 2/16; 1 μg/mL), brefeldin A (Invitrogen; 10 μg/mL); and GolgiStop (BD Biosciences) in a final volume of 200 μL for 4 hours (PMA at 10 ng/mL/ionomycin at 1 μg/mL) or 6 hours (peptide) at 37°C and 5% CO2. Stimulations were performed in untreated, round-bottom, 96-well plates (Corning). Subsequently, cells were incubated with the appropriate tetramers, followed by incubation with CD3 V500, CD8 V450, CD4 PE-Cy5.5, and Live/Dead fixable red cell stain for 30 minutes at 4°C. The cells were then washed twice, fixed, and permeabilized (Cytofix/Cytoperm reagent; BD Biosciences) and subsequently incubated with the following intracellular mAbs: anti-IFNγ APC–Alexa Fluor 750 (Invitrogen) and anti-TNFα Alexa Fluor 700, anti–IL-2 PE, and anti–Mip-1β PE-Cy7 (BD Biosciences) for 30 minutes at 4°C. Cells were washed twice and measured on an LSRFortessa flow cytometer and analyzed with FlowJo Version 9.3.3 software.
Statistical analysis
Statistical analysis of paired samples was done with the 2-tailed Wilcoxon signed-rank test with a 95% confidence interval. Nonpaired samples were analyzed with 2-tailed Mann-Whitney test with a 95% confidence interval.
Results
CMV leaves a fingerprint in the PB CD8+ T-cell compartment
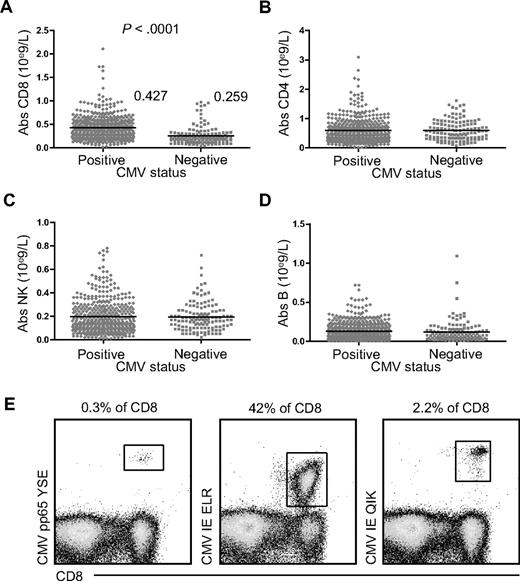
Because CMV has been shown to have a large impact on the composition of the PB CD8+ T-cell compartment,5 we first studied its influence on the total number of CD8+ T cells in the PB. In a large cohort of 560 patients awaiting kidney transplantation, we found nearly twice as many circulating CD8+ T-cell numbers in 430 CMV-seropositive individuals compared with 130 CMV-seronegative ones (0.432 × 109/L vs 0.277 × 109/L; P < .0001, Figure 1A). CD4+ T cells, NK cells, and B cells were not affected (Figure 1B-D, respectively). Even in healthy subjects, up to 40% of the total CD8+ T-cell compartment can be directed against one single peptide-HLA complex (Figure 1E). These findings establish that CMV infection induces a lasting and profound CD8+ T-cell expansion in the PB compartment.
Absolute number of CD8+ T cells, CD4+ T cells, NK cells, and B cells in CMV-seropositive and CMV-seronegative patients awaiting renal transplantation. Comparison of absolute cell numbers in the PB of CMV-seropositive (♦, n = 430) and CMV-seronegative (■, n = 130) patients prior to transplantation. (A) CD8+ T cells; (B) CD4+ T cells; (C) NK cells; and (D) B cells. (E) Percentages of CMV pp65 YSE-, CMV IE ELR-, and CMV IE QIK–specific CD8+ T cells within total CD8+ T cells in 1 healthy subject.
Absolute number of CD8+ T cells, CD4+ T cells, NK cells, and B cells in CMV-seropositive and CMV-seronegative patients awaiting renal transplantation. Comparison of absolute cell numbers in the PB of CMV-seropositive (♦, n = 430) and CMV-seronegative (■, n = 130) patients prior to transplantation. (A) CD8+ T cells; (B) CD4+ T cells; (C) NK cells; and (D) B cells. (E) Percentages of CMV pp65 YSE-, CMV IE ELR-, and CMV IE QIK–specific CD8+ T cells within total CD8+ T cells in 1 healthy subject.
CMV-specific CD8+ T cells are much less dominant in the LNs than in the PB, but percentages of EBV- and FLU-specific CD8+ T cells are similar
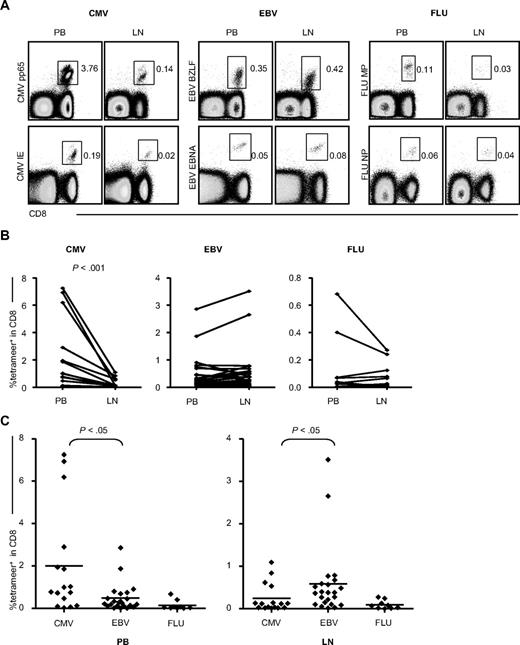
To investigate whether the CMV-specific CD8+ T cells in the LNs were as abundant as in the PB, we compared the percentage of CMV-specific cells in PB CD8+ T cells with those in LN CD8+ T cells (Figure 2). As a control, EBV- and FLU-specific CD8+ T cells were also counted. CMV-specific CD8+ T cells were always found at significantly lower percentages in the LNs than in the PB compartment. However, the presence of EBV- and FLU-specific CD8+ T cells in the PB was similar to that found in the LNs (Figure 2B). Moreover, whereas CMV-specific CD8+ T cells exceeded EBV-specific CD8+ T cells in the PB, in the LNs, this was found to be quite the opposite (Figure 2C). Therefore, in the LNs, CMV-specific CD8+ T cells are only a minority of the total CD8+ T-cell population.
Percentages of CMV-, EBV-, and FLU-specific CD8+ T cells in the PB and LNs. (A) CMV-, EBV-, and FLU-specific CD8+ T cells in the PB compared with the LNs. The dot plots are gated on CD3+ lymphocytes. For each virus, representative plots of 2 different viral epitopes are demonstrated: CMV pp65-epitope and IE-epitope, EBV BZLF-epitope and EBNA epitope, and FLU matrix protein (MP) epitope and nucleoprotein (NP) epitope. PB and LNs are paired samples and all data are derived from 1 patient. (B) Comparison of the percentages of PB- and LN-derived CMV-, EBV-, and FLU-specific cells within total CD8+ T cells. Paired samples were analyzed using the Wilcoxon signed-rank statistical test. (C) Comparison of the mutual proportion of CMV-, EBV-, and FLU-specific cells within the PB and LNs. Statistical analysis was done by the Mann-Whitney U test.
Percentages of CMV-, EBV-, and FLU-specific CD8+ T cells in the PB and LNs. (A) CMV-, EBV-, and FLU-specific CD8+ T cells in the PB compared with the LNs. The dot plots are gated on CD3+ lymphocytes. For each virus, representative plots of 2 different viral epitopes are demonstrated: CMV pp65-epitope and IE-epitope, EBV BZLF-epitope and EBNA epitope, and FLU matrix protein (MP) epitope and nucleoprotein (NP) epitope. PB and LNs are paired samples and all data are derived from 1 patient. (B) Comparison of the percentages of PB- and LN-derived CMV-, EBV-, and FLU-specific cells within total CD8+ T cells. Paired samples were analyzed using the Wilcoxon signed-rank statistical test. (C) Comparison of the mutual proportion of CMV-, EBV-, and FLU-specific cells within the PB and LNs. Statistical analysis was done by the Mann-Whitney U test.
CMV-specific CD8+ T cells and total CD8+ T cells in the LNs are enriched for CCR7 and CXCR3 and almost depleted of CX3CR1-expressing cells
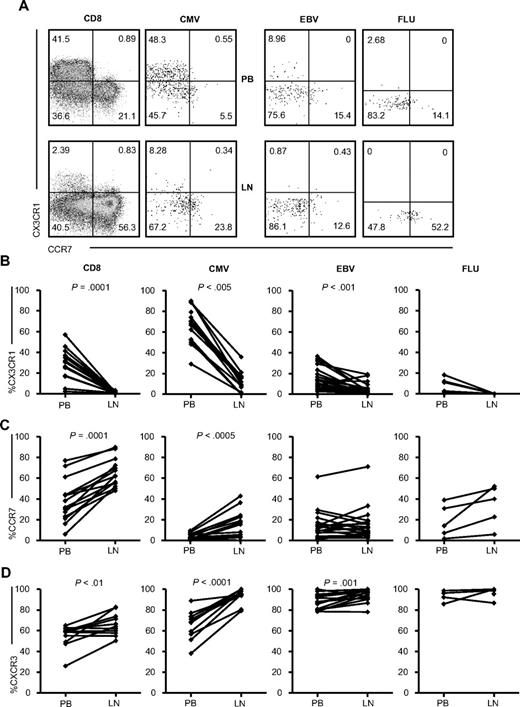
We studied chemokine receptor expression to determine why LN CD8+ T cells contained dramatically fewer CMV-specific CD8+ T cells. One of the chemokines expressed abundantly on PB CMV-specific CD8+ T cells is CX3CR1,15 which allows for homing to inflamed endothelium and tissue via its ligand, fractalkine. The number of cells expressing CX3CR1 in total CD8+ T cells and in CMV- and EBV-specific CD8+ T cells in the LNs was strongly reduced compared with that in the PB (P = .001, P < .005, P < .001, respectively; Figure 3B), suggesting exclusion from the LNs. We found very few CX3CR1-expressing FLU-specific cells in both the PB and LNs. Because CCR7 is required for homing to resting LNs, we next investigated the expression of this receptor. As expected, more CCR7-expressing total CD8+ T cells were found in the LNs (P = .0001, Figure 3C). In addition, CMV-specific CD8+ T cells were highly enriched for CCR7-expressing cells (P < .0005). A similar trend was observed for FLU-specific CD8+ T cells, but not for EBV-specific CD8+ T cells. In mice, CXCR3 has been shown to be involved in the recruitment of T cells toward draining LNs.19 Both total CD8+ T cells and CMV- and EBV-specific CD8+ T cells in the LNs were enriched for CXCR3-expressing cells. Strikingly, LN CMV-specific CD8+ T cells resembled LN EBV-specific CD8+ T cells with respect to all of the chemokine receptors studied. Equally high percentages of FLU-specific cells in the PB and LNs express CXCR3. These data suggest that the lower percentages of CMV-specific CD8+ T cells found in the LNs might be caused by the exclusion of the CX3CR1-positive CMV-specific CD8+ T cells, the major phenotype in the PB, and the recruitment of CCR7-expressing CMV-specific CD8+ T cells into the LNs, a phenotype that is negligibly present in the PB.
Chemokine receptor expression on (virus-specific) CD8+ T cells in the PB and LNs. (A) Representative dot plots of CCR7 and CX3CR1 staining on total (n = 15), CMV-specific (n = 10, 5 donors were analyzed with 2 different tetramers), EBV-specific (n = 7, 4 donors were analyzed with 2 tetramers and 2 with 3 tetramers), and FLU-specific (n = 5) CD8+ T cells. Comparison of percentages of CX3CR1-expressing (B), CCR7-expressing (C), and CXCR3-expressing (D) cells within total and CMV-, EBV-, and FLU-specific CD8+ T cells between PB and LNs. All panels represent from left to right: total, CMV-, EBV-, and FLU-specific CD8+ T cells. Paired samples were analyzed using the Wilcoxon signed-rank test.
Chemokine receptor expression on (virus-specific) CD8+ T cells in the PB and LNs. (A) Representative dot plots of CCR7 and CX3CR1 staining on total (n = 15), CMV-specific (n = 10, 5 donors were analyzed with 2 different tetramers), EBV-specific (n = 7, 4 donors were analyzed with 2 tetramers and 2 with 3 tetramers), and FLU-specific (n = 5) CD8+ T cells. Comparison of percentages of CX3CR1-expressing (B), CCR7-expressing (C), and CXCR3-expressing (D) cells within total and CMV-, EBV-, and FLU-specific CD8+ T cells between PB and LNs. All panels represent from left to right: total, CMV-, EBV-, and FLU-specific CD8+ T cells. Paired samples were analyzed using the Wilcoxon signed-rank test.
Total CD8+ T cells and CMV-specific CD8+ T cells in the LNs contain fewer CD45RA+CD27− effector-type cells
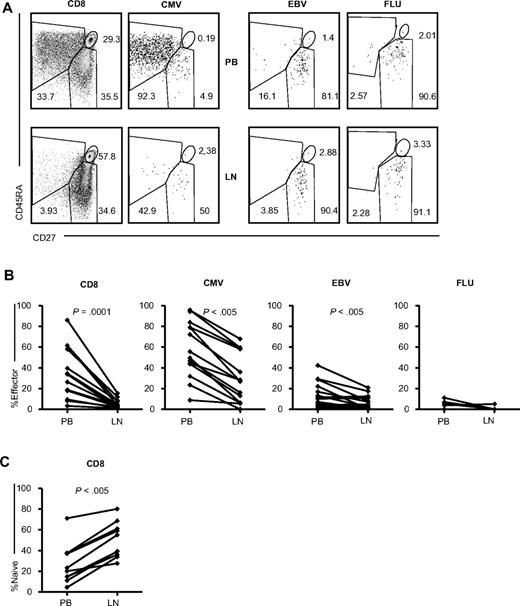
The difference in expression of chemokine receptors between the PB and LN CMV-specific CD8+ T cells prompted us to further investigate other phenotypical differences. Because most CMV-specific cells in the PB are CD45RA+CD27−, markers previously shown to depict an effector phenotype, we studied the LN CMV-specific CD8+ T cells for their expression of these markers (Figure 4). When analyzing the total CD8+ T-cell compartment, the lack of effector type cells in the LNs became readily apparent. The difference between the PB and LN CD8+ T cells was highly significant (P = .0001, Figure 4B). This finding was reflected in the lower percentage of effector-type cells in LN CMV- and EBV-specific CD8+ T cells (both P < .005) compared with the PB. Because almost no PB FLU-specific CD8+ T cells with an effector phenotype could be detected, the difference between the PB and LNs was not significant. In short, a substantially greater number of the LN total CD8+ T cells and CMV- and EBV-specific CD8+ T cells displayed a memory phenotype compared with the PB.
Expression of CD27 and CD45RA on (virus-specific) CD8+ T cells in the PB and LNs. (A) Representative dot plots of CD27 and CD45RA staining, gated on total (n = 15), CMV-specific (n = 10, 5 donors were analyzed with 2 different tetramers), EBV-specific (n = 7, 4 donors were analyzed with 2 tetramers and 2 donors with 3 tetramers), and FLU-specific (n = 5) CD8+ T cells analyzed in paired samples from the PB and LNs. (B) Comparison of percentages of CD27−CD45RA+ (effector-type) cells within total and CMV-, EBV-, and FLU-specific CD8+ T cells between PB and LNs. (C) Comparison of the percentages of CD27+CD45RA+ (naive) cells within total CD8+ T cells between PB and LNs. Paired samples were analyzed using the Wilcoxon signed-rank test.
Expression of CD27 and CD45RA on (virus-specific) CD8+ T cells in the PB and LNs. (A) Representative dot plots of CD27 and CD45RA staining, gated on total (n = 15), CMV-specific (n = 10, 5 donors were analyzed with 2 different tetramers), EBV-specific (n = 7, 4 donors were analyzed with 2 tetramers and 2 donors with 3 tetramers), and FLU-specific (n = 5) CD8+ T cells analyzed in paired samples from the PB and LNs. (B) Comparison of percentages of CD27−CD45RA+ (effector-type) cells within total and CMV-, EBV-, and FLU-specific CD8+ T cells between PB and LNs. (C) Comparison of the percentages of CD27+CD45RA+ (naive) cells within total CD8+ T cells between PB and LNs. Paired samples were analyzed using the Wilcoxon signed-rank test.
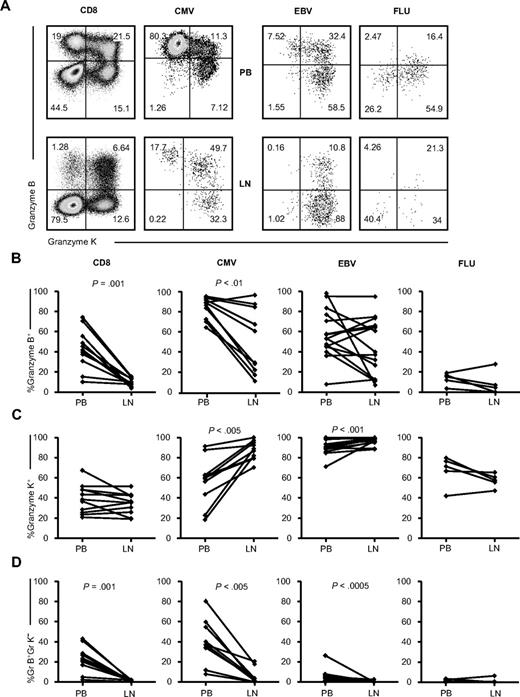
Only few granzyme B+granzyme K− CMV- and EBV-specific CD8+ T cells and total CD8+ T cells are found in the LNs
We next assessed whether, in addition to differences in abundance, homing potential, and effector phenotype, LN CMV-specific CD8+ T cells also differed in function. The cytolytic capacity of the LN CMV-specific CD8+ T cells was investigated by analyzing granzymes. In the PB, the majority of the CMV-specific CD8+ T cells contained vast amounts of granzyme B. However, PB EBV-specific CD8+ T cells usually contained granzyme K instead (Figure 5). In LNs, the percentage of granzyme B–containing CD8+ T cells was significantly lower than the PB CD8+ T cells (P = .001, Figure 5B). This was also the case, albeit to a lower extent, for LN CMV-specific CD8+ T cells (P < .01). LN EBV- and FLU-specific CD8+ T cells consisted of equal amounts of granzyme B–containing cells compared with PB. For granzyme K, no differences between CD8+ T cells from LNs and PB were seen. However, the LN CMV– and EBV-specific CD8+ T cells did comprise more granzyme K–positive cells than the PB CMV– and EBV-specific CD8+ T cells (P < .005 and P < .001, Figure 5C). FLU-specific CD8+ T-cell populations in the LNs showed a tendency toward fewer granzyme B- and granzyme K–containing cells. Most strikingly, however, fewer granzyme B+/granzyme K− cells were found in all LN (virus-specific) CD8+ populations studied (except for the FLU-specific cells, which do not contain detectable amounts of these cells even in the PB; CD8, P = .001; CMV, P < .005; and EBV, P < .0005).
Granzyme expression on (virus-specific) CD8+ T cells in the PB and LNs. (A) Representative dot plots of granzyme K and granzyme B staining on total (n = 11) and CMV-specific (n = 6, 2 donors were analyzed with 2 different tetramers), EBV-specific (n = 10, 3 donors were analyzed with 2 tetramers and 1 donor with 3 tetramers), and FLU-specific (n = 5) CD8+ T cells. (B-D) Comparison of percentages of granzyme-expressing cells within, from left to right: total, CMV-, EBV-, and FLU-specific CD8+ T cells between PB and LNs. (B) Granzyme B–containing cells. (C) Granzyme K–containing cells. (D) Granzyme B–containing and granzyme K–negative cells. Paired samples were analyzed using the Wilcoxon signed-rank test.
Granzyme expression on (virus-specific) CD8+ T cells in the PB and LNs. (A) Representative dot plots of granzyme K and granzyme B staining on total (n = 11) and CMV-specific (n = 6, 2 donors were analyzed with 2 different tetramers), EBV-specific (n = 10, 3 donors were analyzed with 2 tetramers and 1 donor with 3 tetramers), and FLU-specific (n = 5) CD8+ T cells. (B-D) Comparison of percentages of granzyme-expressing cells within, from left to right: total, CMV-, EBV-, and FLU-specific CD8+ T cells between PB and LNs. (B) Granzyme B–containing cells. (C) Granzyme K–containing cells. (D) Granzyme B–containing and granzyme K–negative cells. Paired samples were analyzed using the Wilcoxon signed-rank test.
In summary, with regard to cytolytic function, LN-derived CMV- and EBV-specific and total CD8+ T cells contain fewer effector-type cells.
More polyfunctional CMV-specific CD8+ T cells are found in the LNs
To analyze cytokine secretion capacity, CMV-specific CD8+ T cells were stimulated with their cognate peptides. Strikingly, LN CMV-specific CD8+ T cells needed less peptide to induce the production of IL-2, and a larger percentage of all LN CMV-specific CD8+ T cells were capable of producing IL-2 (Figures 6A). The production of TNFα, IFNγ, and MIP-1β and the expression of CD107a were similar in the LNs and PB CMV-specific CD8+ T cells. Remarkably, the percentage of polyfunctional cells (producing all 4 cytokines and expressing CD107a) was almost solely dependent on the percentage of IL-2–producing cells, because the other cytokines and CD107a each reached a plateau at 1-10 ng/mL of cognate peptide. Stimulation with ionomycin and PMA revealed a substantial increase in IL-2–producing total and CMV-specific CD8+ T cells in the LNs (both P < .005, Figure 6B). For EBV-specific CD8+ T cells, no difference was observed. A lower percentage of IFNγ-producing (P < .001, Figure 6C) LN CD8+ T cells was observed, whereas IFNγ was produced by an equal percentage of LN EBV-, CMV-, and FLU-specific CD8+ T cells compared with their PB counterparts. The same pattern was found for TNFα, Mip-1β, and CD107a (supplemental Figure 2). The lower percentage of IFNγ-, TNFα-, and Mip-1β–producing and CD107a-positive cells was likely because of the higher number of naive cells within the total LN CD8+ T cells (Figure 4C). As a result, total LN CD8+ T cells do not contain more polyfunctional cells. However, LN CMV-specific CD8+ T cells contain more polyfunctional cells (P < .05, Figure 6D) and have a higher responsiveness toward their cognate peptide, suggesting once again that the CMV-specific CD8+ T cells found in the LNs resemble memory phenotype CD8+ T cells. To address the question of whether LN-derived CD8+ T cells are more activated and proliferate better, we did a comparative analysis of the proliferation and activation status of the LN- and PB-derived CD8+ T cells. We did not observe any differences in the expression of the activation markers PD-1, HLA-DR, or CD38 or in the expression of the proliferation marker Ki-67 (data not shown).
Cytokine-producing (virus-specific) CD8+ T cells in the PB and LNs. (A) Percentages of IL-2–, TNFα-, IFNγ-, and MIP-1β–producing, CD107a-expressing, and polyfunctional (PF) cells of CMV-specific CD8+ T cells after stimulation with various concentrations of cognate peptide for 6 hours in the presence of brefeldin A and GolgiStop. The filled circles (●) with the bold line represent the means ± SEM of PB of 4 patients. The open circles (○) with the dotted line represent the means ± SEM of LN cells of the same 4 patients. *P < .05. (B-D) Percentage of IL-2-producing (B), IFNγ-producing (C), and PF cells (D) within, from left to right; total, CMV-, EBV-, and FLU-specific CD8+ T cells after stimulation with PMA and ionomycin for 4 hours in the presence of brefeldin A and GolgiStop. Paired samples were analyzed using the Wilcoxon signed-rank test.
Cytokine-producing (virus-specific) CD8+ T cells in the PB and LNs. (A) Percentages of IL-2–, TNFα-, IFNγ-, and MIP-1β–producing, CD107a-expressing, and polyfunctional (PF) cells of CMV-specific CD8+ T cells after stimulation with various concentrations of cognate peptide for 6 hours in the presence of brefeldin A and GolgiStop. The filled circles (●) with the bold line represent the means ± SEM of PB of 4 patients. The open circles (○) with the dotted line represent the means ± SEM of LN cells of the same 4 patients. *P < .05. (B-D) Percentage of IL-2-producing (B), IFNγ-producing (C), and PF cells (D) within, from left to right; total, CMV-, EBV-, and FLU-specific CD8+ T cells after stimulation with PMA and ionomycin for 4 hours in the presence of brefeldin A and GolgiStop. Paired samples were analyzed using the Wilcoxon signed-rank test.
CMV does not leave a fingerprint in the LN CD8+ T-cell compartment
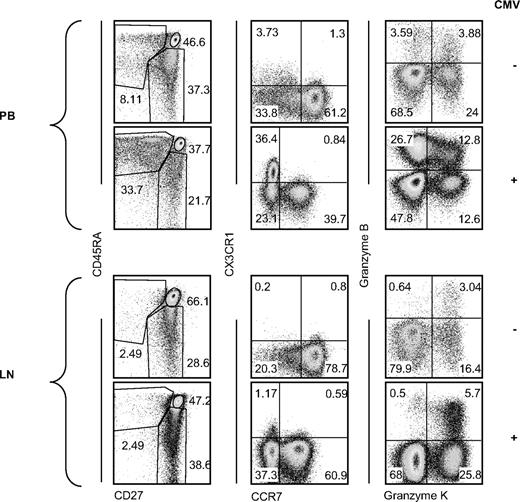
Finally, we studied the impact of CMV on the LN total CD8+ T-cell compartment. When we compared the subsets that in the PB are associated with CMV seropositivity (CD45RA+CD27−; CX3CR1+CCR7−, and granzyme B+ granzyme K−), we observed no apparent differences comparing LNs from CMV-seronegative individuals to LNs from CMV-seropositive ones (Figure 7). Therefore, as a result of the minute amounts of CMV-specific CD8+ T cells in the LNs and their resemblance in both phenotype and function to memory CD8+ T cells, CMV seropositivity does not leave a clear imprint on the total CD8+ T-cell compartment of the LNs.
Summarizing phenotypic comparison of PB- and LN-derived CD8+ T cells. Representative dot plots of CMV-seronegative (n = 4 CD27/CD45RA and CCR7/CX3CR1, n = 2 granzyme B/K, top rows in each panel) and CMV-seropositive (n = 10 CD27/CD45RA and CCR7/CX3CR1, n = 9 granzyme B/K, bottom row in each panel) PB (top panel) and LN (bottom panel) CD8+ T cells analyzed for expression of CD27 and CD45RA, CCR7, CX3CR1, granzyme K, and granzyme B.
Summarizing phenotypic comparison of PB- and LN-derived CD8+ T cells. Representative dot plots of CMV-seronegative (n = 4 CD27/CD45RA and CCR7/CX3CR1, n = 2 granzyme B/K, top rows in each panel) and CMV-seropositive (n = 10 CD27/CD45RA and CCR7/CX3CR1, n = 9 granzyme B/K, bottom row in each panel) PB (top panel) and LN (bottom panel) CD8+ T cells analyzed for expression of CD27 and CD45RA, CCR7, CX3CR1, granzyme K, and granzyme B.
Discussion
Latent CMV infection is characterized by the presence of large numbers of circulating CMV-specific CD8+ T cells. We and others have shown previously that expansion of the CD8+ T-cell pool occurring during primary CMV infection does not affect the absolute numbers of preexisting memory CD8+ T cells directed against other persistent (eg, EBV) or cleared (FLU) viruses.6,11,20 This indicates that the expansion of CMV-specific CD8+ T cells found in the PB compartment does not displace memory CD8+ T cells with other viral specificities, but rather enlarges the total CD8+ T-cell pool. These findings are in agreement with the earlier study in mice demonstrating enlargement of the CD8+ T-cell pool after repetitive Ag challenge.10
Murine naive T cells and central memory T cells expressing CCR7 and L-selectin are known to continuously recirculate through the LNs, where they can encounter Ag and rapidly give rise to effector T cells.21 Human LN-derived CD8+ T cells concordantly appear to contain more naive and memory CD8+ T cells, expressing more CCR7 and CXCR3 and almost no CX3CR1 compared with their PB counterparts. We and others have shown previously that circulating CMV-specific CD8+ T cells in latent virus carriers negligibly express CCR712-14 and highly express CX3CR1,15 which precludes localization to the LNs and favors migration to inflamed endothelium. In the present study, we extend these findings, showing that CMV-specific CD8+ T cells in the LNs are much less dominant than in the PB, whereas the percentages of EBV- and FLU-specific CD8+ T cells in the PB and LNs are similar. To identify the different virus-specific CD8+ T cells, we used tetramers loaded with peptides of the most immunodominant epitopes. The shortcoming of this technique is that it is not possible to analyze the total virus-specific response. Nevertheless, because we analyzed the same tetramer in the PB and LNs, for these epitopes, this is a valid comparison. The few CMV-specific CD8+ T cells that are detected in the LNs express more CCR7 compared with their PB counterparts. However, a significant percentage of LN-derived CMV-specific CD8+ T cells do not express CCR7. This may be because of the down-regulation of CCR7 by ligand-induced internalization once these cells have reached the LNs.22,23 T cells can also make use of other chemokine receptors to home to LNs. During infection and the concurrent inflammation, T cells can reach the draining LNs without making use of CCR7. Instead, inflammatory chemokines and their receptors, such as CXCL10 and CXCR3, play a more important role19 and, indeed, LN-derived total, CMV-specific, and EBV-specific CD8+ T cells express significantly more CXCR3 compared with their PB counterparts. The LNs analyzed in our study were resting LNs. However, humans are constantly exposed to a wide variety of pathogens. The para-iliacal LNs studied here drain the pelvic area and parts of the intestine. Therefore, it is possible that low levels of CXCL10 are continuously produced in the LNs, attracting CXCR3-expressing T cells from the blood.
LN-derived CMV-specific and total CD8+ T cells differ substantially from their PB counterparts regarding their functional capacity. Concordant with the absence of effector-phenotype (CD27−) CD8+ T cells in the LNs, effector molecules are lacking in the LN-derived CD8+ T cells. The functional profile of memory CD8+ T cells is very diverse, including cytolysis and the production of various chemokines and cytokines. Roederer et al developed an assay to simultaneously study 5 CD8+ T-cell functions, including degranulation (CD107a), cytokine (IFNγ and TNFα, IL-2), and chemokine (MIP-1β) production.24 They observed that the presence of polyfunctional HIV-specific CD8+ T cells was inversely correlated with disease progression, and therefore polyfunctional cells can be regarded as “the most fit” cells. In the present study, we demonstrate that LN- and PB-derived CMV-specific CD8+ T cells produce IFNγ, TNFα, and MIP-1β equally and all express CD107a. However, LN-derived CMV-specific CD8+ T cells contain more polyfunctional cells, the difference being in the higher production of IL-2. The fact that LN-derived CMV-specific CD8+ T cells contain more IL-2–producing cells appears to be directly related to the large number of memory-type cells that are known to produce more IL-2 than do effector type cells.25 Furthermore, we observed that LN-derived CMV-specific CD8+ T cells express less granzyme B and more granzyme K, which is a feature of memory phenotype (CD27+CD45RA−) cells in PB CD8+ T cells.25,26
During latency, CMV is known to reside in endothelial cells27,28 and myeloid cells.29 Functionally, PB CX3CR1+ granzyme B+ effector-type CMV-specific CD8+ T cells are equipped to migrate to inflamed endothelium and adequately kill infected cells, preventing further viral reactivation. We speculate that memory phenotype CMV-specific CD8+ T cells patrol LNs and become more important when systemic reactivation occurs. Memory phenotype cells have a superior ability to proliferate30 and produce IL-2,25 and are therefore less dependent on helper signals provided by IL-2–producing CD4+ T cells.
From the results of this study, we conclude that the strong immune response directed against CMV, which leaves a fingerprint in the PB CD8+ T-cell compartment but not in the LNs, is unlikely to restrict immunologic space for naive CD8+ T cells and memory CD8+ T cells with other antigenic specificities. Therefore, the observation that the elderly have an increased susceptibility to infections demands other explanations than the occupation of space by CMV-specific CD8+ T cells. Possible causes are the reduction in absolute numbers of naive T cells due to thymic involution31 or replicative senescence of primed memory T cells. However, these factors cannot entirely explain the concept of the immune risk profile in older people, which has been associated specifically with CMV.32 We have shown previously that CMV induces a sustained chronic inflammation that is apparent from increased serum levels of IFNγ and C-reactive protein.33 In addition to being a very potent activator of the immune system, IFNγ has been reported to exert suppressive actions, such as inhibition of human T-cell responses.34 In a CD70TG-sustained costimulation mouse model, IFNγ has been shown to induce B-cell depletion in the BM, spleen, and LNs. Overexpression of CD70 leads to T-cell differentiation toward an effector phenotype (CD27−).35 Human CMV-seropositive individuals likewise have consistently more circulating CD27− effector-type CD8+ T cells and higher serum levels of IFNγ compared with CMV-seronegative ones. However, we did not observe a lower absolute number of circulating B cells. It is possible that B cells are depleted from other lymphoid compartments, as demonstrated in the mouse study. Furthermore, CMV is famous for having a large number of proteins that subvert immune surveillance in the host.29 One example is the viral IL-10 (hcmvIL-10) ortholog,36 which is able to bind to the human IL-10 receptor37 and can inhibit both innate and adaptive immune responses.38 It is possible that hcmvIL-10 can also more systemically affect innate and adaptive immune responses directed against other infections.
In summary, in the present study, we found that the large numbers of CMV-specific CD8+ T cells generated during latent infection increase the total CD8+ T-cell pool without affecting other virus-specific CD8+ T cells in the PB. Furthermore, we demonstrate that CMV-specific CD8+ T cells are negligibly present in the LNs and thus do not limit immunologic space at sites where immune reactions are initiated. These data argue against the possibility of memory cell attrition due to space competition as a cause for low responses to vaccination in the elderly, who have high percentages of effector CD8+ T cells in the PB compartment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.B.M.R. designed, performed, and analyzed the experiments and wrote the manuscript; S.H.C.H. designed and analyzed the experiments and wrote the manuscript; M.M.I., K.A.M.I.v.D., and F.J.B. cared for patients and coordinated the gathering of patient samples; E.M.M.v.L. analyzed the experiments and edited the manuscript; N.v.d.B.-B. collected and processed the patient samples; A.t.B. contributed reagents; and R.A.W.v.L. and I.J.M.t.B. designed the experiments, interpreted the data, wrote and edited the manuscript, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr R. J. M. ten Berge, Academisch Medisch Centrum F4-215, PO Box 22660, 1100 DD Amsterdam, The Netherlands; e-mail: r.j.tenberge@amc.uva.nl.
References
Author notes
E.B.M.R. and S.H.C.H. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal