Abstract
R-Ras is a member of the RAS superfamily of small GTP-binding proteins. The physiologic function of R-Ras has not been fully elucidated. We found that R-Ras is expressed by lymphoid and nonlymphoid tissues and drastically up-regulated when bone marrow progenitors are induced to differentiate into dendritic cells (DCs). To address the role of R-Ras in DC functions, we generated a R-Ras-deficient mouse strain. We found that tumors induced in Rras−/− mice formed with shorter latency and attained greater tumor volumes. This finding has prompted the investigation of a role for R-Ras in the immune system. Indeed, Rras−/− mice were impaired in their ability to prime allogeneic and antigen-specific T-cell responses. Rras−/− DCs expressed lower levels of surface MHC class II and CD86 in response to lipopolysaccharide compared with wild-type DCs. This was correlated with a reduced phosphorylation of p38 and Akt. Consistently, R-Ras–GTP level was increased within 10 minutes of lipopolysaccharide stimulation. Furthermore, Rras−/− DCs have attenuated capacity to spread on fibronectin and form stable immunologic synapses with T cells. Altogether, these findings provide the first demonstration of a role for R-Ras in cell-mediated immunity and further expand on the complexity of small G-protein signaling in DCs.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells with key roles in initiating antigen-specific immune responses.1 Although numerous reports have implicated members of the RAS superfamily of GTP-binding proteins in DC biology,2 a complete understanding of their individual roles is still lacking. Small GTPases traditionally operate as molecular switches, being inactive in the GDP-bound state and active in the GTP-bound state, therein conferring signaling function through interactions with their downstream effectors.3 Indeed, several small GTPases have been shown to play critical roles in immune cell functions by regulating actin cytoskeleton-dependent processes.4-10
Small GTPases Rap1, Rac1/2, and Cdc42, with the latter 2 being part of the RHO family of GTPases, have been demonstrated to be important in DC biology. Active Cdc42 can reactivate endocytosis in mature DCs, whereas microinjection of a dominant negative Cdc42N17 mutant abrogates the uptake of dextran.9 Interestingly, it was demonstrated in DCs that levels of active Cdc42 and Rac were not increased in response to lipopolysaccharide (LPS).11 However, using mature DCs from Rac1 and Rac2 double-knockout mice (Rac1/2−/−), Rac1 and Rac2 were shown to be critical for dendrite formation and T-cell activation.12 In addition, RAPL, a downstream effector of Rap1, has been shown to be critical for both lymphocyte and DC migration and adhesion.5,6 Although both Rap1 and Cdc42/Rac GTPases are clearly involved in DC biology, little is known concerning the role of other key members of the Ras subfamily.
R-Ras is a small GTPase that belongs to the Ras subfamily and shares approximately 55% amino acid sequence similarity with the RAS proto-oncogenes, H-RAS, N-RAS, and K-RAS (collectively referred to as Ras herein).13,14 Distinctively, R-Ras possesses a unique 26-amino acid sequence in the amino-terminus that is necessary for Rac activation and Rac-dependent cell spreading.15 Furthermore, active R-Ras in macrophages has been shown to positively regulate phagocytosis of C3bi-opsonized particles via the integrin M2.16
Insights into the physiologic function of R-Ras were revealed through analyzing a mouse strain in which the R-Ras gene was disrupted by gene-trap methodology.17 Here, R-Ras expression was restricted to endothelial and vascular smooth muscle cells, and R-Ras is a negative regulator of vascular proliferation and angiogenesis. Furthermore, these studies suggested that R-Ras has a negligible role in inflammatory responses, as similar levels of F4/80-positive macrophages were seen in atherosclerotic lesions in both wild-type (WT) and R-Ras–deficient mice. More recently, TC21, a closely related small GTPase of R-Ras, has been shown to bind both T- and B-cell antigen receptors and play a role in homeostatic proliferation and survival of these immune cell types.18
In this study, we provide evidence of R-Ras expression in multiple murine immune cells that include DCs. Characterization of an Rras−/− mouse line revealed multiple defects in DCs, including impaired maturation and T-cell priming, attenuated activation of intracellular signaling pathways downstream of Toll-like Receptor 4 (TLR4), and reduced formation of stable immunologic synapses between DCs and T cells. This report represents the first demonstration of a physiologic function of R-Ras in cell-mediated immune responses.
Methods
Mice
Mice deficient for R-Ras were generated by homologous recombination of ES cells in 129SvEvBrd (129Sv) background by deleting a 1.2-kb region spanning exons 2 to 4 (inGenious Targeting Laboratory). Rras+/− mice in a C57BL/6 background were generated by 10 generations of backcrossing. Rras−/− mice were obtained by intercrossing Rras+/− animals. The genotypes of litters were determined by PCR of genomic DNA with primers specific for WT R-Ras (MRg1, 5′-aaagatctgcactgtggacggcat-3′; MRg2, 5′-ttctccagatctgccttgttccca-3′) and knockout (UNI, 5′-agcgcatcgccttctatcgccttc-3′; A4, 5′-cactccccgaggctctaatctcctg-3′) alleles. At an age of 6 to 10 weeks, littermates or age- and sex-matched mice in 129Sv background were used in all experiments, unless otherwise stated. Balb/c and OT-II mice were purchased from The Jackson Laboratory. Mice were maintained under pathogen-free conditions in accordance with Institutional Guidelines, and all mice experiments were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin and the Mount Sinai School of Medicine.
Reagents
A rabbit polyclonal antiserum against the N-terminal 23 amino acid of R-Ras was generated in the author's laboratory. Antibodies for p-Akt Ser473 (clone 587F11, #4051), Akt (#9272), p-p38 Thr180/182 (clone 28B10, #9216), p38 (#9212), p-IκBα Ser32/36 (clone 5A5, #9246), IκBα (#4812), p-Erk1/2 (E10, #9106), and Erk1/2 Thr202/Tyr204 (#9102) were obtained from Cell Signaling Technology. Antibodies for Ras (clone 10, #05-516; Millipore), Rap1 (#610195; BD Biosciences), and Rac (#05–389; Millipore) were purchased from commercial sources. HRP-conjugated anti-actin (#sc-1616), HRP-anti–rabbit IgG (#sc-2313), and HRP-anti–mouse IgG (#sc-2314) were from Santa Cruz Biotechnology. Antibodies for flow cytometric analysis, including phycoerythrin-Cy7 (PE-Cy7)–CD11c, PE-CD86, FITC-MHC class II, and allophycocyanin (APC)–CD40, were obtained from BD Biosciences. Data were collected using a FACS LSR II (BD Biosciences). The following items were from Invitrogen: RPMI 1640 media, FBS, 2mM glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 20mM HEPES buffer, 2-mercaptoethanol, penicillin-streptomycin, and carboxyfluorescein diacetate succinimidyl (CFSE). LPS (Escherichia coli 055:B5) and ovalbumin (OVA) were from Sigma-Aldrich.
Tumorigenicity assay
Tumor induction was performed as previously described.19 Briefly, 105 B16-F10 cells were injected subcutaneously in a volume of 100 μL of PBS. Tumor growth was monitored once weekly, and tumor volume was measured with a caliper. Tumor volume was calculated by the formula: π/6(width × length × height).
BM-DC generation
Primary immature DC cultures were generated by culturing bone marrow precursors for at least 6 days in GM-CSF containing media, with nonadherent cells replated in fresh media every 2 days, as previously described.20 Maturation was induced by 12-hour treatment with either 0.1 or 1 μg/mL LPS, unless stated otherwise. Splenic DCs (sDCs) were derived from spleens isolated from naive mice and digested in RPMI media containing 10% FBS and collagenase (1 mg/mL) for 30 minutes at 37°C. After digestion, sDCs were isolated using anti-CD11c antibody-coupled magnetic beads, according to the manufacturer's protocol (Miltenyi Biotec). GM-CSF stock vials were kindly provided by Dr Julie Blander (Mt Sinai School of Medicine, New York, NY).
Immunoblotting
DCs were solubilized in RIPA buffer. Protein extracts were subjected to SDS-PAGE on a 12.5% gel. After transferring onto nitrocellulose membranes, filters were blocked in 5% milk in TTBS, and proteins of interest were detected by incubating with primary antibodies overnight, followed by 1-hour incubation with HRP-conjugated secondary antibodies. Bound antibodies were detected by standard ECL detection method (Pierce Chemical) and exposed to radiographic films.
Antigen presentation assay
T-cell depleted splenoctyes (APCs) from Rras+/+ mice or Rras−/− mice (129Sv) were isolated, irradiated (3000 cGy), and used as stimulating cells. T cells (2 × 105) from the spleens of Balb/c mice were isolated by Mouse T-cell Enrichment Columns (R&D Systems) and mixed in triplicates with APCs in different ratios for 4 days. Proliferation was monitored by adding [H3]-labeled thymidine (1 μCi/well in a 96-well plate) for the last 16 hours. Cells were harvested, and the amount of radioactive materials incorporated was counted in a scintillation counter.
Allogeneic and antigen-specific T-cell assays were performed using Thy1.2+ T cells from naive Rras+/+ mice purified by AutoMACS (Miltenyi Biotec). For these assays, T cells (2 × 105) were mixed with either bone marrow–derived DCs (BM-DCs) or sDCs in different ratios as indicated for 4 to 5 days. Allogeneic or antigen-specific T-cell proliferation was monitored either by [H3]-labeled thymidine incorporation or CFSE staining, respectively. For CFSE labeling, freshly isolated T cells (1 × 107 cells/mL) were stained for 10 minutes at 37°C with 10μM CFSE in PBS with 0.1% FBS. Reactions were quenched by the addition of an equal volume of 100% FBS followed by 3 washes in PBS with 10% FBS to ensure complete CFSE removal.
Cell spreading assay
BM-DCs from Rras+/+ and Rras−/− mice were plated at variable concentrations (0.57 × 104, 1.7 × 104, and 5 × 104 cells in 50 μL per well) on fibronectin (10 μg/mL) or BSA-coated 96-well plates. BM-DCs were allowed to attach for 10 to 20 minutes at 37°C. Bright-field images were taken from 5 random fields, and the percentage of spread cells was scored. Similar experiments were performed in 8-well chamber slides (Nalge Nunc) by plating 5 × 103 cells per well in triplicates for immunofluorescence analysis. Briefly, cells were fixed in 3% paraformaldehyde and permeabilized with 0.2% Triton X-100 followed by incubation in Texas Red–conjugated phalloidin (1:500; Invitrogen). For quantifying cell spreading, fluorescence images were first captured with an Axiocam digital camera (Carl Zeiss) using a 20× objective. Round cells lacking membrane protrusions were counted as nonspread cells and used for calculating the percentage of cell spreading. To quantify mean cell length, the maximal width of individual cell was measured by the ruler tool of the ImageJ 1.43 software and expressed as an arbitrary unit. A mean cell length was assigned to each field. For each experimental condition, 9 fields from triplicate wells with an average of 62 cells per field were analyzed.
R-Ras-GTP pull-down assay
For each pull-down reaction, approximately 30 μg of GST-RalGDS-RBD fusion protein was coupled onto 25 μL of glutathione-Sepharose 4B beads (GE Healthcare) as previously described.21 DCs were solubilized in lysis buffer containing 25mM HEPES-KOH (pH 7.4), 200mM NaCl, 1% NP-40, 2mM EDTA (pH 8.0), 5mM MgCl2, and 0.25% NaDOC. A total of 1 mg of protein lysates was added to loaded beads and incubated for 1 hour at 4°C. Reactions were washed 3 times and boiled in 60 μL of 2× Laemmli buffer.
Microscopy
Differential-interference contrast images (1024 × 1024 pixels) monitoring DC–T-cell interactions were acquired using a 63×/1.4 NA objective, Leica SP5 DMI Inverted Confocal microscope (Leica Microsystems) equipped with a temperature and CO2 controlled incubation chamber. For these studies, 1 × 104 BM-DCs were activated with LPS (0.1 μg/mL) in high optical grade chambers (Ibidi) for 12 hours. Four hours before beginning image-sequence capture, DCs were loaded with OT-II peptide. During this time, fresh OT-II T cells were purified by antibody depletion (B220+, DX5+, CD8+, CD11b+) from the spleens of naive OT-II mice. Before mixing, DCs and T cells were washed 3 times. Next, 2 × 105 T cells were added to the DC-containing chambers. One minute after addition (t = 0), z-stack images were collected on different focal planes for which DC dendrites traversed. This resulted on average one image per focal plane, every 15 seconds for 45 minutes. Videos were generated using Leica Application Suite (Version 1.8.2 build 1465) software, using in-focused images from z-stack accelerated 300×, with the total time length of each video being 5 to 9 seconds. To quantify the number of DC–T-cell contacts, we followed the fate of a single DC along the length of the movie, by scrolling images one by one. Repetitive contacts were scored as 1 interaction, and a DC–T-cell synapse was defined as T-cell in contact with a DC membrane for more than 4 frames (60 seconds). For positive synapses, the time duration of each DC–T-cell synapse was quantified by frame counts as defined earlier in this paragraph. All data shown was collected from 2 randomly selected fields and averaged from 3 independent experiments.
Statistical analysis
Statistical analysis was performed using unpaired Mann-Whitney U test to compare groups of WT and knockout mice. Results are expressed as mean (SE) values. One-tail P value < .05 is considered statistically significant.
Results
Generation of Rras−/− mouse strain
To address the physiologic role of R-Ras, Rras−/− mice were generated by homologous recombination, deleting an intragenic 1.2-kbp region. This region spans exons 2 to 4, which included the switch I and II regions encompassing amino acids 52 to 151 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Intragenic deletion of R-Ras was confirmed by Southern blotting and PCR-based analysis of genomic DNAs (supplemental Figure 1B-C). The lack of R-Ras expression was revealed by Western blotting analysis (supplemental Figure 1D). Similar to a previous report,17 Rras−/− mice generated in this study have the predicted Mendelian ratio of inheritance and lacked gross morphologic aberrations. In addition, Rras−/− mice in both 129Sv or C57BL/6 genetic background showed no significant defects in T-cell development in the thymus or in CD11c+, B220+, CD8+, or CD4+ cells in the spleen (data not shown).
Enhanced tumor growth in Rras−/− mice
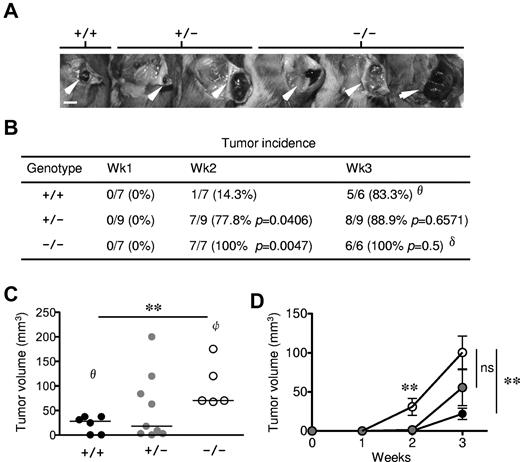
Previous studies have uncovered a lack of R-Ras expression in epithelial cells but readily detectable in various stromal cell types.17 As an initial attempt to evaluate the physiologic responses to exogenously introduced tumor, 1 × 105 B16-F10 (C57BL/6 background) mouse melanoma cells were injected subcutaneously in Rras−/− mice of mixed B6;129Sv genetic background. By week 2, tumor incidence in Rras−/− (100%) and Rras+/− (77.8%) mice was significantly higher than in Rras+/+ (14.3%) mice (Figure 1A-B). The mean volume of B16-F10 tumors induced in Rras−/− mice (30.9 ± 10.9 mm3) was significantly greater than in Rras+/− mice (1.5 ± 0.6 mm3). By week 3, almost all mice have palpable tumors. However, the mean tumor volume induced in Rras−/− mice (100.5 ± 21.1 mm3) was approximately 5-fold and approximately 2-fold greater than in Rras+/+ (21.9 ± 7.2 mm3) and Rras+/− (55.6 ± 22.9 mm3) mice, respectively (Figure 1C-D). In addition, several Rras+/− mice possessed tumors of similar size as in Rras−/− mice, suggesting dose dependency on the observed phenotypic differences (Figure 1C). These data were in contrast with those obtained from Komatsu and Ruoslahti,17 where they have not observed differences in tumor incidence and sizes using F3 to F5 mice backcrossed to a C57BL/6 background. These differences may be attributed to the fact that we injected 5 times less cells and animals of B6;129Sv genetic background were used instead. Although these results do not necessarily identify the involvement of immune cells, defects in DCs are known to subvert tumor immune surveillance.22 Furthermore, the fact that less was known concerning R-Ras in the immune system has prompted us to investigate the role of this Ras-related protein in immune cells.
Enhanced tumor growth in Rras−/− mice. (A) Approximately 105 B16-F10 cells were injected subcutaneously into Rras+/+ (n = 7), Rras+/− (n = 9), and Rras−/− (n = 7) mice in a B6;129Sv genetic background. Images from representative tumors induced after 3 weeks are shown. White arrowheads indicate tumors. Bar represents 0.5 cm. (B) Tumor incidence (%) is shown with the number of animals with palpable tumors indicated. Statistical analysis was performed using Fisher χ2 (P values). (C) Tumor volumes were measured at week 3. Data derived from 2 independent experiments with 3 to 6 mice each. Bars represent median. **P < .005. (D) The kinetics of tumor growth is shown. Bars represent SE. **P < .005. ns indicates not significant. θOne Rras+/+ animal was removed because of mortality. δOne Rras−/− animal was removed from incidence analysis because of damaged tumor. φTwo Rras−/− mice with either tumors that have exceeded humane endpoint (> 2 cm in diameter) or damaged were excluded from tumor volume analysis.
Enhanced tumor growth in Rras−/− mice. (A) Approximately 105 B16-F10 cells were injected subcutaneously into Rras+/+ (n = 7), Rras+/− (n = 9), and Rras−/− (n = 7) mice in a B6;129Sv genetic background. Images from representative tumors induced after 3 weeks are shown. White arrowheads indicate tumors. Bar represents 0.5 cm. (B) Tumor incidence (%) is shown with the number of animals with palpable tumors indicated. Statistical analysis was performed using Fisher χ2 (P values). (C) Tumor volumes were measured at week 3. Data derived from 2 independent experiments with 3 to 6 mice each. Bars represent median. **P < .005. (D) The kinetics of tumor growth is shown. Bars represent SE. **P < .005. ns indicates not significant. θOne Rras+/+ animal was removed because of mortality. δOne Rras−/− animal was removed from incidence analysis because of damaged tumor. φTwo Rras−/− mice with either tumors that have exceeded humane endpoint (> 2 cm in diameter) or damaged were excluded from tumor volume analysis.
R-Ras expression in immune cells
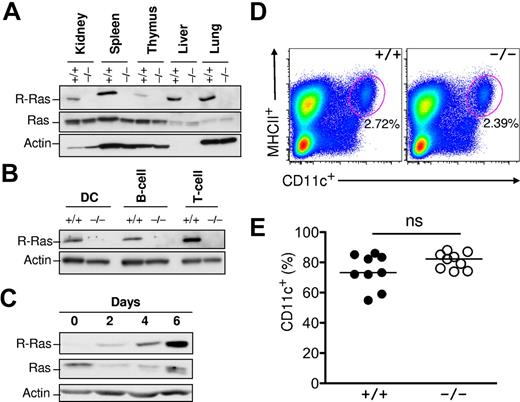
To characterize the role of R-Ras in the immune system, its expression in both lymphoid and nonlymphoid tissues was examined. Previous studies have uncovered R-Ras expression restricted to the lung, spleen, and intestine but almost undetectable in the bone marrow.17 As shown in Figure 2A, R-Ras was readily detected in the lung, as previously reported in addition to the spleen, liver, and kidney. Low levels of expression were observed in primary lymphoid organs, such as the thymus (Figure 2A). Next, splenic B220+ B cells, CD3+ T cells, and CD11c+ DCs from Rras+/+ and Rras−/− mice were isolated with more than 99% purity by FACS and analyzed for R-Ras expression by Western blotting analysis. DCs isolated from Rras+/+ mice expressed an approximately 23-kDa protein species that was absent in Rras−/− cells (Figure 2B). A similar expression pattern was also observed for both B cells and T cells. To validate R-Ras expression in DCs, BM-DCs were cultured in the presence of GM-CSF. From day 0 to day 6, R-Ras levels progressively increased more than 20-fold during differentiation (Figure 2C). On the contrary, the levels of the prototypic Ras proteins were reduced. However, this drastic increase in R-Ras expression was unlikely to play a role in DC development in vivo as Rras+/+ and Rras−/− mice had similar percentages of splenic resident MHC class II+CD11c+ DCs (Figure 2D). In addition, there were no differences in the percentage of CD11c+ in day 6 BM-DC cultures from Rras+/+ and Rras−/− mice (Figure 2E).
R-Ras expression in immune cells. (A) WT (+/+) and R-Ras knockout (−/−) organs were isolated, and total cell extracts were subjected to Western blotting analysis with the indicated antibodies. (B) WT (+/+) and R-Ras knockout (−/−) spleen was dissociated and subjected to cell sorting with DC-, B cell–, and T cell– specific markers. Western blotting analysis was carried out using an R-Ras specific antibody. (C) Bone marrow progenitors from 6- to 8-week-old mice were cultured in the presence of GM-CSF for 6 days. Cells were monitored throughout the DC differentiation process for R-Ras expression by Western blotting. Parallel membranes were probed with an anti–Ras monoclonal antibody (Millipore). Actin was used as a protein loading control. (D) Age-matched spleens from Rras+/+ and Rras−/− mice were harvested and analyzed for the percentage of MHC class II+CD11c+ DCs. (E) Day 6 BM-DC cultures from Rras+/+ (n = 9) and Rras−/− (n = 9) mice were analyzed for the percentage of CD11c+ DCs. ns indicates not significant. Results are representative of 3 independent experiments.
R-Ras expression in immune cells. (A) WT (+/+) and R-Ras knockout (−/−) organs were isolated, and total cell extracts were subjected to Western blotting analysis with the indicated antibodies. (B) WT (+/+) and R-Ras knockout (−/−) spleen was dissociated and subjected to cell sorting with DC-, B cell–, and T cell– specific markers. Western blotting analysis was carried out using an R-Ras specific antibody. (C) Bone marrow progenitors from 6- to 8-week-old mice were cultured in the presence of GM-CSF for 6 days. Cells were monitored throughout the DC differentiation process for R-Ras expression by Western blotting. Parallel membranes were probed with an anti–Ras monoclonal antibody (Millipore). Actin was used as a protein loading control. (D) Age-matched spleens from Rras+/+ and Rras−/− mice were harvested and analyzed for the percentage of MHC class II+CD11c+ DCs. (E) Day 6 BM-DC cultures from Rras+/+ (n = 9) and Rras−/− (n = 9) mice were analyzed for the percentage of CD11c+ DCs. ns indicates not significant. Results are representative of 3 independent experiments.
R-Ras controls the ability of DC to activate allogeneic T cells
The observed expression of R-Ras in DCs and lymphocytes has prompted us to examine potential defects in immune response in Rras−/− mice. To determine whether Rras−/− DCs are defective in their ability to prime naive T cells, irradiated Rras+/+ and Rras−/− splenic cells were cocultured with allogeneic Balb/c T cells for 4 days. T-cell proliferation was significantly reduced by more than 70% when allogeneic T cells were cocultured with Rras−/− splenocytes when compared with Rras+/+ cells (Figure 3A). Similar T-cell proliferation assays were performed using LPS-activated splenic DCs from Rras+/+ and Rras−/− mice. As expected, the ability of Rras−/− DCs in stimulating T-cell proliferation was impaired by 25% to 45% compared with Rras+/+ DCs (Figure 3B). These results were further validated by mixing similar numbers of Rras−/− BM-DCs (129Sv) with Balb/c T cells. In this case, the ability of Rras−/− BM-DCs to stimulate T-cell proliferation was decreased by 62% to 89% compared with Rras+/+ (Figure 3C). These data suggest that R-Ras plays a role in mounting an allogeneic response.
Rras−/− splenic APCs and BM-DCs show impaired T-cell proliferation capacity. (A) Bulk splenic APCs (129Sv; n = 3) or (B) splenic DCs (129Sv; n = 4) from Rras+/+ or Rras−/− mice in different ratios were comixed with 2 × 105 T cells from WT Balb/c for 96 hours. Allogeneic T-cell proliferation was measured by adding [H3]-labeled thymidine for the last 16 hours, and the amount of incorporation was measured by a scintillation counter. Results represent triplicate measurements from a single experiment with data in panel A reproduced in 2 additional experiments. (C) A similar proliferation assay using WT T cells was conducted as in panels A and B, except that BM-DCs (129Sv) were used. Bars represent SD. (D) In vivo T-cell proliferation assays were performed. Rras+/+ or Rras−/− mice (129Sv) were injected intraperitoneally with PBS, 10 μg LPS, or 10 μg LPS and 1 mg OVA. Six hours after injection, splenic DCs were isolated and comixed with naive CFSE-labeled polyclonal syngeneic splenic T cells (129Sv) for 96 hours. CFSE+ staining was analyzed by flow cytometry to measure T-cell proliferation. All plots are gated for TCR+CD4+ cells. (E) Percentages from panel D for CFSE+ proliferating T cells are plotted. Results represent data from 2 independent experiments with Rras+/+ (n = 5) and Rras−/− (n = 6) mice. Bars represent median. ***P < .0005.
Rras−/− splenic APCs and BM-DCs show impaired T-cell proliferation capacity. (A) Bulk splenic APCs (129Sv; n = 3) or (B) splenic DCs (129Sv; n = 4) from Rras+/+ or Rras−/− mice in different ratios were comixed with 2 × 105 T cells from WT Balb/c for 96 hours. Allogeneic T-cell proliferation was measured by adding [H3]-labeled thymidine for the last 16 hours, and the amount of incorporation was measured by a scintillation counter. Results represent triplicate measurements from a single experiment with data in panel A reproduced in 2 additional experiments. (C) A similar proliferation assay using WT T cells was conducted as in panels A and B, except that BM-DCs (129Sv) were used. Bars represent SD. (D) In vivo T-cell proliferation assays were performed. Rras+/+ or Rras−/− mice (129Sv) were injected intraperitoneally with PBS, 10 μg LPS, or 10 μg LPS and 1 mg OVA. Six hours after injection, splenic DCs were isolated and comixed with naive CFSE-labeled polyclonal syngeneic splenic T cells (129Sv) for 96 hours. CFSE+ staining was analyzed by flow cytometry to measure T-cell proliferation. All plots are gated for TCR+CD4+ cells. (E) Percentages from panel D for CFSE+ proliferating T cells are plotted. Results represent data from 2 independent experiments with Rras+/+ (n = 5) and Rras−/− (n = 6) mice. Bars represent median. ***P < .0005.
R-Ras controls the ability of DCs to prime antigen-specific T cells
To further study the role of R-Ras in T-cell priming, ex vivo antigen specific T-cell responses were assayed. To this end, Rras+/+ or Rras−/− mice were injected intraperitoneally with PBS, LPS, or LPS plus OVA to allow for efficient DC maturation and loading with OVA antigen. Subsequently, splenic DCs were isolated and cocultured with CFSE-labeled polyclonal syngeneic T cells isolated from naive Rras+/+ mice. Consistent with a reduced capacity in activating allogeneic T cells, splenic DCs isolated from Rras−/− mice injected with LPS and OVA were approximately 73% less effective in priming a T-cell response (Figure 3D-E). These data establish that R-Ras expression in DCs is necessary for the priming of allogeneic and antigen-specific autologous responses.
Impaired LPS induced DC maturation in Rras−/− DCs
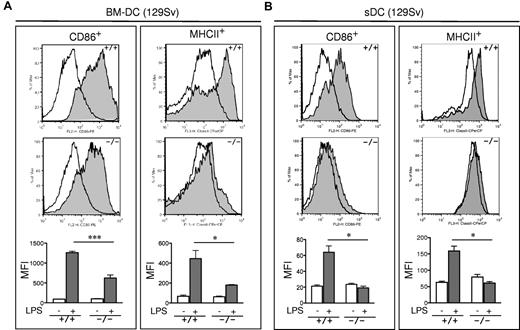
The observed defects in T-cell priming led us to further explore the role of R-Ras in additional DC functions. DC maturation is critical for mounting an efficient T-cell response. To examine the role of R-Ras in DC maturation, the levels of CD86 and MHC class II after LPS stimulation were compared between BM-DCs derived from Rras+/+ or Rras−/− mice. As shown in Figure 4A, unstimulated Rras+/+ and Rras−/− BM-DCs showed low levels of CD86 and MHC class II. Exposure to 0.1 μg/mL of LPS increased surface expression of both CD86 and MHC class II in Rras+/+ DCs. In contrast, the surface expression levels of CD86 and MHC class II were significantly reduced in Rras−/− DCs by 50.6% and 59.6%, respectively (Figure 4A; supplemental Figure 3A). This defect was observed over a broad range of LPS concentrations from 0.001 to 1 μg/mL (supplemental Figure 2A). These differences were unlikely the result of reduced TLR4 expression in Rras−/− mice as FACS analysis revealed comparable expression with that of Rras+/+ mice (supplemental Figure 2B). In addition, similar defects in up-regulating surface CD86 and MHC class II were observed in BM-DCs isolated from mice in C57BL/6 genetic background (supplemental Figure 3B). In addition, Rras−/− BM-DCs also displayed attenuated up-regulation of maturation markers when stimulated with other TLR ligands, such as CpG, polyIC, and flagellin (supplemental Figure 4). These findings suggest that R-Ras may mediate the signaling events emanated from different TLRs. Consistently, on systemic LPS injection, Rras+/+ splenic DCs have increased CD86 and MHC class II expression compared with untreated controls, whereas Rras−/− splenic DCs showed significant decreases in these maturation events (Figure 4B). The impaired maturation seen in BM-DCs and splenic DCs demonstrates that R-Ras is required for DC maturation, and this may account for the defects in T-cell priming observed in Rras−/− mice.
Impaired maturation of Rras−/− BM-DC. (A) BM-DCs in culture for 6 days from Rras+/+ (n = 3) and Rras−/− mice (n = 3) in 129Sv strain were treated without (opened areas) or with (shaded areas) LPS (0.1 μg/mL) for 12 hours. Maturation markers were plotted as histograms on CD11c+ gated cells. MFI values are quantified in the bottom panels. Bars represent SE. ***P < .0005. *P < .05. Similar results were reproduced in an independent experiment (supplemental Figure 3A). (B) Rras+/+ (n = 3) and Rras−/− (n = 3) mice were intraperitoneally injected with 10 μg LPS (0.45 mg/kg). After 6 to 8 hours, the levels of maturation markers were analyzed on CD11c+ gated cells as described in panel A. Bars represent SE. *P < .05. Similar results were obtained in at least 2 independent experiments (supplemental Figure 3C).
Impaired maturation of Rras−/− BM-DC. (A) BM-DCs in culture for 6 days from Rras+/+ (n = 3) and Rras−/− mice (n = 3) in 129Sv strain were treated without (opened areas) or with (shaded areas) LPS (0.1 μg/mL) for 12 hours. Maturation markers were plotted as histograms on CD11c+ gated cells. MFI values are quantified in the bottom panels. Bars represent SE. ***P < .0005. *P < .05. Similar results were reproduced in an independent experiment (supplemental Figure 3A). (B) Rras+/+ (n = 3) and Rras−/− (n = 3) mice were intraperitoneally injected with 10 μg LPS (0.45 mg/kg). After 6 to 8 hours, the levels of maturation markers were analyzed on CD11c+ gated cells as described in panel A. Bars represent SE. *P < .05. Similar results were obtained in at least 2 independent experiments (supplemental Figure 3C).
R-Ras is required for TLR4 signaling
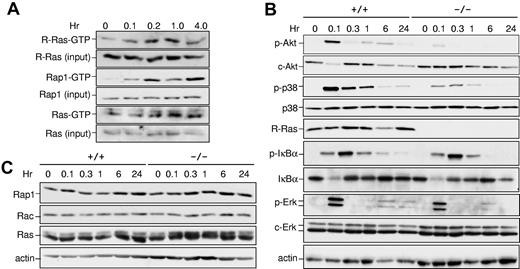
Results in Figure 4 suggest that R-Ras controls the ability of DCs to respond to TLR activation. To test whether R-Ras was activated in response to TLR4 activation, the kinetics of R-Ras activation were monitored by affinity pull-down assays using the Ras binding domain of Ral-GDS (RalGDS-RBD) as a probe that could bind the active form of R-Ras. As demonstrated in Figure 5A, the levels of R-Ras-GTP were increased by approximately 3.5-fold within 10 minutes of LPS stimulation and returned to basal levels by 6 hours. This magnitude of change in GTP loading was commonly observed for R-Ras, as has been shown in semaphorin4D-treated primary neurons.23 Similarly, Rap1 was maximally activated by LPS by 10 minutes but distinct from R-Ras in having a biphasic activation profile. On the contrary, the prototypic Ras proteins were not activated to an appreciable level until 1 hour after LPS addition (Figure 5A). These findings demonstrate that R-Ras can be acutely activated in response to TLR4 stimulation.
Impaired signaling in Rras−/− BM-DCs. (A) Rras+/+ BM-DCs were exposed to LPS (1 μg/mL) for the indicated duration, and the levels of GTP-bound R-Ras were measured by an affinity pull-down assay using GST-RalGDS-RBD as a probe. The total levels of R-Ras in the corresponding lysates were detected using an anti–R-Ras specific antibody. Similar experiments were performed for Rap1 and Ras using GST-RalGDS-RBD and GST-Raf-RBD as probes, respectively. (B-C) BM-DCs were treated with LPS (1 μg/mL) for the indicated duration, and total cell lysates were prepared. Western blotting analysis was carried out using the indicated antibodies. Similar results were obtained from 2 additional experiments.
Impaired signaling in Rras−/− BM-DCs. (A) Rras+/+ BM-DCs were exposed to LPS (1 μg/mL) for the indicated duration, and the levels of GTP-bound R-Ras were measured by an affinity pull-down assay using GST-RalGDS-RBD as a probe. The total levels of R-Ras in the corresponding lysates were detected using an anti–R-Ras specific antibody. Similar experiments were performed for Rap1 and Ras using GST-RalGDS-RBD and GST-Raf-RBD as probes, respectively. (B-C) BM-DCs were treated with LPS (1 μg/mL) for the indicated duration, and total cell lysates were prepared. Western blotting analysis was carried out using the indicated antibodies. Similar results were obtained from 2 additional experiments.
To further delineate the relevance of R-Ras in the downstream signaling of TLR4, the activation states of several key signaling kinases in Rras+/+ and Rras−/− BM-DCs were examined. In Rras+/+ DCs, a biphasic response for Akt activation was observed with robust activation within 5 minutes and less so after 6 hours of TLR4 stimulation (Figure 5B). Under similar conditions, the magnitude of Akt activation in Rras−/− DCs was reduced by approximately 5-fold and the biphasic response was not appreciable. The PI3K/Akt pathway has been shown to positively regulate DC activation through NF-κB and p38.24 On TLR4 stimulation, Rras+/+ DCs displayed robust activation of p38 and IκBα that peaked at 6 and 18 minutes, respectively, followed by their down-modulation (Figure 5B). In contrast, Rras−/− DCs activated with LPS displayed an approximately 7-fold decrease in p38 activation. However, no significant differences were observed in IκBα or ERK1/2 activation (Figure 5B). Furthermore, there were no drastic changes in the levels of other related small GTPases, such as Rap1, Rac, and Ras (Figure 5C). Together, these data implicate R-Ras as a mediator of TLR4 signaling through Akt and p38 MAPK.
Rras−/− DCs are impaired in forming T-cell synapses
Dynamic changes in actin cytoskeleton regulate DCs ability to form immune synapses with T cells. These complex processes are coordinated by small GTPases of the Ras and Rho subfamilies.25,26 Indeed, defects in Wiscott-Aldrich syndrome are attributed to failure of the Rho family of small GTPases to coordinate actin cytoskeleton in DCs, resulting in reduced cellular protrusions.27,28 In light of the observed defects in DC maturation and previous data suggesting R-Ras being upstream of Rac and Rap1,16,29-31 it was possible that R-Ras may play a role in actin cytoskeletal rearrangement in DCs.
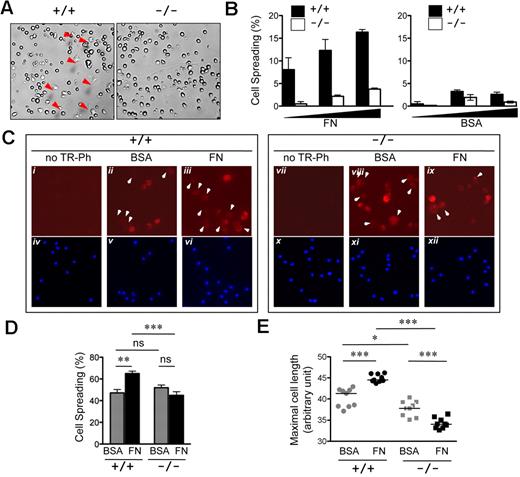
The ability to spread on defined extracellular matrix is indicative of a dynamic actin cytoskeleton. To study the role of R-Ras in DC spreading, the relative ability of Rras+/+ and Rras−/− DCs to spread on fibronectin was compared. For this, BM-DCs cultured in vitro for 6 days were used. Within 20 minutes of plating, spreading was evidenced in Rras+/+ DCs with the formation of the characteristic stellate morphology (Figure 6A). In contrast, Rras−/− DCs retained a mostly rounded morphology with an approximately 75% decrease in cell spreading (Figure 6B). To test whether similar observations could be detected in DCs propagated for longer time, BM-DCs cultured for 9 days were also examined. For these experiments, the actin cytoskeletal structures were analyzed by staining with phalloidin. Overall, these cultures harbored greater propensity to spread on either BSA- or fibronectin-coated wells, with more than 40% of cell spreading (Figure 6D). Intense staining of cortical actin structure was evidenced in rounded Rras+/+ BM-DCs plated on BSA (Figure 6Cii). Plating on fibronectin induced a modest but significant 37.7% increase in the number of cells with elongated morphology (Figure 6Ciii,D). On the contrary, the extent of cell spreading was not altered for Rras−/− BM-DCs plated either on BSA or fibronectin (Figure 6Cviii-ix,D). More important, the extent of cell spreading on fibronectin was significantly higher for Rras+/+ BM-DCs compared with Rras−/− (Figure 6D). As an independent mean to assess cell spreading, the mean cell length was determined. As shown in Figure 6E, plating on fibronectin significantly increased the mean cell length of Rras+/+ BM-DCs. On the contrary, Rras−/− BM-DCs have reduced cell size, and fibronectin did not stimulate an increase in mean cell length.
Attenuated cell spreading in Rras−/− DCs. (A) Day 6 BM-DCs from Rras+/+ (n = 1) or Rras−/− (n = 1) mice were plated on fibronectin (FN; 10 μg/mL) or BSA-coated plates and allowed to attach for 20 minutes. Images were taken from 5 random fields, and the percentage of spread cells (red arrows) was scored. (B) Results are from a single experiment using increased concentrations (▴) of BM-DCs and have been repeated once with similar results. Bars represent SD. (C) Immunofluorescence analysis of day 9 Rras+/+ (n = 1) or Rras−/− (n = 1) BM-DCs using Texas Red-conjugated phalloidin (TR-Ph; ii-iii,viii-ix, red) to stain F-actin filaments. Cell nuclei were visualized by 4,6-diamidino-2-phenylindole (iv-vi,x-xii, blue). Arrowhead indicates spread cells. (D) The percentages of spread cells were quantified as described in “Cell spreading assay.” ns indicates not significant. (E) The maximal cell length was determined by ImageJ software, with each data point representing the mean cell length for a single field of approximately 62 cells. (C-E) Results were from a single experiment, and 9 fields were selected from triplicate wells with an average of 550 cells being analyzed per group. Bars represent SE. ***P < .0005. **P < .005. *P < .05.
Attenuated cell spreading in Rras−/− DCs. (A) Day 6 BM-DCs from Rras+/+ (n = 1) or Rras−/− (n = 1) mice were plated on fibronectin (FN; 10 μg/mL) or BSA-coated plates and allowed to attach for 20 minutes. Images were taken from 5 random fields, and the percentage of spread cells (red arrows) was scored. (B) Results are from a single experiment using increased concentrations (▴) of BM-DCs and have been repeated once with similar results. Bars represent SD. (C) Immunofluorescence analysis of day 9 Rras+/+ (n = 1) or Rras−/− (n = 1) BM-DCs using Texas Red-conjugated phalloidin (TR-Ph; ii-iii,viii-ix, red) to stain F-actin filaments. Cell nuclei were visualized by 4,6-diamidino-2-phenylindole (iv-vi,x-xii, blue). Arrowhead indicates spread cells. (D) The percentages of spread cells were quantified as described in “Cell spreading assay.” ns indicates not significant. (E) The maximal cell length was determined by ImageJ software, with each data point representing the mean cell length for a single field of approximately 62 cells. (C-E) Results were from a single experiment, and 9 fields were selected from triplicate wells with an average of 550 cells being analyzed per group. Bars represent SE. ***P < .0005. **P < .005. *P < .05.
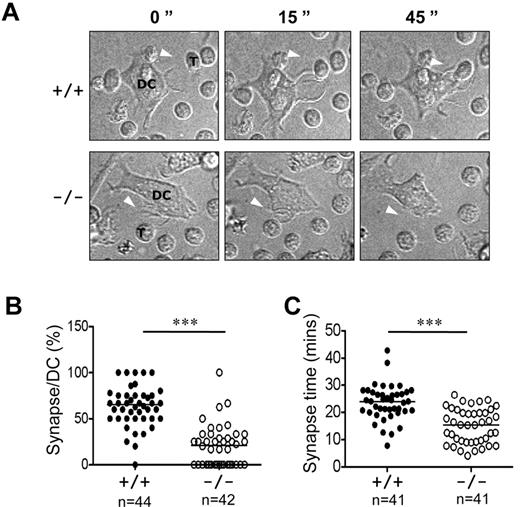
DCs undergo significant actin reorganization during maturation, therein projecting long and motile membrane extensions. These processes are necessary for DCs to form stable interactions with T cells during priming.32 We used time-lapse video microscopy to visualize synapses between antigen-loaded mature DCs and naive antigen-specific TCR transgenic T cells. Whereas immature Rras+/+ and Rras−/− DCs did not display dendritic protrusions and migrated slowly, LPS efficiently stimulated peripheral extensions and the acquisition of a stellate morphology (Figure 7A; supplemental Videos 1-4). In addition, increased DC migration distances were observed. The dendrites on mature DCs would actively capture T cells with the formation of multiple synapses (supplemental Video 3). Consistent with previous findings, the percentage of synapses observed between mature Rras+/+ DCs and naive T cells was measured to be 62.9% ± 21.9% (Figure 7B).12 In contrast, the percentage of Rras−/− DCs forming stable synapse was significantly decreased, reaching only 20.5% ± 22.3%. Analysis of individual positive synaptic events revealed an average interaction duration of 23.6 ± 6.5 minutes for Rras+/+ DCs and 15.0 ± 6.3 minutes for Rras−/− DCs (Figure 7C). Furthermore, although mature DCs migrated more than immature DCs, differences in total distance traveled were noted between mature Rras+/+ and Rras−/− DCs (data not shown), consistent with the observation that Rras−/− DCs were defective in maturation. Taken together, these results demonstrate the importance of R-Ras in DC function that can be attributed to a failure in establishing stable immune synapse with T cells.
Attenuated immune synapse formation in Rras−/− DCs. (A) Time-lapse confocal images at different time points are shown, illustrating an example of a synapse between a mature Rras+/+ or Rras−/− DCs with a naive T cell. White arrows indicate DC protrusions. DCs and T cells (T) are indicated. (B) The percentage of DC–T-cell synapses were quantified (n > 40 for each genotype) as described in “Microscopy.” (C) The duration (minutes) of T-cell synapses (n = 41) with either WT- or KO-activated DCs was quantified. ***P < .0005. Bars represent median.
Attenuated immune synapse formation in Rras−/− DCs. (A) Time-lapse confocal images at different time points are shown, illustrating an example of a synapse between a mature Rras+/+ or Rras−/− DCs with a naive T cell. White arrows indicate DC protrusions. DCs and T cells (T) are indicated. (B) The percentage of DC–T-cell synapses were quantified (n > 40 for each genotype) as described in “Microscopy.” (C) The duration (minutes) of T-cell synapses (n = 41) with either WT- or KO-activated DCs was quantified. ***P < .0005. Bars represent median.
Discussion
In this study, we characterized an R-Ras knockout mouse strain for defects in immune responses. Our results represent the first demonstration of the ability of R-Ras in controlling DC maturation in response to TLR4 ligand. Consistently, Rras−/− DCs are impaired in their ability to prime allogeneic and antigen specific T cells and compromised in forming stable immunologic synapses with antigen specific T cells.
Our findings are reminiscent of CD4+ T-cell priming defects observed in Mindin-null mice that are associated with a reduction in Rac1 expression.33 These studies have highlighted the importance of Rac in controlling immunologic functions of DCs, T cells, or B cells through its ability to regulate actin-dependent cytoskeletal processes.12,34,35 However, the fact that Rac expression was not altered in Rras−/− BM-DCs would suggest alternative mechanisms being involved. Future studies using gene expression profiling may identify R-Ras regulated genes that are critical for DC maturation.
BM-DCs lacking R-Ras have impaired LPS-induced surface up-regulation of CD86 and MHC class II. A possible explanation could be related to the role of R-Ras in vesicular trafficking. It has been reported that, although R-Ras is inactive on the plasma membrane, it is more active in endosomes.36,37 Indeed, blocking R-Ras in PC12 cells significantly perturbs calcium-mediated exocytosis. It remains to be determined whether the observed decreased surface maturation markers in Rras−/− DCs are the result of an attenuated transport of these receptors from the endoplasmic reticulum to the plasma membrane.
Ligand binding to TLRs triggers the coupling of adaptor molecules, such as MyD88 and IRAKs, to the activation of 3 major signaling cascades, which include NF-κB, p38, and JNK.38 Alternatively, LPS activates Akt in DCs through the PI3K signaling pathway.39 These signaling pathways play critical roles in regulating DC maturation, endocytosis, cytokine production, gene expression, and survival. To our knowledge, the increase in GTP-bound R-Ras in response to TLR4 stimulation is the first demonstration that a member of the Ras subfamily can be activated by this receptor class in DCs. Indeed, the activation of the classic Ras was only observed 60 minutes after TLR4 stimulation. Akt has been shown to be important for DC maturation and survival.40-43 Although unknown in immune cells, the ability of R-Ras in activating Akt has been extensively demonstrated in fibroblasts and epithelial cells, presumably through its interaction with the p110α subunit of PI3K.44,45 Consistently, LPS-activated Rras−/− BM-DCs revealed reduced Akt activation. It was reported that PI3K regulates a negative feedback pathway limiting IL-12 production and curtailing excessive TH1 polarization in DCs.46 It will be of interest to investigate whether this negative feedback pathway is disrupted in Rras−/− DCs.
p38 MAPK signaling is critical for actin reorganization and MHC class II up-regulation during DC maturation,11 whereas NF-κB is thought to be important for cytokine release.47 The initial burst in endocytosis on LPS stimulation in DCs is p38 dependent, as treatment with the p38 inhibitor SB203580 inhibits this uptake.11 In addition, p38 signaling is linked to DC adhesion and spreading, as Vav1 was shown to be important in adhesion-dependent p38 activation. As reported previously, p38 is mapped upstream of Rap1 in J774.A1.16,30 Thus, it is tempting to speculate a potential signaling pathway of R-Ras > p38 > Rap1 in regulating specific DC functions. Validating this hypothesis will require the demonstration of direct interaction between components of the TLR4 signaling pathway and R-Ras. Furthermore, as LPS-treated cells have increased complex formation between PI3K and components of the TLR4 signaling cascade, including MyD88 and TRAF6,48 we hypothesize that R-Ras may also bind to members of this complex as well.
By live cell imaging techniques, we uncovered that LPS-activated Rras−/− DCs were impaired in forming immune synapses with naive T cells. Mechanistically, it is possible that R-Ras plays a positive role in synapse formation through either a direct or an indirect mechanism. For example, the failure of Rras−/− BM-DCs to spread on fibronectin is indicative of R-Ras having a positive role in modulating actin dynamics, as previously suggested.49 It has been shown that DC dendrites could isotropically extend their cell body until they entangled a T cell and formed a stable DC–T-cell contact. Alternatively, the clustering of adhesion molecules, such as MHC class II, CD86, and CD40, at the c-SMAC and p-SMAC could be positively regulated by R-Ras. In this case, the reduction of these maturation markers on LPS-stimulated Rras−/− DCs might explain the impaired immune synapse formation. Our results are, however, contrary to studies of DCs from Rac1/2−/− mice. Although Rac1/2−/− DCs were significantly impaired in synapse formation, their maturation in response to LPS was normal.12 Thus, it is tempting to speculate that R-Ras may act upstream of Rac in the hierarchy of TLR4 signaling. In conclusion, our results reveal previously unknown functions of R-Ras in DCs. By modulating the Akt and p38 signaling in response to TLR4 activation in DCs, R-Ras influences actin cytoskeleton-dependent processes and plays a role in immune synapse formation and mounting T-cell responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Thomas Moran, Gwendalyn Randolph, Adrian Ting, Jay Unkeless, Julie Blander, and Stuart Aaronson (Mount Sinai School of Medicine), Drs Bonnie Dittel and Demin Wang (Medical College of Wisconsin), and Dr Michael Dustin (New York University) for helpful advice; and Dr Rumana Huq (Microscopy Core, Mount Sinai) in assisting with live-cell imaging.
This work was supported by the National Institutes of Health (CA78509 and MH59771), Midwest Athletes Against Childhood Cancer (A.M.C.), Advancing a Healthier Wisconsin (A.M.C.), Wisconsin Breast Cancer Showhouse (A.M.C.), and the Children's Research Institute of the Children's Hospital of Wisconsin. G.S. was supported by the National Cancer Institute (predoctoral training grant T32CA078207).
National Institutes of Health
Authorship
Contribution: G.S. designed experiments, conducted experimental procedures, analyzed the data, and wrote the manuscript; D.H., X.Y., J.H., P.J.Y.P., G.M., R.F.Q., and C.R.K. conducted experimental procedures and analyzed the data; and S.-H.C., M.M., and A.M.C. designed experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew M. Chan, Department of Pediatrics, Division of Hematology and Oncology, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: axchan@mcw.edu.
References
Author notes
S.-H.C., M.M., and A.M.C. contributed equally to this study as last co-authors.

![Figure 3. Rras−/− splenic APCs and BM-DCs show impaired T-cell proliferation capacity. (A) Bulk splenic APCs (129Sv; n = 3) or (B) splenic DCs (129Sv; n = 4) from Rras+/+ or Rras−/− mice in different ratios were comixed with 2 × 105 T cells from WT Balb/c for 96 hours. Allogeneic T-cell proliferation was measured by adding [H3]-labeled thymidine for the last 16 hours, and the amount of incorporation was measured by a scintillation counter. Results represent triplicate measurements from a single experiment with data in panel A reproduced in 2 additional experiments. (C) A similar proliferation assay using WT T cells was conducted as in panels A and B, except that BM-DCs (129Sv) were used. Bars represent SD. (D) In vivo T-cell proliferation assays were performed. Rras+/+ or Rras−/− mice (129Sv) were injected intraperitoneally with PBS, 10 μg LPS, or 10 μg LPS and 1 mg OVA. Six hours after injection, splenic DCs were isolated and comixed with naive CFSE-labeled polyclonal syngeneic splenic T cells (129Sv) for 96 hours. CFSE+ staining was analyzed by flow cytometry to measure T-cell proliferation. All plots are gated for TCR+CD4+ cells. (E) Percentages from panel D for CFSE+ proliferating T cells are plotted. Results represent data from 2 independent experiments with Rras+/+ (n = 5) and Rras−/− (n = 6) mice. Bars represent median. ***P < .0005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/7/10.1182_blood-2011-05-357319/4/m_zh89991185620003.jpeg?Expires=1769161883&Signature=trP5q0I2dtevEf2P9SRpnkvCG6-H2qmjJlgToICZ1UWRokwULMJIUv~m1cFUBWiXIagh53BWLkvqPs3TvsPcqO7LLxTWVWPjfOQ92a-QI4EUgxeD0qunxp5GB74fJJZEOmld2eslZI0KFSUZ6Q5aGX93VoSaEhPdOZS3xe0kNbF-zAJR~PIPTq1kMc2ByfVtKMxmVbhFUfrBod7amyzqyjZbOixt6SrTblpt5AjonoxPQ4XdF0WVh86M~LypQhEbgiLpphYpeCJUFRFNmmMRKPo3C~hvHgkpD9CAbRV~nwrc9xwPd7Q3oa202Qjov1rCxlFWPaIDpkAPa2zb0nZKng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal