Abstract
Large cell transformation (LCT) in mycosis fungoides (MF) is generally associated with an aggressive clinical course and poor survival, requiring aggressive therapeutic approach. However, a proportion of cases may follow an indolent clinical course. To identify prognostic factors, we analyzed the prognostic relevance of clinical, histologic, and immunophenotypical features in a large cohort of transformed MF patients, including 75 patients with only skin lesions, 19 patients with LCT in skin and lymph nodes, and 6 patients with LCT in lymph nodes only. Multivariate analysis of the total group showed that CD30 negativity, folliculotropic MF, extent of skin lesions and extracutaneous transformation were associated with reduced disease-specific survival (DSS) and, except for CD30 negativity and folliculotropic MF, also overall survival. In a multivariate analysis of 75 patients with only skin lesions at the time of LCT, CD30 negativity, folliculotropic MF and extent of skin lesions were independent parameters for both DSS and overall survival. Using the most discriminating parameters as a prognostic index, in both study groups differences in DSS between patients with 0-1 unfavorable prognostic factor(s) and ≥ 2 unfavorable prognostic factors were statistically significant (P < .001). This prognostic index may be helpful in predicting prognosis and selecting the most appropriate treatment in patients with transformed MF.
Introduction
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma, clinically characterized by the slow progression from patches to plaques and in a proportion of patients to tumors. In a minority of patients, dissemination to nodal sites, other extracutaneous sites, or both may occur. Although patients with early patch or plaque stage MF generally run an indolent course with a 10-year disease-specific survival (DSS) over 80%, patients developing skin tumors or extracutaneous disease have a reduced 10-year DSS of 42% and < 20%, respectively.1,2 Apart from clinical stage, large cell transformation (LCT) in MF has been associated with an aggressive clinical course and a poor survival.3-10 Most studies report a median survival between 2 and 36 months (Table 1). Data on prognostic factors within these studies are however inconsistent and often conflicting and are probably related to the small size of the groups studied thus far (12-45 patients; median, 22 patients). Evaluation of these previous studies is further hampered by the inclusion of not only patients with MF but also variable numbers of patients with Sézary syndrome (SS) in 5 of 7 studies, and by the variable proportions (17%-92%) of patients with transformation at extracutaneous sites.
Studies on patients with transformed CTCL (MF/SS syndrome)
| . | MF patients with LCT . | LCT at first diagnosis MF (%) . | Stage at time LCT (%) . | Median survival, mo . | ||
|---|---|---|---|---|---|---|
| IA-IIA . | IIB . | IV . | ||||
| Dmitrovsky et al8 | 12 | 0/12 (0) | 1/12 (8) | 11/12 (92) | 2 | |
| Salhany et al4 | 17 | 7/17 (41) | 6/17 (35) | 11/17 (65) | 12 | |
| Greer et al3 | 22 | 9/22 (41) | 9/22 (41) | 13/22 (59) | 12 | |
| Diamandidou et al7 | 26 | 9/26 (35) | 7/26 (27) | 10/26 (38) | 7/26 (27) | 19 |
| Vergier et al9 * | 45 | 8/45 (18) | 2/45 (4) | 24/45 (53) | 20/45 (44) | 36 |
| Barberio et al6 * | 17 | 2/17 (12) | 7/17 (41) | 7/17 (41) | 3/17 (18) | 27 |
| Arulogun et al5 | 22 | 7/22 (32) | 3/22 (14) | 11/22 (50) | 7/22 (32) | 27 |
| Agar et al1 | 70 | 70/70 (100) | NR | NR | NR | 100 |
| This study* | 100 | 42/100 (42) | 10 (10) | 65 (65) | 25 (25) | 24 |
| . | MF patients with LCT . | LCT at first diagnosis MF (%) . | Stage at time LCT (%) . | Median survival, mo . | ||
|---|---|---|---|---|---|---|
| IA-IIA . | IIB . | IV . | ||||
| Dmitrovsky et al8 | 12 | 0/12 (0) | 1/12 (8) | 11/12 (92) | 2 | |
| Salhany et al4 | 17 | 7/17 (41) | 6/17 (35) | 11/17 (65) | 12 | |
| Greer et al3 | 22 | 9/22 (41) | 9/22 (41) | 13/22 (59) | 12 | |
| Diamandidou et al7 | 26 | 9/26 (35) | 7/26 (27) | 10/26 (38) | 7/26 (27) | 19 |
| Vergier et al9 * | 45 | 8/45 (18) | 2/45 (4) | 24/45 (53) | 20/45 (44) | 36 |
| Barberio et al6 * | 17 | 2/17 (12) | 7/17 (41) | 7/17 (41) | 3/17 (18) | 27 |
| Arulogun et al5 | 22 | 7/22 (32) | 3/22 (14) | 11/22 (50) | 7/22 (32) | 27 |
| Agar et al1 | 70 | 70/70 (100) | NR | NR | NR | 100 |
| This study* | 100 | 42/100 (42) | 10 (10) | 65 (65) | 25 (25) | 24 |
MF indicates mycosis fungoides; LCT, large cell transformation; and NR, not reported.
Studies that included only patients with MF.
Remarkably, in a recent study of 1502 patients with MF/SS, including 70 patients with transformed MF at the time of first diagnosis, the median survival was 8.3 years and the 5-year overall and DSSs were 63% and 65%, respectively.1 Although not described in detail, these survival data suggest a much better prognosis of transformed MF than reported in all previous studies (Table 1).
To better define the prognosis and prognostic factors in patients with transformed MF, we evaluated the clinical, histologic, and immunophenotypical features of a large cohort of 100 patients with transformed MF. This study reveals independent prognostic factors and provides a prognostic index that may be helpful in the clinical management of patients with transformed MF.
Methods
Patient selection
One hundred thirty patients with a diagnosis of transformed MF or a histologic diagnosis of (early) blastic transformation were retrieved from the cutaneous lymphoma database of the Leiden University Medical Center. For each case clinical records, skin biopsies (2-6 biopsies/case) and lymph node biopsies, if applicable, were reviewed. Hundred cases were included because they had clinical and histologic features consistent with MF, and at least 1 biopsy showing LCT according to the criteria described previously: the presence of large T cells exceeding 25% of the total lymphoid infiltrate or forming microscopic nodules.4 This study group did not contain cases with coexisting lymphomatoid papulosis (LyP) or primary cutaneous anaplastic large cell lymphoma (C-ALCL).
In a previous study, no difference in survival was found between patients presenting with tumor-stage MF (stage IIB) with or without LCT.11 For that reason, 25 patients with stage IIB MF with LCT at first presentation were compared with 27 patients presenting with stage IIB MF without LCT at first presentation, including 15 patients who developed LCT during follow-up and 12 patients who did not meet the criteria of LCT and were therefore excluded from the main study.
Clinicopathologic evaluation
All patients were classified according to the new criteria proposed by the International Society for Cutaneous Lymphomas and European Organization of Research and Treatment of Cancer.12 Staging included complete physical examination; blood cell count and chemistry; and in most cases, CT scan of abdomen, chest, neck, and head (in cases of head lesions, neck or head lesions); and 1 or multiple skin biopsies. In all patients with clinically significant adenopathy, a lymph node biopsy had been performed.
For each patient, the following clinical data were recorded: sex, age at diagnosis of MF and age at diagnosis of LCT, duration of skin lesions before MF, clinical stage at diagnosis of MF, time interval between MF and LCT, clinical stage at LCT, extent of skin tumors at the time of LCT, first treatment after LCT, result of treatment, duration of follow-up, and survival status. For the first skin biopsy showing transformation, folliculotropic MF and the percentage of large T cells (nodules of large cells but < 25%, 25%-75%, or > 75%) were recorded. Immunohistochemical stainings against T-cell antigens (CD2, CD3, CD4, CD5, and CD8), B-cell antigens (CD20 and CD79a), histiocytes (CD68), and CD30 were studied to determine the phenotype of the atypical cells, to differentiate large T cells from admixed histiocytes, and to determine the percentage of large T-cells expressing CD30 (0%-25%, 26%-50%, 51%-75%, or > 75%). In the MF stage IIB group without transformation, only age, sex, folliculotropic MF, extent of skin lesions, duration of follow-up, and survival status were recorded.
Prognostic factors
The following parameters were analyzed for their prognostic significance in transformed MF: sex, age at diagnosis of LCT (≤ 60 years vs > 60 years), time interval between MF and LCT (≤ 24 months after diagnosis MF vs > 24 months after diagnosis MF), clinical stage at the time of LCT, folliculotropic MF, CD30 expression by > 50% of the neoplastic T cells, and the extent of skin tumors. Extent of skin lesions was scored as solitary, regional (multiple skin lesions within 1 anatomic region), or generalized (multiple lesions in > 1 anatomic region). In a separate analysis of the 75 patients who presented with only skin lesions at the time of transformation, the percentage of large T cells (< 25% but clusters, 25%-75%, or > 75%) was included in the analysis as well.
Statistical analysis
All statistical calculations were performed using SPSS Version 17.0 (SPSS Inc). DSS was calculated from the date of first biopsy showing LCT until death as result of lymphoma or date of last follow-up. Overall survival (OS) was calculated from the date of first biopsy showing transformation until the patient's death or date of last follow-up. Survival curves were estimated by the method of Kaplan and Meier, and comparison between curves was done by log-rank testing. Univariate analysis of parameters with possible prognostic significance for DSS and OS was performed using Cox proportional hazards regression analysis. Factors significant at the .25 level in univariate analysis were included in a multivariate analysis model. In this model, P values below .05 were considered significant, and all parameters included were categoric.
Results
Clinical characteristics and follow-up data
The main clinical and histologic characteristics and follow-up data of the 100 patients with transformed MF are summarized in Table 2. The study group included 64 males and 36 females (male:female ratio, 1.8:1), with a median age at transformation of 68 years (range, 33-90 years). The median time between the initial biopsy-proven diagnosis of MF and transformation was 10 months (range, 0-222 months). Forty-two patients had transformation at first presentation. The other 58 patients developed LCT 1 to 222 months (median, 44 months) after the diagnosis MF had been made. At the time of LCT, 75 patients had transformation only in skin lesions; 19 patients had transformation in both skin and lymph nodes (n = 19), in 2 of them also in bone marrow or tongue; and 6 patients had transformation only in lymph nodes but not in concurrent skin lesions (stage IVA). Altogether, 10 patients had MF stage IB, 65 had stage IIB, 24 had stage IVA, and 1 patient had stage IVB. Initial treatment after transformation, listed in Table 2, resulted in a complete remission in only 18 of 100 patients; the remission was however generally short-lived. Median survival after transformation was 24 months (range, 1-235 months). DSS after 2, 5, and 10 years was 62%, 38%, and 36%, respectively, and OS was 57%, 33%, and 24%, respectively.
Clinical and histologic characteristics of 100 patients with transformed MF
| Characteristic . | Value . |
|---|---|
| Male:female | 64:36 |
| Median duration skin lesions before diagnosis MF, mo (range) | 33 (1-480) |
| Median interval between diagnosis MF and LCT, mo (range) | 10 (0-222) |
| Median age at diagnosis MF, y (range) | 64 (29-90) |
| Median age at diagnosis MF with LCT, y (range) | 68 (33-90) |
| Site of LCT | |
| Only skin | 75 |
| Only lymph nodes | 6 |
| Skin + lymph nodes | 19 |
| Stage at MF with LCT | |
| IB | 10 |
| IIB | 65 |
| IV | 25 |
| Folliculotropic MF | |
| Absent | 69 |
| Present | 31 |
| Percent of blast cells | |
| < 25% (clusters, %) | 9 (10) |
| 25%-75% | 39 (41) |
| > 75% | 46 (49) |
| CD30 expression* | |
| 0%-25% | 53 |
| 26%-50% | 0 |
| 51%-75% | 8 |
| 76%-100% | 39 |
| First therapy after LCT | |
| Local radiotherapy† | 44 |
| Total skin electron beam therapy | 12 |
| Polychemotherapy | 28 |
| Other (eg, local steroids, excision, photochemotherapy) | 16 |
| Median duration follow-up after | |
| Start skin lesions, mo (range) | 100 (15-640) |
| Diagnosis MF, mo (range) | 54 (5-318) |
| Transformation, mo (range) | 24 (1-235) |
| Current status | |
| Alive without disease | 6 |
| Alive with disease | 25 |
| Died of lymphoma | 55 |
| Died of other cause | 14 |
| Survival | |
| 2-y DSS/OS, % | 62/57 |
| 5-y DSS/OS, % | 38/33 |
| 10-y DSS/OS, % | 36/24 |
| Characteristic . | Value . |
|---|---|
| Male:female | 64:36 |
| Median duration skin lesions before diagnosis MF, mo (range) | 33 (1-480) |
| Median interval between diagnosis MF and LCT, mo (range) | 10 (0-222) |
| Median age at diagnosis MF, y (range) | 64 (29-90) |
| Median age at diagnosis MF with LCT, y (range) | 68 (33-90) |
| Site of LCT | |
| Only skin | 75 |
| Only lymph nodes | 6 |
| Skin + lymph nodes | 19 |
| Stage at MF with LCT | |
| IB | 10 |
| IIB | 65 |
| IV | 25 |
| Folliculotropic MF | |
| Absent | 69 |
| Present | 31 |
| Percent of blast cells | |
| < 25% (clusters, %) | 9 (10) |
| 25%-75% | 39 (41) |
| > 75% | 46 (49) |
| CD30 expression* | |
| 0%-25% | 53 |
| 26%-50% | 0 |
| 51%-75% | 8 |
| 76%-100% | 39 |
| First therapy after LCT | |
| Local radiotherapy† | 44 |
| Total skin electron beam therapy | 12 |
| Polychemotherapy | 28 |
| Other (eg, local steroids, excision, photochemotherapy) | 16 |
| Median duration follow-up after | |
| Start skin lesions, mo (range) | 100 (15-640) |
| Diagnosis MF, mo (range) | 54 (5-318) |
| Transformation, mo (range) | 24 (1-235) |
| Current status | |
| Alive without disease | 6 |
| Alive with disease | 25 |
| Died of lymphoma | 55 |
| Died of other cause | 14 |
| Survival | |
| 2-y DSS/OS, % | 62/57 |
| 5-y DSS/OS, % | 38/33 |
| 10-y DSS/OS, % | 36/24 |
CD30 expression by more than 50% of the cells was taken as prognostic factor for statistical analysis.
Often combined with other skin-directed therapies such as local steroids or photochemotherapy.
Histopathologic and immunophenotypical features
Biopsies from tumorous lesions generally showed a dense and diffuse infiltrate throughout the entire dermis, whereas the infiltrates in biopsies from patients with plaque-stage (stage IB) disease were generally confined to the upper dermis. In this latter group, 3 patients showed a predominantly intraepidermal accumulation of large CD30+ (1 case) or CD30− (2 cases) T cells simulating pagetoid reticulosis. However, the clinical presentation and the presence of part of these large blast cells in the superficial dermal compartment ruled out this diagnosis. In the majority of cases, the large cell population consisted of a mixture of large T cells with cerebriform nuclei, blast cells with prominent nucleoli (T immunoblasts), intermediate forms, and variable numbers of large anaplastic cells. Cases with a monotonous population of either large cerebriform cells without prominent nucleoli (n = 3) or T immunoblasts (n = 4) or large anaplastic cells (n = 8) comprising > 75% of the total lymphoid infiltrate were less commonly observed. In 31 cases, the histologic (and clinical) features were consistent with the diagnosis of folliculotropic MF. The percentages of large T cells of the total lymphoid population in the skin biopsies showing transformation are presented in Table 2. Immunophenotypic analysis showed that 70 cases had a CD3+CD4+CD8− T-cell phenotype, 7 cases had a CD3+CD4−CD8+ T-cell phenotype, and 19 cases a CD3+CD4−CD8− T-cell phenotype. In 4 cases, the phenotype could not be assessed. In many cases, there was (partial) loss of 1 or more pan-T-cell antigens, whereas 4 cases showed aberrant expression of CD20, CD79a, or both. CD30 was expressed by > 75% of the large T cells in 39 cases, by 50% to 75% in 8 cases, and between 5% and 20% in 8 cases. In the other 45 cases, CD30 staining was either completely negative or expressed by only very few (< 5%) large T cells.
Prognostic factors
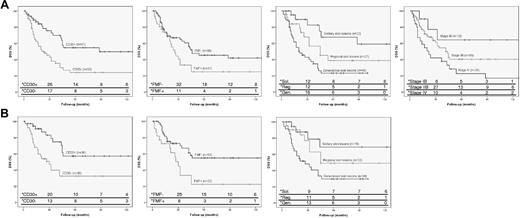
In the total group of patients (n = 100), both univariate and multivariate analysis established that extent of skin lesions, extracutaneous transformation, negative staining for CD30, and folliculotropic MF were associated with a reduced DSS (Table 3). Further analysis showed that the difference in survival between stage IB versus IIB was not significant but that the differences between stage IV versus IIB and stage IV versus combined stage IB and IIB were significant for DSS (P = .048 and P = .018, respectively). Multivariate analysis for OS showed that extent of skin lesions and extracutaneous transformation, but not folliculotropic MF and CD30 negativity, were independent parameters for reduced OS (Table 3).
Univariate and multivariate analysis of prognostic factors in 100 patients with transformed MF
| Characteristic . | No. . | Median survival, mo . | DSS, % . | Univariate analysis DSS . | Multivariate analysis DSS . | OS, % . | Univariate analysis OS . | Multivariate analysis OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Sex | .418 | .980 | ||||||||||||||
| Male | 64 | 25 (1-235) | 65 | 38 | 38 | 1 | 59 | 31 | 21 | 1 | ||||||
| Female | 36 | 20 (2-160) | 56 | 38 | 32 | 1.3 (0.7-2.2) | 54 | 37 | 30 | 1 (0.6-1.6) | ||||||
| Age | .395 | .043 | .071 | |||||||||||||
| < 60 y | 34 | 42.5 (3-215) | 70 | 40 | 40 | 1 | 70 | 40 | 36 | 1 | 1 | |||||
| ≥ 60 y | 66 | 19 (1-235) | 57 | 38 | 33 | 1.3 (0.7-2.2) | 47 | 29 | 16 | 1.7 (1.0-2.9) | 1.9 (0.9-4.0) | |||||
| Time interval between MF and LCT | .089 | .322 | .246 | .475 | ||||||||||||
| ≤ 24 mo | 63 | 30 (2-215) | 71 | 44 | 39 | 1 | 1 | 66 | 36 | 25 | 1 | 1 | ||||
| > 24 mo | 37 | 17 (1-235) | 46 | 29 | 29 | 1.6 (0.9-2.7) | 1.4 (0.7-2.5) | 42 | 26 | 23 | 1.3 (0.8-2.2) | 1.3 (0.7-2.3) | ||||
| Clinical stage at LCT | .021 | .046 | .224 | .002 | ||||||||||||
| Skin disease (stage IB + stage IIB) | 75 | 26 (1-235) | 69 | 44 | 44 | 1 | 1 | 59 | 36 | 26 | 1 | 1 | ||||
| Extracutaneous disease (stage IV) | 25 | 20 (3-215) | 42 | 23 | 17 | 1.9 (1.1-3.3) | 1.9 (1.0-3.7) | 42 | 23 | 17 | 1.4 (0.8-2.3) | 3.2 (1.5-6.6) | ||||
| CD30 expression | .003 | .039 | .007 | .068 | ||||||||||||
| Positive | 47 | 38 (1-215) | 78 | 55 | 50 | 1 | 1 | 70 | 49 | 33 | 1 | 1 | ||||
| Negative | 53 | 20 (2-235) | 48 | 24 | 24 | 2.3 (1.3-4.1) | 2.0 (1.0-3.7) | 46 | 19 | 16 | 2.0 (1.2-3.2) | 1.8 (1.0-3.3) | ||||
| Folliculotropic MF | .060 | .048 | .318 | |||||||||||||
| Absent | 69 | 25 (1-235) | 67 | 45 | 42 | 1 | 1 | 57 | 37 | 25 | 1 | |||||
| Present | 31 | 22 (2-134) | 52 | 25 | 25 | 1.7 (1.0-3.0) | 1.9 (1.0-3.4) | 50 | 24 | 24 | 1.3 (0.8-2.2) | |||||
| Extent of skin lesions | .005 | .034 | .002 | .023 | ||||||||||||
| Solitary | 22 | 43 (5-164) | 89 | 68 | 59 | 1 | 1 | 81 | 61 | 54 | 1 | 1 | ||||
| Regional | 27 | 23 (1-235) | 74 | 39 | 39 | 1.9 (0.8-4.9) | 1.7 (0.7-4.4) | 62 | 29 | 29 | 2.0 (0.9-4.5) | 1.7 (0.6-4.4) | ||||
| Generalized | 45 | 20 (2-100) | 46 | 23 | 3.6 (1.6-8.5) | 3.0 (1.2-7.2) | 44 | 20 | – | 3.5 (1.7-7.4) | 3.2 (1.3-7.7) | |||||
| Characteristic . | No. . | Median survival, mo . | DSS, % . | Univariate analysis DSS . | Multivariate analysis DSS . | OS, % . | Univariate analysis OS . | Multivariate analysis OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Sex | .418 | .980 | ||||||||||||||
| Male | 64 | 25 (1-235) | 65 | 38 | 38 | 1 | 59 | 31 | 21 | 1 | ||||||
| Female | 36 | 20 (2-160) | 56 | 38 | 32 | 1.3 (0.7-2.2) | 54 | 37 | 30 | 1 (0.6-1.6) | ||||||
| Age | .395 | .043 | .071 | |||||||||||||
| < 60 y | 34 | 42.5 (3-215) | 70 | 40 | 40 | 1 | 70 | 40 | 36 | 1 | 1 | |||||
| ≥ 60 y | 66 | 19 (1-235) | 57 | 38 | 33 | 1.3 (0.7-2.2) | 47 | 29 | 16 | 1.7 (1.0-2.9) | 1.9 (0.9-4.0) | |||||
| Time interval between MF and LCT | .089 | .322 | .246 | .475 | ||||||||||||
| ≤ 24 mo | 63 | 30 (2-215) | 71 | 44 | 39 | 1 | 1 | 66 | 36 | 25 | 1 | 1 | ||||
| > 24 mo | 37 | 17 (1-235) | 46 | 29 | 29 | 1.6 (0.9-2.7) | 1.4 (0.7-2.5) | 42 | 26 | 23 | 1.3 (0.8-2.2) | 1.3 (0.7-2.3) | ||||
| Clinical stage at LCT | .021 | .046 | .224 | .002 | ||||||||||||
| Skin disease (stage IB + stage IIB) | 75 | 26 (1-235) | 69 | 44 | 44 | 1 | 1 | 59 | 36 | 26 | 1 | 1 | ||||
| Extracutaneous disease (stage IV) | 25 | 20 (3-215) | 42 | 23 | 17 | 1.9 (1.1-3.3) | 1.9 (1.0-3.7) | 42 | 23 | 17 | 1.4 (0.8-2.3) | 3.2 (1.5-6.6) | ||||
| CD30 expression | .003 | .039 | .007 | .068 | ||||||||||||
| Positive | 47 | 38 (1-215) | 78 | 55 | 50 | 1 | 1 | 70 | 49 | 33 | 1 | 1 | ||||
| Negative | 53 | 20 (2-235) | 48 | 24 | 24 | 2.3 (1.3-4.1) | 2.0 (1.0-3.7) | 46 | 19 | 16 | 2.0 (1.2-3.2) | 1.8 (1.0-3.3) | ||||
| Folliculotropic MF | .060 | .048 | .318 | |||||||||||||
| Absent | 69 | 25 (1-235) | 67 | 45 | 42 | 1 | 1 | 57 | 37 | 25 | 1 | |||||
| Present | 31 | 22 (2-134) | 52 | 25 | 25 | 1.7 (1.0-3.0) | 1.9 (1.0-3.4) | 50 | 24 | 24 | 1.3 (0.8-2.2) | |||||
| Extent of skin lesions | .005 | .034 | .002 | .023 | ||||||||||||
| Solitary | 22 | 43 (5-164) | 89 | 68 | 59 | 1 | 1 | 81 | 61 | 54 | 1 | 1 | ||||
| Regional | 27 | 23 (1-235) | 74 | 39 | 39 | 1.9 (0.8-4.9) | 1.7 (0.7-4.4) | 62 | 29 | 29 | 2.0 (0.9-4.5) | 1.7 (0.6-4.4) | ||||
| Generalized | 45 | 20 (2-100) | 46 | 23 | 3.6 (1.6-8.5) | 3.0 (1.2-7.2) | 44 | 20 | – | 3.5 (1.7-7.4) | 3.2 (1.3-7.7) | |||||
MF indicates mycosis fungoides; LCT, large-cell transformation; DSS, disease specific survival; OS, overall survival; HR, hazard ratio; and CI, confidence interval.
Missing data: extent of skin lesions: 6.
In the group with only transformation in skin lesions (stage IB-IIB; n = 75) multivariate analysis showed that CD30 negativity, folliculotropic MF and extent of skin tumors were independent parameters for reduced DSS and OS (Table 4). Further analysis showed no significant differences between solitary and regional skin tumors or between regional and generalized skin tumors. However, patients with generalized skin tumors had a significantly worse DSS (P = .005) and OS (P = .002) compared with the combined group of solitary and regional skin tumors. DSS curves of all independent parameters in the total group of patients (n = 100) and in the patients with only skin lesions and LCT (n = 75) are shown in Figure 1.
Univariate and multivariate analysis of prognostic factors in 75 transformed MF patients presenting with only skin lesions
| Characteristic . | No. . | Median survival, mo . | DSS, % . | Univariate analysis DSS . | Multivariate analysis DSS . | OS, % . | Univariate analysis OS . | Multivariate analysis OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Sex | .390 | .962 | ||||||||||||||
| Male | 51 | 27 (1-235) | 73 | 46 | 46 | 1 | 60 | 36 | 22 | 1 | ||||||
| Female | 24 | 22.5 (2-160) | 60 | 39 | 39 | 1.4 (0.7-2.7) | 56 | 37 | 37 | 1 (0.6-1.9) | ||||||
| Age | .515 | .084 | .012 | |||||||||||||
| < 60 y | 19 | 45 (4-164) | 48 | 48 | 48 | 1 | 42 | 42 | 42 | 1 | 1 | |||||
| ≥ 60 y | 56 | 21 (1-235) | 65 | 42 | 42 | 1.3 (0.6-2.8) | 53 | 32 | 20 | 1.9 (0.9-3.7) | 2.8 (1.8-6.1) | |||||
| Time interval between MF and LCT | .050 | .830 | .180 | .537 | ||||||||||||
| ≤ 24 mo | 48 | 31 (2-164) | 80 | 51 | 51 | 1 | 1 | 67 | 49 | 21 | 1 | 1 | ||||
| > 24 mo | 27 | 20 (1-235) | 51 | 31 | 31 | 2.0 (1.0-3.8) | 1.1 (0.5-2.4) | 45 | 27 | 22 | 1.5 (0.8-2.6) | 1.2 (0.6-2.5) | ||||
| Clinical stage at LCT | .205 | .220 | .207 | .127 | ||||||||||||
| IB | 10 | 48 (11-160) | 77 | 64 | 64 | 1 | 1 | 77 | 64 | 38 | 1 | 1 | ||||
| IIB | 65 | 25 (1-235) | 68 | 40 | 40 | 2.2 (0.7-7.0) | 2.2 (0.6-6.4) | 57 | 32 | 25 | 1.8 (0.7-4.6) | 2.1 (0.8-5.6) | ||||
| CD30 expression | .008 | .006 | .026 | .027 | ||||||||||||
| Positive | 36 | 38 (1-160) | 90 | 57 | 57 | 1 | 1 | 76 | 50 | 33 | 1 | 1 | ||||
| Negative | 39 | 21 (2-235) | 54 | 33 | 33 | 2.6 (1.3-5.6) | 3.2 (1.4-7.1) | 45 | 24 | 21 | 1.9 (1.0-3.5) | 2.1 (1.1-3.9) | ||||
| Folliculotropic MF | .014 | < .001 | .178 | .001 | ||||||||||||
| Absent | 53 | 27 (1-235) | 73 | 55 | 55 | 1 | 1 | 60 | 43 | 29 | 1 | 1 | ||||
| Present | 22 | 23.5 (2-134) | 61 | 22 | 22 | 2.3 (1.2-4.6) | 4.0 (1.9-8.2) | 58 | 21 | 21 | 1.5 (0.8-2.8) | 3.4 (1.7-7.0) | ||||
| % large cells in infiltrate | .784 | .789 | ||||||||||||||
| < 25 (nodules) | 7 | 26 (11-44) | 100 | 1 | 80 | 1 | ||||||||||
| 25-75 | 32 | 27 (2-235) | 71 | 49 | 49 | 0.67 (0.2-2.1) | 60 | 41 | 29 | 0.74 (0.3-2.0) | ||||||
| > 75 | 36 | 24.5 (1-164) | 62 | 46 | 46 | 0.75 (0.2-2.2) | 55 | 38 | 28 | 0.71 (0.3-1.9) | ||||||
| Extent of skin tumors | .024 | .024 | .009 | .002 | ||||||||||||
| Solitary | 19 | 30 (5-164) | 87 | 69 | 69 | 1 | 1 | 78 | 62 | 62 | 1 | 1 | ||||
| Localized | 22 | 29 (1-235) | 84 | 49 | 49 | 1.6 (0.5-5.0) | 1.2 (0.4-4.0) | 69 | 35 | 35 | 1.7 (0.7-4.4) | 0.9 (0.3-2.6) | ||||
| Generalized | 34 | 20.5 (2-100) | 52 | 30 | 3.4 (1.3-9.5) | 3.4 (1.2-9.7) | 44 | 25 | 3.4 (1.5-7.8) | 3.3 (1.3-8.0) | ||||||
| Characteristic . | No. . | Median survival, mo . | DSS, % . | Univariate analysis DSS . | Multivariate analysis DSS . | OS, % . | Univariate analysis OS . | Multivariate analysis OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | 2 y . | 5 y . | 10 y . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| Sex | .390 | .962 | ||||||||||||||
| Male | 51 | 27 (1-235) | 73 | 46 | 46 | 1 | 60 | 36 | 22 | 1 | ||||||
| Female | 24 | 22.5 (2-160) | 60 | 39 | 39 | 1.4 (0.7-2.7) | 56 | 37 | 37 | 1 (0.6-1.9) | ||||||
| Age | .515 | .084 | .012 | |||||||||||||
| < 60 y | 19 | 45 (4-164) | 48 | 48 | 48 | 1 | 42 | 42 | 42 | 1 | 1 | |||||
| ≥ 60 y | 56 | 21 (1-235) | 65 | 42 | 42 | 1.3 (0.6-2.8) | 53 | 32 | 20 | 1.9 (0.9-3.7) | 2.8 (1.8-6.1) | |||||
| Time interval between MF and LCT | .050 | .830 | .180 | .537 | ||||||||||||
| ≤ 24 mo | 48 | 31 (2-164) | 80 | 51 | 51 | 1 | 1 | 67 | 49 | 21 | 1 | 1 | ||||
| > 24 mo | 27 | 20 (1-235) | 51 | 31 | 31 | 2.0 (1.0-3.8) | 1.1 (0.5-2.4) | 45 | 27 | 22 | 1.5 (0.8-2.6) | 1.2 (0.6-2.5) | ||||
| Clinical stage at LCT | .205 | .220 | .207 | .127 | ||||||||||||
| IB | 10 | 48 (11-160) | 77 | 64 | 64 | 1 | 1 | 77 | 64 | 38 | 1 | 1 | ||||
| IIB | 65 | 25 (1-235) | 68 | 40 | 40 | 2.2 (0.7-7.0) | 2.2 (0.6-6.4) | 57 | 32 | 25 | 1.8 (0.7-4.6) | 2.1 (0.8-5.6) | ||||
| CD30 expression | .008 | .006 | .026 | .027 | ||||||||||||
| Positive | 36 | 38 (1-160) | 90 | 57 | 57 | 1 | 1 | 76 | 50 | 33 | 1 | 1 | ||||
| Negative | 39 | 21 (2-235) | 54 | 33 | 33 | 2.6 (1.3-5.6) | 3.2 (1.4-7.1) | 45 | 24 | 21 | 1.9 (1.0-3.5) | 2.1 (1.1-3.9) | ||||
| Folliculotropic MF | .014 | < .001 | .178 | .001 | ||||||||||||
| Absent | 53 | 27 (1-235) | 73 | 55 | 55 | 1 | 1 | 60 | 43 | 29 | 1 | 1 | ||||
| Present | 22 | 23.5 (2-134) | 61 | 22 | 22 | 2.3 (1.2-4.6) | 4.0 (1.9-8.2) | 58 | 21 | 21 | 1.5 (0.8-2.8) | 3.4 (1.7-7.0) | ||||
| % large cells in infiltrate | .784 | .789 | ||||||||||||||
| < 25 (nodules) | 7 | 26 (11-44) | 100 | 1 | 80 | 1 | ||||||||||
| 25-75 | 32 | 27 (2-235) | 71 | 49 | 49 | 0.67 (0.2-2.1) | 60 | 41 | 29 | 0.74 (0.3-2.0) | ||||||
| > 75 | 36 | 24.5 (1-164) | 62 | 46 | 46 | 0.75 (0.2-2.2) | 55 | 38 | 28 | 0.71 (0.3-1.9) | ||||||
| Extent of skin tumors | .024 | .024 | .009 | .002 | ||||||||||||
| Solitary | 19 | 30 (5-164) | 87 | 69 | 69 | 1 | 1 | 78 | 62 | 62 | 1 | 1 | ||||
| Localized | 22 | 29 (1-235) | 84 | 49 | 49 | 1.6 (0.5-5.0) | 1.2 (0.4-4.0) | 69 | 35 | 35 | 1.7 (0.7-4.4) | 0.9 (0.3-2.6) | ||||
| Generalized | 34 | 20.5 (2-100) | 52 | 30 | 3.4 (1.3-9.5) | 3.4 (1.2-9.7) | 44 | 25 | 3.4 (1.5-7.8) | 3.3 (1.3-8.0) | ||||||
MF indicates mycosis fungoides; LCT, large-cell transformation; DSS, disease specific survival; OS, overall survival; and HR, Hazard ratio
DSS curves of independent parameters in patients with transformed MF. (A) Curves for total group of patients with transformed MF (n = 100), indicating CD30 expression, folliculotropic MF (FMF), extent of skin lesions, and clinical stage, respectively. (B) Curves for subanalysis of patients with only skin lesions and LCT (n = 75), indicating CD30 expression, FMF and extent of skin lesions. * indicates number of persons at risk at the specified times after diagnosis of transformed MF.
DSS curves of independent parameters in patients with transformed MF. (A) Curves for total group of patients with transformed MF (n = 100), indicating CD30 expression, folliculotropic MF (FMF), extent of skin lesions, and clinical stage, respectively. (B) Curves for subanalysis of patients with only skin lesions and LCT (n = 75), indicating CD30 expression, FMF and extent of skin lesions. * indicates number of persons at risk at the specified times after diagnosis of transformed MF.
Prognostic index
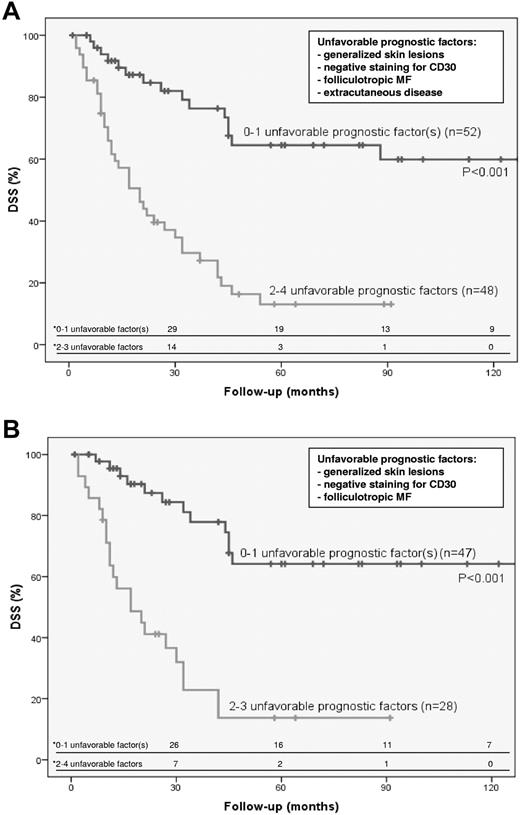
Because patients may have a combination of favorable and unfavorable prognostic factors, we developed a prognostic index that may better predict prognosis and be an useful tool in selecting appropriate treatment. For that purpose, the most discriminating independent prognostic factors for DSS were selected. For the total group of patients with transformed MF, these were the presence of generalized skin lesions, extracutaneous transformation, CD30 negativity, and folliculotropic MF. Patients with 0 (16 cases), 1 (36 cases), 2 (31 cases), 3 (14 cases), or 4 (3 cases) unfavorable prognostic factors had a 2-year DSS of 83%, 85%, 52%, 14%, and 33%, respectively. Subanalysis showed that the difference in DSS between the 52 patients with 0 to 1 unfavorable prognostic factor and the 48 patients with 2 to 4 unfavorable prognostic factors was statistically significant (P < .001; Figure 2A). For the group of patients with only transformed skin lesions, CD30 negativity, folliculotropic MF, and the presence of generalized skin lesions were selected. Patients with 0 (14 cases), 1 (33 cases), 2 (21 cases), or 3 (7 cases) unfavorable prognostic factors had a 5-year DSS of 73%, 61%, 19%, and 0%, respectively. The difference in DSS between the 47 patients with 0 to 1 unfavorable prognostic factor and the 28 patients with 2 to 3 unfavorable prognostic factors was statistically significant (P < .001; Figure 2B).
Prognostic index indicating differences in DSS. Prognostic index indicating differences in DSS in total group of patients with transformed MF (n=100; A) and in patients with only skin lesions and LCT (n = 75; B). * indicates number of persons at risk at the specified times after diagnosis of transformed MF.
Prognostic index indicating differences in DSS. Prognostic index indicating differences in DSS in total group of patients with transformed MF (n=100; A) and in patients with only skin lesions and LCT (n = 75; B). * indicates number of persons at risk at the specified times after diagnosis of transformed MF.
Comparison between patients with tumor-stage MF (stage IIB) with or without transformation at first presentation
Although transformed MF is generally associated with a poor prognosis, in a previous study no difference in survival was found between patients with stage IIB with or without LCT at the time of first presentation.11 In our study, 65 patients had stage IIB at time of transformation. Within this group, 25 patients had LCT at first presentation. These 25 patients were compared with 27 patients with stage IIB MF without LCT at first presentation. Differences in DSS and OS between both groups were not statistically significant (Table 5). Further analysis between those patients who presented with stage IIB without LCT but who developed LCT during follow-up (n = 15) and those patients who did never develop LCT (n = 12) also showed no statistically significant differences in survival (data not shown).
Summary of clinical characteristics and survival data of patients with tumor-stage MF (stage IIB) with or without lLCT at presentation
| . | MF stage IIB with LCT at presentation (n = 25) . | MF stage IIB without LCT at presentation (n = 27) . |
|---|---|---|
| Male/female | 16/9 | 23/4 |
| Median age, y (range) | 72 (42-90) | 63 (40-82) |
| CD30 expression | ||
| Positive (> 50% of blast cells, %) | 13/25 (52) | |
| Negative (< 50% of blast cells, %) | 12/25 (48) | —* |
| Folliculotropic MF (%) | 10/25 (40) | 13/27 (59) |
| Extent of skin lesions | ||
| Solitary (%) | 8/25 (32) | 8/27 (30) |
| Regional/localized (%) | 9/25 (36) | 8/27 (30) |
| Generalized (%) | 8/25 (32) | 11/27 (40) |
| Median survival, mo (range) | 42 (5-153) | 53 (12-262) |
| 2-y DSS/OS, % | 95/75 | 88/84 |
| 5-y DSS/OS, % | 56/40 | 54/48 |
| 10-y DSS/OS, % | 56/30 | 29/16 |
| P value DSS/OS | .644/.721 | |
| . | MF stage IIB with LCT at presentation (n = 25) . | MF stage IIB without LCT at presentation (n = 27) . |
|---|---|---|
| Male/female | 16/9 | 23/4 |
| Median age, y (range) | 72 (42-90) | 63 (40-82) |
| CD30 expression | ||
| Positive (> 50% of blast cells, %) | 13/25 (52) | |
| Negative (< 50% of blast cells, %) | 12/25 (48) | —* |
| Folliculotropic MF (%) | 10/25 (40) | 13/27 (59) |
| Extent of skin lesions | ||
| Solitary (%) | 8/25 (32) | 8/27 (30) |
| Regional/localized (%) | 9/25 (36) | 8/27 (30) |
| Generalized (%) | 8/25 (32) | 11/27 (40) |
| Median survival, mo (range) | 42 (5-153) | 53 (12-262) |
| 2-y DSS/OS, % | 95/75 | 88/84 |
| 5-y DSS/OS, % | 56/40 | 54/48 |
| 10-y DSS/OS, % | 56/30 | 29/16 |
| P value DSS/OS | .644/.721 | |
In most cases scattered CD30+ blast cells (< 1%-5% of total infiltrate).
Discussion
Previous studies demonstrated that LCT in MF is associated with an aggressive clinical course and a poor survival.3-10 Notwithstanding, in the past 20 years we have regularly seen MF patients with transformation in skin and even lymph node biopsies who after treatment followed an indolent course for many years. This is reflected in the present study, in which 23 of 100 patients, including 19 with LCT in the skin and 4 with LCT in lymph nodes, had an indolent course for 5 to almost 20 years (median, 99 months; range, 60-235 months) after LCT. Only 4 of these 23 long survivors died of lymphoma, 88 to 215 months after transformation. These observations underscore the need to define parameters that may help to predict which patients will have an aggressive and which a more indolent clinical course. Data on prognostic factors from previous studies are however conflicting, perhaps largely because of the small and heterogeneous groups studied. For that purpose, we analyzed the clinical, histologic, and immunophenotypical features of the largest cohort of transformed MF patients studied thus far.
The median survival of the 100 patients was 24 months (range, 1-235 months) and the 5-year DSS and OS were 38% and 33%, respectively. These results are similar to those of previous studies (median survival, 12-36 months; 5-year OS, 11%-32%) but contrast with those of Agar et al, who reported a median survival of 100 months and a 5-year DSS and OS of 65% and 63%, respectively, in a group of 70 patients with LCT at first diagnosis.1 In this latter study,1 survival data and prognostic factors, including LCT, were analyzed on a group of 1502 patients with MF or SS. Because further details on the group of 70 patients with LCT are not provided, the difference in survival between this recent study and all other studies in transformed MF cannot be explained.
The most important prognostic factors in patients with transformed MF from the present and previous studies include advanced stage at transformation, CD30 expression, folliculotropic MF, and increased extent of skin lesions.
Advanced stage at transformation
Several studies reported that patients with extracutaneous transformation have a shorter median survival than patients with transformation limited to the skin.3,4 In contrast, in the study by Diamandidou et al, no difference in survival was found between patients with cutaneous and extracutaneous transformation.7 In fact, patients with transformed stage IIB (tumor stage) did as poorly as or even worse than patients with extracutaneous transformation (stage IV). Patients with transformed stage I-IIA (plaques) did significantly better than the combined transformed IIB-IV groups.7 In the present study, more advanced stage was significantly associated with reduced survival (Table 3). Subanalysis showed that extracutaneous transformation (stage IV) had a significantly poorer survival than cutaneous disease transformation (combined IB-IIB vs IV; P = .018). No significant difference in survival was found between stage IB and stage IIB, indicating that extracutaneous transformation is the main factor responsible for shorter survival.
CD30 expression
Several studies have suggested that CD30 expression in transformed MF may be associated with a better prognosis.5,6,9 However, in none of the published studies was a significant difference between CD30+ and CD30− cases found, probably because of the small size of the study groups. In the present study, CD30 expression proved a strong and independent predictor of improved survival, both in the total group of patients and in patients with transformation limited to the skin. The favorable prognosis of the patients with CD30+-transformed MF raises the question whether part of them might not have a primary cutaneous CD30+ lymphoproliferative disorder (LyP of C-ALCL) coexisting with MF. Indeed differentiation between transformed MF and these primary cutaneous CD30+ lymphoproliferative disorder may be difficult, in particular in case the infiltrate contains > 75% CD30+ blast cells, as observed in 39 of 100 cases (Table 2). However, none of our patients had a chronic recurrent self-healing papulonodular eruption consistent with LyP. In all CD30+ cases, a diagnosis of C-ALCL was unlikely, because (1) tumors developed in areas with preexistent patches or plaques; (2) tumors showed an admixture with small- to medium-sized atypical T cells with cerebriform nuclei (characteristic of MF); or (3) tumors showed significant folliculotropism, a condition that is rarely seen in C-ALCL. Although it cannot be excluded, we do not believe our study contained patients with combined MF and C-ALCL.
Folliculotropic MF
The significance of folliculotropic MF in the prognosis of transformed MF has not been examined in previous studies. Although included as a distinct variant of MF in recent classifications for cutaneous lymphomas, cases of folliculotropic MF were included in the present study to allow comparison with previously published series. Both in the total group and in patients with transformation in only skin lesions, folliculotropic MF was a strong and independent predictor of reduced survival. Similarly, in other studies on large cohorts of MF patients, folliculotropic MF was associated with a poorer survival.1,2
Extent of skin tumors
In the group with transformation only in skin lesions, we investigated the prognostic significance of the extent of transformed skin lesions. It must be emphasized that transformation was generally demonstrated in only 1 lesion, assuming that other, clinically similar lesions showed the same histology. The presence of generalized skin tumors was strongly associated with reduced DSS and OS, compared with the combined group of solitary and localized skin tumors. However, no significant differences were found between solitary and localized skin tumors or between localized and generalized skin tumors.
Percentage of blast cells
Progression from plaque- to tumor-stage MF is accompanied by a gradual increase of blast cells and a decrease of atypical T cells with cerebriform nuclei and of inflammatory cells, and associated with a reduced prognosis. One might therefore expect that high percentages of blast cells (> 75%), reflecting further tumor progression, are associated with a worse prognosis. However, no association between the percentage of blast cells and survival was found, consistent with the results of a previous study.9 In addition, when stratified for CD30 expression, an association was not found (data not shown).
In conclusion, previous studies of transformed MF emphasized above all the aggressive clinical behavior and poor prognosis of these patients. As a result, recent National Comprehensive Cancer Network guidelines suggest that a more aggressive therapeutic approach should be considered in patients with transformed MF compared with patients with the same stage of MF without LCT.14 However, some caution is warranted. First, consistent with previous studies we did not find a difference in survival between patients with stage IIB with or without LCT at first diagnosis.11,13 There were no major differences in age, percentage of folliculotropic MF, and extent of skin lesions between both groups (Table 5). In fact, prognostic unfavorable factors (eg, folliculotropic MF, generalized skin lesions) were even slightly more frequent in the MF stage IIB group without LCT, strengthening our conclusions that MF stage IIB with LCT at presentation do not necessarily worse than MF stage IIB without LCT at presentation. This observation is important for clinical management and suggests that patients with stage IIB with LCT should not be treated more aggressively than patients presenting with stage IIB without LCT, as suggested by recent National Comprehensive Cancer Network guidelines.14 Second, although the median and 5-year survival in the present study were similar to that of previous studies, the results of this study clearly demonstrate that not all patients with transformed MF run an aggressive clinical course. The prognostic index described herein may be a valuable tool to predict which patients will have an aggressive and which a more indolent clinical course, and they may aid to select the most appropriate treatment in patients with transformed MF. However, the applicability and usefulness of this new tool needs to be validated by future studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Ron Wolterbeek for providing statistical advice.
Authorship
Contribution: R.W., P.M.J., and M.H.V. designed research; R.W., P.M.J., and M.F.B. collected data; R.W., P.M.J., M.F.B., and M.H.V. analyzed and interpreted data; M.F.B. performed statistical analysis; and R.W. and M.F.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marchina F. Benner, Department of Dermatology, B1-Q, Leiden University Medical Center, P O Box 9600, 2300 RC Leiden, The Netherlands; e-mail: m.f.benner@lumc.nl.