Abstract
Although platelets are the smallest cells in the blood, they are implied in various processes ranging from immunology and oncology to thrombosis and hemostasis. Many large-scale screening programs, genome-wide association, and “omics” studies have generated lists of genes and loci that are probably involved in the formation or physiology of platelets under normal and pathologic conditions. This creates an increasing demand for new and improved model systems that allow functional assessment of the corresponding gene products in vivo. Such animal models not only render invaluable insight in the platelet biology, but in addition, provide improved test systems for the validation of newly developed anti-thrombotics. This review summarizes the most important models to generate transgenic platelets and to study their influence on platelet physiology in vivo. Here we focus on the zebrafish morpholino oligonucleotide technology, the (platelet-specific) knockout mouse, and the transplantation of genetically modified human or murine platelet progenitor cells in myelo-conditioned mice. The various strengths and pitfalls of these animal models are illustrated by recent examples from the platelet field. Finally, we highlight the latest developments in genetic engineering techniques and their possible application in platelet research.
Introduction
Blood platelets play part in a myriad of processes, such as inflammation, tumor growth and metastasis, immunology and, of course, thrombosis and blood clotting where they provide a first and crucial line of defense against vascular injury, thus maintaining normal hemostasis.1,2 Primary hemostasis starts when platelets recognize a site of vascular injury where the subendothelial matrix is exposed, bind to collagen, and become activated.3 The subsequent rise in intracellular calcium triggers conformational changes in integrin receptors, degranulation, exposition of a procoagulant surface, and generation and release of secondary agonists resulting in a thrombus that will cover the site of injury and prevent further blood loss.4 Platelets are furthermore an important factor in thrombotic events, such as stroke and myocardial infarction.5 To identify more proteins regulating platelet function that may serve as new targets for the development of anti-thrombotics or in the prevention of bleeding, the platelet research community has seen the completion of several large-scale screening programs and the spectacular rise in the “platelet-omics” field. Several genome-wide association studies and subsequent meta-analysis in patients with coronary artery disease and healthy volunteers identified numerous genetic loci that are possibly involved in regulating platelet formation, count, volume, and function and might confer a risk for coronary artery disease.6-11 On the other hand, gene expression profiling of healthy volunteer platelets, in combination with comparative microarray analysis between in vitro differentiated megakaryocytes (MKs) and closely related cell types, established a comprehensive platelet transcriptome.6,12-19 Finally, advanced proteomics studies identified proteins of the platelet sheddome, secretome, interactome, kinome, and phosphoproteome potentially involved in platelet function.20 The overall result is a large number of newly identified gene products for which we are only beginning to understand their role in platelet formation and physiology.17,21-23 It seems reasonable to assume that our current knowledge about platelets is only the tip of the iceberg and that functional characterization of all these gene products will revolutionize our view on platelet function.
The study of these genes by classical molecular biologic means is hampered by the anucleate nature of the platelet and the physiologic alterations, possibly resulting from direct permeabilization, thus causing an experimental bias.24-26 This creates a growing demand for new techniques and model systems, which allow the study of genes in platelets in vivo. Recent advances in platelet functional genomics techniques are now galvanizing this field. This review discusses the latest animal models for in vivo generation of genetically modified platelets along with an extensive overview of current and future developments.
Fishing for gene function
The zebrafish Danio rerio has only been introduced in 1999 in the platelet functional genomics field, when zebrafish thrombocytes were first described as the primary actors in teleost hemostasis sharing features with mammalian platelets and MKs in structure, formation, and function.27 On the one hand, zebrafish thrombocytes resemble mammalian MKs because they both are nucleated. Furthermore, transcription factors, such as GATA-1, Ets, and Fli1, are active in teleost thrombocytes during specific timeframes, hereby mimicking mammalian megakaryopoiesis. Remarkably, thrombocytes can also increase in size, another hallmark of mammalian MK maturation.28 On the other hand, thrombocytes share structural features with platelets, such as the open canalicular system and the presence of α and dense granules. Like mammalian platelets, D rerio thrombocytes also form pseudopodia-like extensions upon activation.27,29 Finally, along with the presence of most of the coagulation factors in D rerio,30,31 thrombocytes contain homologues of important mammalian platelet proteins, such as VWF, glycoprotein (GP) Ibα, GPIbβ, integrin αIIb, protease-associated receptors, cyclooxygenase, and putative ADP receptors.27,31-35 Taken together, these findings support the concept of the zebrafish thrombocyte as a suitable model for mammalian megakaryopoiesis and hemostasis.
Zebrafish have also gained interest as a model for mammalian hematopoiesis and hemostasis from a practical viewpoint: zebrafish are not only easy to house and raise in a laboratory environment, their high fecundicity along with the external fertilization and development of transparent embryos allows easy visual inspection of the major developmental processes, including organogenesis, hematopoiesis, and analysis of early disease-related phenotypes without the need for invasive imaging techniques. Furthermore, zebrafish survive up to 7 days without blood circulation via passive diffusion of oxygen, permitting the study of genes whose absence might result in severe vascular defects and early embryonic lethality in mammals.36 The latest assembly of the zebrafish genome combined with fast methods of genetic modification will furthermore push the identification of zebrafish orthologs of human (platelet) genes forward (Genome Reference Consortium; www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/).37
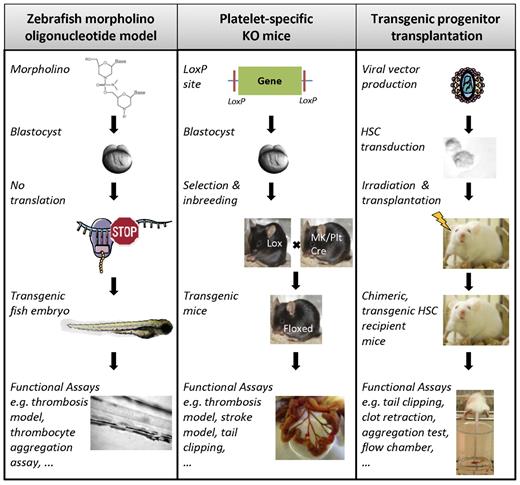
Studying thrombocyte function is now facilitated by the existence of certain transgenic zebrafish lines having fluorescently labeled thrombocytes or vasculature, allowing easy visualization.28,35 The observation and transplantation of 2 distinct populations of CD41-GFP–positive cells has recently led to identification of zebrafish hematopoietic stem cells (HSCs), thus permitting to screen for genes or proteins involved in thrombocyte formation, such as the zebrafish orthologue of the thrombopoietin receptor c-Mpl, MASTL kinase, and cleavage and polyadenylation specificity factor 1.35,38-40 Genetically modified zebrafish can be generated either randomly by exposure to ethylnitrosourea or by using morpholino technology (Figure 1).41-43 Exposure to ethylnitrosourea is a forward genetics approach that induces random mutations in the DNA of premeiotic germ cells at a rate of approximately 100 to 200 per fish, which after mating with unexposed zebrafish results in mutant heterozygotes and further inbreeding results in the isolation of the carriers of the mutations and eventually the mutations themselves (Table 1). Although this approach has led to several relevant disease models, such as the fade out mutant resembling the Hermansky-Pudlak syndrome phenotype, it is time-consuming because of the random nature of the mutations, which each have to be characterized and identified.44 Morpholino oligonucleotides (MOs), on the other hand, are short anti–sense RNA oligomers designed to bind to a specific mRNA at the translation initiation site or at an intron-exon boundary resulting in either translational inhibition or incorrect splicing yielding a nonfunctional protein (Figure 1). Classic MO technology involves microinjection of the oligomer in zebrafish embryos at the one-cell stage, leading to spreading throughout the body of the developing embryo and a whole-organism knockdown (Table 1). Although the life span of a typical MO is only 4 to 5 days, it allows direct assessment of platelet adhesion and visualization of the thrombus size in, for example, FeCl3- or laser-induced thrombosis models in the arterial and venous circulation (Figure 1).29,36 As such, well-known human disorders, such as von Willebrand disease and c-Mpl deficiency, could be successfully reproduced in D rerio.32,35
Overview of in vivo systems for generating transgenic platelets. Experimental design to generate transgenic zebrafish embryos using MO technology (left), platelet-specific KO mice with the Cre-LoxP method (middle), or genetically modified human or murine platelets by transduction of hematopoietic progenitor cells followed by transplantation in myelo-conditioned recipient mice (right). (Figure was produced using Servier Medical Art; http://www.servier.com/servier-medical-art.) MK/Plt indicates megakaryocyte or platelet-specific promoter.
Overview of in vivo systems for generating transgenic platelets. Experimental design to generate transgenic zebrafish embryos using MO technology (left), platelet-specific KO mice with the Cre-LoxP method (middle), or genetically modified human or murine platelets by transduction of hematopoietic progenitor cells followed by transplantation in myelo-conditioned recipient mice (right). (Figure was produced using Servier Medical Art; http://www.servier.com/servier-medical-art.) MK/Plt indicates megakaryocyte or platelet-specific promoter.
The real benefit of the zebrafish model lies mainly in its use as a rapid screening method for platelet genes with a hitherto unknown function. This is illustrated by a recent paper in which genetic screenings and association studies in healthy volunteers as well as patients with myocardial infarction suggested a putative role for LRRFIP1, which was next shown to be a positive regulator of thrombus formation in a zebrafish MO model. Further proteomic analysis confirmed a possible role for LRRFIP1 as a component of the platelet cytoskeleton where it can interact with 2 actin-remodeling proteins Flightless-1 and Drebrin.6 In addition, zebrafish are also used to identify genes responsible for certain pathologic phenotypes. Recently, an exome sequencing study published back to back with proteomic analysis and genomic DNA sequencing in a patient suggested that NBEAL2 is the causative gene for Gray platelet syndrome, which was then confirmed in zebrafish.23,45,46 In another study, genetic linkage analysis of 2 case reports suggested mutations in a 5 million-bp locus on chromosome 10p11-12, where a.o., the microtubule-associated Ser/Thr-like (MASTL) kinase gene, is located, as responsible for an autosomal dominant inherited thrombocytopenia.47,48 Transient MO knockdown of MASTL kinase in zebrafish indeed results in thrombocytopenia and correlates with decreased expression of c-mpl and itga2B, thus identifying the cause of the disease.39 Perhaps the power of D rerio in discovering and characterizing the function of a gene can be best illustrated by a study in which comparative whole-genome expression analysis of the major blood cells and MKs and erythroblasts resulted in identification of 279 MK-specific transcripts, which contained approximately 35 putative transmembrane proteins, 4 of which were knocked down in zebrafish resulting in modified kinetics of laser-induced thrombus formation that revealed a role of BAMBI and LRRC32 as positive regulators and DCBLD2 and ESAM as inhibitors of thrombus formation.16,17 The latter was confirmed in ESAM knockout (KO) mice, which also develop larger thrombi compared with controls, thus again providing strong evidence for the relevance of the zebrafish MO model as a reverse genetics screen for novel proteins involved in thrombus formation.49
Although all the aforementioned studies used whole embryo micro-injections to deliver MOs, a recent development in the chemical structure has resulted in cell permeability of MOs.42 These so-called Vivo-MOs have already successfully been used to inhibit the function of VWF and αIIb in adult zebrafish thrombocytes thereby further expanding the potential of MO technology.32,50
One of the major pitfalls of using MO technology is that it inevitably results in a transient knockdown and more importantly in a knockdown in all cells, thus resulting in a global instead of a thrombocyte-specific phenotype. Addition of a thrombocyte-specific promoter to the recently developed Cre-LoxP system in zebrafish can help to solve this problem. Finally, there is inevitably a relatively large evolutionary distance between mammals and fish, which results in severaldifferences between mammalian platelets and MKs on the one hand and zebrafish thrombocytes on the other, as illustrated by the similar yet different coagulation pathways in zebrafish and other vertebrates resulting from a genome duplication after the 2 evolutionary lineages had diverged.31 Nevertheless, these recent data clearly illustrate that D rerio can be used successfully as a high-throughput early screening method to establish the relevance of newly identified platelet proteins in thrombosis and hemostasis.
Mice that will knock you out
A classic approach to generate large amounts of genetically modified platelets is breeding mutant mice. These mice can be either knock-in or KO animals. The use of knock-in mice, expressing a transgene or mutant form of a target protein, has led to a better understanding of, for example, regulators of G-protein–mediated signaling in modulating platelet responsiveness and of protein kinase D2 in dense granule secretion.51,52 The generation of KO mice lacking one or more proteins is nevertheless a more common approach. However, it takes several generations of mouse crossing and genotyping before a stable genotype and phenotype is achieved (Table 1). The time-consuming and laborious nature is therefore the major limiting factor in the application of KO mouse models in basic and translational research. The benefit of mutant mice mainly lies in the fact that they serve as an unlimited source of uniformly modified platelets or platelet progenitors, which can either be used for in vitro studies (eg, in aggregation tests or flow chamber models) or for in vivo studies (eg, in thrombosis models).53 The technique has been widely applied to investigate the role of certain proteins in platelet functioning (eg, arrestin-2, kindlin-3, CalDAG-GEFI, and Orai1) and also to successfully reproduce several monogeneic platelet disorders, such as Glanzmann thrombasthenia (GT) and the Bernard-Soulier syndrome (BSS).54-58 The latter is one of the most extensively studied platelet disorders in KO mice.59-63 Data in KO mice not only confirmed that absence or dysfunction of the GPIbα or GPIbβ subunit, but not GPV, is sufficient to cause prolonged bleeding time and macrothrombocytopenia, as observed in human BSS patients, but in addition provides evidence for a role of the GPIb/V/IX complex during megakaryopoiesis upon detection of an abnormal demarcation membrane system and enlarged peripheral zone.59,60,62-64 Furthermore, the GPIbα−/− bleeding phenotype could be rescued by expression of wild-type human GPIbα in GPIbα−/− mice, thus establishing BSS as a potential candidate disease for gene therapy.63 A recent paper extends these findings by showing that lentiviral-mediated platelet-specific GPIbα expression in GPIbα−/− HSCs followed by transplantation in GPIbα−/− mice results in a correction of the tail bleeding time and significant amelioration of the macrothrombocytopenia.65
A major limiting factor of the classic approach of whole-organism KO is the difficulty to ascribe the observed phenotype to the intended cell type and the possible generation of a lethal phenotype as seen with, for example, talin, filamin A, or the novel platelet receptor CLEC-2, which nevertheless resulted in the assignment of a role for platelets in embryonic development.66-68
These problems can nowadays be circumvented quite elegantly using inducible or cell type specific promoters in combination with the Cre-loxP KO technique, allowing spatiotemporal KO or targeted expression in a particular cell type, thereby avoiding developmental effects or effects of gene knockdown in nontarget cell types, respectively. A frequently used inducible system consists of MX1-Cre-LoxP mice in which the target allele can be effectively eliminated by injection of polyinosinic-polycytidylic acid.69 Such an approach has led to the appreciation of the role of AML-1 and SCL in megakaryopoiesis, which had previously gone unnoticed because of embryonic lethality.70,71 More recently, the use of cell type-specific or tissue-specific promoters to control Cre cDNA has gained a lot of interest (Table 1; Figure 1).72,73 The most frequently used promoters are the Vav promoter, which is specific for hematopoiesis, the GATA-1 promoter specific for the erythrocytic/megakaryocytic lineage, and finally the platelet factor 4 (PF4) and αIIb promoters, which are both specific for the megakaryocytic lineage, although the latter also shows basal expression in HSCs and is active throughout the entire differentiation process contrary to PF4, which reaches maximal activity only in the later stages.72-75 To date, there is a growing interest in the PF4-Cre mice, through which transgenic mice with MK and platelets devoid of proteins, such as talin, vinculin, focal adhesion kinase, survivin, and STIM1, have been bred.18,76-80 One of the most elegant demonstrations of the potential of the Cre-LoxP method was by Tiedt et al who applied a constitutive, a hematopoiesis-specific, and an inducible promoter to generate transgenic mice with different levels of mutant JAK2-V617F relative to wild-type JAK2, thus for the first time providing evidence that the ratio of mutant to wild-type JAK2 is a determining factor in the development of either essential thrombocythemia or polycythemia vera.75
Despite its broad applicability, the Cre-LoxP technique is not without pitfalls. A general concern is the existence of so-called endogenous pseudo-LoxP sites, which can be targeted by Cre as well.81 In addition, the choice of the promoter controlling the Cre gene is of critical importance in achieving the desired KO. First, as stated above, some promoters are only active during the later stages of megakaryopoiesis, thereby possibly missing out on an early effect of the target gene.82,83 Second, promoter specificity is not always fully restricted to the intended cell type as demonstrated by the leakiness of the erythrocyte/MK-specific GATA-1 promoter resulting in ectopic expression.84 Third, promoters driving expression of Cre recombinase should be strong enough to obtain complete DNA recombination to generate a complete KO organism and avoid mosaicism. Fourth, transgene expression from a 5′-promoter element can differ from the endogenous expression pattern depending on, for example, the length of the cloned fragment, as illustrated by the differential activity of αIIb promoter 5′ deletion mutants.85
Finally, a general concern lies in the species differences between humans and mice as not all counterparts of human platelet proteins are represented in mice as is the case for the protease-associated receptors.18,19,86 In addition, a significant difference in the induction of disease models has been reported; for example, attempts to establish a mouse thrombotic thrombocytopenic purpura model showed that ADAMTS13 KO mice require an additional trigger with shigatoxin to produce a thrombocytopenic purpura phenotype.87 However, when taking proper care of these possible pitfalls, the Cre-LoxP method is a great tool to generate MK or platelet-specific KO mice as proven by the number of reports published over the past few years.
Transplantation models
Several groups have focused on (xeno)transplantation of ex vivo genetically modified hematopoietic progenitor cells in murine recipients to rapidly generate a transgenic HSC transplantation model or in a gene therapy setting. In this model, mouse or human HSCs are modified by, for example, the introduction of foreign DNA on transduction with a lentiviral vector (LV), allowing stable integration of the transgene in the host genome (Figure 1).88,89 After transplantation into myelo-conditioned recipient mice, they will repopulate the bone marrow and give rise to all differentiated blood lineages (Table 1; Figure 1). One of the major drawbacks concerning this technique is the relatively low permissiveness of progenitor cells to LV, which results in transduction efficiencies ranging from 19% of the murine MK progeny successfully expressing a therapeutic transgene to 71% of mouse platelets expressing eGFP and from 16% of human CD34+ HSCs containing a GFP sequence to 62% of human platelets expressing eGFP.88,90-98 Transduction efficiencies can be improved, for example, using higher multiplicities of infection, however, with concomitant increased risk of insertional mutagenesis and lentiviral-mediated cytotoxicity. Therefore, other ways of improving transduction efficiency are currently being explored (see “Perspectives”).99,100 As shown with a GFP reporter gene, the activity of a given promoter can vary greatly depending on the cell type and even the source of human progenitor cells (bone marrow, cord, or peripheral blood); therefore, the choice of promoter driving transgene expression is of particular importance.88,93,94,101 Like Cre-LoxP KO mice, transplantation approaches use either ubiquitous or lineage-restricted promoters. Ubiquitous promoters, such as cellular polypeptide chain elongation factor 1α, human cytomegalovirus, phosphoglycerate kinase, ubiquitin C, and simian virus 40, have the obvious benefit of driving transgene expression in all blood lineages, albeit not necessarily at comparable levels among the different cell types.101 However, ubiquitous expression is not always desired as it may cause adverse effects or difficulties in assigning an observed effect to a certain cell type.102 Therefore, MK or platelet-specific promoters to down-regulate or drive expression of a target gene have been added to the transduction and transplantation toolbox. One of the most successfully used promoters is the MK specific αIIb promoter, which displays basal expression in HSCs and gets up-regulated during megakaryopoiesis reaching up to 100% expression in platelets, hereby allowing the effect of the genetic modification to be studied during all stages of platelet development.103,104 Alternatively, 2 late MK specific promoters (ie, the PF4 promoter, used to express FVIII variants in platelets in a therapeutic approach, and the GPIbα promoter) have been reported to restrict transgene expression to platelets.92-94,105,106 A comparative study by Lavenu-Bombled et al nevertheless demonstrated significantly lower expression levels throughout MK differentiation for a transgene under control of the GPIbα promoter compared with the αIIb promoter.93 Finally, the murine and human-Mpl promoter permits MK-restricted expression in differentiating murine BM cells. Surprisingly, the human c-Mpl promoter allowed higher expression levels compared with its murine counterpart but was still outweighed by the GPIbα promoter.92 Interestingly, in contrast to all previously described promoters, transgene expression under control of a c-Mpl promoter gradually declined during MK differentiation. Therefore, not only cell types but also the time frame of transgene expression is an important factor to take into consideration, especially when studying megakaryopoiesis and thrombopoiesis.
The main benefit of the transplantation approach lies in the fact that it is a less laborious and time-consuming process compared with the generation of mutant mice (Table 1). Therefore, this technique not only allows in-depth functional studies but also permits rapid screening of numerous genes using for instance libraries of short hairpin RNA (shRNA) in RNA interference (RNAi).107 It can furthermore provide information regarding the optimal conditions and transgenic protein levels required for correction or establishment of a certain phenotype, thereby paving the way for (human) gene therapy. One of the earliest disease candidates for gene therapy has been hemophilia A, which is caused by a deficiency in factor VIII (FVIII). A lot of work in this field has been performed by the groups of Robert Montgomery and David Wilcox in Milwaukee, who, using the αIIb promoter, were able to specifically target production and storage of FVIII to platelet α granules along with its carrier protein VWF, thereby promoting local release at the site of injury and establishing the platelet as a possible carrier vehicle for the delivery of therapeutical proteins.108 Moreover, in FVIII−/− mice, correction of the bleeding phenotype was shown even in the presence of inhibitory antibodies and in FVIII−/− mice, which had been previously immunized against FVIII.109,110 Similar studies have been performed on BM cells from integrin β3−/− mice presenting with a GT bleeding phenotype, where transgenic expressed human β3 efficiently paired with murine αIIb and restored platelet function in transplanted β3−/− mice.90 Using the same β3 construct, the platelet functionality of 2 GT patients could be restored after in vitro transduction of autologous CD34+ cells.111 Recently, hemostatic functions in αIIbβ3-deficient dogs presenting with a nearly identical phenotype to human GT patients could be restored, resulting in markedly decreased bleeding times and reduced blood loss for up to 5 years after transduction and transplantation of canine CD34+ HSCs with a LV containing the full-length αIIb subunit under control of the αIIb promoter.112 The successful long-term correction of the bleeding phenotype in such a large mammalian model paves the way toward gene therapy and will undoubtedly provide a wealth of information for future studies. This breakthrough illustrates the potential of transplantation-based studies in the gene therapy context. Other examples of this approach are reported in a variety of settings ranging from correction of aplastic anemia caused by deficiency in the TPO receptor c-Mpl to functional studies of the receptor VPAC1 and from rescue of the aforementioned BSS mouse to the establishment of platelet-mediated FIX gene therapy as a possible treatment for hemophilia B, thus spanning the research field from thrombopoiesis to primary hemostasis and coagulation.63,92,113,114
Perspectives: platelets on demand, the quest for the Holy Grail?
Although the previous sections illustrated the potential of several model systems, they all remain limited by either interspecies differences, transient effects (eg, limited MO life span), safety issues (eg, the use of viral vectors), and the incompleteness of nucleic acid delivery or knockdown (all RNAi-based approaches). Therefore, the development of novel techniques and approaches is still ongoing and should ultimately result in model systems that allow safe, complete, and stable manipulation of organisms, platelets, and platelet progenitor cells in a short time, thus providing the researcher with a (transgenic) model that mimics human platelet behavior under normal and pathologic conditions as close as possible. Significant progress is currently being made in terms of (1) improved efficiency in the generation of transgenic animals and reducing off-target effects, (2) an improved safety profile of gene delivery techniques, and (3) more precisely targeted gene delivery.
A new powerful tool in genetic engineering comes from the field of nuclease technology in which 2 related yet distinct techniques are drawing a lot of attention these days. The first strategy involves zinc finger nuclease (ZFN) technology to achieve highly efficient and specific genetic engineering. These endonucleases contain a zinc finger domain, designed to recognize a specific DNA triplet sequence, and the nuclease domain of nonspecific FokI nuclease. Specificity comes from a combination of several zinc fingers to recognize a specific DNA sequence and has improved recently by the strict requirement of heterodimerization of the FokI domains, thus relying on 2 independent DNA-binding events. The ZFN is delivered to a target cell by standard lipofection methods, lentiviral transduction, or micro-injection of either DNA or mRNA depending on the target cell type. Its action results in a double-stranded break, which can then be repaired via nonhomologous end joining in the absence of donor DNA or via homology directed repair in the presence of donor DNA. The former process efficiently ligates double-stranded breaks in all eukaryotes but is prone to errors, thus leading to genetic disruption. Addition of a custom-designed homologous donor DNA can serve as a template to repair the double-stranded break and be exploited to mutate either specific nucleotides (gene correction) or even introduce a whole gene at a specific site (gene addition). This technique is currently successfully used to disrupt or replace genes in human and mouse embryonic stem cells, zebrafish, and rats to generate transgenic cells or whole organisms.115 A related technique uses transcription activator-like effector nucleases (TALEN) to silence a gene of interest. Again, the nuclease activity is provided by the FokI domain, but in this case specificity comes from transcription factor-like effectors, a family of virulence factors originally found in a genus of plant pathogens. Although the possible applications of TALENs are much the same as for ZFN, the TAL effectors recognize single nucleotides and not triplets, which significantly simplifies their design and broadens the target range to virtually any sequence. TALENS are the latest addition to the genetic engineering toolbox and have already been used successfully in gene disruption in human cells, somatic zebrafish cells, and in the generation of knockout rats.116-119 Nuclease technology in general can provide a complete transformation of the target cell, but in practice the applicability can be restricted by the local DNA structure with the presence of chromatin and local genetic complexity. Because of the novelty of ZFN and TALEN, development of algorithms for the design of DNA binding motifs and improvements in terms of specificity is still ongoing.
A more established technique to knock a target gene down is RNAi, which is achieved by introduction of either small interfering RNA (siRNA), short hairpin RNA (shRNA), or artificial micro-RNA in a target cell.94,120-126 The low transfection efficiency in combination with the altered platelet physiology on permeabilization hinders direct application of basic siRNA on platelets. The limited life span of siRNAs furthermore hampers the generation of transgenic platelets by genetic modification of progenitor cells.24-26,122 Alternatively, lentiviral delivery of shRNA has been used to stably modify HSCs and generate transgenic platelets.94 However, shRNA transcription from polymerase III promoters precludes platelet specific expression, thus possibly inducing off-target effects in other HSC-derived cell types. Moreover, several reports showed cytotoxic effects induced by shRNA.127-130 Therefore, artificial microRNA seem to be the preferred tool to knock down a target gene because they permit inducible or tissue specific expression from polymerase II promoters and have not been associated with any cytotoxic effects to date.129,131,132 Such a platelet specific approach, however, has currently not been reported on.
As mentioned previously, efficient delivery of DNA or RNA to a target cell is often a limiting factor in genetic engineering, functional genomics, and gene therapy studies. Therefore, efforts to improve the design of (lenti)viral vectors are still ongoing. An interesting development is the pseudotyping of LV with more specific envelope proteins or even cell-type specific envelopes by incorporating specific ligands or antibodies in the viral envelope, whereas another approach focuses on using various biomaterials as a means for more efficient gene delivery by, for example, tethering of viral particle and/or target cell.131,133,134 Taken together, this shows that there are several exciting new developments that can provide more efficient delivery or effectiveness of a transgene to and in a target cell.
A completely different delivery approach involves the use of (hyperactive) transposons as a means to stably introduce DNA in target (hematopoietic progenitor) cells.135-137 Although hyperactive sleeping beauty transposons have not yet achieved significantly higher transfection efficiencies than lentiviral vectors, they do have an improved safety profile avoiding viral particle-mediated cellular toxicity or immune responses, a reduced risk of insertional mutagenesis or oncogenesis. Furthermore, the application of different promoters, the discovery of potential enhancer elements, and the exploration of different transfection methods will possibly improve transgene integration and expression levels in the near future.
Finally, to further diminish the differences between small animal models and humans, xenotransplantation models and the use of humanized mice have attracted attention in the past decade (Table 1).138 The nonobese diabetic/severe combined immunodeficiency mice (NOD.CB17-Prkdcscid/J or NOD/SCID) already allowed stable engraftment of human CD34+ HSCs originating from either mobilized peripheral blood or umbilical cord blood.95,139,140 The presence of all blood cell types in the bone marrow was confirmed; and although MK represented only a small fraction, successful platelet production was achieved. In addition, these platelets not only get activated in response to known agonists but also incorporated in a (predominantly) mouse thrombus on perfusion of transplanted mouse peripheral blood over a collagen-coated surface.139 The current generation of immunodeficient mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ – or NSG) with virtual absence of natural killer cell activity already allows higher engraftment; and with numerous other immunodeficient mouse strains still under development, it is likely to see a novel strain with even better production of human platelets emerging in the ensuing years.141 Combining this transplantation model with safer and improved delivery and genetic engineering systems can hopefully provide the research community with a small animal model in which transgenic human MKs and platelets can be successfully generated and used in either functional genomics studies, thus aiding in expanding our basic knowledge of platelets, their formation and behavior or as superior models of human pathologies that allow faster and better screening and testing of novel compounds acting to improve platelet production or survival, or to prevent bleeding or thrombosis.
Acknowledgments
T.T. is a PhD fellow of the Agency for Innovation by Science and Technology in Flanders. This work was further supported by KU Leuven (concerted action grant GOA 2009/829) and the Fund for Scientific Research, Flanders (grant FWO G.0564.08).
Authorship
Contribution: T.T. designed and wrote the manuscript and created Figure 1; K.B. conceived and edited the manuscript and Figure 1; and H.D. critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hans Deckmyn, Laboratory for Thrombosis Research, KU Leuven campus Kortrijk, E Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: hans.deckmyn@kuleuven-kortrijk.be.