Abstract
Dendritic cell (DC) homeostasis, like all mature blood cells, is maintained via hierarchal generation from hematopoietic precursors; however, little is known about the regulatory mechanisms governing DC generation. Here, we show that prostaglandin E2 (PGE2) is required for optimal Flt3 ligand–mediated DC development and regulates expression of the Flt3 receptor on DC-committed progenitor cells. Inhibition of PGE2 biosynthesis reduces Flt3-mediated activation of STAT3 and expression of the antiapoptotic protein survivin, resulting in increased apoptosis of DC-committed progenitor cells. Reduced DC development caused by diminished PGE2 signaling is reversed by overexpression of Flt3 or survivin in DC progenitors and conversely is mimicked by STAT3 inhibition. PGE2 regulation of DC generation is specifically mediated through the EP1 and EP3 G protein PGE2 receptors. These studies define a novel DC progenitor regulatory pathway in which PGE2 signaling through EP1/EP3 receptors regulates Flt3 expression and downstream STAT3 activation and survivin expression, required for optimal DC progenitor survival and DC development in vivo.
Introduction
Dendritic cells (DCs) are specialized Ag-presenting immune cells that induce adaptive immune responses and maintain self-tolerance and are attractive targets for therapeutic manipulation of the immune system.1,2 Two main DC subsets have been identified on the basis of their location, phenotype, and function: Ag-presenting classic DC (cDC) and type I IFN-producing plasmacytoid DC (pDC),3,4 and are generated from Flt3-expressing hematopoietic progenitor cells in the BM.5,6 Recent studies identified a common DC, monocyte and macrophage precursor designated as macrophage DC progenitor cell (MDP)7,8 and a common DC progenitor (CDP) that is restricted to DC development.9 MDPs differentiate to CDPs, which in turn give rise to cDCs and pDCs, but not monocytes, and finally to pre-cDCs,10 a committed precursor of cDCs.11,12 Unlike MDPs and CDPs that differentiate within the BM, pre-cDCs traffic to secondary lymphoid organs where they further differentiate into cDCs.4,13 In addition, monocytes can also develop a DC phenotype under inflammatory conditions.14,15
Flt3 (also known as Flk2 and CD135) is a receptor tyrosine kinase that is broadly expressed on early BM hematopoietic progenitor cells (HPCs), including DC progenitor cells.5,9,16 Mice with a deficiency in Flt3 or Flt3 ligand (Flt3L) have reduced DC numbers,13,17 whereas administration of Flt3L dramatically increases BM and peripheral DCs.18 Although Flt3 signaling is crucial for DC generation from their progenitor cells at steady state, the normal physiologic mechanisms that control Flt3 expression and regulate Flt3L-dependent DC differentiation remain poorly understood.
Prostaglandin E2 (PGE2) is the predominant metabolite of arachidonic acid metabolism produced through the sequential action of phospholipase A2, cyclooxygenases (COXs), and PGE synthase.19,20 There are 2 functionally distinct COX enzyme isoforms, COX1 and COX2, that are encoded by different genes.20 PGE2 has been shown to modulate HPC differentiation, inhibiting CFU-GM21-23 but promoting erythroid burst-forming unit and granulocyte, erythroid, megakaryocyte, and macrophage CFU.24,25 The long-acting PGE2 analog, 16,16-dimethyl-PGE2 (dmPGE2) was shown to increase HSC frequency and engraftment26,27 and to enhance HSC survival, proliferation, and homing to the BM.27 Thus, PGE2 regulates self-renewal and differentiation of HSCs and HPCs, and its effects can differ, depending on hematopoietic cell type and stage of differentiation.
The role of PGE2 in DC maturation and migration under inflammatory conditions is well known. PGE2 promotes the migration of monocyte-derived and Langerhans DCs, increases their expression of costimulatory molecules, and enhances their ability to stimulate T cells after exposure to proinflammatory cytokines and Ags.28-30 However, the role of PGE2 in regulation of DC differentiation from their specific progenitor cells and maintenance of DC homeostasis at steady state has not been explored. We now report that PGE2 is an important regulator of Flt3L-dependent DC development. PGE2 regulates DC development by modulating Flt3 expression on DC-committed progenitor cells, resulting in increased STAT3-mediated survivin expression that enhances survival of DC-committed progenitor cells. The effect of PGE2 on DC progenitor cells is specifically mediated via signaling through the EP1 and EP3 G protein–coupled PGE2 receptors, and DC production was significantly lower in EP1, EP3, and EP1/EP3 knockout mice. Our results are the first to define a role of PGE2 in normal Flt3L-dependent DC differentiation and describe a novel regulatory pathway for DC generation that can be pharmaceutically manipulated.
Methods
Mice and human cord blood
C57BL/6 mice were purchased from Harlan Laboratories or The Jackson Laboratory and maintained in the Indiana University School of Medicine animal facility. EP1, EP3, and EP1/EP3 knockout mice were obtained from Dr Richard M. Breyer (Vanderbilt University), all on the C57BL/6 background. All mouse experiments were approved by the Indiana University Institutional Animal Care and Use Committee. Human umbilical cord blood (UCB) was obtained from Wishard Hospital with approval from the institutional review board.
Inhibition of PGE2 synthesis
Mice were injected subcutaneously with indomethacin (2.5 mg/kg twice daily; Sigma-Aldrich) or indomethacin plus PGE2 (1 mg/kg) for 6 days. BM and spleen were harvested, and single-cell suspensions were prepared in IMDM (Lonza Biologics) supplemented with 2% heat-inactivated FBS (Hyclone). For in vitro experiments, lineage-negative (Linneg) BM cells (CD5, B220, CD11b, Gr-1,7-4, and Ter-119)neg or CDPs (Linneg c-Kitint Flt3+ CD115+) or CD34+ human UCB cells that underwent FACS were treated with 1μM indomethacin, 10nM SC560 (COX1-specific inhibitor), or 1μM NS-398 (COX2-specific inhibitor; Cayman Chemical). Ethanol was used as a solvent for indomethacin, SC560, and NS-398 at a final concentration of 0.01%.
In vitro DC generation
Mouse BM cells were enriched for the Linneg cell population with the MACS lineage depletion kit (Miltenyi Biotec). Linneg marrow cells or CDPs that had undergone FACS were cultured in 10% FBS, and a predetermined optimal concentration of recombinant human Flt3L (100 ng/mL, Amgen)9 supplemented with IMDM for 9 days in the presence of Indomethacin, SC560, NS-398, PGE2 (0.1nM-1000nM), or vehicle control. Medium was replaced every 3 days. For some studies, Linneg BM cells were cultured for 5 days in supplemented complete medium (10% FBS) with 10 ng/mL recombinant mouse GM-CSF to induce DC generation. For DC generation from human UCB, CD34+ cells were purified from low-density UCB with the use of CD34 Ab-conjugated magnetic beads (Miltenyi Biotec) and cultured for 10 days in Flt3L (100 ng/mL), SCF (50 ng/mL), IL-3 (10 ng/mL), and IL-6 (10 ng/mL) supplemented with STEM Pro-CD34 (Invitrogen) medium as described31 in the presence or absence of indomethacin.
Cell sorting and flow cytometric analysis
BM and spleen were harvested, and single-cell suspensions were prepared in IMDM with 2% FBS. Total nucleated cell counts were obtained with a Hemavet-950 (Drew Scientific Inc). Cells were incubated for 15 minutes with 0.5 μg of FcR block per million cells (BD Biosciences). To purify CDPs, BM cells were first immunomagnetically pre-enriched for Linneg cells, then stained with fluorochrome-conjugated anti–IL-7R, anti–c-Kit, anti-Flt3R, and anti-CD115 and sorted on a FACSAria (BD Biosciences). For phenotypic analysis of DC subsets (CD3, CD19, NK1.1, Ter119)neg cells were analyzed for CD11c, MHCII, CD11b, and B220 expression. All Abs were purchased from BD Biosciences or eBioscience. Dead cells were excluded by staining the cells with LIVE/DEAD Fixable Violet Dead Cell Staining dye (Invitrogen). For DC, pre-cDC, and CDP analysis, at ≥ 0.1, 0.25, and 1 × 106 million events were acquired, respectively.
DC and DC progenitor cell analysis
Total BM and spleen DC frequency was determined by gating on the CD3/CD19/NK1.1/Ter119neg CD11c+ and MHC class II+ cell population. The frequency of cDCs and pDCs was determined by gating on live CD3/CD19/NK1.1/Ter119neg CD11c+ B220− and CD11c+ B220+ cells, respectively.9 The percentage of CDPs was determined by gating on Linneg IL-7R− c-Kitint CD115+ Flt3+ cells9 and pre-cDCs by gating on [Ter119, CD19, CD3, NK1.1]neg MHC II− CD11cint/hi SIRPαint Flt3+ cells.10 Surface Flt3 expression on CDP and pre-cDC gated cell populations was determined by flow cytometry.
DC progenitor proliferation and survival
Indomethacin- or vehicle-treated mice were injected with BrdU (1 mg intraperitoneally). Mice were killed 20 hours after BrdU injection, and BM cells were harvested and stained with cell-surface Abs for CDPs and pre-cDCs. Cells were then fixed and permeabilized for intracellular BrdU staining according to the manufacturer's instructions (BD Biosciences). For in vitro analysis of DC-committed progenitor cell proliferation, CDPs that had undergone FACS were labeled with CFDA (1μM) and were cultured in Flt3L-supplemented medium in the presence or absence of indomethacin for 3 days. At the end of incubation, CDP proliferation was determined by measuring intracellular CFDA intensity by flow cytometry.13 To examine the effect of blockade of PG synthesis on DC progenitor survival, CDPs that had undergone FACS were cultured for 5 days in Flt3L-supplemented complete medium in the presence or absence of indomethacin, and survival of Linneg CD11c+ MHCII− Flt3+ pre-cDCs was determined by annexin V staining. In addition, phospho-STAT3, activated caspase-3, and survivin protein in pre-cDCs and/or CDPs were measured by flow cytometry after intracellular staining.
PGE2 ELISA
PGE2 in the culture supernatant fluids of Flt3L-stimulated Linneg BM cells was measured with a PGE2-specific ELISA kit (Neogen Co), according to the manufacturer's instructions.
PGE2 receptor analysis
To determine the expression of PGE2 receptors (EP1-4) on CDP and pre-cDC progenitors, Linneg BM cells were stained with EP receptor–specific Abs (all from Cayman Chemical), and EP receptor expression on gated cell populations was determined by flow cytometry. Analysis of EP receptor mRNA was performed by quantitative RT-PCR with the use of primer sequences as we previously described.27 To determine the EP receptor involved in DC development, Linneg BM cells were cultured for 9 days in Flt3L plus-indomethacin–supplemented medium and treated with butaprost (EP2 agonist), 17-phenyl-trinor prostaglandin E2 (17ptPGE2; EP1, and EP3 agonist), each at 1μM, sulprostone (EP3 agonist; 50nM), and 50nM PGE2, all from Cayman Chemical or with L-902,688 (EP4 agonist; 1μM), a kind gift from Merck-Frost during culture. For some experiments, Linneg BM cells were cultured for 9 days in Flt3L-supplemented medium with indomethacin, SC-51322 (EP1 antagonist), L798,106 (EP3 antagonist), or PGE2 plus indomethacin during culture.
Flt3 overexpression in DC progenitor cells
The retroviral MSCV-IRES-EGFP vector containing Flt3 was prepared as described previously.32 Twenty-four micrograms of purified Flt3 in MSCV-IRES-EGFP or empty vector were transfected into Phoenix Eco cells with the use of Lipofectamine LTX and plus reagent (Invitrogen), according to the manufacturer's instructions. Flt3 was overexpressed in CDP-enriched Linneg BM cells by retroviral transduction as described previously.32
Statistical analysis
All values are reported as mean ± SEM. Statistical significance was determined by paired or unpaired Student t test or 1-way ANOVA with Bonferroni posthoc analysis, as appropriate.
Results
Inhibition of PGE2 biosynthesis reduces in vivo DC development
We have previously shown that PGE2 differentially regulates myeloid and erythroid hematopoietic lineage differentiation21-25 and is an important regulator of stem cell function.27 To investigate the role PGE2 plays in regulation of DC production, we inhibited in vivo PGE2 biosynthesis in mice by treatment with indomethacin, a dual COX1 and COX2 inhibitor, for 6 days and quantitated DCs in BM and spleen by flow cytometry. The frequency and total number of BM DCs (CD3/CD19/NK1.1/Ter119neg CD11c+ MHCII+) were 3.2- ± 0.6-fold lower in indomethacin-treated mice compared with vehicle-treated mice (Figure 1A). Mature T cells, natural killer cells, and B cells showed no change after indomethacin treatment; whereas, as we reported previously,22,33 monocytes were increased after blockade of PGE2 biosynthesis (Table 1). Spleen DC numbers were also significantly decreased after indomethacin treatment, although to a lesser degree than BM (1.8- ± 0.2-fold; Figure 1A). Analysis of DC subsets showed that the total number of cDCs (CD11c+ B220−) and pDCs (CD11c+ B220+) are significantly decreased in BM and spleen when PGE2 biosynthesis is blocked (Figure 1B). The reduction in cDCs was significantly greater than pDCs in the BM (69% ± 8% vs 29% ± 3%; P < .01); however, in the spleen, cDC and pDC reduction was equivalent (43.6% ± 12.8% vs 35.5% ± 13.1%; P = .64). Coadministration of PGE2 with indomethacin completely blocked the reduction in BM and spleen DCs (not shown) produced by indomethacin treatment (Figure 1C), confirming a specific requirement for PGE2 in DC development and suggesting that PGE2 acts as a positive regulator of DC development. As previously reported,18 in vivo administration of Flt3L in mice dramatically increased total DCs (cDCs and pDCs) in the BM. Similar to that observed for steady state mice, blockade of PGE2 synthesis during in vivo Flt3L treatment resulted in significantly reduced DC generation (Figure 1D).
Effects of PGE2 inhibition on bone marrow cellularity
| Group* . | WBC/femur† . | Neutrophils‡ . | Monocytes‡ . | B cells‡ . | T + NK cells‡ . |
|---|---|---|---|---|---|
| Vehicle | 17.0 ± 0.9 | 6. ± 0.3 (37.6 ± 1.0) | 1.8 ± 0.2 (10.0 ± 0.7) | 4.0 ± 0.2 (27.7 ± 0.3) | 3.7 ± 0.5 (27.6 ± 1.0) |
| Indomethacin | 17.3 ± 1.3 | 6.3 ± 0.4 (38.0 ± 1.1) | 2.5 ± 0.2§ (14.6 ± 0.3) | 3.4 ± 0.4 (21.6 ± 2.4) | 4.0 ± 0.5 (25.6 ± 1.2) |
| Group* . | WBC/femur† . | Neutrophils‡ . | Monocytes‡ . | B cells‡ . | T + NK cells‡ . |
|---|---|---|---|---|---|
| Vehicle | 17.0 ± 0.9 | 6. ± 0.3 (37.6 ± 1.0) | 1.8 ± 0.2 (10.0 ± 0.7) | 4.0 ± 0.2 (27.7 ± 0.3) | 3.7 ± 0.5 (27.6 ± 1.0) |
| Indomethacin | 17.3 ± 1.3 | 6.3 ± 0.4 (38.0 ± 1.1) | 2.5 ± 0.2§ (14.6 ± 0.3) | 3.4 ± 0.4 (21.6 ± 2.4) | 4.0 ± 0.5 (25.6 ± 1.2) |
WBC indicates white blood cell; and NK, natural killer.
Groups of 5 mice were treated with vehicle (0.01% ethanol in PBS) or indomethacin (2.5 mg/kg) twice daily for 6 days.
BM cells were harvested from vehicle control and indomethacin-treated mice, and WBCs were determined on individual mice with the use of a Hemavet 950 analyzer. WBCs are expressed as mean ± SEM total nucleated cells per femur × 106, based on counts from individual mice. Data are representative of 5 experiments.
Harvested BM cells from treated mice were stained with antibodies for GR-1, Mac1, B220, CD3, and NK1.1 and analyzed by flow cytometry. Data are expressed as mean ± SEM total cells per femur × 106 (mean percentage of WBCs ± SEM), based on analysis of individual mice. Data are representative of 3 experiments.
P < .05
Inhibition of PGE2 biosynthesis reduces DC and DC-committed progenitor cells in mouse BM and spleen. (A left) Representative flow plots show gating strategy to determine DCs in BM (top) and spleen (bottom) of mice treated with indomethacin. (Right) DC frequency of nucleated cells and total DCs per femur (top) and spleen (bottom; mean ± SEM; n = 6 mice in 2 experiments, each assayed individually). (B) Representative flow plots of cDCs (CD11c+ B220−) and pDCs (CD11c+ B220+) in BM. The bar graphs show (left) total cDCs and pDCs per femur, and (right) total cDCs and pDCs in spleen (mean ± SEM; m = 3 mice per experiment, each assayed individually; 2 experiments). (C) Total DCs per femur in mice after treatment with indomethacin with or without dmPGE2 (mean ± SEM; n = 4 mice per group, each assayed individually). (D) Total DCs per femur in mice after treatment with Flt3L with or without dmPGE2 (mean ± SEM; n = 4 mice per group, each assayed individually). (E) Representative dot plots of CDP frequency and average total CDPs per femur in the BM after 6 days of indomethacin treatment (mean ± SEM; n = 3 mice/group in each of 2 experiments). (F) Representative dot plots of pre-cDC frequency and total pre-cDCs per femur (mean ± SEM; n = 3 mice/group in each of 2 experiments). V indicates vehicle; Indo, indomethacin. *P < .05.
Inhibition of PGE2 biosynthesis reduces DC and DC-committed progenitor cells in mouse BM and spleen. (A left) Representative flow plots show gating strategy to determine DCs in BM (top) and spleen (bottom) of mice treated with indomethacin. (Right) DC frequency of nucleated cells and total DCs per femur (top) and spleen (bottom; mean ± SEM; n = 6 mice in 2 experiments, each assayed individually). (B) Representative flow plots of cDCs (CD11c+ B220−) and pDCs (CD11c+ B220+) in BM. The bar graphs show (left) total cDCs and pDCs per femur, and (right) total cDCs and pDCs in spleen (mean ± SEM; m = 3 mice per experiment, each assayed individually; 2 experiments). (C) Total DCs per femur in mice after treatment with indomethacin with or without dmPGE2 (mean ± SEM; n = 4 mice per group, each assayed individually). (D) Total DCs per femur in mice after treatment with Flt3L with or without dmPGE2 (mean ± SEM; n = 4 mice per group, each assayed individually). (E) Representative dot plots of CDP frequency and average total CDPs per femur in the BM after 6 days of indomethacin treatment (mean ± SEM; n = 3 mice/group in each of 2 experiments). (F) Representative dot plots of pre-cDC frequency and total pre-cDCs per femur (mean ± SEM; n = 3 mice/group in each of 2 experiments). V indicates vehicle; Indo, indomethacin. *P < .05.
Because DCs develop in the BM from DC lineage–committed HPCs7,9,16 and we previously demonstrated that PGE2 can differentially regulate HPCs depending on lineage,21-25,34 we examined whether the attenuated development of DCs observed on inhibition of PGE2 biosynthesis results from an effect on the DC-committed progenitor cell compartment. In mice treated with indomethacin for 6 days, the early BM common DC progenitors, CDP (Linneg c-Kitint Flt3+ CD115+; Figure 1E), and immediate cDC precursors, pre-cDC (CD3/CD19/NK1.1/Ter119neg CD11c+ MHCII− SIRPαint Flt3+; Figure 1F), were significantly reduced compared with control. In contrast, phenotypically defined myeloid-committed progenitor cells, KL (Linneg c-Kithigh Sca-1−) increased in indomethacin-treated mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), which is consistent with the increase in CFU-GM we previously reported.33 The total number of MDPs per femur was not statistically different from control in indomethacin-treated mice, although they did trend lower (supplemental Figure 1B). These findings expand the scope of regulation of hematopoiesis by PGE2 to include the DC-committed progenitor cell compartment.
PGE2 is a positive regulator of Flt3L-dependent DC generation from hematopoietic progenitor cells
At steady state, DC development mainly depends on Flt3 signaling.13,17,18 To evaluate the role PGE2 plays in DC development from hematopoietic progenitor cells, we evaluated Flt3L-dependent generation of DCs from BM cells in vitro. Lineage-depleted mouse BM cells enriched for CDP were cultured for 9 days in Flt3L-supplemented medium with indomethacin, with or without dmPGE2, and DCs enumerated by flow cytometry with the use of a previously defined phenotypic determination.9 Generation of CD11c+ B220− cDCs and CD11c+ B220+ pDCs was substantially reduced in the presence of indomethacin (cDC, 48% ± 3.1%; pDC, 27% ± 1.4%; Figure 2A). The addition of exogenous PGE2 completely reversed the indomethacin-mediated reduction in DC differentiation, indicating that PGE2 signaling is required for optimal DC generation. Consistent with the requirement of PGE2 for optimal DC generation, PGE2 levels were virtually absent in cultures grown in the presence of indomethacin (Figure 2B). To test whether endogenous PGE2 biosynthesis by DC-committed progenitor cells directly regulates DC differentiation and to rule out effects mediated through accessory cells, including stromal cells, we evaluated the effects of PGE2 inhibition on DC generation from highly purified DC progenitors. BM CDPs underwent FACS (> 98% purity) and were cultured for 9 days in Flt3L-supplemented media with or without indomethacin. Significantly fewer DCs were generated from purified CDP in the presence of indomethacin compared with control (Figure 2C), strongly suggesting autocrine secretion.
PGE2 positively regulates Flt3L-dependent DC development from hematopoietic progenitor cells. (A) Total cDC (CD11c+ CD11b+ B220−) and pDC (CD11c+ CD11b− B220+) generation from Linneg BM cells in Flt3L cultures in presence of indomethacin or indomethacin plus dmPGE2 (mean ± SEM; n = 3 mice/group in each of 2 experiments). (B) Detection of PGE2 at indicated time points during Flt3L-mediated DC differentiation in culture from panel A. (C) Total DC generation from CDPs (103 cells/well) that underwent FACS and cultured for 9 days in Flt3L-supplemented media in the presence/absence of indomethacin (mean ± SEM; n = 5 mice; 2 experiments). (D) Total DC generation from Linneg BM cells (2 × 105 cells/well) cultured for 9 days with Flt3L and SC560 (COX1 inhibitor) or NS398 (COX2 inhibitor) or different dose of PGE2 (0.1nM-100nM; mean ± SEM; n = 6 mice; 2 experiments). (E) Effect of indomethacin treatment on CD34+ CD11a+ CD14− DC precursor and CD11c+ CD14− myeloid DC generation from purified UCB CD34+ cells (5 × 105 cells/well; mean ± SEM; n = 3 cord blood samples). (F) Total DC generation from mouse Linneg BM cells cultured with GM-CSF with or without indomethacin (mean ± SEM; n = 5 mice). *P < .05. V indicates vehicle; and Indo, indomethacin.
PGE2 positively regulates Flt3L-dependent DC development from hematopoietic progenitor cells. (A) Total cDC (CD11c+ CD11b+ B220−) and pDC (CD11c+ CD11b− B220+) generation from Linneg BM cells in Flt3L cultures in presence of indomethacin or indomethacin plus dmPGE2 (mean ± SEM; n = 3 mice/group in each of 2 experiments). (B) Detection of PGE2 at indicated time points during Flt3L-mediated DC differentiation in culture from panel A. (C) Total DC generation from CDPs (103 cells/well) that underwent FACS and cultured for 9 days in Flt3L-supplemented media in the presence/absence of indomethacin (mean ± SEM; n = 5 mice; 2 experiments). (D) Total DC generation from Linneg BM cells (2 × 105 cells/well) cultured for 9 days with Flt3L and SC560 (COX1 inhibitor) or NS398 (COX2 inhibitor) or different dose of PGE2 (0.1nM-100nM; mean ± SEM; n = 6 mice; 2 experiments). (E) Effect of indomethacin treatment on CD34+ CD11a+ CD14− DC precursor and CD11c+ CD14− myeloid DC generation from purified UCB CD34+ cells (5 × 105 cells/well; mean ± SEM; n = 3 cord blood samples). (F) Total DC generation from mouse Linneg BM cells cultured with GM-CSF with or without indomethacin (mean ± SEM; n = 5 mice). *P < .05. V indicates vehicle; and Indo, indomethacin.
To determine the COX enzyme involved in PGE2 biosynthesis by DC-committed progenitor cells, we induced Flt3L-dependent DC differentiation in vitro in the presence of a selective COX1 (SC560) or COX2 (NS-398) inhibitor. The COX2-specific inhibitor NS-398 substantially reduced DC generation equivalent to that observed with indomethacin, whereas the COX1-selective inhibitor, SC560, was substantially less effective (Figure 2D), suggesting that PGE2 production primarily via the COX2 isoform is required for optimal DC generation. The addition of exogenous PGE2 during Flt3L-induced DC development enhanced DC generation particularly at 0.1nM and 10nM (Figure 2D), further supporting a role for PGE2 in DC development.
Similar to the mouse model, inhibition of PGE2 synthesis impairs Flt3L, SCF, IL-3, and IL-6–induced CD1a+ CD14− DC progenitor cell generation and CD11c+ CD14− myeloid DC generation from human CD34+ UCB cells (Figure 2E), showing that PGE2 regulation of DC generation from HPCs is conserved across species.
Although the numbers of DCs generated in the presence of indomethacin are reduced, these DCs do up-regulate MHC-II, CD40, and CD86 on lipopolysaccharide stimulation to a similar degree compared with DCs generated with Flt3L alone (supplemental Figure 2A), with no difference in IL-6 or IL-12 cytokine production (supplemental Table 1). Similarly, DCs generated from indomethacin-treated C57Bl/6 mice cultured with CFDA-SE–labeled CD4+ T cells from BALB/c mice in a MLR assay induced a similar level of T-cell proliferation compared with control (supplemental Figure 2B). These results indicate that, although inhibition of PGE2 signaling during DC development reduces total DC progenitor number and impairs optimal DC generation, those DCs that are produced appear to function normally at least in vitro.
DC differentiation from progenitor cells can also be induced by GM-CSF,35 although it is not required for in vivo DC development.13 To determine whether inhibiting PGE2 biosynthesis exclusively reduces Flt3L-dependent DC development or globally reduces DC differentiation, we induced mouse DC differentiation with GM-CSF in vitro in the presence or absence of indomethacin. In contrast to Flt3L-induced DC generation, GM-CSF–mediated DC development was significantly higher after indomethacin treatment compared with control (Figure 2F), indicating that PGE2 differentially regulates Flt3L-dependent and GM-CSF–dependent DC development.
PGE2 protects DC progenitors from apoptosis
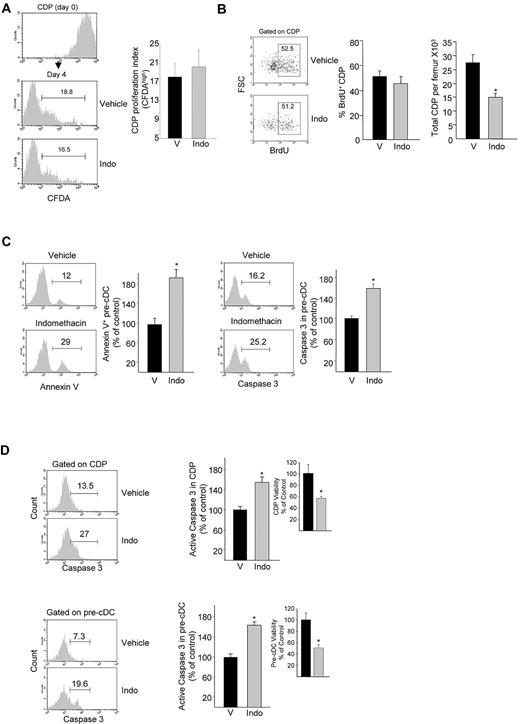
Attenuated DC generation as a result of reduced PGE2 signaling could result from a defect in DC-committed progenitor cell survival or as a consequence of effects on DC progenitor cell proliferation. To explore the functional consequences of inhibition of PGE2 biosynthesis on DC progenitor cell proliferation, we first examined the effect of indomethacin treatment on BM CDP proliferation. Analysis of Flt3L-stimulated CDP proliferation in vitro was unaffected by indomethacin (Figure 3A). To further explore the effects of PGE2 biosynthesis inhibition on DC progenitor cell proliferation in vivo, mice were treated with indomethacin for 6 days and pulsed with BrdU for the last 20 hours before being killed. BrdU incorporation in CDPs was unchanged after indomethacin treatment, whereas the total number of CDPs was decreased as we described (Figure 3B). These results show that reduced PGE2 signaling does not alter DC progenitor cell proliferation.
Effect of PGE2 biosynthesis inhibition on DC progenitor proliferation and survival. (A) CFDA dilution 3 days after in vitro culture of CDPs that had undergone FACS in Flt3L-supplemented medium with or without indomethacin (mean ± SEM; n = 4 mice). (B) Representative dot plots of BrdU incorporation in CDPs of BrdU-treated mice after indomethacin treatment. (Left) Average frequency of BrdU+ CDPs; (right) total CDPs per femur (mean ± SEM, 2 experiments; n = 3 mice/group/experiment). (C) CDP-enriched Linneg BM cells were cultured for 5 days in Flt3L-supplemented medium with or without indomethacin, and annexin V (left) and active caspase-3 (right) expressions were determined in pre-cDC gated cell population (mean ± SEM; 2 experiments; n = 3 mice/experiment). (D) Active caspase-3 expression in BM CDPs and pre-cDCs, and CDP and pre-cDC viability (insets) in mice treated for 6 days with indomethacin (mean ± SEM from 5 mice per group assayed individually; expressed as percentage of control). *P < .05. V indicates vehicle; and Indo, indomethacin.
Effect of PGE2 biosynthesis inhibition on DC progenitor proliferation and survival. (A) CFDA dilution 3 days after in vitro culture of CDPs that had undergone FACS in Flt3L-supplemented medium with or without indomethacin (mean ± SEM; n = 4 mice). (B) Representative dot plots of BrdU incorporation in CDPs of BrdU-treated mice after indomethacin treatment. (Left) Average frequency of BrdU+ CDPs; (right) total CDPs per femur (mean ± SEM, 2 experiments; n = 3 mice/group/experiment). (C) CDP-enriched Linneg BM cells were cultured for 5 days in Flt3L-supplemented medium with or without indomethacin, and annexin V (left) and active caspase-3 (right) expressions were determined in pre-cDC gated cell population (mean ± SEM; 2 experiments; n = 3 mice/experiment). (D) Active caspase-3 expression in BM CDPs and pre-cDCs, and CDP and pre-cDC viability (insets) in mice treated for 6 days with indomethacin (mean ± SEM from 5 mice per group assayed individually; expressed as percentage of control). *P < .05. V indicates vehicle; and Indo, indomethacin.
To investigate whether impaired DC development after inhibition of PGE2 biosynthesis resulted from an effect on DC progenitor cell survival, we first evaluated survival of pre-cDCs generated from CDP-enriched BM cells in the presence of Flt3L plus indomethacin in vitro. Pre-cDC survival was significantly reduced, measured by both annexin V staining (Figure 3C left) and expression of activated caspase-3 (Figure 3C right). Analysis of active caspase-3 expression in BM CDPs and pre-cDCs after treatment of mice with indomethacin also showed significantly higher levels of active caspase-3 in CDPs and pre-cDCs of indomethacin-treated mice, consistent with fewer viable CDPs and pre-cDCs (Figure 3D). These data suggest that PGE2 regulates DC production primarily by attenuating apoptosis, thus promoting DC progenitor cell survival and are consistent with several studies that indicate that PGE2 protects many cell types of hematopoietic origin from apoptosis.27,36
PGE2 regulation of Flt3 expression enhances DC progenitor cell survival via STAT3-mediated elevation in survivin
STAT3 is a known transcription factor downstream of Flt3 signaling that is required for DC development.37,38 PGE2 has been shown to regulate STAT3 phosphorylation and to protect cardiomyocytes from doxorubicin-induced apoptosis.39,40 We therefore examined the effect of inhibiting PGE2 biosynthesis on STAT3 phosphorylation in pre-cDCs generated from Linneg BM cells in vitro. Inhibition of PGE2 signaling during Flt3L-induced DC differentiation resulted in significantly reduced phospho-STAT3 levels in pre-cDCs (Figure 4A). The STAT3 inhibitor stattic mimicked the reduction in DC generation seen with indomethacin and the combination of stattic plus indomethacin did not result in further reduction in DC differentiation compared with stattic alone (Figure 4B), suggesting that PGE2/Flt3 regulates the level of STAT3 activation during DC differentiation. Identical reduction in DC generation from highly purified CDPs was observed in the presence of indomethacin and stattic alone and in combination, suggesting that both indomethacin and stattic are acting on the same DC progenitor cell population (supplemental Figure 3).
PGE2 regulation of Flt3 expression enhances DC progenitor cell survival through STAT3-mediated elevation of survivin. (A) Representative FACS plot of pre-cDC phospho-STAT3 expression generated from CDPs cultured in Flt3L with or without indomethacin and relative phospho-STAT3 expression in pre-CDC (mean ± SEM; n = 3 mice pooled per experiment; 3 experiments). (B) Total DC generation from Linneg BM cells (2 × 105 cells/well) cultured for 9 days with Flt3L with or without indomethacin and/or stattic (mean ± SEM; 2 experiments; n = 3 mice/group/experiment; *P < .05 compared with vehicle; #P > .05 compared with indomethacin). (C) Survivin expression in pre-cDCs generated in vitro from Flt3L-cultured CDPs with or without indomethacin (mean ± SEM; n = 3 mice pooled per experiment; 3 experiments). (D) Total DC generation from Linneg BM cells nontransduced (NT) or transduced with survivin overexpression or control vector (mean ± SEM; n = 3 mice/group/experiment; 2 experiments; *P < .05 compared with vehicle, †P < .05 compared with vector control). (E) Total DC generation from Linneg BM cells (2 × 105 cells/well) cultured for 9 days with or without Flt3L and/or indomethacin. (F) Mean fluorescence intensity (MFI) of Flt3 receptor on CDPs and pre-cDCs (mean ± SEM; n = 4 mice). (G) Linneg BM cells transduced with Flt3 MSCV-IRES-EGFP or control vector and cultured with or without indomethacin. (Left) DC generation after 9 days of culture (mean ± SEM; N = 6 mice assayed individually; 2 experiments); (middle) MFI of phospho-STAT3 expression; (right) survivin expression in pre-cDCs after 5 days of culture (mean ± SEM; N = 3 mice). *P < .05. V indicates vehicle; and Indo, indomethacin.
PGE2 regulation of Flt3 expression enhances DC progenitor cell survival through STAT3-mediated elevation of survivin. (A) Representative FACS plot of pre-cDC phospho-STAT3 expression generated from CDPs cultured in Flt3L with or without indomethacin and relative phospho-STAT3 expression in pre-CDC (mean ± SEM; n = 3 mice pooled per experiment; 3 experiments). (B) Total DC generation from Linneg BM cells (2 × 105 cells/well) cultured for 9 days with Flt3L with or without indomethacin and/or stattic (mean ± SEM; 2 experiments; n = 3 mice/group/experiment; *P < .05 compared with vehicle; #P > .05 compared with indomethacin). (C) Survivin expression in pre-cDCs generated in vitro from Flt3L-cultured CDPs with or without indomethacin (mean ± SEM; n = 3 mice pooled per experiment; 3 experiments). (D) Total DC generation from Linneg BM cells nontransduced (NT) or transduced with survivin overexpression or control vector (mean ± SEM; n = 3 mice/group/experiment; 2 experiments; *P < .05 compared with vehicle, †P < .05 compared with vector control). (E) Total DC generation from Linneg BM cells (2 × 105 cells/well) cultured for 9 days with or without Flt3L and/or indomethacin. (F) Mean fluorescence intensity (MFI) of Flt3 receptor on CDPs and pre-cDCs (mean ± SEM; n = 4 mice). (G) Linneg BM cells transduced with Flt3 MSCV-IRES-EGFP or control vector and cultured with or without indomethacin. (Left) DC generation after 9 days of culture (mean ± SEM; N = 6 mice assayed individually; 2 experiments); (middle) MFI of phospho-STAT3 expression; (right) survivin expression in pre-cDCs after 5 days of culture (mean ± SEM; N = 3 mice). *P < .05. V indicates vehicle; and Indo, indomethacin.
STAT3 modulates transcription of the antiapoptotic protein survivin,41 and we recently reported that PGE2 increases survivin transcription and decreases apoptosis in HSCs.27 To determine whether PGE2/Flt3 signaling regulates DC progenitor survival through a STAT3-mediated increase in survivin expression, survivin protein was measured in pre-cDCs generated from CDPs during in vitro culture with Flt3L with or without indomethacin. Intracellular flow cytometry showed that survivin was significantly lower in pre-cDCs when PGE2 biosynthesis was inhibited (Figure 4C). Retroviral overexpression of survivin in CDP-enriched Linneg BM cells showed that the indomethacin-mediated defect in DC differentiation was completely reversed by ectopic survivin (Figure 4D), confirming that PGE2/Flt3-mediated survivin expression is required for optimal DC generation.
Because both PGE2 and Flt3 regulate phospho-STAT3 and survivin expression, we tested whether PGE2 regulates DC differentiation directly downstream of its EP receptors or indirectly by modulating Flt3 receptor signaling. We first examined DC generation from CDP-enriched Linneg BM cells cultured with or without Flt3L and/or indomethacin. Robust DC differentiation was observed in the presence of Flt3L, which was significantly reduced when PGE2 synthesis was inhibited. DC generation was not observed in the absence of Flt3L regardless of the presence or absence of indomethacin (Figure 4E). This suggests that PGE2 by itself is not sufficient for DC differentiation but regulates DC development in conjunction with Flt3 receptor signaling. We next analyzed the effects of inhibition of PGE2 biosynthesis on expression of Flt3 receptor on DC-committed progenitor cells. Flt3 expression was significantly decreased on BM CDPs (40.0% ± 7.07%; P < .01) and on pre-cDCs (42.5% ± 3.72%; P < .01) after treatment of mice with indomethacin (Figure 4F), indicating that PGE2 signaling regulates Flt3 receptor expression. Similarly, Flt3 mRNA was significantly reduced (35% ± 4.8%; P < .01) in pre-cDCs that underwent FACS after indomethacin treatment. In contrast, Flt3 expression on Linneg c-Kithigh Sca-1− myeloid-committed progenitor cells was not decreased in indomethacin-treated mice (supplemental Figure 4).
To confirm that the reduced Flt3 expression on DC progenitor cells is responsible for the reduction in DC generation after PGE2 biosynthesis inhibition, we overexpressed Flt3 in CDP-enriched Linneg BM cells and induced Flt3L-dependent DC development. Ectopic Flt3 expression resulted in enhanced DC generation as expected and completely reversed the defect in DC generation as a consequence of indomethacin treatment (Figure 4G left). Similarly, the reduction in phospho-STAT3 and survivin in DC-lineage progenitors was reversed by Flt3 overexpression (Figure 4G middle and right, respectively). These data indicate that reduced DC generation as a result of inhibition of PGE2 biosynthesis/reduced PGE2 signaling is because of a reduction in Flt3 receptor expression on DC progenitor cells rather than direct reduction of EP receptor regulation of STAT3 activation and survivin expression.
PGE2 regulation of DC generation is mediated by EP1 and EP3 receptor signaling
PGE2 signals through 4 different G protein–coupled receptors, EP1-4, with unique, similar, or opposing intracellular signaling pathways.42 As a consequence, the effects of PGE2 result from the net effect of signals generated at all 4 receptors. To identify the specific pathways involved in regulation of DC progenitor cell differentiation by PGE2 and to define a potential pharmaceutic target for modulation of DC generation, we investigated which EP receptors mediate DC development. All 4 EP receptors were found to be expressed on CDPs and pre-cDCs (data not shown) by flow cytometric analysis. In addition, mRNAs for all 4 receptors were detected in CDPs and pre-cDCs. To determine which EP receptor(s) regulates DC generation, DC cultures were treated with selective EP receptor agonists to reverse the inhibitory effects of indomethacin. DC generation from Linneg BM cells was significantly reduced in the presence of indomethacin as expected, but it could be completely rescued by the addition of PGE2 or by the dual EP1/EP3 agonist 17ptPGE2 and partially rescued by the EP3 agonist sulprostone (Figure 5A). In contrast, the EP2 specific agonist butaprost and the EP4-specific agonist L-902688 failed to rescue DC generation. We also evaluated the ability of EP receptor selective antagonists to inhibit DC development. Antagonism of EP1 receptor signaling with the EP1-specific antagonist SC51322 or EP3 receptor signaling with the EP3-specific antagonist L-798106 resulted in significantly reduced DC differentiation both in vitro (Figure 5B left) and in vivo (Figure 5B right). Consistent with the effect of EP1 and EP3 receptor–specific agonists to rescue DC generation when PGE2 biosynthesis/signaling is inhibited, 17ptPGE2 and sulprostone reversed the indomethacin-mediated reduction in survivin in pre-cDCs generated from CDPs (Figure 5C). These data show that PGE2 regulates DC differentiation through EP1 and EP3 receptor signaling.
EP1 and EP3 receptors regulate Flt3L-mediated DC development. (A) Linneg BM cells cultured for 9 days in Flt3L-supplemented media with indomethacin alone or with indomethacin plus selective EP receptor agonists, and DC generation was measured by flow cytometry (mean ± SEM; n = 6 mice assayed individually; from 2 experiments). (B left) In vitro, Flt3L-dependent DC generation from Linneg BM cells in the presence of EP1 and EP3 receptor antagonist (mean ± SEM; n = 8 mice assayed individually; from 3 experiments). (Right) Total DC number per femur in mice treated with indomethacin or EP1 or EP3 antagonists in vivo (mean ± SEM; n = 4 mice assayed individually). (C) Linneg BM cells cultured for 5 days in Flt3L-supplemented media with indomethacin alone or with selective EP receptor agonists. Surviving expression in pre-cDC gated cells was determined by flow cytometry (mean ± SEM; n = 3 mice assayed individually). *P < .05 compared with vehicle; †P < .05 compared with indomethacin. (D) Total CD11c+ MHCll+ DCs in the BM and spleen of EP1 and EP3 knockout mice and EP1/EP3 double knockout mice (mean ± SEM; n = 3 mice assayed individually). *P < .05 compared with wild-type control. V indicates vehicle; and Indo, indomethacin.
EP1 and EP3 receptors regulate Flt3L-mediated DC development. (A) Linneg BM cells cultured for 9 days in Flt3L-supplemented media with indomethacin alone or with indomethacin plus selective EP receptor agonists, and DC generation was measured by flow cytometry (mean ± SEM; n = 6 mice assayed individually; from 2 experiments). (B left) In vitro, Flt3L-dependent DC generation from Linneg BM cells in the presence of EP1 and EP3 receptor antagonist (mean ± SEM; n = 8 mice assayed individually; from 3 experiments). (Right) Total DC number per femur in mice treated with indomethacin or EP1 or EP3 antagonists in vivo (mean ± SEM; n = 4 mice assayed individually). (C) Linneg BM cells cultured for 5 days in Flt3L-supplemented media with indomethacin alone or with selective EP receptor agonists. Surviving expression in pre-cDC gated cells was determined by flow cytometry (mean ± SEM; n = 3 mice assayed individually). *P < .05 compared with vehicle; †P < .05 compared with indomethacin. (D) Total CD11c+ MHCll+ DCs in the BM and spleen of EP1 and EP3 knockout mice and EP1/EP3 double knockout mice (mean ± SEM; n = 3 mice assayed individually). *P < .05 compared with wild-type control. V indicates vehicle; and Indo, indomethacin.
To further validate the involvement of EP1/EP3 signaling in DC development, we evaluated total DC number in EP1 and EP3 receptor single knockout mice and EP1/EP3 double knockout mice. Total DC cell number was significantly lower in the BM and spleen of the EP1 and EP3 receptor single knockout mice compared with wild-type control mice (Figure 5D). In addition, double EP1/EP3 receptor knockout mice showed significantly more reduction in DC number in BM and spleen, mimicking results with indomethacin, and confirming agonist/antagonist data, showing a role of both EP1 and EP3 receptors in DC-progenitor cell function.
Discussion
Although PGE2 has been implicated in the regulation of hematopoietic stem and progenitor cells, its role in steady state DC development is largely unknown. We now demonstrate that PGE2 is a positive physiologic regulator of Flt3L-dependent DC development and is required for optimal DC-committed progenitor cell differentiation by regulating progenitor cell Flt3 expression and survival. Inhibition of PGE2 biosynthesis and signaling reduces Flt3 expression on DC progenitors, resulting in suboptimal activation of the Flt3 signaling cascade, decreased activation of STAT3, and reduced production of the antiapoptotic protein survivin, causing DC progenitor apoptosis. Although inhibition of PGE2 signaling reduces DC production, it does not impair the immune function of those DCs generated.
PGE2 has distinct effects on different types of HPCs, inhibiting granulocyte/monocyte,21,22,25 B-cell,43,44 and T-lymphoid precursors,45 while enhancing erythropoiesis.24,25 Pharmacologic doses of PGE2 have been shown to impair GM-CSF–mediated DC development from BM-derived hematopoietic progenitors and from monocytes.46 Steady state DC development, however, depends primarily on Flt3L not GM-CSF, thus the impairment of Flt3L-mediated DC development in the absence of endogenous PGE2 synthesis/signaling suggests that physiologic levels of PGE2 are necessary for normal steady state DC development. The variable effects of PGE2 on different HPCs may occur because of expression of different EP receptors and/or differential coupling of these receptors to intracellular signaling pathways. DC-committed progenitors originate in the BM from HSCs after multiple lineage-restricting differentiation processes. Because PGE2 enhances HSC proliferation and survival, it is reasonable to speculate that its effect on DC differentiation is a consequence of its effects on HSCs. However, generation of fewer DCs after indomethacin treatment of highly purified CDP cultures, which lack HSCs, indicates that PGE2 regulation of DC differentiation is mediated by direct effects on DC-committed progenitor cells.
At least 2 cellular processes, proliferation and apoptosis, can control mature blood cell generation from HPCs. In contrast to the effects of PGE2 on myeloid, erythroid, and multipotential progenitor cell proliferation,22-25,34 inhibition of PGE2 biosynthesis did not affect BrdU incorporation in DC-committed progenitor cells, suggesting that PGE2 does not primarily regulate DC-lineage progenitor cell proliferation. Because BrdU incorporation measures DNA replication not cell division, we confirmed this finding by in vitro CFDA staining that measures cell division. Similar CFDA dilution in both vehicle- and indomethacin-treated DC progenitor cells indicates that PGE2 does not regulate DC progenitor cell proliferation; rather, its primary effects are on progenitor cell survival. In support of this hypothesis, recent studies have also shown that Flt3 signaling can regulate progenitor cell survival without affecting proliferation47,48
PGE2 is a lipid messenger that regulates the expression of effector genes downstream of G protein–coupled receptors. PGE2 signaling through EP receptors is known to directly promote survival of colon cancer cells and myocardium by up-regulation of STAT3 and survivin.40,49 Because both Flt3 and PGE2 signaling pathways can activate STAT3 and induce survivin expression, it is possible that Flt3 signaling may synergize with PGE2 signaling to modulate the survival of DC progenitors through optimal survivin expression. PGE2 signaling alone was not sufficient to protect DC progenitors from apoptosis. and it completely failed to generate DCs in the absence of Flt3L, suggesting that PGE2 regulates DC differentiation primarily by modifying Flt3 signaling.
Flt3 is expressed on DC-committed progenitor cells and is essential for DC development in lymphoid organs. The presence of Flt3 on these progenitor cells directly correlates with their steady state differentiation into DCs.5,6 Despite its importance in DC development, little is known about how Flt3 expression is regulated. Reduced Flt3 expression on DC-committed progenitors as a result of PGE2 biosynthesis inhibition and complete reversal of reduced DC generation in the absence of PGE2 biosynthesis/signaling by overexpression of Flt3 argue that PGE2 regulates DC development indirectly by modulating Flt3 signaling. A recent study reported the involvement of PU.1 in Flt3 gene expression on DC-committed progenitors and its role in DC development.50 PU.1 expression and activation is controlled by several intracellular signal transduction events, including induction of PI3K/AKT and Src signaling pathways.51,52 Because these pathways are activated by EP1 and EP3 signaling,53,54 and because our data identified that PGE2 regulates Flt3L-dependent DC development through the EP1 and EP3 receptors, it is possible that PGE2-mediated induction of PI3K/AKT and Src signaling pathways regulates PU.1 expression in DC-committed progenitors that leads to increased expression of Flt3. However, in other progenitor lineages change in transcription factor activities in the absence of change in expression has been reported.55 Further study is needed to define the transcriptional events that regulate PGE2-induced Flt3 expression in DC progenitor cells.
On the basis of these in vitro and in vivo findings, we propose a model in which PGE2 signaling through the EP1 and EP3 receptors regulates Flt3 expression on DC progenitors. Flt3 receptor signaling activates STAT3 and enhances survivin expression in DC progenitors, which protects them from apoptosis, resulting in optimal DC generation, and that inhibition of PGE2 signaling impairs DC generation (Figure 6). Because of its potent Ag presentation properties, DCs are required for induction of immune responses at the onset of infection and quantitative, and functional loss of DCs has been attributed to severity of infectious diseases.56,57 In this context, our studies raise the possibility that nonsteroidal anti-inflammatory agents inhibiting PGE2 biosynthesis, particularly when taken over extended periods of time, may exacerbate disease as a consequence of reduced DC generation. In addition, several experimental and clinical studies support the use of DC vaccines58 ; however, current DC vaccines are not optimal for the treatment of human diseases. One of the main hurdles for DC vaccine development is a generation of large numbers of DCs that can be used to deliver target Ags to generate antitumor or anti-infective immune responses. Understanding DC differentiation mechanisms would be an essential step to achieve this goal. Our study shows that physiologic doses of PGE2 are crucial for optimal DC generation and may prove useful for efficient DC generation for vaccine development. Furthermore, recent studies suggest a role for PGE2 in self-renewal and expansion of HSCs26,27 and, although the up-regulation of PGE2 production after total body irradiation59 may be beneficial for HSC homing, engraftment, and expansion in the short term, our studies suggest that prolonged elevated levels of PGE2 may lead to altered hematopoietic progenitor function and DC generation. Further studies to evaluate the regulatory role of PGE2 in hematopoietic generation of immune cells after transplantation will identify how best to therapeutically modulate PGE2 signaling during and after hematopoietic transplantation for maximal benefit.
Model for the regulation of DC development by PGE2. In this model, we propose that PGE2 signaling through the PGE2 G protein EP1 and EP3 receptors regulates Flt3 expression on DC-committed progenitors (CDPs and pre-cDCs) and modifies downstream phopho-STAT3 activation and survivin expression. Elevated survivin expression protects DC progenitors from caspase-3–mediated apoptotic death and results in optimal DC development.
Model for the regulation of DC development by PGE2. In this model, we propose that PGE2 signaling through the PGE2 G protein EP1 and EP3 receptors regulates Flt3 expression on DC-committed progenitors (CDPs and pre-cDCs) and modifies downstream phopho-STAT3 activation and survivin expression. Elevated survivin expression protects DC progenitors from caspase-3–mediated apoptotic death and results in optimal DC development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Mark Kaplan, Janice Blum, and Randy Brutkiewicz for critical comments on the manuscript.
This work was supported by the National Institutes of Health (NIH; grants HL069669, HL079654, and HL096305, L.M.P.), partially supported by the National Institute of Diabetes and Digestive and Kidney Disease Center for Excellence in Molecular Hematology (grant P30 DK090948), and by the NIH (training grants DK07519 and HL007910, J.H.) and (grants DK37097 and DK46205 for EP1 KO and EP3 KO mice generation, R.M.B.). Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (NCI P30 CA082709).
National Institutes of Health
Authorship
Contribution: P.S. designed the study, performed the experiments, interpreted data, and wrote the manuscript; J.H. participated in designing the experiments and interpreting data and wrote the manuscript; P.H. participated in performance of research, collection, and analysis of data; J.M.S. maintained the mice colony, performed experiments, and critically read the manuscript; S.F. prepared survivin and Flt3-expressing plasmids and critically read the manuscript; R.M.B. provided EP1 and EP3 and EP1/EP3 double knockout mice and participated in design of experiments; and L.M.P. participated in designing the study, interpreting data, and coordinating and performing the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Louis M. Pelus, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 W Walnut St, Indianapolis, IN 46202; e-mail: lpelus@iupui.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal