Mesenchymal stromal cells (MSCs) overexpressing the Wnt3a gene support hematopoiesis at least in part via Wnt-dependent production of extracellular matrix proteoglycans. Canonical Wnt signaling is known to have direct effects on hematopoietic stem cell (HSC) function.
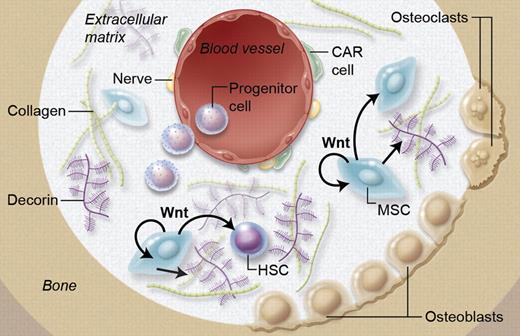
It is well established that Wnt can directly influence HSCs; however Wnt also regulates MSCs, which become more supportive of hematopoiesis. Ichii and coworkers show that this is at least in part mediated by Wnt-dependent production of extracellular matrix component Decorin, thereby proving a Wnt-dependent cross-talk between MSCs and HSCs. Professional illustration by A. Y. Chen.
It is well established that Wnt can directly influence HSCs; however Wnt also regulates MSCs, which become more supportive of hematopoiesis. Ichii and coworkers show that this is at least in part mediated by Wnt-dependent production of extracellular matrix component Decorin, thereby proving a Wnt-dependent cross-talk between MSCs and HSCs. Professional illustration by A. Y. Chen.
In this issue of Blood, Ichii et al demonstrate that Wnt signaling also affects stromal components, thereby regulating the architecture of the hematopoietic niche.1 They demonstrate that human HSCs are retained, while B-cell development is inhibited and lineage-committed progenitors can dedifferentiate when exposed to MSC lines that express the canonical Wnt gene Wnt3a.1 The opposite occurred when the noncanonical Wnt5a gene was used.
Wnt signaling is required for several basic developmental processes. Wnt signaling plays a crucial role during hematopoiesis and development of myeloid and T lymphoid cells (reviewed in Staal et al2 ). There are at least three different Wnt pathways: the canonical Wnt pathway, which involves β-catenin and members of the T-cell factor (Tcf)/lymphocyte-enhancer binding factor (Lef) family; the planar cell polarity (PCP) pathway; and the Wnt-Ca2+ pathway. The latter two pathways are referred to as noncanonical Wnt pathways. It is generally accepted that canonical Wnt signaling is one of the key factors underlying self-renewal of many types of stem cells, including HSCs. Fetal liver HSCs deficient for Wnt3a lack self-renewal because of a complete loss of canonical Wnt signaling.3 Likewise, adult HSCs that lack Wnt signaling by overexpressing DKK1, a Wnt sequestering and inhibitory protein, are unable to self-renew.4
The role of canonical Wnt signaling in HSCs has been quite controversial because of conflicting results in various gain- and loss-of-function studies. By carefully studying the levels of Wnt signaling in these various models, it has now become clear that Wnt is regulated in a dosage-dependent fashion at key checkpoints in various lineages of the hematopoietic system.5 HSCs both require and tolerate only relatively low levels of Wnt signaling, compared with developing T cells or other types of stem cells, for instance, those in the intestine.5 On the other hand, complete absence of Wnt signaling in HSCs severely impairs their repopulating capacity.3,4
While most attention has been on direct effects of Wnt proteins on HSCs, the work reported here by Ichii et al shows that Wnt mediates cross-talk of MSCs and HSCs via Wnt-dependent production of Decorin, an important extracellular matrix component (see figure).1 Decorin “decorates” collagen and other molecules with proteoglycans, thereby shaping the niche. It should be noted that these findings were generated using MSC lines, not primary MSCs. Yet the incredibly high up-regulation of Decorin in MSC lines was followed by studies checking various primary niche cells, again showing high Decorin expression in MSCs but not hematopoietic cells or CXCL12 abundant reticular cells. Indeed, Decorin-deficient mice where shown to have a complex hematopoietic phenotype, with extramedullar hematopoiesis and increased numbers of CD150+ LSK, the phenotype of LT-HSC in the mouse.
HSC self-renewal, specification and differentiation are complex processes regulated by intricate networks of signals that have to be tightly orchestrated and “fine-tuned” to correctly drive these processes. Recent work by Schaniel and coworkers shows that changes in Wnt signaling in the HSC niche also influence the fine-tuning of other pathways including the hedgehog pathway.6 This pathway is no less controversial than Wnt in regulating HSCs. Similar to Wnt, it is also involved in leukemia development.7 Interestingly, in the normal stem cell niche, Wnt signaling restricts self-renewal by providing a local dosage-dependent gradient of Wnt proteins, akin to the function of Wnts in lower vertebrates as true morphogens. In contrast, in the AML niche Wnt can no longer exert this type of regulation as the leukemic stem cells have undergone mutations rendering them independent of extracellular Wnt proteins.8 It will be of interest to compare the contributions of cell autonomous versus extracellular signals in normal and leukemic stem cell niches for various important self-renewal signals.
The current work also points to the possibility that changes in the microenvironment, including MSCs, can contribute to dysregulated signals leading to leukemia. It has been previously suggested that altered gene expression in osteoprogenitors may induce bone marrow dysfunction with myelodysplasia and subsequently leukemias.9 Such proleukemic alterations in MSCs are therefore not unprecedented and point to many possibilities of future research on both normal and malignant hematopoiesis. Finally, as Ichii and coworkers demonstrate, the genetic modification of MSCs can alter their functional and differentiation properties dramatically. Because MSCs are used clinically to successfully treat various diseases, including GVHD10 and provide hematopoietic support, use of genetically altered MSCs offers a potential novel treatment modality.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal