In this issue of Blood, Toy et al identify recipient and blood product factors associated with increased risk of transfusion-associated acute lung injury (TRALI) using data derived from the largest active surveillance, prospective, case-controlled study.1 This study provides important new insights into our mechanistic understanding of an important clinical problem in transfusion medicine and validates the effectiveness of TRALI mitigating strategies that have been implemented.
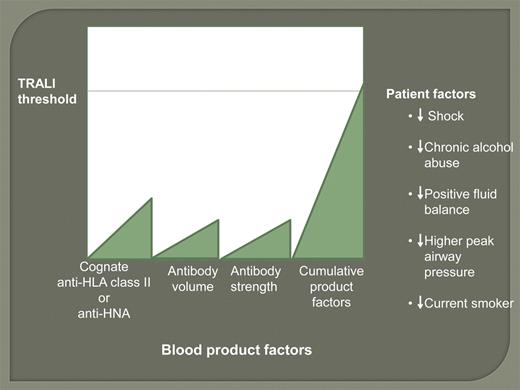
Threshold model of TRALI: On the y-axis the recipient's threshold for developing TRALI is lowered in the presence of shock, chronic alcohol abuse, intravascular volume overload, higher peak airway pressure while being mechanically ventilated, and current smoker. On the x-axis are the blood product factors (volume of high titer cognate HLA class II antibody or sufficient volume of high-titer HNA antibody), which are cumulative and together may trigger TRALI in the recipient if above the recipient threshold.
Threshold model of TRALI: On the y-axis the recipient's threshold for developing TRALI is lowered in the presence of shock, chronic alcohol abuse, intravascular volume overload, higher peak airway pressure while being mechanically ventilated, and current smoker. On the x-axis are the blood product factors (volume of high titer cognate HLA class II antibody or sufficient volume of high-titer HNA antibody), which are cumulative and together may trigger TRALI in the recipient if above the recipient threshold.
With the decrease of transfusion-transmitted diseases, TRALI is the most frequent cause of transfusion-associated mortality reported to the US Food and Drug Administration (FDA).2 Recipient and blood product factors have been implicated in TRALI in earlier studies.3 The present comprehensive study by Toy et al has shown that the recipient factors include high IL-8 levels, liver surgery, chronic alcohol abuse, shock, higher peak airway pressure while being mechanically ventilated, current smoking practice, and positive intravascular fluid balance. Blood product factors include plasma or whole blood from female donors, volume of high-titer HLA class II antibody, and volume of anti-HNA (human neutrophil antigen). Interestingly, blood factors not associated with increased risk of TRALI were noncognate or weak anti-HLA class II antibody, HLA class I antibody, and red blood cell products stored for long duration.
The decrease in TRALI reported in the US and throughout the world during the past few years is the result of implementing TRALI mitigation strategies focusing on eliminating transfusion of high-volume plasma products, usually platelets and plasma products, from donors at risk for or with leukocyte antibodies (HLA class I and II and HNA antibodies). These strategies include use of plasma and apheresis platelets from only males, never pregnant females, and HLA antibody negative females and use of solvent detergent plasma.3 In this study by Toy et al, with implementation of plasma TRALI mitigation strategies, the incidence decreased by ∼ 70% (1:4000 to 1:12 000 units transfused). Similar decreases have been reported from the United Kingdom and Germany through their biovigilance systems and also in a number of US institutions; some of which have reported no incidents of TRALI in recent years.4 TRALI-related fatalities reported to the FDA have decreased from 29 (16 associated with plasma) in 2005 to 13 (3 associated with plasma) in 2009.2 Although the downward trend is consistent, the risk per unit is higher in the study by Toy et al because of active surveillance compared with the other studies that used passive reporting systems, thus highlighting the significant underreporting of TRALI (and other transfusion reactions) to the transfusion service (in the Toy el al study, 40% were reported to the blood bank).
One significant message from the present study is that we need to move away from universal TRALI mitigation strategies to personalized strategies that include not only the product but, importantly, the patient in the risk calculations, and prioritize which factors to focus on. In the threshold model a smaller amount of cognate HLA class II antibody would trigger TRALI in a more susceptible recipient, such as someone with chronic alcohol abuse, yet a larger amount of cognate antibody is required to trigger TRALI in a patient without risk factors (see figure). As mitigation requires multiple strategies, the transfusion medicine community has to adapt to development of new approaches for patient-specific transfusion practice. For example, these strategies may include decreasing the amount of cognate antibody transfused to a higher-risk recipient, such as testing the donor for high titer antibody and the recipient for the corresponding antigen or eliminating the transfusion of red blood cells or other low-volume plasma products with HLA class II or HNA antibodies; and screening patients for their risk and preemptively taking action to decrease their risk, such as improving nutrition for alcoholics or decreasing airway pressures in patients on mechanical ventilation. These steps would require careful, individualized patient intervention and subsequent monitoring of the outcome. In the current environment with improved electronic medical records, the necessary data can be collected and effective protocols can be developed to further decrease the risk of TRALI and, if TRALI did occur, result in rapid identification and treatment.
Toy et al's study demonstrates that patient-focused strategies can decrease the risk of TRALI. Appropriate management of critically ill patients decreases their risk of acute lung injury. In addition to implementing process improvements to decrease TRALI, Mayo Clinic Rochester implemented measures to decrease risk of ALI (nontransfusion-related) in its critical-care population (from 82 to 39 cases per 100 000 person-years), including intensivist 24-hour coverage of the intensive care unit, decrease and ensure appropriate transfusion, ventilator, and sepsis management, pneumonia treatment, critical care protocols, and electronic medical records.5 These are the initial steps to mitigating TRALI from both sides—patient and product.
The study by Toy et al leaves to further investigation the 25% to 50% of cases that appear not to be related to leukocyte antibodies in the blood products. Are these patients at high risk of acute lung injury with requirement of minimal additional neutrophil stimulation from a blood product (or another coincidental event) or are there other product factors that this study was unable to identify?
The allure of this study is the incorporation of the patient into the TRALI model in a systematic approach. The study enables the transfusion community to realize how far we have come and where we need to go to fully mitigate TRALI. It is time for us to refine our testing, improve our algorithms, and include the patient.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■