Even in the setting of intensive, multiagent chemotherapy treatment, interpatient variability in pharmacokinetics and adequate dosing are keenly important to the successful treatment of acute lymphoblastic leukemia (ALL). In this issue of Blood, Kawedia et al report the findings of their pharmacologic studies of dexamethasone and asparaginase, which highlight the significant impact that interpatient variability in dexamethasone and asparaginase drug exposure has on response to treatment and risk of relapse.1
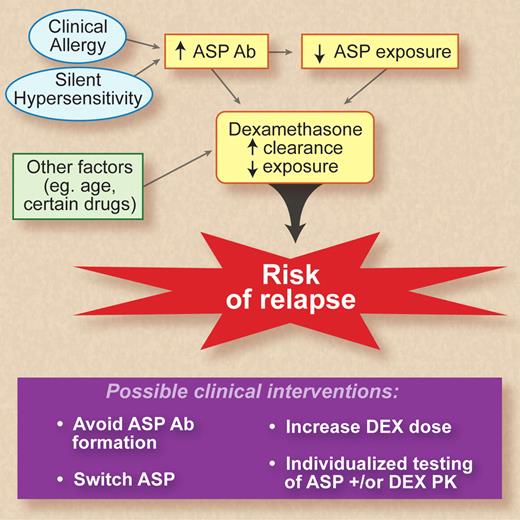
This schematic diagram demonstrates the relationships between asparaginase (ASP) and dexamethasone (DEX) pharmacokinetics and risk of relapse as described by Kawedia et al.1 Clinical allergy and silent hypersensitivity are known to result in formation of anti-asparaginase antibodies (Abs). The authors also identify older age and several drugs that are associated with increased clearance of dexamethasone. The purple box details clinical strategies that may compensate for the altered pharmacokinetics (PK) and thus avoid the deleterious effects in terms of treatment outcome. Professional illustration by Debra T. Dartez.
This schematic diagram demonstrates the relationships between asparaginase (ASP) and dexamethasone (DEX) pharmacokinetics and risk of relapse as described by Kawedia et al.1 Clinical allergy and silent hypersensitivity are known to result in formation of anti-asparaginase antibodies (Abs). The authors also identify older age and several drugs that are associated with increased clearance of dexamethasone. The purple box details clinical strategies that may compensate for the altered pharmacokinetics (PK) and thus avoid the deleterious effects in terms of treatment outcome. Professional illustration by Debra T. Dartez.
The story of corticosteroids and asparaginase as part of successful therapy for childhood leukemia dates back 50 years to a time when ALL in children was always fatal. Fast forward to 2011, cure rates approach 90%, and dexamethasone and asparaginase are now essential components of every successful treatment regimen for ALL. Limited numbers of pharmacokinetic studies of dexamethasone in children demonstrate marked intra- and interpatient variability,2 but the impact of such variations in systemic drug exposure on treatment outcomes has not been studied. Asparaginase studies have identified development of anti-asparaginase antibodies related to clinical allergy or “silent hypersensitivity” as a potential cause of diminished drug exposure, but the clinical consequences of such interpatient variations remain controversial.3-5
Kawedia and colleagues report their investigations that demonstrate an association among dexamethasone clearance, development of anti-asparaginase antibodies, the consequent decreased drug exposure to both agents, and a detrimental effect on clinical efficacy in terms of risk of relapse. Based on their previous observation of an association between lower asparaginase (either because of lower dose, after allergy, and/or development of neutralizing antibodies), higher serum albumin, higher clearance rate of dexamethasone and consequently lower exposure to dexamethasone,6 the investigators prospectively evaluate the clinical consequences of these individual variations in drug exposure in terms of treatment outcome. As hypothesized, they show that development of anti-asparaginase antibodies results in increased dexamethasone clearance. Those patients with lower exposure had an increased risk of relapse; specifically, risk of any relapse and risk of CNS relapse. Development of asparaginase antibodies was independently associated with increased risk of CNS relapse. To emphasize the relationship between systemic drug exposure of dexamethasone and asparaginase and treatment outcome, the figure shows these findings as a schematic diagram.
Given the novel asparaginase-dexamethasone pharmacokinetic interaction described by Kawedia et al, there are several simple strategies that may minimize allergic reactions to asparaginase and have the double benefit of improved effectiveness of both asparaginase and dexamethasone therapy. Pretreatment with glucocorticoids is expected to decrease the likelihood of development of anti-asparaginase antibodies. Regimens that use a “continuous” asparaginase dosing schedule (eg, weekly Escherichia coli at 25 000 IU/M2) rather than “pulse” dosing (eg, E coli 10 000 IU/M2 3 doses/wk for 6 doses/course repeated over 6 weeks apart) are less immunogenic.7 Change to an alternate asparaginase preparation (eg, E coli or pegaspargase to Erwinia) is beneficial especially if done early with any suggestion of allergy either by clinical symptoms, measurement of asparaginase antibody, enzyme activity level, or a surrogate marker of asparaginase effect such as albumin.7-10 Development of a simple, inexpensive assay for anti-asparaginase antibody or asparaginase enzyme activity that could be readily accessible to clinicians would allow prospective modifications of both asparaginase and dexamethasone dosages to optimize exposure for both drugs in a single patient. Such assays would be particularly useful to identify patients who are at risk for diminished asparaginase effect as well as increased dexamethasone clearance and decreased dexamethasone exposure because of “silent hypersensitivity.” The phrase “silent hypersensitivity” is used to describe the phenomena of development of anti-asparaginase antibodies and frequently increased clearance rate of asparaginase without overt signs of allergic reaction.7 These approaches, however, have not been evaluated in a prospective trial.
Dexamethasone and asparaginase together during induction therapy have proven very effective. Because of significant toxicities, however, the use of this combination has been limited to our youngest and lower-risk patients. In fact, our higher-risk patients including adolescents and young adults as well as patients with T-cell disease stand to benefit the most from this combination as long as the risks could be minimized. The data suggest that re-examination of induction regimens that use asparaginase and dexamethasone is warranted to test if optimal asparaginase effect (because asparaginase antibody formation is rare during induction) would allow a lower dexamethasone dose to be used. The lower dose of dexamethasone would be feasible if it provided systemic exposure that was efficacious yet not toxic in terms of increased risk of infection and severe osteonecrosis. As shown in the figure, there are additional factors such as age and concomitant treatment with certain drugs that also influence drug exposure to dexamethasone. The major challenge will be in defining the optimal dexamethasone exposure that balances efficacy and toxicity within the context of concurrent asparaginase therapy for all age groups, risk categories, and immunophenotypes.
Despite the numerous advances in diagnosis, intensive risk-directed therapy, and supportive care measures, some children are not cured. This clinical pharmacologic study underscores the importance of individualized therapy: the right dose for the right patient. One size does not fit all.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal