Abstract
The Really Interesting New Gene (RING) Finger Protein 4 (RNF4) represents a class of ubiquitin ligases that target Small Ubiquitin-like Modifier (SUMO)–modified proteins for ubiquitin modification. To date, the regulatory function of RNF4 appears to be ubiquitin-mediated degradation of sumoylated cellular proteins. In the present study, we show that the Human T-cell Leukemia Virus Type 1 (HTLV-1) oncoprotein Tax is a substrate for RNF4 both in vivo and in vitro. We mapped the RNF4-binding site to a region adjacent to the Tax ubiquitin/SUMO modification sites K280/K284. Interestingly, RNF4 modification of Tax protein results in relocalization of the oncoprotein from the nucleus to the cytoplasm. Overexpression of RNF4, but not the RNF4 RING mutant, resulted in cytoplasmic enrichment of Tax. The RNF4-induced nucleus-to-cytoplasm relocalization was associated with increased NF-κB–mediated and decreased cAMP Response Element-Binding (CREB)–mediated Tax activity. Finally, depletion of RNF4 by RNAi prevented the DNA damage–induced nuclear/cytoplasmic translocation of Tax. These results provide important new insight into STUbL-mediated pathways that regulate the subcellular localization and functional dynamics of viral oncogenes.
Introduction
Human T-cell Leukemia Virus Type 1 (HTLV-1) is the etiological agent for adult T-cell leukemia.1 Immortalization and transformation of T lymphocytes can be attributed to the expression and activity of the viral oncoprotein Tax.2 Although the exact mechanism of Tax-mediated transformation is unknown, studies indicate that Tax expression results in genomic instability via chronic disruption of the cellular DNA damage response.3 It is generally accepted that cellular transformation is a by-product of the long period of genomic instability.
Tax exhibits pleiotropic functionality, which is at least partly regulated by subcellular localization to nuclear or cytoplasmic compartments.3,4 We demonstrated previously that Tax shuttles between the nucleus and cytoplasm.5 The mechanism for the regulation of Tax localization is unknown, although localization-specific structural elements have been uncovered. The Tax protein contains a nuclear localization signal,6 and we recently identified a specific signal sequence that targets Tax to nuclear bodies.7 We and others have described a nuclear export signal.5,8 In addition, Lamsoul et al showed that ubiquitylation of Tax is correlated with its accumulation in the cytoplasm, whereas sumoylation of Tax is required for nuclear localization.9 Therefore, the molecular switch for nuclear versus cytoplasmic localization of Tax at least in part depends on the protein modifiers Small Ubiquitin-like Modifier (SUMO) and ubiquitin, but the mechanism underlying this switch is unknown.
Coincident sumoylation and ubiquitylation of a substrate protein is common, and in some cases, these modifications work cooperatively to regulate specific biologic processes (for review, see Ulrich10 ). The recent discovery of a novel class of Really Interesting New Gene (RING)–domain proteins called SUMO-targeted ubiquitin ligases (STUbLs) has contributed to our understanding of how ubiquitylation of proteins is regulated. STUbL proteins contain SUMO-interacting motifs (SIMs) to interact with the SUMO or SUMO-like domains of their ubiquitylation targets. Therefore, STUbLs are well suited to play an important role in the cross-talk between SUMO and ubiquitin pathways.11 The role of STUbLs was first realized by studying 2 RING domain–containing proteins, Slx5 and Slx8, in budding and fission yeast.12-15 The Slx5/Slx8 complex was subsequently found to mediate quality control of a transcriptional regulator, Mot1, and degradation of the MATalpha2 repressor in vivo.16,17 More recently, the human STUbL protein RNF4, the ortholog to Slx5/Slx8, was found to degrade the sumoylated PML-RAR oncoprotein.18,19 The previously reported effects of ubiquitin and SUMO on Tax localization prompted our analysis of a role for RNF4 in this process.
In the current study, we demonstrate that RNF4 plays an important role in regulating nuclear-cytoplasmic localization of Tax. Specifically, we show that RNF4 interacts with Tax and that Tax is a substrate for ubiquitylation by RNF4. We also demonstrate that RNF4 expression alters the functional profile of Tax by inducing nuclear-to-cytoplasmic relocalization. Furthermore, antisense suppression of RNF4 inhibits the damage-induced nuclear export of Tax. Our data support a model by which RNF4 regulates the subcellular localization and function of HTLV-1 Tax in response to genotoxic stress.
Methods
Plasmids and Abs
The HpX, S-TaxGFP, and SGFP expression vectors (where GFP indicates green fluorescent protein) and Tax deletion mutants have been described previously.7,20 Tax double-point mutant S-TaxK280/284R-GFP was created using site-directed mutagenesis of S-TaxGFP with the QuickChange kit (Stratagene) and primers TCCTCCTTTATATTTCACAGATTTCAA and GGGGTGGTAGGCCCTGGTTTGAAA. RNF4-GFP and RNF4-mCherry were purchased from Genecopoeia. MBP-RNF4 was created by PCR-mediated amplification of the RNF4 open reading frame and subcloning into EcoRI/HindIII restriction of the pMALcTH vector (a gift from Sean Prigge, Johns Hopkins School of Public Health, Baltimore, MD). MBP-RNF4 RING Mutant and RNF4-mcherry RING Mutant were generated through site-directed substitution of cystines 132 and 135 to serines using the primers GGTACTGTCAGTTCTCCGATATCCATGGACGGATACTCAGC and GCTGCGTATCCGT CCATGGATATCGGAGAACTGACAGTACC. The SUMO-T7-Tax vector was created by cloning the PCR-amplified tax open reading frame into gapped pET-SUMO vector (Invitrogen). This construct was then modified by addition of a T7 tag using site-directed mutagenesis with primers GAACAGATTGGTATGGCTAGCATGACTGGTGGACAGCAAAATGGGTATGGCCCACTTC and GAAGTGGGCCATACCCATTTGCTGTCCACCAGTCATGCTAGCCATACCAATATGTTC. The following Abs were used for immunofluorescence analysis: anti-Tax rabbit polyclonal Ab21 (1:1000) and anti-SC35 mouse mAb (1:1000; Invitrogen). The following Abs were used for immunoblot analysis: anti-GFP rabbit polyclonal Ab (1:1000; Santa Cruz Biotechnology); anti-RNF4 mouse polyclonal Ab (1:1000; Gene Tex), anti-Tax rabbit polyclonal Ab (1:1000), and anti-hemagglutinin (anti-HA) rabbit polyclonal Ab (1:4000; Abcam); and anti-MBP rabbit polyclonal Ab (1:10 000; New England Biolabs).
Cell culture and transient transfections
The HTLV-1–infected T-cell lines C8166 and HUT102 were maintained in RPMI 1640 medium and human embryonic kidney 293T (HEK 293T) cells (which express SV40 T-Ag) were maintained in DMEM, each supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2. Transfections of adherent cells were performed with the standard calcium phosphate precipitation method. T-lymphocyte cell lines were transfected with the Neon Microporation System (Invitrogen) according to the manufacturer's instructions. For S-bead purification and Western blotting assays, cells were plated in 100-mm plates at 2 × 106 cells per plate. At 48 hours after transfection, cells were washed once in PBS and lysed with 400 μL of mammalian protein extraction reagent (M-PER; Pierce) with protease inhibitor cocktail and immediately frozen at −80°C. For transcriptional activation assays, cells were grown in 6-well plates at 2 × 105 cells per well. At 48 hours after transfection, cells were washed once in PBS and harvested for luciferase assays.
Immunofluorescence confocal microscopy
HEK 293T cells were seeded at 1 × 105 cells/well on ethanol-washed 22-mm diameter coverslips in 6-well plates. After 48 hours, the cells were washed 3 times with ice-cold PBS and fixed in 4% paraformaldehyde/PBS for 12 minutes at room temperature. Coverslips were washed twice with PBS, permeabilized with methanol for 2 minutes at room temperature, washed 3 times with PBS, and incubated overnight in a humidifying chamber at 4°C with primary Abs diluted in 3% BSA-PBS. Cells were washed twice with PBS/0.1% Tween 20 (PBS-Tween) and twice with PBS, and then incubated for 1 hour at room temperature with Alexa Fluor 488/594 secondary Abs and TO-PRO-3′ iodide (Molecular Probes) diluted 1:1000 with BSA-PBS. Coverslips were washed twice with BSA-PBS and twice with PBS and then mounted on glass slides using Vectashield with 4′,6-diamidino-2-phenylindole (Vector Laboratories). Confocal fluorescent images were acquired on an LSM 510 confocal microscope (Carl Zeiss) using argon (488 nm), HeNe1 (543 nm), and HeNe2 (633 nm) lasers with a 63× objective oil lens and a 2× zoom and imaged with LSM Image Browser Version 4.2 software.
S-TaxGFP and RNF4 binding assay
Lysates from HEK 293T cells expressing GFP-RNF4 and coexpressing either S-TaxGFP, S-TaxGFP Mutants, or SGFP were normalized to total protein using the Bradford protein assay. Lysates were performed in M-PER with added protease inhibitors. Next, 500 μL of normalized extract was combined with 150 μL of a 50/50 slurry of S-protein agarose beads (Novagen). Beads were incubated for 30 minutes at room temperature with rotation and centrifuged at 500g for 5 minutes at 4°C. Supernatants were removed and the beads washed 3 times with 1 mL of washing buffer (20mM Tris-HCl, pH 7.5, 150mM NaCl, and 0.1% Triton X-100). Purified S-tagged protein complexes were eluted from the beads by the addition of Laemmli buffer and incubation at 100°C for 10 minutes. Supernatants containing the isolated protein complexes were separated via 8%-12% gradient SDS-PAGE, transferred to Immobilon-P membrane (Millipore), and subjected to immunoblot analysis.
Immunoblot analysis
Protein-impregnated membranes were blocked for 1 hour at room temperature in Odyssey blocking buffer (LI-COR Biosciences). Primary Abs diluted in blocking buffer were applied to membranes and incubated at 4°C overnight on an orbital shaker. Membranes were washed 4 times for 5 minutes with PBS-Tween. LI-COR Odyssey secondary Abs diluted in Odyssey blocking buffer with 0.5% SDS and 0.5% Tween 20 were applied at a concentration of 1:20 000 and incubated with the membranes for 1 hour at room temperature in the dark with shaking. Membranes were washed 4 times with PBS-Tween and scanned with a LI-COR Odyssey scanner.
Transcriptional transactivation assay
HEK 293T cells were transiently transfected with 1 μg of plasmid DNA for either pHTLV-LTR-Luciferase or pNF-κB-Luciferase (Clontech) reporter constructs. In some cases, the same cells were cotransfected with 0-5μg of pRNF4-GFP and/or 1 μg of S-TaxGFP plasmid DNA. Total DNA per transfection was normalized to 6μg per well with the addition of pTriEx4-Neo (Novagen). Cells were harvested 48 hours after transfection by washing once with ice-cold PBS and lysing in 400 μL of reporter/lysis buffer (Promega). Lysates were immediately frozen at −80°C. Samples were thawed on ice and the protein concentration was determined. A total of 1 μg of protein from each sample was applied to 100 μL of luciferase assay substrate and luciferase activity was measured in a Turner TD 20/20 luminometer. Transcriptional activation was analyzed and is expressed as the fold activation over reporter alone. All assays were performed in triplicate.
shRNA knockdown of RNF4
HEK 293T cells were seeded at a density of 2 × 105 cells per well 24 hours before infection. Cells were infected with lentiviral particles containing either control shRNA or shRNA against RNF4 in serum-free medium supplemented with Polybrene. Infection medium was replaced with complete medium 24 hours after infection. Cells were split at 48 hours after infection, and those expressing shRNA were selected by the addition of 10mM puromycin on the day 4 after infection. Knockdown of RNF4 expression was verified by Western blotting. Clones displaying highly efficient knockdown of RNF4 were expanded under selection with puromycin.
In vivo ubiquitylation assay
Plasmids expressing S-TaxGFP and HA-ubiquitin were transiently transfected into either control or RNF4-depleted cells with or without the RNF4-mcherry or RNF4-mcherry RING mutant. Forty-eight hours after transfection, cells were treated with 10μM MG132 (AG Scientific) and 5mM N-ethylmaleimide for 30 minutes followed by harvesting in M-PER with 5mM N-ethylmaleimide. Lysates were subjected to S-bead purification and immunoblot analysis as described previously. Ubiquitylated Tax was detected by polyclonal anti-Tax Ab and polyclonal anti-HA Ab.
In vitro ubiquitylation assay
Plasmids expressing Maltose Binding Protein-RNF4 (MBP-RNF4), MBP-RNF4 RING mutant (C132/153S), or SUMO-T7-Tax were transformed into BL21 cells under selection by kanamycin or ampicillin. Transformants were grown to the log phase in 200 mL of SOC medium with the appropriate antibiotics, induced with 0.4mM isopropyl β-D-1-thiogalactopyranoside, and harvested 3 hours after induction. Cells were pelleted in PBS plus protease inhibitors, flash frozen, sonicated, and the proteins were affinity purified. MBP-RNF4 and MBP-RNF4 RING mutant were purified on amylase resin. SUMO-T7-Tax was purified on Talon affinity resin following the manufacturer's protocol (Invitrogen) and quantified with the Bradford assay. The E2 ligase Ubc4 was induced and purified as described previously.14 Poly-SUMO-2 chains were purchased from Boston Biochem. Ubiquitylation reactions were performed in the presence or absence of ATP, Ubc4 (E2 ligase), RNF4 (E3 ligase), and SUMO-T7-Tax (substrate), and reactions were allowed to proceed for 3 hours at 30°C. Ubiquitylation reactions were stopped by the addition of 2× SUTEB buffer (1% SDS, 8M urea, 10mM Tris, pH 8, 10mM EDTA, and 0.01% bromophenol blue) and incubation at 65°C for 10 minutes. Proteins in the reactions were separated by 4%-12% gradient SDS-PAGE followed by immunoblot analysis. Modification of Tax by addition of ubiquitin was detected with anti-Tax (1:1000) or anti-ubiquitin (1:4000) polyclonal Ab (Enzo Biochem). Modification of poly–SUMO-2 chains was detected with polyclonal anti–SUMO-2 (1:3300) Ab (Enzo Biochem).
Induction of DNA damage by ionizing radiation
HEK 293T cells expressing RNF4 shRNA or control shRNA were seeded onto glass coverslips at a density of 1 × 106 cells per well and transiently transfected with pS-TaxGFP. Cells expressing RNF4 shRNA were also transfected with pS-TaxGFP and pRNF4-mCherry. Cells were subjected to a single dose of 10 Gy ionizing radiation at 48 hours after transfection, allowed to recover for 30 minutes, and fixed as described previously for confocal microscopy analysis. Tax localization was observed for 100 Tax-expressing cells for each data point.
Results
Expression of RNF4 results in enrichment of Tax in the cytoplasm
Tax is predominantly localized to nuclear bodies that we previously termed Tax speckled structures (TSSs).21 Tax is also found in the cytoplasmic space and subcellular localization is influenced by ubiquitylation. Because it has been demonstrated that the subcellular distribution of Tax is affected by both sumoylation and ubiquitylation,9,22 we reasoned that RNF4, a member of the recently identified STUbL family of proteins, might be involved in regulating Tax ubiquitylation. To test this hypothesis, we used confocal microscopy to observe the cellular localization of Tax after overexpression of RNF4. Before exogenous expression of RNF4, Tax localization was predominately within nuclear TSS's overlapping with the splicing factor SC35 (Figure 1 row 1). As observed by microscopy, 38% of cells displayed an exclusive TSS localization, approximately 46% of cells displayed localization in TSS with partial cytoplasmic localization (mixed), and the remaining 16% of cells displayed an exclusive cytoplasmic localization (Figure 2A-B). Herein, we refer to cytoplasmic bundles, perinuclear staining, and diffuse cytoplasmic staining as cytoplasmic localization. Consistent with a previous study,23 RNF4 was localized to the nucleus (Figure 1 rows 2-4). Interestingly, when RNF4 was coexpressed with Tax, there was an approximately 5-fold increase in cytoplasmic-only expression, with a concomitant 10-fold decrease in TSS-only expression of Tax (Figure 1 third row and Figure 2B). The localization of RNF4 remained diffusely nuclear. The observed RNF4-dependent relocalization was specific to Tax, as evidenced by the localization of the TSS-resident protein sc35, which remained unaltered (Figure 1 row 2). The RNF4-dependent cytoplasmic enrichment of Tax required an intact RING domain of RNF4. Overexpression of the RNF4 RING mutant, in which cysteines 132 and 135 of RNF4 were altered to serines, ablating RNF4 ubiquitin ligase activity failed to initiate cytoplasmic redistribution (Figure 1 bottom row and Figure 2B). Interestingly, overexpression of the RING mutant was associated with a loss of the cytoplasmic-only Tax localization pattern, suggesting a transdominant phenotype. These data suggest that RNF4 expression regulates Tax localization.
RNF4 expression induces cellular redistribution of Tax. HEK 293T cells were transiently transfected to express RNF4-GFP (RNF4), RNF4-mCherry RING Mutant (RNF4 RM), and/or native Tax (Tax). At 48 hours after transfection, cells were fixed and subjected to immunofluorescence confocal microscopy. RNF4-GFP and RNF4 RM-GFP were detected by direct fluorescence. Tax and sc35 were detected by indirect immunofluorescence using the appropriate anti–rabbit or anti–mouse Alexa Fluor–conjugated secondary Abs. Row 1 shows the expression of native Tax (Tax, green) and endogenous SC35 (sc35, red). Row 2 shows the expression of RNF4-GFP (RNF4, green) and endogenous SC35 (sc35, red). Row 3 shows the expression of RNF4-GFP (RNF4, green) and native Tax (red). Row 4 shows the expression of RNF4 RING mutant (green) and native Tax (Tax, red). Nuclei of cells were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO (blue). Each row shows expression of the individual protein and an overlay image (Merged).
RNF4 expression induces cellular redistribution of Tax. HEK 293T cells were transiently transfected to express RNF4-GFP (RNF4), RNF4-mCherry RING Mutant (RNF4 RM), and/or native Tax (Tax). At 48 hours after transfection, cells were fixed and subjected to immunofluorescence confocal microscopy. RNF4-GFP and RNF4 RM-GFP were detected by direct fluorescence. Tax and sc35 were detected by indirect immunofluorescence using the appropriate anti–rabbit or anti–mouse Alexa Fluor–conjugated secondary Abs. Row 1 shows the expression of native Tax (Tax, green) and endogenous SC35 (sc35, red). Row 2 shows the expression of RNF4-GFP (RNF4, green) and endogenous SC35 (sc35, red). Row 3 shows the expression of RNF4-GFP (RNF4, green) and native Tax (red). Row 4 shows the expression of RNF4 RING mutant (green) and native Tax (Tax, red). Nuclei of cells were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO (blue). Each row shows expression of the individual protein and an overlay image (Merged).
Quantitation of RNF4-induced Tax cellular redistribution. (A) HEK 293T cells were transiently transfected to express TaxGFP and at 48 hours after transfection, Tax localization was classified into 3 groups (cytoplasmic, mixed, and TSS). Shown are representative examples of each expression phenotype. (B) Quantitation of Tax distribution. Shown is the relative distribution of Tax-GFP localization alone, after coexpression of Tax-GFP and RNF4-mCherry (+ RNF4), and after coexpression of Tax-GFP and RNF4-mCherry RM (+ RNF4 RM). (C) The effect of RNF4 overexpression on the basal activity of the HTLV-1–LTR and the NF-κB–responsive promoter was assessed. Results are reported as the relative activity of the reporter alone and in the presence of RNF4 as determined by the luciferase enzyme assay. Also analyzed was the effect of RNF4 expression on Tax transcriptional activation of the HTLV-1–LTR (D) and the NF-κB–responsive promoter (E). Results are reported as the fold activation by Tax over basal transcription. (F) The impact of RNF4 expression on the activation of both HTLV-1–LTR and NF-κB–responsive promoters in HUT102 cells by endogenous Tax. Error bars indicate the SD of experiments performed in triplicate.
Quantitation of RNF4-induced Tax cellular redistribution. (A) HEK 293T cells were transiently transfected to express TaxGFP and at 48 hours after transfection, Tax localization was classified into 3 groups (cytoplasmic, mixed, and TSS). Shown are representative examples of each expression phenotype. (B) Quantitation of Tax distribution. Shown is the relative distribution of Tax-GFP localization alone, after coexpression of Tax-GFP and RNF4-mCherry (+ RNF4), and after coexpression of Tax-GFP and RNF4-mCherry RM (+ RNF4 RM). (C) The effect of RNF4 overexpression on the basal activity of the HTLV-1–LTR and the NF-κB–responsive promoter was assessed. Results are reported as the relative activity of the reporter alone and in the presence of RNF4 as determined by the luciferase enzyme assay. Also analyzed was the effect of RNF4 expression on Tax transcriptional activation of the HTLV-1–LTR (D) and the NF-κB–responsive promoter (E). Results are reported as the fold activation by Tax over basal transcription. (F) The impact of RNF4 expression on the activation of both HTLV-1–LTR and NF-κB–responsive promoters in HUT102 cells by endogenous Tax. Error bars indicate the SD of experiments performed in triplicate.
RNF4-induced relocalization alters Tax activity
The expression of Tax in the cytoplasm and in the nucleus of cells has been associated with distinct activities. Activation of the HTLV-1 long-terminal repeat (HTLV-LTR), a viral promoter, provides a convenient measure of the “nuclear” activity of Tax. Likewise, Tax-mediated activation of an NF-κB–responsive promoter is an indicator of “cytoplasmic” activity, because the ability of Tax to activate the NF-κB–responsive promoters is dependent on an initial cytoplasmic interaction with NEMO.24-27 We reasoned that if RNF4 mediates the relocalization of Tax to the cytoplasm, then we should observe a reduction in the activation of the HTLV-1–LTR with a concomitant increase in activation of the NF-κB–responsive promoter. RNF4 expression had little or no effect on the basal activity of the HTLV-LTR (Figure 2C). However, we observed a dose-dependent decrease in basal transcription of the NF-κB–responsive promoter. We next examined the effect of RNF4 on Tax-induced transactivation of each promoter. Consistent with previous reports, Tax expression resulted in a 40-fold activation of the HTLV-1–LTR. Interestingly, coexpression of RNF4 led to a dose-dependent decrease in Tax-induced activation of the HTLV-LTR (Figure 2D). The activation of the HTLV-LTR by Tax was reduced by 30-fold in the presence of RNF4. In contrast, when we measured the ability of Tax to transactivate the NF-κB–Luc reporter, we observed a more than 2-fold increase in activation (Figure 2E). Figure 2C shows the RNF4-induced 10-fold decrease in basal activity of the NF-κB–responsive promoter (Figure 2C). When this basal activity is used the estimated fold activation of the NF-κB–responsive promoter by Tax is approximately 20-fold, comparable to the decrease in activity from the HTLV-LTR.
We also examined the impact of RNF4 expression in the Tax-expressing HTLV-1–transformed cell line HUT102. In these experiments, we relied on the endogenous expression of Tax protein for activation of the luciferase reporter constructs. As reported elsewhere, we observed only background activity from the HTLV-1–LTR (< 300 units) and NF-κB promoter (< 70 units) when transfected into the T-cell line Jurkat (data not shown). In the Tax-expressing background of HUT102 cells, each reporter was reproducibly activated (Figure 2F). The coexpression of RNF4 resulted in a dose-dependent decrease in HTLV-1–LTR activity and a concomitant dose-dependent increase in NF-κB activity. These results demonstrated that the activity of Tax is compartmentally regulated and that RNF4-induced relocalization results in a shift in the balance between “nuclear” and “cytoplasmic” activities.
RNF4 binds to Tax
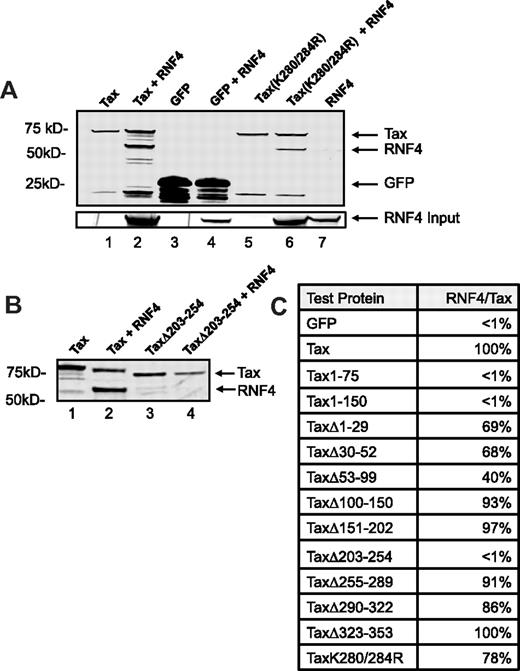
One possible mechanism for the RNF4-dependent relocalization of Tax may involve protein-protein interaction. Therefore, we tested the ability of RNF4 to bind Tax. Cells were transiently transfected to express S-GFP, S-Tax-GFP, or S-TaxK280/284R-GFP either alone or cotransfected with RNF4-GFP. S-tagged proteins complexes were subsequently purified from cell extracts, separated by SDS-PAGE, and subjected to immunoblot analysis using anti-GFP. Isolated Tax protein coprecipitated with RNF4, indicating that they interact in the same complex, whereas control GFP protein failed to interact with RNF4 (Figure 3A lanes 1-4). Similar results were obtained using S-Tax (data not shown). We also examined the ability of RNF4 to bind to a Tax mutant containing the double substitution K280/284R. These 2 lysine residues have been reported to be important sites for ubiquitin modification of Tax.9,28 Interestingly, the Tax double-point mutant retained the ability to interact with RNF4 (Figure 3A lanes 5-6), indicating that the interaction between RNF4 and Tax does not require prior modification of these sites. RNF4-GFP did not precipitate in the absence of S-tagged protein (Figure 3A lane 7).
RNF4 binds Tax. HEK 293T cells were transiently transfected to express SGFP, S-TaxGFP, or S-TaxGFP mutants with RNF4-GFP. At 48 hours after transfection, S-tagged protein complexes were isolated on S-protein agarose, separated by SDS-PAGE, and subjected to immunoblot analysis with a anti-GFP polyclonal Ab. (A) Affinity precipitation of S-TaxGFP (lanes 1 and 2), SGFP (lanes 3 and 4), or S-TaxK280/284RGFP (lanes 5 and 6) in the presence or absence of cotransfected RNF4-GFP as indicated are shown. As a control for binding specificity, we also incubated S-beads with RNF4-GFP alone (lane 7). Shown is the immunoblot analysis with a polyclonal anti-GFP Ab. The migration of each GFP-fusion protein is indicated (arrows). Whole-cell lysates were normalized for expression of RNF4 (RNF4 input). (B) Affinity purification of S-TaxGFP and deletion mutant S-TaxΔ203-254GFP in the presence or absence of cotransfected RNF4-GFP. Indicated are the Tax/Tax mutant and RNF4 (arrows). (C) Relative binding efficiency of Tax and Tax mutants to RNF4. Densitometry analysis was used to determine the relative amount of RNF4 that coprecipitated with each of the Tax mutants. Binding efficiency was expressed as the ratio between recovered RNF4 and Tax or Tax mutant after subtraction of background. The binding of wild-type Tax to RNF4 was set at 100% and all other measures of binding efficiency were compared with this ratio.
RNF4 binds Tax. HEK 293T cells were transiently transfected to express SGFP, S-TaxGFP, or S-TaxGFP mutants with RNF4-GFP. At 48 hours after transfection, S-tagged protein complexes were isolated on S-protein agarose, separated by SDS-PAGE, and subjected to immunoblot analysis with a anti-GFP polyclonal Ab. (A) Affinity precipitation of S-TaxGFP (lanes 1 and 2), SGFP (lanes 3 and 4), or S-TaxK280/284RGFP (lanes 5 and 6) in the presence or absence of cotransfected RNF4-GFP as indicated are shown. As a control for binding specificity, we also incubated S-beads with RNF4-GFP alone (lane 7). Shown is the immunoblot analysis with a polyclonal anti-GFP Ab. The migration of each GFP-fusion protein is indicated (arrows). Whole-cell lysates were normalized for expression of RNF4 (RNF4 input). (B) Affinity purification of S-TaxGFP and deletion mutant S-TaxΔ203-254GFP in the presence or absence of cotransfected RNF4-GFP. Indicated are the Tax/Tax mutant and RNF4 (arrows). (C) Relative binding efficiency of Tax and Tax mutants to RNF4. Densitometry analysis was used to determine the relative amount of RNF4 that coprecipitated with each of the Tax mutants. Binding efficiency was expressed as the ratio between recovered RNF4 and Tax or Tax mutant after subtraction of background. The binding of wild-type Tax to RNF4 was set at 100% and all other measures of binding efficiency were compared with this ratio.
The region in Tax responsible for interaction with RNF4 was determined by examining a series of Tax deletion mutants for their ability to bind RNF4. As described in the previous paragraph, the S-tagged Tax proteins were isolated and examined by SDS-PAGE and immunoblot analysis for RNF4 binding. As expected, we detected efficient copurification of RNF4 with Tax (Figure 3B lane 2); however, the deletion mutant TaxΔ203-254 failed to bind RNF4 (Figure 3B lane 4,) suggesting that this region is required to mediate the interaction between Tax and RNF4. Similar experiments were performed with a full panel of Tax deletion mutants. Densitometry analysis was used to determine the binding efficiency between RNF4 and each of the deletion mutants, and this binding was reported as a percentage of the binding observed for wild-type Tax and RNF4 (Figure 3C). Whereas several deletion mutants were reduced for RNF4 binding, only the mutant deleted for amino acids 203-254 displayed a total loss of RNF4 interaction. Likewise, Tax constructs expressing only the N-terminus of Tax, Tax1-75 and Tax1-150, failed to bind RNF4. These results define a Tax region, amino acids 203-254, that is required for interaction between Tax and RNF4.
Tax is ubiquitylated by RNF4
RNF4 is an E3 ubiquitin ligase that preferentially targets sumoylated proteins.13 Earlier studies have demonstrated that Tax can be both sumoylated and ubiquitylated, and that ubiquitylation is associated with cytoplasmic localization of Tax whereas sumoylation drives nuclear localization.9,28-30 Our findings that overexpression of RNF4 resulted in egress of Tax from the nucleus suggested that Tax may be a substrate for ubiquitylation by RNF4. To test this theory directly, we examined the requirement of RNF4 for in vivo ubiquitylation of Tax. We used lentiviral transduction of shRNA to establish a stable cell line in which RNF4 expression was reduced 3-fold (Figure 4A). We coexpressed S-Tax and HA-ubiquitin into the stable RNF4-knockdown or shRNA-expressing control cell lines. The S-Tax protein complex was isolated, separated on SDS-PAGE, and immunoprobed with anti-Tax. Tax protein isolated from the control cell line showed incorporation of ubiquitin, as determined by the presence of higher molecular weight bands cross-reactive with Tax Ab (Figure 4B lane 1). However, Tax protein isolated from the RNF4 knockdown cell line showed no evidence of incorporation of ubiquitin (Figure 4B lane 3). Overexpression of exogenous RNF4 resulted in rescue of the ubiquitylation phenotype in the RNF4 knockdown cells (Figure 4B lane 2). However, overexpression of the RNF4 RING mutant failed to rescue ubiquitylation of Tax in the knockdown line (Figure 4B lane 4). The same blot was stripped and then reprobed with an Ab against HA to visualize ubiquitylation. The presence of higher molecular weight HA-reactive smears was observed for Tax-associated protein complexes isolated from control cells (Figure 4C lanes 1-2), but not when derived from cells lacking RNF4 (Figure 4C lane 3), confirming that RNF4 expression is directly correlated with ubiquitylation in vivo. In lane 4, which represents knockdown cells transfected with the RNF4 RING mutant, we noted a lighter smear, possibly indicating coprecipitation of ubiquitylated cellular proteins. These data demonstrate that Tax can be ubiquitylated by RNF4 in vivo.
RNF4-mediated ubiquitylation of Tax in vivo. (A) Western blot analysis of endogenous RNF4 expression in RNF4-specific shRNA and control shRNA stably expressing cell lines. The electrophoretic migration of RNF4 is indicated. (B) Analysis of purified S-TaxGFP from HA-ubiquitin–expressing cells. S-TaxGFP was affinity precipitated from transiently transfected control shRNA and RNF4-specific shRNA (RNF4 knockdown) cells. Also shown are samples from Tax-expressing RNF4 knockdown cells that have been cotransfected with RNF4-mCherry or with RNF4-mCherry RM. (C) The same blot was probed with anti-HA Ab. (D) In vitro ubiquitylation of Tax by RNF4. Ubiquitylation reactions were assembled using recombinant, purified MBP-RNF4, SUMO-Tax, and Ubc4. The ubiquitylation assays were halted and separated by SDS PAGE and probed with anti-Tax Ab. Control reactions were assembled in the absence of ATP (−ATP), Ubc4 (−E2), MBP-RNF4 (−E3), or SUMO-Tax (−SUMO-Tax) as indicated. An ubiquitylation reaction with all components (Complete rxn) was also conducted. High-molecular weight adducts corresponding to ubiquitylated Tax is indicated (Ubn-Tax). Unmodified Tax protein (Tax) and molecular weights (in kDa) are indicated.
RNF4-mediated ubiquitylation of Tax in vivo. (A) Western blot analysis of endogenous RNF4 expression in RNF4-specific shRNA and control shRNA stably expressing cell lines. The electrophoretic migration of RNF4 is indicated. (B) Analysis of purified S-TaxGFP from HA-ubiquitin–expressing cells. S-TaxGFP was affinity precipitated from transiently transfected control shRNA and RNF4-specific shRNA (RNF4 knockdown) cells. Also shown are samples from Tax-expressing RNF4 knockdown cells that have been cotransfected with RNF4-mCherry or with RNF4-mCherry RM. (C) The same blot was probed with anti-HA Ab. (D) In vitro ubiquitylation of Tax by RNF4. Ubiquitylation reactions were assembled using recombinant, purified MBP-RNF4, SUMO-Tax, and Ubc4. The ubiquitylation assays were halted and separated by SDS PAGE and probed with anti-Tax Ab. Control reactions were assembled in the absence of ATP (−ATP), Ubc4 (−E2), MBP-RNF4 (−E3), or SUMO-Tax (−SUMO-Tax) as indicated. An ubiquitylation reaction with all components (Complete rxn) was also conducted. High-molecular weight adducts corresponding to ubiquitylated Tax is indicated (Ubn-Tax). Unmodified Tax protein (Tax) and molecular weights (in kDa) are indicated.
We next examined the ability of RNF4 to modify Tax in vitro. Because RNF4 is known to target sumoylated proteins for ubiquitylation, we used an in vitro ubiquitylation assay in which purified recombinant SUMO-Tax protein was subjected to ubiquitylation in the presence of purified recombinant RNF4. The ubiquitin ligase activity of purified recombinant RNF4 was confirmed in the in vitro assay by its ability to auto-ubiquitylate and ubiquitylate poly-SUMO chains (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). When Tax was tested as the substrate, we observed that Tax was able to form high molecular weight adducts corresponding to ubiquitin modification (Figure 4D). The efficient ubiquitylation required SUMO-Tax and the presence of ATP and was mediated through Ubc4, a ubiquitin E2 ligase. Subsequent experiments substituting the RNF4 RING mutant as the E3 ligase demonstrated that ubiquitylation of Tax by RNF4 required an intact RING domain (supplemental Figure 1). These data demonstrate that Tax is a substrate for RNF4 STUbL E3 ligase.
RNF4 is required for Tax nuclear egress after DNA damage
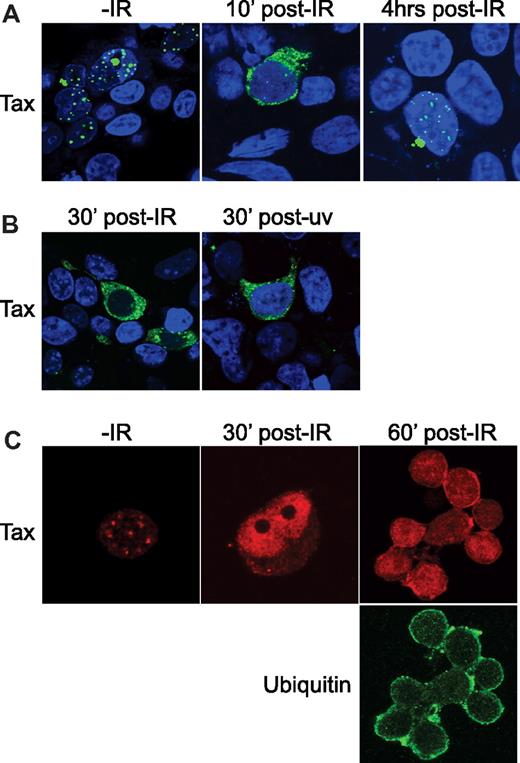
In previous studies by Marriott et al, DNA damage–induced genotoxic stress resulted in egress of Tax from the nucleus.22 Therefore, we investigated whether ubiquitylation by RNF4 was required to mediate nuclear egress. In our model, ionizing radiation (IR) was used, which we show is as effective as UV at inducing a rapid and transient nuclear egress of Tax (Figure 5A). We determined the optimal time point for observation of cytoplasmic Tax to be 30 minutes after damage (Figure 5B). We also demonstrate that exposure of the HTLV-1–transformed T-cell line C8166 to IR resulted in the relocalization of Tax from nuclear TSS to the cytoplasm (Figure 5C). Therefore, consistent with earlier observations, at least 2 different DNA-damaging agents elicit nuclear egress of Tax protein, and this response can be observed in multiple cell types, including HTLV-1–transformed T cells.
Ionizing radiation induces cytoplasmic accumulation of Tax. (A) HEK 293T cells were transfected to express Tax-GFP (green). Shown is a representative of Tax expression in cells at 40 hours after transfection before exposure (−IR) and at 10 minutes and 4 hours after exposure to 10 Gy of ionizing radiation (post-IR). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO and the images merged. (B) Tax-expressing HEK 293T cells were exposed to optimal doses of either UV or IR. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO and the images merged. (C) The HTLV-1–infected T-cell line C8166 was grown to the log phase, plated onto polylysine-coated slides, and prepared for immunofluorescence confocal microscopy. Endogenous Tax (red) was visualized by indirect immunofluorescence confocal microscopy before exposure to IR (−IR) and at 30 minutes and 60 minutes after exposure to 10 Gy (post-IR). At the 60-minute time point, cells were costained for ubiquitin (green).
Ionizing radiation induces cytoplasmic accumulation of Tax. (A) HEK 293T cells were transfected to express Tax-GFP (green). Shown is a representative of Tax expression in cells at 40 hours after transfection before exposure (−IR) and at 10 minutes and 4 hours after exposure to 10 Gy of ionizing radiation (post-IR). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO and the images merged. (B) Tax-expressing HEK 293T cells were exposed to optimal doses of either UV or IR. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO and the images merged. (C) The HTLV-1–infected T-cell line C8166 was grown to the log phase, plated onto polylysine-coated slides, and prepared for immunofluorescence confocal microscopy. Endogenous Tax (red) was visualized by indirect immunofluorescence confocal microscopy before exposure to IR (−IR) and at 30 minutes and 60 minutes after exposure to 10 Gy (post-IR). At the 60-minute time point, cells were costained for ubiquitin (green).
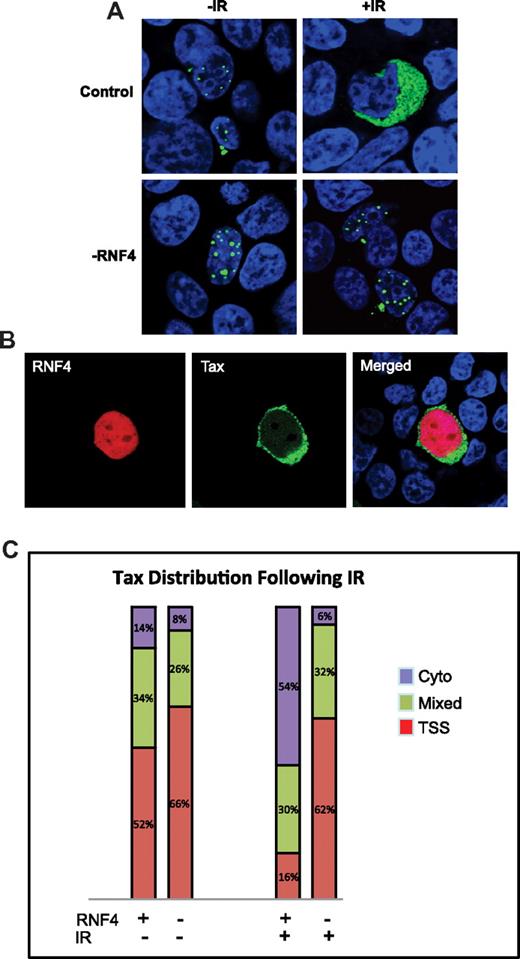
We further investigated whether this stress-induced rapid nuclear egress of Tax was mediated by RNF4. Stable RNF4 knockdown and control cells were transiently transfected to express Tax-GFP, treated with a single dose of IR, and allowed to recover for 30 minutes. Cells were then fixed, permeabilized, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO 3′ iodide. We observed the damage-induced nuclear-to-cytoplasmic redistribution of Tax in control shRNA cells. In the RNF4-depleted cell line, however, Tax did not relocalize after IR treatment and retained a normal expression pattern (Figure 6A,C). To validate that the results achieved were due explicitly to repression of RNF4, we rescued RNF4 by transient expression of exogenous RNF4 into the knockdown line. In these cells we observed that overexpression of RNF4 restored Tax relocalization to the cytoplasm after IR treatment (Figure 6B). The results from tabulating 50 for each phenotype are shown in Figure 6C. Expression of Tax in control and RNF4-depleted cells in the absence of IR treatment resulted in a Tax distribution comparable to that of parental cells (compare Figure 6C with Figure 2B). Interestingly, depletion of RNF4 alone resulted in an almost 50% reduction in cytoplasmic Tax and an approximately 20% increase in TSS-localized Tax. As expected, IR treatment of control shRNA cells resulted in a 4-fold increase in cells with exclusively cytosolic expression of Tax. These results demonstrate that cytoplasmic relocalization of Tax after DNA damage is regulated by physiologic levels of RNF4.
RNF4 expression required for DNA damage–induced relocalization of Tax. (A) RNF4-depleted cells (RNF4) or control cells (control) were transfected with S-TaxGFP. At 48 hours after transfection, the cells were either left untreated (−IR) or irradiated with 10 Gy (+IR) before recovery for 30 minutes. Cells were then fixed, counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO (blue), and Tax (green) was visualized using confocal microscopy. Shown is the overlay image. (B) RNF4-depleted cells were cotransfected with S-TaxGFP (green) and RNF4-mCherry (red). Cells were fixed and analyzed by confocal microscopy. Shown are the individual and overlay (Merged) images. (C) Quantitation of Tax redistribution after IR. The graph represents the relative cellular distribution of Tax within the control cells or the RNF4-depleted cells before and after ionizing radiation.
RNF4 expression required for DNA damage–induced relocalization of Tax. (A) RNF4-depleted cells (RNF4) or control cells (control) were transfected with S-TaxGFP. At 48 hours after transfection, the cells were either left untreated (−IR) or irradiated with 10 Gy (+IR) before recovery for 30 minutes. Cells were then fixed, counterstained with 4′,6-diamidino-2-phenylindole/TO-PRO (blue), and Tax (green) was visualized using confocal microscopy. Shown is the overlay image. (B) RNF4-depleted cells were cotransfected with S-TaxGFP (green) and RNF4-mCherry (red). Cells were fixed and analyzed by confocal microscopy. Shown are the individual and overlay (Merged) images. (C) Quantitation of Tax redistribution after IR. The graph represents the relative cellular distribution of Tax within the control cells or the RNF4-depleted cells before and after ionizing radiation.
Discussion
Before the previous study, there have been a few mammalian RNF4 substrates identified. One such substrate is the oncogenic fusion protein PML-RAR, in which the stability of PML/PML-RAR during cellular stress is regulated by RNF4-mediated ubiquitylation.18,19 More recently, poly(ADP-ribose) polymerase 1 (PARP-1a), centromere protein 1 (CENP-I), and hypoxia inducible factor 2α (HIF-2α) were identified as RNF4 substrates,31-33 solidifying an emerging role for STUbLs in cell homeostasis. RNF4 is known to preferentially ubiquitylate poly-SUMO2/3 chains and proteins modified with poly-SUMO2/3 chains via K48 ubiquitin linkage, thus targeting the substrate to the proteasome.18 All of the known RNF4 substrates have in common that their ubiquitylation results in proteasome-mediated degradation. In contrast, our results establish Tax as the first protein substrate of RNF4 for which modification regulates subcellular localization.
Tax is a potent regulator of a wide variety of cellular activities mediated through specific protein-protein interactions. One avenue for regulation of differential activities of this oncoprotein appears to be subcellular compartmentalization, for example, the localization of Tax to TSS nuclear bodies.21 These TSS's are populated with cellular proteins known to interact with Tax and to be involved in numerous cellular functions, including transcriptional activation and regulation of the DNA damage response.34-37 Tax is also present in the cytoplasm and has been observed to localize to IKK signalosomes within cytoplasmic structures.9,25,28-30 In the cytosol, Tax interacts with the IKK complex to trigger degradation of IκB and nuclear translocation of RelA.24,26,38-40 Tax also shepherds RelA into the nucleus,25 where RelA promotes the transcription of anti-apoptotic genes (for review, see Newmann and Naumann41 ). Clearly, the mechanisms that regulate subcellular localization of Tax will have profound impact on the functional consequences of expression.
Specific sequences within Tax that serve to signal nuclear localization,6 nuclear export,5,8 specific targeting to TSS's,7 and cellular secretory activity have been identified.42 It is also clear that posttranslational modification of Tax provides a rapid way to regulate subcellular localization, possibly via modification of the presentation of these defined localization signals. Most prominent of the reported Tax modifications with respect to subcellular localization is ubiquitylation/sumoylation.9,28,43,44 Although polyubiquitylation via K48 linkages signals degradation of Tax,45 the formation of ubiquitin chains via K63 is associated with cytosolic subcellular localization.29 Tax has also been shown to be modified with multiple SUMO moieties and possibly SUMO chains,9 and fusion of SUMO2 targeted Tax to the TSS nuclear bodies.22 We demonstrated previously that Tax shuttles between the nucleus and cytoplasm5 and, although NLS and NES signals may mediate the transport processes, it seems clear that ubiquitin modification provides the activating signal. Our present study establishes that RNF4, a SUMO-targeted ubiquitin ligase, provides a molecular regulatory switch between nuclear and cytoplasmic localization of Tax.
The process of substrate selection by RNF4, and STUbLs in general, is not precisely known,11,12 but it appears that the topography presented by regions known as SIMs is involved. The consensus sequence for a SIM is either fairly broad or not completely understood, but seems to include V/I:X:V/I:V/I or similar hydrophobic stretches.46 In our current study, we mapped Tax amino acids 202-254 as critical for interaction with RNF4. A structural examination of this region reveals a sequence (LIIL208) with homology to known SIM's. This region is separate from and just upstream of the lysine residues K280/K284, which were shown previously to be sites of sumoylation, ubiquitylation, and localization of Tax.9 Interestingly, mutation of these 2 residues, which prevents sumoylation of Tax, had no effect on Tax binding. In addition, an RNF4 RING mutant lacking ubiquitin ligase activity retained the ability to bind Tax. However, the RNF4 RING mutant failed to ubiquitylate Tax or rescue IR-induced nuclear egress, suggesting that RNF4 binding to its target substrate and modification of that substrate are independent and separable events. We were also able to demonstrate the dependence of RNF4 modification, at least in vitro, on sumoylation of Tax. However, we have not determined the site of Tax ubiquitylation or whether this modification depends on Tax sumoylation/polysumoylation. Likewise, our observation of discrete high molecular weight Tax products in the presence of RNF4 is supportive of polyubiquitylation, although the molecular details for Tax polyubiquitylation structure are still to be determined. Nevertheless, because cytosolic Tax has been shown to consist of K63-linked polyubiquitin chains, it is tempting to speculate that RNF4-mediated K63 polyubiquitylation is involved in the dynamic localization of Tax. It is also interesting to speculate whether Tax becomes disengaged from TSS's before RNF4-mediated ubiquitylation or if ionizing radiation serves to recruit RNF4 to chromatin-bound nuclear bodies.
Viruses maintain a fine line between manipulating cellular processes to ensure viral survival and preserving viability of their cellular hosts. HTLV-1 is an extremely successful virus in terms of long-term maintenance of this virus/host balance. Less than 3% of infected individuals will become symptomatic, and usually the virus and host coexist.47 RNF4-dependent Tax nuclear export may provide a survival advantage for both the virus and the host during cellular stress. Independent of DNA damage, we have shown that Tax sequesters several DDR proteins into TSS's, including Chk2, DNA-PK, BRCA1, and MDC1, presumably as a means of regulating cellular response to stress.34,36,37,48 The recruitment of these DDR proteins to TSS's impairs the cellular response to DNA damage repair. Therefore, activation of RNF4 and ubiquitylation and relocalization of Tax during cellular stress could serve to release these DDR proteins in an attempt to restore the cellular DNA damage response. Tax relocalization to the cytoplasm may also induce an anti-apoptotic response through activation of the NF-κB pathway. This would push the host cell toward survival despite DNA insult and cell-cycle arrest. A recent study found that USP20 deubiquitylates Tax49 and this process may work in tandem with RNF4 to regulate Tax nuclear-cytoplasmic balance. We present in Figure 7 a model for RNF4 regulation of Tax.
Model of RNF4-mediated Tax relocalization. The HTLV1 Tax protein can sequester DNA-repair proteins (eg, DNA-PK and Chk2) into TSS's and also transactivate the promoters of host genes (eg, those responsive to CREB, AP-1, or p300/CBP). Tax enters the nucleus as a multimer, coincident with sumoylation, and forms TSS. After DNA damage, RNF4 targets sumoylated Tax for ubiquitylation resulting in nuclear egress of Tax. In the cytoplasm, interactions between Tax and NEMO lead to activation of the NF-κB pathway. Our work shows that this virus has adopted a sophisticated anti-apoptotic approach that depends on the STUbL RNF4.
Model of RNF4-mediated Tax relocalization. The HTLV1 Tax protein can sequester DNA-repair proteins (eg, DNA-PK and Chk2) into TSS's and also transactivate the promoters of host genes (eg, those responsive to CREB, AP-1, or p300/CBP). Tax enters the nucleus as a multimer, coincident with sumoylation, and forms TSS. After DNA damage, RNF4 targets sumoylated Tax for ubiquitylation resulting in nuclear egress of Tax. In the cytoplasm, interactions between Tax and NEMO lead to activation of the NF-κB pathway. Our work shows that this virus has adopted a sophisticated anti-apoptotic approach that depends on the STUbL RNF4.
A stress-induced protein relocalization strategy would not be unique to HTLV-1–infected cells. The cyclin-dependent kinase inhibitor p21cip1 is relocalized and degraded in response to cellular stress induced by exposure to reactive oxygen species. In cells exposed to H2O2, p21CIP1 is rapidly relocalized to the cytoplasm, where it undergoes ubiquitin-mediated proteasomal degradation.50 This reactive oxygen species–induced nucleocytoplasmic shuttling of p21CIP1 is also NES/Crm-1 dependent. In our experiments, RNF4-induced cytoplasmic relocalization of Tax was correlated with decreased transcriptional transactivation of the HTLV-LTR, a nuclear function of Tax, and increased transactivation of NF-κB, which is at least initiated in the cytoplasmic space. This finding suggests that RNF4-mediated cytosolic enrichment of Tax serves an anti-apoptotic, cytoprotective function. Our studies are the first to implicate a cellular STUbL in the regulation of a viral protein.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all members of the Kerscher and Semmes laboratories for stimulating discussions concerning this work.
This study was supported by the National Institutes of Health (grants GM085792 to O.K. and CA76959 to O.J.S.), and the American Recovery and Reinvestment Act (supplement CA76959-S1 to O.J.S. and supplement GM085792-01S1 to O.K.).
National Institutes of Health
Authorship
Contribution: K.A.F. conducted all of the experiments, assisted in the design and interpretation of the study, and contributed to all aspects of manuscript preparation; X.G. assisted in conducting the experiments and in data interpretation; and O.K. and O.J.S. conceived and designed the study, interpreted the data, and contributed to all aspects of manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: O. John Semmes, Dept of Microbiology and Molecular Cell Biology, The Leroy T. Canoles Cancer Research Center, Eastern Virginia Medical School, Norfolk, VA 23508; e-mail: semmesoj@evms.edu; or Oliver Kerscher, Biology Dept, Integrated Science Center, College of William & Mary, Williamsburg, VA 23186; e-mail: opkers@wm.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal