Abstract
Sema4D, also known as CD100, is a constitutively expressed immune semaphorin on T cells and NK cells. CD100 has important immune regulatory functions that improve antigen-specific priming by antigen-presenting cells, and can also act as a costimulatory molecule on T cells. We investigated the consequence of HIV-1 infection on CD100 expression by T cells, and whether CD100 expression signifies functionally competent effector cells. CD100 expression on T cells from healthy individuals was compared with HIV-1–infected subjects including elite controllers, noncontrollers, and patients receiving antiretroviral therapy. The frequency and fluorescence intensity of CD100 on CD8+ and CD4+ T cells were decreased during HIV-1 infection. Furthermore, the absolute number of CD100-expressing CD8+ T cells was positively associated with the magnitude of HIV-1–specific T-cell responses. CD8+ T cells lacking CD100 expression were functionally impaired and present in increased numbers in HIV-1–infected individuals. The number of CD100−CD8+ T cells positively correlated with T-cell immunosenescence, immune activation, and viral load. Loss of CD100 expression appears to result from direct antigen stimulation, as in vitro cytokine exposure and viral replication did not significantly impact CD100 expression. These data suggest that loss of CD100 expression probably plays an important role in dysfunctional immunity in HIV-1 infection.
Introduction
Semaphorins are a family of proteins that are traditionally associated with neuronal development and guidance. Immune semaphorins represent a small number of semaphorins expressed on immune cells. Sema4D, also called CD100, was the first immune semaphorin discovered and is abundantly expressed by resting T cells and NK cells.1 CD100 is a 150 kDa transmembrane glycoprotein that can be proteolytically cleaved into a soluble form.2 CD100 uses a dual receptor system where it binds Plexin-B1 in nonlymphoid tissues3 and CD72 in the immune system.4 CD72 is present on the surface of most antigen-presenting cells (APCs) and B cells, and interaction with CD100 leads to dendritic cell maturation and cytokine production, and enhanced B-cell activation.5,6 Studies in CD100−/− mice have demonstrated the importance of CD100 for both humoral and cellular immune responses. CD100−/− mice have normal T cell and B cell numbers, but specific effector functions are impaired including T-cell priming and B-cell responsiveness.7 Interestingly, T cells from CD100−/− mice respond normally after mitogen or anti-CD3 antibody stimulation7 suggesting that CD100-CD72 interaction is not essential for direct T-cell receptor (TCR) stimulation, but is required for effective APC presentation of peptide to antigen-specific T cells. This is further supported by the physical interaction of CD100 and CD45 during T-cell activation where CD100 potentially acts as a costimulatory molecule.8 In addition, CD100 appears to be important for differentiation into effector T cells.5,8,9
The importance of CD8+ T-cell immunity during HIV-1 infection is well established. As HIV-1–specific CD8+ T cells emerge during acute infection, plasma viremia rapidly decreases.10-12 Lymphocytes isolated from HIV-1–infected individuals with high viral loads have decreased effector functions (ie, lack of detectable HIV-1–specific cytotoxicity, cytokine production, and the ability to proliferate).13-16 However, a rare subset of HIV-1–infected individuals, termed elite controllers, is capable of durably suppressing viremia below the level of detection without antiretroviral therapy.17 The mechanisms behind nonprogressive HIV-1 infection in elite controllers are still not clear, but appear to be genetically linked with an over-representation of HLA B57 and HLA B27 alleles,18-20 and more responsive CD8+ T cells.21-23 These genetic and functional associations with HIV-1 control further support the importance of CD8+ T cells during HIV-1 infection.
Substantial evidence indicates that HIV-1–associated chronic immune activation and continuous antigen exposure are associated with profound dysfunction of all immune cell subsets including HIV-1–specific CD8+ T cells. Two markers, HLA-DR and CD38, are reliable surrogates of immune activation and are stronger predictors of disease progression than a viral load.24-27 However, these markers do not measure the functional capacity of CD8+ T cells. Instead, PD-1 and CD57 have been used to define terminally differentiated, exhausted, or dysfunctional T cells,28-31 although correlations between PD-1 and polyfunctionality of antigen-specific CD8+ T cells have not always been observed.31 Therefore, additional markers capable of correlating with T-cell function are urgently needed to monitor immune function, treatment responses, and T cell-mediated vaccines in HIV-1–infected persons (eg, measles, mumps, rubella, varicella, and possibly in the future, therapeutic HIV-1 vaccines).
There are undoubtedly several factors involved in HIV-1–related immune dysfunction, but accumulating evidence indicates that CD100 plays a relevant role in immune regulation and enhancement of effector functions.5,8,9,32,33 We hypothesized that lower levels of CD100 reduce effector functions that render T cells incapable of optimally responding to pathogens. We used subjects at different stages of HIV-1 infection to determine the effect of infection on CD100 expression and to assess the potential role of CD100 for CD8+ T cell responses.
Methods
Study subjects
Cryopreserved peripheral blood mononuclear cells (PBMCs) from 138 HIV-1–infected subjects from 3 different cohorts (University of California San Francisco [UCSF] Options cohort, UCSF SCOPE cohort, and the pediatric HIV-1 clinic at Jacobi Medical Center, Bronx, NY) were used in this study. PBMCs from 30 healthy blood donors from the Stanford Blood Center, Palo Alto, CA, and 10 from San Francisco, CA were included as controls. Additional information on the cohorts can be found in supplemental Methods and supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These studies were approved by the UCSF Committee on Human Research and all subjects gave informed consent to participate in accordance with the Declaration of Helsinki.
Flow cytometry and TruCount bead assay
PBMCs (5 × 105) were stained with antibody cocktails in FACS buffer (PBS containing 0.5% BSA and 2mM EDTA) on ice for 30 minutes. Antibody information is summarized in supplemental Table 2. Aqua amine reactive dye (Invitrogen) was used for dead cell exclusion. Samples were analyzed on a 4-laser LSR II flow cytometer (BD Biosciences). Data analysis was performed using FlowJo Version 8.8.6 software (TreeStar). Boolean gating was used to evaluate multiparametric expression and visualized using PESTLE and SPICE Version 4.3 software.34 Absolute counts for healthy donors were performed using Trucount tubes (BD Biosciences) per the manufacturer's instructions.
IFN-γ ELISPOT assay
T-cell responses were assessed by IFN-γ ELISPOT as previously described.35 The HIV-1 Gag p24 15-mer peptide pool (2 μg/mL; National Institutes of Health [NIH] AIDS Research and Reference Reagents Program) or phytohaemagglutinin (PHA; 1 μg/mL; Sigma-Aldrich) were used for stimulation. FACS sorted cells were stimulated with phorbol myristate acetate (PMA; 50 ng/mL, Sigma-Aldrich) and ionomycin (1 μg/mL, Sigma-Aldrich). Average spot counts for duplicate wells were calculated and background from wells with cells in media only was subtracted. Wells containing more than 50 spot-forming units after subtraction of 2 times background were considered a positive response.
Cell sorting
PBMCs from healthy individuals were sort-purified based on CD100 staining on CD8+ T cells using a BD FACS Aria (BD Biosciences). Purity of sorted cells was consistently > 97%.
Pentamer staining
Antigen-specific CD8+ T cells were assessed for CD100 expression using biotinylated MHC class I pentamers (ProImmune) per manufacturer's instructions.
Multiplex cytokine array
CD100+ or CD100− CD8+ T cells were cocultured with autologous peptide-pulsed PBMCs. Peptide-pulsing was performed with peptides from CMV (NLVPMVATV [HLA A2-restricted], QIKVRVDMV [HLA B8–restricted], or TPRVTGGGAM [HLA B7–restricted]) at 10 μg/mL at 37°C for 1 hour followed by irradiation (Gammacell 3000; MDS Nordion) at 5000 rads. PBMCs were washed extensively with complete culture medium. Supernatants were collected after 24 hours of coculture. In addition, each FACS sorted cell population was stained with pentamer to verify the frequency of antigen-specific CD8+ T cells. Production of IL-2, IFN-γ, TNFα, MIP-1β, perforin and granzyme B was assayed using multiplex cytokine arrays (Biolegend) per the manufacturer's protocols. Samples were acquired on a Labscan 100 analyzer (Luminex) using Bio-Plex manager 6.0 software (Bio-Rad). Background levels were determined by coculturing nonpeptide stimulated autologous PBMCs with FACS sorted cells. Cytokine production was normalized to 1000 antigen-specific T cells in the well calculated from the frequency of pentamer-positive cells determined by flow cytometry.
In vitro HIV-1 infection of PBMCs
PBMCs from HIV-1–negative donors were stimulated with 2 μg/mL PHA and infected on day 3 with NL4-3 by spinoculation (1200g for 2 hours) at a multiplicity of infection of 0.05 per 5 × 105 cells. PBMCs were maintained in culture in the presence of 50 IU/mL IL-2 and were assessed for changes in CD100 expression on day 7 after infection.
Intracellular cytokine staining
PBMCs (5 × 105) were stimulated with PHA (1 μg/mL), CEF (5 μg/mL), p24 peptide pools (5 μg/mL), 9-mer HIV or 9-mer CMV peptides (5 μg/mL) in the presence of anti-CD49d and anti-CD28 (clone 9F10 and L293, respectively, 1 μg/mL each; BD Biosciences). After 1 hour brefeldin A was added (10 μg/mL) and the cells incubated for an additional 5 hours. T-cell expression of IFNγ (clone B27; BD Biosciences) was performed by intracellular cytokine staining.
Cytokine stimulation
Fresh PBMCs (5 × 105 cells) were stimulated in a 96 well round-bottom plate with the following conditions: IL-6 (10 ng/mL), IFNγ (50 ng/mL), TNFα (10 ng/mL), IL-12/IL-18 (50 ng/mL each), IL-15 (25 ng/mL), IL-7 (25 ng/mL), IL-1β (50 pg/mL), and anti-CD3/anti-CD28 (clone Hit3α and L293, respectively, 1 μg/mL of each). After 24 hours of incubation, cells were assessed for the frequency and gMFI of CD100 expression by flow cytometry.
Statistical analysis
All statistical analyses were performed using Prism Version 4.0 software (GraphPad). Flow cytometry data were analyzed using student t test or Kruskal-Wallis test followed by the Dunn posttest as indicated. Correlation coefficients were determined by Spearman rank correlation. Area under the curve (AUC) analysis was calculated by the trapezoidal method. P values based on 2-tailed tests were considered statistically significant when P < .05.
Results
CD100 frequency and fluorescence intensity is decreased on CD8+ and CD4+ T cells during HIV-1 infection
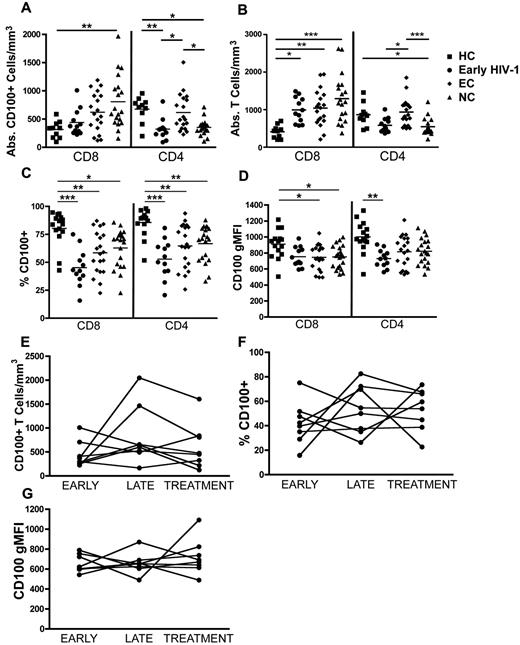
CD100 is highly expressed on resting T cells in healthy individuals, however the effect of HIV-1 infection on CD100 expression has never been studied. A cross-sectional analysis of PBMCs from individuals with early HIV-1 infection (within first year of infection), elite controllers (EC), and noncontrollers (NC; supplemental Table 1) were surface stained for CD100 and analyzed by flow cytometry. The mean absolute number of CD100+CD8+ T cells was significantly higher in the NC group than healthy controls (Figure 1A). In contrast, the mean absolute number of CD100+CD4+ T cell numbers was lower in both the early HIV-1–infected and NC groups compared with either the healthy controls or EC. The absolute numbers of CD100+CD4+ T cells in the EC group remained similar to healthy controls. Changes in the absolute number of CD4+ and CD8+ T cells expressing CD100 appear to reflect the changes in absolute CD4+ and CD8+ T cells observed during HIV-1 infection (Figure 1B). To account for these changes in absolute CD4+ and CD8+ T-cell counts that occur during HIV-1 infection, we assessed the percentage of each T-cell population expressing CD100. Despite the increase in absolute CD8+ T cells and loss of CD4+ T cells, the percentage of both CD4+ and CD8+ T cells expressing CD100 were significantly lower in all HIV-1–infected groups compared with healthy controls (Figure 1C). Furthermore, the geometric mean fluorescence intensity (gMFI) of CD100 on CD8+ T cells was significantly decreased in both the EC (mean gMFI = 746.5) and NC (mean gMFI = 751) compared with healthy controls (mean gMFI = 910.1; Figure 1D). The mean CD100 gMFI on CD4+ T cells was significantly decreased only in early HIV-1–infected subjects (Figure 1D). Taken together, CD100 expression on CD4+ and CD8+ T cells, and the percentage of CD100+ T cells decline during HIV-1 infection.
CD100 T cell expression in HIV-1–infected subjects is altered compared with healthy controls. PBMCs from early HIV-1–infected patients (n = 12), elite controllers (EC, n = 20), viremic noncontrollers (NC, n = 20), and healthy controls (HCs, n = 15) were surface stained for CD100 expression. (A) Absolute numbers of CD8+ T cells and CD4+ T cells expressing CD100. (B) Absolute CD8+ and CD4+ T cells. (C) Percentage of CD8+ T cells or CD4+ T cells expressing CD100. (D) Geometric mean fluorescence intensity (gMFI) of CD100 on CD8+ T cells and CD4+ T cells. (E-G) CD100 expression on CD8+ T cells from early HIV-infected subjects at time points, (i) within the first year of HIV-1 infection (early), (ii) 1 to 5 years after infection (late), and (iii) within 3 months after starting ART (treatment). (E) Absolute numbers of CD100+CD8+ T cells, (F) percentage of CD100 expression, and (G) CD100 gMFI. Statistical analysis was performed using (A-D) Kruskal Wallis tests with Dunn posttest; (E-G) paired t tests (*P < .05, **P < .01, and ***P < .001).
CD100 T cell expression in HIV-1–infected subjects is altered compared with healthy controls. PBMCs from early HIV-1–infected patients (n = 12), elite controllers (EC, n = 20), viremic noncontrollers (NC, n = 20), and healthy controls (HCs, n = 15) were surface stained for CD100 expression. (A) Absolute numbers of CD8+ T cells and CD4+ T cells expressing CD100. (B) Absolute CD8+ and CD4+ T cells. (C) Percentage of CD8+ T cells or CD4+ T cells expressing CD100. (D) Geometric mean fluorescence intensity (gMFI) of CD100 on CD8+ T cells and CD4+ T cells. (E-G) CD100 expression on CD8+ T cells from early HIV-infected subjects at time points, (i) within the first year of HIV-1 infection (early), (ii) 1 to 5 years after infection (late), and (iii) within 3 months after starting ART (treatment). (E) Absolute numbers of CD100+CD8+ T cells, (F) percentage of CD100 expression, and (G) CD100 gMFI. Statistical analysis was performed using (A-D) Kruskal Wallis tests with Dunn posttest; (E-G) paired t tests (*P < .05, **P < .01, and ***P < .001).
CD100 expression on total CD8+ T cells is disrupted during early HIV-1 infection, persists through chronic infection, and is not restored upon ART initiation
To determine whether CD100 expression changed over the course of infection, we performed a longitudinal analysis on 8 early HIV-1–infected patients with the following time points: within 1 year of infection (early); 1.3 to 5 years after infection (late); and within 3 months of initiating ART (treatment). No significant changes in absolute numbers (Figure 1E) or frequency (Figure 1F) of CD100-expressing CD8+ T cells were observed, although 2 patients had a noticeable increase in absolute CD100+CD8+ T-cell numbers as their infections progressed. CD100 gMFI on CD8+ T cells within individuals remained relatively unchanged over time after infection (Figure 1G). Importantly, initiation of ART did not appear to have a significant effect on the frequency or gMFI of CD100 expression. These data suggest that CD100 expression is altered during acute infection and persists through chronic infection despite effective ART viral suppression.
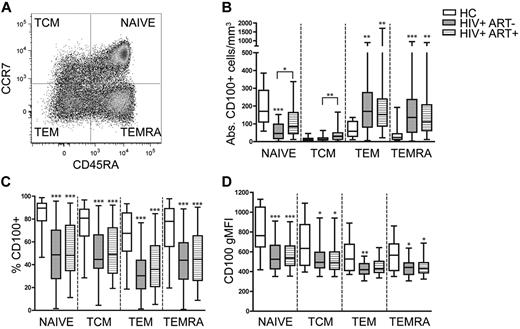
Sustained ART partially restores the number of naive CD100+CD8+ T cells and increases the number of CD100+CD8+ TCM cells
CD100 expression on CD8+ T cells is disrupted during early HIV-1 infection and is not significantly improved during the first 3 months of ART. HIV-1 infection commonly skews the ratio of naive and memory T-cell populations in the periphery toward greater frequencies of the latter subsets.36,37 Therefore, we hypothesized that sustained ART for greater than one year would significantly shift the proportion of naive and memory T cells and restore the frequency of CD100 expression. We analyzed CD100 expression on naive, central memory (TCM), effector memory (TEM), and terminally differentiated effector memory (TEMRA) CD8+ T cells using CCR7 and CD45RA (Figure 2A) in chronic HIV-1 noncontrollers with (HIV+ ART+, N = 40), and without therapy (HIV+ ART−, N = 40). The mean CD4+ T cell counts did not differ between the treated (mean = 418, range 249-590 cells/mm3) and untreated (mean = 411, range 350-499 cells/mm3) groups although most subjects receiving ART had undetectable HIV-1 RNA plasma viral loads. The number of naive T cells expressing CD100 significantly decreased in untreated patients compared with healthy controls (Figure 2B). ART significantly increased the number of CD100+ naive T cells, although this was nearly 2-fold less than healthy controls. Treatment had a small, but significant, effect on the number of CD100+ TCM compared with untreated subjects (42 vs 17 cells/mm3). In contrast, the absolute numbers of CD100-expressing TEM and TEMRAs increased 3.6- and 4.6-fold, respectively in untreated subjects, and 3.5- and 4-fold in the ART group compared with healthy controls. To account for the typical expansions in total CD8+ T cell counts that occur during HIV-1 infection and ART, we assessed the percentage of each CD8+ T-cell subset expressing CD100. Despite a significant increase in the absolute number of CD100-expressing TEM and TEMRA in untreated and ART subjects, the mean CD100 frequency was significantly decreased within all CD8+ T-cell subsets in both untreated and ART individuals compared with healthy individuals (Figure 2C). Similarly, the mean gMFI was also reduced in all CD8+ T-cell subsets for both patient groups compared with healthy controls (Figure 2D). These data indicate that loss of CD100 expression on CD8+ T cells during HIV-1 infection is not simply because of the redistribution of memory phenotypes as mean gMFI, and the frequency of CD100 expression are decreased on all CD8+ T-cell subsets. Furthermore, the expansion of the absolute number of CD100-expressing CD8+ T cells observed during chronic infection (Figure 1A) results from the expanded CD100-expressing TEM and TEMRA subsets. Despite ART-induced viral control, ART has minimal impact on improving CD100-expressing CD8+ T cells.
CD100 frequency and gMFI is not significantly modified by sustained ART but reconstitutes CD100+ naive CD8+ T cells. (A) Representative dot plot depicting the different CD8+ T-cell memory populations (TCM indicates central memory T cell; TEM, effector memory T cell; and TEMRA, terminally differentiated effector memory T cell). (B) Absolute numbers of CD100+CD8+ T cells, (C) percentage of CD100 expression, and (D) CD100 gMFI within each memory subset in HCs, untreated HIV-1–infected subjects (HIV+ ART−), and treated HIV-1–infected subjects (HIV+ ART+). Statistical analysis was performed using Kruskal Wallis tests with Dunn posttest (*P < .05, **P < .01, and ***P < .001).
CD100 frequency and gMFI is not significantly modified by sustained ART but reconstitutes CD100+ naive CD8+ T cells. (A) Representative dot plot depicting the different CD8+ T-cell memory populations (TCM indicates central memory T cell; TEM, effector memory T cell; and TEMRA, terminally differentiated effector memory T cell). (B) Absolute numbers of CD100+CD8+ T cells, (C) percentage of CD100 expression, and (D) CD100 gMFI within each memory subset in HCs, untreated HIV-1–infected subjects (HIV+ ART−), and treated HIV-1–infected subjects (HIV+ ART+). Statistical analysis was performed using Kruskal Wallis tests with Dunn posttest (*P < .05, **P < .01, and ***P < .001).
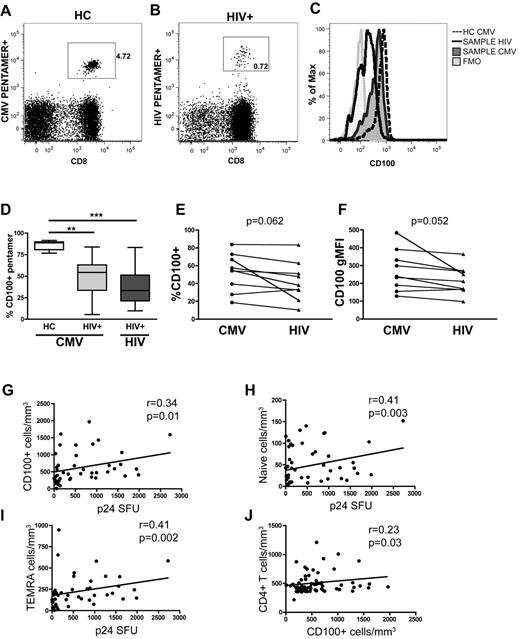
The magnitude of the HIV-1–specific T-cell response is related to CD100 expression
CD100 is important for T cell costimulation and differentiation into effector cells.5,8,9,32 Therefore we hypothesized that the number of CD100+CD8+ T cells would directly relate to the magnitude of the antigen-specific T-cell responses. To elucidate whether antigen-specific CD8+ T cells in general, or if HIV-1–specific CD8+ T cells in particular, had decreased CD100 expression in HIV-1–infected patients, pentamer staining was performed on PBMCs from seronegative and chronically HIV-1–infected individuals (Figure 3A-B). CMV or HIV-1 pentamer positive cells were then assessed for CD100 expression (Figure 3C). Overall, the frequency of CMV and HIV-1–specific CD8+ T cells expressing CD100 was significantly lower in HIV-1–infected subjects compared with healthy individuals (Figure 3D). In a subset of HIV-1–infected individuals (n = 9), we measured the frequency of both HIV-1 and CMV-specific CD100-expressing T cells by pentamer staining within the same individual. We observed a trend toward a lower frequency and gMFI of CD100 expression on HIV-1–specific T cells, compared with CMV-specific T cells within the same individual (Figure 3E-F). This suggests that although the frequency and gMFI of CD100-expressing T cells generally appears to be lower in HIV-1–infected patients, CD100 expression on different antigen-specific T cells within the same HIV-1–infected individual may be different.
Antigen-specific CD8+ T-cell expression of CD100 is decreased and the absolute numbers of CD100 expressing total CD8+ T cells, naive cells and TEMRAS correlate with p24-specific T-cell responses in untreated, viremic chronically infected individuals. Representative dot plots of (A) CMV pentamer-specific T cells in HC and (B) HIV-1–specific CD8+ T cells in a chronically HIV-1–infected individual. (C) Histogram of CD100 expression by pentamer-specific CD8+ T cells. (D) Summary of CD100 expression on antigen-specific CD8+ T cells in HCs and HIV-1 patients. Comparison of the (E) percentage of CD100 expression and (F) CD100 gMFI in HIV-1 infected subjects with both CMV and HIV-1–specific CD8+ T cells within the same individual. Correlation of absolute numbers of CD100 expressing (G) CD8+ T cells, (H) naive CD8+ T cells, and (I) CD8+ TEMRA cells with p24-specific T-cell responses in untreated, viremic chronically infected HIV-1 patients (n = 53). (J) Correlation of CD100+ CD8+ T cells with CD4+ T-cell count in both untreated early and chronically infected patients (n = 84). Statistical analysis was performed using (D) Kruskal Wallis tests with Dunn posttest, and (E-F) 2-tailed paired Student t tests. (G-J) Correlations were determined by 2-tailed nonparametric Spearman correlations.
Antigen-specific CD8+ T-cell expression of CD100 is decreased and the absolute numbers of CD100 expressing total CD8+ T cells, naive cells and TEMRAS correlate with p24-specific T-cell responses in untreated, viremic chronically infected individuals. Representative dot plots of (A) CMV pentamer-specific T cells in HC and (B) HIV-1–specific CD8+ T cells in a chronically HIV-1–infected individual. (C) Histogram of CD100 expression by pentamer-specific CD8+ T cells. (D) Summary of CD100 expression on antigen-specific CD8+ T cells in HCs and HIV-1 patients. Comparison of the (E) percentage of CD100 expression and (F) CD100 gMFI in HIV-1 infected subjects with both CMV and HIV-1–specific CD8+ T cells within the same individual. Correlation of absolute numbers of CD100 expressing (G) CD8+ T cells, (H) naive CD8+ T cells, and (I) CD8+ TEMRA cells with p24-specific T-cell responses in untreated, viremic chronically infected HIV-1 patients (n = 53). (J) Correlation of CD100+ CD8+ T cells with CD4+ T-cell count in both untreated early and chronically infected patients (n = 84). Statistical analysis was performed using (D) Kruskal Wallis tests with Dunn posttest, and (E-F) 2-tailed paired Student t tests. (G-J) Correlations were determined by 2-tailed nonparametric Spearman correlations.
In addition, we assessed the role of CD100 in the generation of total CD4+ and CD8+ T-cell responses on restimulation with HIV-1 antigens in untreated chronically-infected subjects by IFN-γ ELISPOT after Gag p24 peptide pool stimulation. In patients with detectable viremia (n = 53), we observed a positive correlation between the number of CD100-expressing CD8+ T cells and spot-forming units (Figure 3G). This association was predominantly attributed to the presence of CD8+CD100+ naive and TEMRA T cells (Figure 3H-I). In addition, in untreated HIV-1–infected patients, including early, EC and NC individuals, a modest, but significant correlation between CD4+ T cell counts and the number of CD100+CD8+ T cells was observed (Figure 3J).
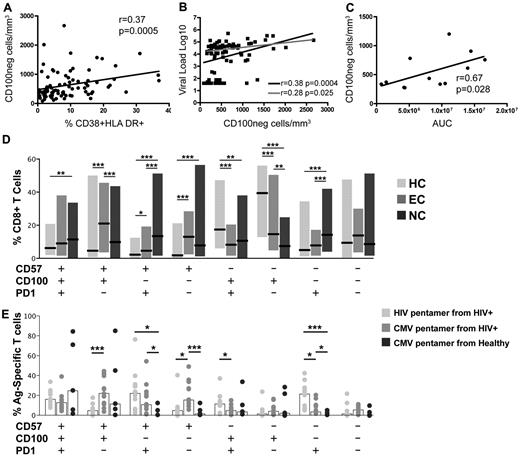
Loss of CD100 is related to immune activation status and viral loads
CD100+ T-cell frequencies are significantly decreased in all HIV-1–infected groups, however the absolute number of CD100-expressing cells in the total CD8+ T-cell population is significantly increased in noncontrollers (Figure 1A-C). Therefore, an increased population of CD100−CD8+ T cells should be present in the periphery. Indeed, the mean frequency (data not shown) and absolute number (supplemental Figure 1) of CD100−CD8+ T cells was significantly expanded in early and chronically infected noncontrollers compared with healthy controls. Although the mean absolute number of CD100−CD8+ T cells in EC was also increased compared with healthy controls, it was approximately 2-fold less than the NC group. Because CD100 can be proteolytically cleaved during activation,2 we proposed that the immune activating milieu created by HIV-1 infection would be associated with an expanded CD100−CD8+ T-cell population. We observed that in untreated patients the number of CD100−CD8+ T cells correlated with the frequency of CD38+HLA-DR+CD8+ T cells (Figure 4A), as well as with viral loads (Figure 4B black line). The correlation with viral load remained statistically significant after exclusion of the EC group (Figure 4B gray line). Interestingly, calculation of the AUC for viral load before ART in the 12 early HIV-1–infected subjects showed that the number of CD100−CD8+ T cells present during the first year of infection was associated with viral control in the early stages of infection (Figure 4C).
Expansion of CD100−CD8+ T cells coexpressing CD57 and PD-1 in HIV-1–infected individuals. Correlation of the number of CD100−CD8+ T cells with (A) immune activation as assessed by percentage of CD38+HLA-DR+CD8+ T cells of all untreated subject groups (n = 84), or (B) viral loads of all untreated subject groups (black line; n = 84) or only untreated viremic patients (gray line; n = 65). (C) Correlation of the number of CD100−CD8+ T cells during the first year of infection and the AUC for viral loads over time (n = 12). D) Frequency of CD100 coexpression with CD57 and PD-1 in the total CD8+ T cell population of ECs (n = 20) and untreated, chronically infected NCs (n = 60) compared with HCs. Shaded bars represent minimum-maximum range and the black middle bar indicates the median. (E) Frequency of CD100, CD57, and PD-1 coexpression in HIV (lightest gray) and CMV (medium gray) pentamer-specific CD8+ T-cell population of HIV-1–infected subjects and CMV pentamer-specific CD8+ T cell population of healthy controls (darkest gray). Open bars represent mean and symbols represent individual subjects. Statistical analysis: (A-C) Correlations were determined by 2-tailed nonparametric Spearman correlations; (D-E) Student t tests (*P < .05, **P < .01, and ***P < .001).
Expansion of CD100−CD8+ T cells coexpressing CD57 and PD-1 in HIV-1–infected individuals. Correlation of the number of CD100−CD8+ T cells with (A) immune activation as assessed by percentage of CD38+HLA-DR+CD8+ T cells of all untreated subject groups (n = 84), or (B) viral loads of all untreated subject groups (black line; n = 84) or only untreated viremic patients (gray line; n = 65). (C) Correlation of the number of CD100−CD8+ T cells during the first year of infection and the AUC for viral loads over time (n = 12). D) Frequency of CD100 coexpression with CD57 and PD-1 in the total CD8+ T cell population of ECs (n = 20) and untreated, chronically infected NCs (n = 60) compared with HCs. Shaded bars represent minimum-maximum range and the black middle bar indicates the median. (E) Frequency of CD100, CD57, and PD-1 coexpression in HIV (lightest gray) and CMV (medium gray) pentamer-specific CD8+ T-cell population of HIV-1–infected subjects and CMV pentamer-specific CD8+ T cell population of healthy controls (darkest gray). Open bars represent mean and symbols represent individual subjects. Statistical analysis: (A-C) Correlations were determined by 2-tailed nonparametric Spearman correlations; (D-E) Student t tests (*P < .05, **P < .01, and ***P < .001).
Decreased CD100 expression is associated with CD8+ T-cell immunosenescence and exhaustion
Based on our observation that expansion of CD100−CD8+ T cells is associated with higher levels of immune activation and higher viral loads, we investigated coexpression of CD100 with the immunosenescence marker CD57 and the T cell regulatory marker PD-1. CD57 and PD-1 increase during HIV-1 infection and are associated with immune dysfunction.28,29,38,39 We hypothesized that increased frequencies of CD100−CD8+ T cells coexpressing CD57 and PD-1 would be elevated in NC and represent highly dysfunctional T cells. Indeed, the majority of the increased expression of CD57 and PD-1 coexpression, and CD57 or PD-1 mono-expression was observed on CD100−CD8+ T cells in the NC group. Interestingly, EC also had a small but significant expansion of CD100−CD57+PD-1+CD8+ T cells, although the frequency was significantly lower compared with NC (Figure 4D). In healthy donors, CD8+ T cells were predominantly CD100+CD57negPD-1neg (Figure 4D). A positive correlation between the absolute numbers of CD100−CD8+ T cells and CD57+ or PD-1+CD8+ T cells was also observed (supplemental Figure 2A-B). Using HIV and CMV-specific pentamers, we assessed coexpression of CD100, CD57, and PD-1 on antigen-specific CD8+ T cells. In HIV-1–infected subjects we observed ∼ 20% of the HIV-specific CD8+ T cells were CD100−CD57+PD-1+ compared with ∼ 10% of CMV-specific CD8+ T cells, although this difference did not reach statistical significance (Figure 4E). Interestingly, CD57 expression was observed on a significantly higher proportion of CMV-specific CD8+ T cells in the presence or absence of CD100 compared with HIV-1–specific CD8+ T cells. In contrast, ∼ 20% of the HIV-1–specific CD8+ T cells were CD100−CD57negPD-1+ in comparison to only ∼ 5% of CMV-specific CD8+ T cells and less than 5% of CMV-specific CD8+ T cells in uninfected controls. Taken together, these data indicate an emergence of a highly dysfunctional CD8+ T cell subset characterized by mainly PD-1, and to lesser extent CD57 coexpression in the absence of CD100 that appears to be particularly elevated on HIV-1–specific CD8+ T cells.
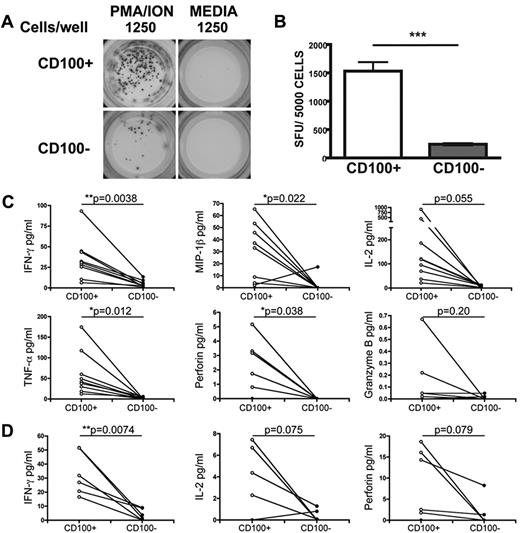
CD100−CD8+ T cells are functionally different in response to stimulation
To address the functional implications of altered CD100 expression on T cells, PBMCs from healthy individuals were FACS sorted into CD100+ or CD100−CD8+ T-cell populations and assessed by ELISPOT assay. Compared with CD100+ T cells, the number of CD100− cells that produced IFN-γ after PMA/ionomycin stimulation was significantly lower (Figure 5A-B). In addition, Luminex analysis of PMA/ionomycin stimulated CD100+ and CD100− cell supernatants indicated a significant difference in the levels of IFN-γ, MIP-1β, TNF-α, and perforin production between CD100+CD8+ and CD100−CD8+ T cells (Figure 5C). A trend toward greater IL-2 production from CD100+ cells was also observed. Similarly, after peptide stimulation, CD100+ cells produced significantly higher levels of IFN-γ compared with CD100− cells (P = .0074; Figure 5D). Both IL-2 and perforin showed comparable, although not significant, patterns. No antigen-specific induction of MIP-1β, TNF-α, or granzyme B was detected. These data suggest that CD100−CD8+ T cells have an impaired ability to secrete cytokines after stimulation.
CD100−CD8+ T cells are functionally impaired after stimulation. (A) Representative ELISPOT wells (left column) illustrating IFN-γ production in response to PMA/ionomycin in FACS sorted CD100+ or CD100− CD8+ T cells from HCs (n = 3). Control wells containing cells in media only are shown on the right column of wells. (B) Summary of 3 independent ELISPOT experiments. (C) Assessment of IFNγ, MIP-1β IL-2, TNF-α, perforin, and granzyme B production by Luminex assay after PMA/ionomycin stimulation representing cytokine production per 1000 cells (n = 9). D) Assessment of IL-2, IFN-γ, perforin production by Luminex assay after CMV antigen-specific stimulation with CMV peptide-pulsed autologous PBMCs representing cytokine production per 1000 antigen-specific T cells (n = 6). Statistical analysis was performed using (B) Student t tests (***P < .001), and (C-D) 2-tailed paired t tests.
CD100−CD8+ T cells are functionally impaired after stimulation. (A) Representative ELISPOT wells (left column) illustrating IFN-γ production in response to PMA/ionomycin in FACS sorted CD100+ or CD100− CD8+ T cells from HCs (n = 3). Control wells containing cells in media only are shown on the right column of wells. (B) Summary of 3 independent ELISPOT experiments. (C) Assessment of IFNγ, MIP-1β IL-2, TNF-α, perforin, and granzyme B production by Luminex assay after PMA/ionomycin stimulation representing cytokine production per 1000 cells (n = 9). D) Assessment of IL-2, IFN-γ, perforin production by Luminex assay after CMV antigen-specific stimulation with CMV peptide-pulsed autologous PBMCs representing cytokine production per 1000 antigen-specific T cells (n = 6). Statistical analysis was performed using (B) Student t tests (***P < .001), and (C-D) 2-tailed paired t tests.
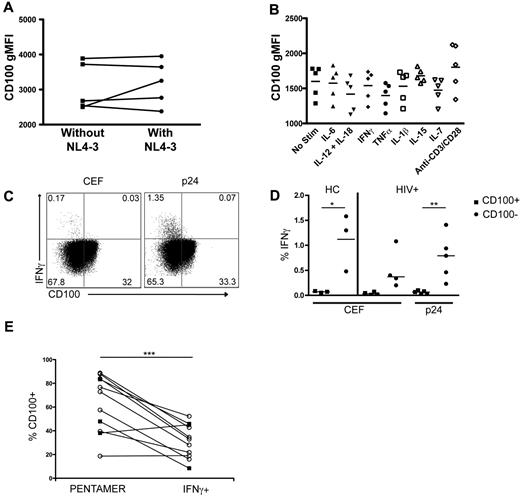
Viral replication and pro-inflammatory cytokines do not induce the loss of CD100 expression on CD8+ T cells
To assess the potential mechanism(s) resulting in diminished CD100 expression on T cells, we assessed the influence of in vitro HIV-1 infection, cytokine administration, and antigen-specific stimulation on CD100 expression using both HIV-1–infected and uninfected donors. PBMCs infected with NL4–3 for 7 days did not exhibit a significant change in the frequency of CD100+CD8+ T cells (data not shown), or the gMFI of CD100 expression (Figure 6A). Similarly, culturing PBMCs with pro-inflammatory (IL-1β, IL-6, IL-12 and IL-18, IFNγ, TNFα), and homeostatic cytokines (IL-7, IL-15) did not statistically change the frequency (data not shown) or gMFI of CD100 expression (Figure 6B). Direct TCR stimulation using anti-CD3 and anti-CD28 antibodies also did not significantly change the frequency (data not shown) or gMFI of CD100 expression (Figure 6B). To determine whether antigen-specific stimulation affected CD100 expression, PBMCs from healthy controls or HIV-1–infected subjects were stimulated with CEF (healthy and HIV-1–infected subjects) or p24 (HIV-1–infected only) peptides in an intracellular cytokine-staining assay. Peptide stimulation induced IFN-γ expression from CD100−CD8+ T cells (Figure 6C-D). This result is in direct contrast to our findings that CD100+CD8+ T cells respond with greater production of cytokines after PMA/ionomycin or peptides (Figure 5), and suggests peptide stimulation induces a rapid loss of CD100 expression on responding T cells. To address this further, we measured CD100 expression on epitope-specific CD8+ T-cell populations using MHC class I pentamers (unstimulated condition) compared with CD100 expression on IFNγ+CD8+ T cells after stimulation with the matched pentamer-specific peptide (stimulated condition). CD100 expression was significantly lower on IFNγ+CD8+ T cells compared with the matched pentamer-specific CD8+ T cells, further supporting that peptide stimulation results in down-regulation of CD100 in responding cells (Figure 6E).
CD100 expression is down-regulated on antigen-specific T cells after peptide stimulation but not after in vitro cytokine culture or HIV-1 infection. (A) CD100 gMFI after 7 days of culture in the absence or presence of NL4–3 infection. (B) CD100 gMFI after 24 hours of incubation in the presence of proinflammatory or homeostatic cytokines or anti-CD3 plus anti-CD28. (C) Representative dot plots illustrating IFN-γ expression in CD100− CD8+ T cells after CEF or p24 peptide pool stimulation. (D) Percentage of CD100+ and CD100− CD8+ T cells expressing IFN-γ after CEF or p24 stimulation. HCs were assessed for CD100 expression after CEF stimulation. HIV-1 infected subjects were assessed for CD100 expression after p24 or CEF stimulation. CEF and p24 responses in HIV+ subjects are not necessarily matched from the same individual. (E) CD100 frequency on CMV pentamer-specific CD8+ T cells (open circles) in HC and HIV-1 infected individuals and HIV-1 pentamer-specific CD8+ T cells (closed square) in HIV-1 infected individuals compared with IFN-γ producing cells after PBMC peptide-stimulation with the peptide corresponding to the pentamer within the same individual. Statistical analysis was performed using (D) Student t tests between CD100+ and CD100− cells, and (E) 2-tailed, paired Student t tests (*P < .05 and **P < .01). CEF = pool of peptides from cytomegalovirus, Epstein-Barr, and influenza viruses.
CD100 expression is down-regulated on antigen-specific T cells after peptide stimulation but not after in vitro cytokine culture or HIV-1 infection. (A) CD100 gMFI after 7 days of culture in the absence or presence of NL4–3 infection. (B) CD100 gMFI after 24 hours of incubation in the presence of proinflammatory or homeostatic cytokines or anti-CD3 plus anti-CD28. (C) Representative dot plots illustrating IFN-γ expression in CD100− CD8+ T cells after CEF or p24 peptide pool stimulation. (D) Percentage of CD100+ and CD100− CD8+ T cells expressing IFN-γ after CEF or p24 stimulation. HCs were assessed for CD100 expression after CEF stimulation. HIV-1 infected subjects were assessed for CD100 expression after p24 or CEF stimulation. CEF and p24 responses in HIV+ subjects are not necessarily matched from the same individual. (E) CD100 frequency on CMV pentamer-specific CD8+ T cells (open circles) in HC and HIV-1 infected individuals and HIV-1 pentamer-specific CD8+ T cells (closed square) in HIV-1 infected individuals compared with IFN-γ producing cells after PBMC peptide-stimulation with the peptide corresponding to the pentamer within the same individual. Statistical analysis was performed using (D) Student t tests between CD100+ and CD100− cells, and (E) 2-tailed, paired Student t tests (*P < .05 and **P < .01). CEF = pool of peptides from cytomegalovirus, Epstein-Barr, and influenza viruses.
Discussion
We observed a significant reduction in the frequency and gMFI of CD100 on both CD4+ and CD8+ T cells during early and chronic HIV-1 infection, despite an overall increased absolute number of CD100+CD8+ T cells during chronic HIV-1 infection. Reduced CD100 expression was particularly evident in naive and effector memory T cells. Furthermore, lower numbers of CD100-expressing T cells were related to reduced HIV-1–specific T-cell responses. The presence of CD100−CD8+ T cells among untreated HIV-1–infected individuals was generally associated with higher viral loads, T-cell activation and absolute numbers of dysfunctional and/or senescent T cells. Although ART can reverse some immunologic abnormalities, the effect of sustained ART on CD100 expression was often incomplete. Long-term elite control of HIV-1 infection was associated with preservation of CD100 on CD57+CD8+ T cells. HIV-1–specific CD8+ T cells exhibited a more dysfunctional (ie, CD100−PD-1+) profile compared with CMV-specific CD8+ T cells from HIV-1–infected subjects or healthy controls. Functionally, CD100−CD8+ T cells were shown to have an impaired capacity to produce cytokines in response to stimulation. Importantly, direct stimulation of antigen-specific T-cell responses resulted in a rapid loss of CD100 in responding cells. Pro-inflammatory and homeostatic cytokines as well as HIV-1 replication did not appear to significantly change CD100 expression. Collectively, these data suggest that CD100 may have an important role in HIV-1–related T-cell dysfunction, which can potentially be an important coindicator when monitoring T-cell immune function.
In HIV-1–negative individuals CD100 is highly expressed on resting T cells. A previous study indicated that CD100 expression can be differentially modulated by various stimuli; anti-CD3 antibody or PHA result in rapid up-regulation of CD100, whereas PMA decreased CD100 expression.32 We observed that HIV-1 infection significantly decreased the frequency and fluorescence intensity of CD100 on both CD8+ and CD4+ T cells. Interestingly, our data indicate that there was either no re-expression or the rate of re-expression was outweighed by the loss of CD100, because CD100 was maintained at reduced levels over time. Furthermore, we demonstrated that CD100 expression was reduced not only on total CD8+ T cells, but also on antigen-specific CD8+ T cells in HIV-1–infected subjects. The increased numbers of total CD100−CD8+ T cells was positively associated with the number of senescent and exhausted CD8+ T cells as measured by CD57 and PD-1 expression, respectively. Although the loss of CD100 expression appeared to cut across all CD8+ T cells in HIV-1–infected subjects, there was a greater frequency of CD100− cells expressing CD57, PD-1, or both markers on HIV-1–specific compared with CMV-specific CD8+ T cells. This is an interesting observation as CMV-specific CD8+ T cells typically remain at high numbers and functional throughout HIV-1 infection.40,41 CD57 and PD-1 expression on HIV-1–specific T cells are reported to be associated with HIV-1–related immune dysfunction and thus are proposed to contribute to lack of viral control.29,30 These data would therefore suggest that monitoring the coexpression of CD100, CD57, and PD-1 on HIV-1–specific T cells may be a better measure of the functionality of these cells than using any of these markers alone.
CD100 expression is regulated, at least partially, by proteolytic cleavage and activated T cells have been reported to be one source of soluble CD100.2,42 Although soluble CD100 was not measured in the sera of these cohorts, the rapid loss of CD100 after peptide stimulation suggests that CD100 is probably cleaved from the surface of activated T cells. Interestingly, in vitro anti-CD3/anti-CD28 stimulation did not significantly change CD100 expression, however stimulation with peptides resulted in significant CD100 loss. This suggests that cell-cell contact, which occurs during peptide presentation, is required for loss of CD100 surface expression. This is further supported by the observation that individual cytokines did not significantly change CD100 expression, although it is possible that the complex cytokine milieu in addition to chronic HIV-1 antigenemia in vivo enhances cleavage of CD100 or inhibits the re-expression of CD100 on antigen-stimulated cells. Subjects assessed within 3 months of initiating ART, which decreases immune activation and plasma viral load, did not exhibit changes in the frequency or fluorescence intensity of CD100 expression. This is probably because even in the presence of ART, antigens are still present at significant levels either because of trapped virions in secondary lymphoid tissues or low-level viral replication in tissue sanctuaries. However, sustained ART for greater than 1 year did partially restore the number of CD100-expressing naive CD8+ T cells, although not to the levels observed in healthy individuals. These data illustrate the long-term damage induced by HIV-1 infection on T-cell phenotype and function, and suggest that complete reconstitution of immune cell function requires more than viral suppression alone.
CD100-CD72 signaling has been primarily focused on the CD72-induced signals after engagement with CD100. The functional significance of CD100 as a receptor on T cells is relatively limited. Studies found that membrane-bound CD100 is associated with serine kinase activity after antibody cross-linking,43 that augments proliferation in the presence of submitogenic levels of anti-CD3 and anti-CD2.32 In addition, CD100 was discovered to associate with CD45, an essential component for T-cell activation via the TCR.44 Collectively, these studies suggest that CD100 acts as a costimulatory molecule to enhance T-cell responses. In agreement with these studies, we observed that HIV-1–specific IFN-γ production was associated with the absolute number of CD100+CD8+ T cells, in particular naive and TEMRA cells, suggesting that CD100 is important for antigen-specific responses. In addition, we demonstrated that CD100−CD8+ T-cell responses to PMA/ionomycin stimulation were impaired. Conversely, T cells from CD100−/− mice have been described to respond normally to PHA and anti-CD3 stimulation, and were concluded to have no intrinsic deficiency.7 However, differences in PHA and PMA signaling pathways have been described,45 which could potentially account for the divergent results. Our studies of antigen-specific responses also showed very low levels of cytokine production in the sort-purified CD100−CD8+ T-cell population, which suggests a potentially intrinsic deficiency in these cells. Alternatively, it is possible that part of the decreased cytokine levels in the cells lacking CD100 may be because of antigen-induced apoptosis since CD100 has been implicated in leukemic prosurvival properties.46,47 Moreover, both CD57 and PD-1–expressing cells are described as dysfunctional and exhibit a higher level of spontaneous apoptosis in vitro.30,48,49 There are fractions of CD57+ and PD-1+ T cells within the sorted CD100− population that could potentially contribute to antigen-induced cell death. Nevertheless, this does not account for the noticeable difference in cytokine production between the CD100+ and CD100− populations, because we have shown that in healthy individuals, CD57 and PD-1 were coexpressed to some extent with CD100, and there were cells lacking expression of all 3 markers. Consequently, further studies are necessary to completely understand the underlying mechanisms for the impairment of CD100−CD8+ T cells.
In conclusion, we observed that the CD8+ T-cell population in HIV-1–infected subjects is altered from a highly CD100-expressing cell population to a population with increased CD100−CD8+ T cells. Our observations in elite controllers support the hypothesis that preservation of CD100 is a contributing component for enhanced viral control and T-cell function in untreated individuals as elite controllers have lower numbers of CD100−CD8+ T cells compared with noncontrollers. Interestingly, one of the key differences between elite and noncontrollers is CD57 coexpression with CD100, which was found at higher frequency in elite controllers than in noncontrollers. Although CD57+CD8+ T cells are replicatively senescent cells, they are able to produce high levels of IFN-γ in response to TCR stimulation.50 This indicates that CD57+CD8+ T cells can be subdivided, and that loss of CD100 in combination with CD57 expression describes a subset of dysfunctional T cells. In summary, these findings suggest that CD100 can potentially be a novel marker, that when used in combination with CD57 and PD-1, may provide greater insight into the functional integrity of the T-cell response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by NIH grant AI68498 (D.F.N.) and WIHS ARRA supplement, U01-AI-034993 (P.J.N). The SCOPE cohort was supported in part by the National Institute of Allergy and Infectious Diseases (RO1 AI087145, K24AI069994), the University of California–San Fransisco (UCSF) CFAR (P30 AI27763), the UCSF CTSI (UL1 RR024131), the Cleveland Immunopathogenesis Consortium (AI 76 174), and CFAR Network of Integrated Systems (R24 AI067039; S.G.D, J.N.M., and P.W.H.).
E.M.E. was supported by a fellowship from the American-Scandinavian Foundation and a grant from the NIH, UCSF–Gladstone Institute of Virology & Immunology Center for AIDS Research, P30 AI27763. E.L.H. received a Clinical Research Training Fellowship from the American Academy of Neurology. C.E.K. was supported, in part, by National Institute of Allergy and Infectious Diseases grant 2T32AI060530-06. J.M.M. was supported, in part, by Department of Health and Human Services funding under NIH grant number 5T32HL007185.
National Institutes of Health
Authorship
Contribution: E.M.E. designed and performed experiments, analyzed data, and wrote the paper; J.M.M. assisted in experimental design, performed experiments, and wrote the revised paper; E.L.H. and M.D.B. designed and performed experiments, and provided critical revision of the paper; C.E.K. performed experiments and provided critical revision of the paper; S.J.H. and S.K. performed experiments; P.J.N. assisted in experimental design, and provided critical revision of the paper; S.G.D., F.M.H., and J.N.M provided reagents and critical revision of the paper; P.W.H. and M.G.R. provided reagents; and D.F.N. supervised research and provided critical revision of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.L.H. is the Department of Neurology, University of Washington, Seattle, WA.
Correspondence: Emily M. Eriksson and Douglas F. Nixon, 1001 Potrero Ave, Bldg 3, Rm 603, San Francisco, CA 94110; e-mail: erikssonemy@gmail.com and douglas.nixon@ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal