Abstract
CXCL12 and VCAM1 retain hematopoietic stem cells (HSCs) in the BM, but the factors mediating HSC egress from the BM to the blood are not known. The sphingosine-1-phosphate receptor 1 (S1P1) is expressed on HSCs, and S1P facilitates the egress of committed hematopoietic progenitors from the BM into the blood. In the present study, we show that both the S1P gradient between the BM and the blood and the expression of S1P1 are essential for optimal HSC mobilization by CXCR4 antagonists, including AMD3100, and for the trafficking of HSCs during steady-state hematopoiesis. We also demonstrate that the S1P1 agonist SEW2871 increases AMD3100-induced HSC and progenitor cell mobilization. These results suggest that the combination of a CXCR4 antagonist and a S1P1 agonist may prove to be sufficient for mobilizing HSCs in normal donors for transplantation purposes, potentially providing a single mobilization procedure and eliminating the need to expose normal donors to G-CSF with its associated side effects.
Introduction
Hematopoietic stem cells (HSCs) normally reside in a microenvironment within the BM called the stem cell niche, which is essential for the regulation of stem cell self-renewal and cell-fate decisions.1 A small but significant number of HSCs regularly exit the BM as part of a regulated physiologic process and transit through the blood.2 The egress of HSCs from the BM can be dramatically increased via several mechanisms, collectively referred to as HSC mobilization.3 This process is used clinically to provide HSCs for transplantation purposes, and forms an essential component for the treatment of an increasing number of malignant and nonmalignant conditions.4 Despite the clinical success of peripheral blood stem cell (PBSC) transplantation, considerable problems remain, including inadequate collections in a substantial minority of cases and significant side effects for normal donors.5 Improving HSC mobilization procedures requires a better fundamental understanding of the physiology of HSC mobilization.
Sphingosine-1-phosphate (S1P) is a biologically active phospholipid produced by sphingosine kinases (SK1 and SK2). S1P is a chemoattractant for hematopoietic cells, and binds to a series of 5 cell-surface receptors.6,7 S1P levels are high in the blood, but are extremely low in tissues because of degradation by S1P lyase. These differences in S1P levels lead to the formation of a gradient used by lymphoid cells to exit tissues.8 HSCs also express S1P receptors, undergo chemotaxis in response to S1P, and have recently been shown to use a S1P gradient to emigrate from peripheral tissues to the lymphatic system.9,10 The egress of B-cell progenitors from the BM is dependent on S1P/S1P1 interactions.11 Mobilization of hematopoietic progenitor cells (HPCs) with the CXCR4 antagonist AMD3100 in mice can be inhibited by prior treatment with an S1P lyase inhibitor,12 suggesting that S1P may also be involved in the egress of HPCs from the BM into the peripheral blood (PB).
In the present study, we examined the role of S1P in the egress of HSCs/HPCs after 2 different methods of mobilization: with the CXCR4 antagonists TC1401213 and AMD310014 and with the cytokine G-CSF.15 Using pharmacologic disruption of the S1P/S1P1 axis, Sphk1 deleted (SK1−/−), and S1pr1 conditional knockout (KO) mice (S1P1−/−), we show that S1P plays a significant role in the egress of HSCs/HPCs from the BM after mobilization with the CXCR4 antagonists AMD3100 and TC14012. In contrast, G-CSF–mediated mobilization was independent of the S1P/S1P1 axis. More importantly, we demonstrate that S1P1 is required for the steady-state trafficking of HSCs. Furthermore, we show that plasma S1P levels do not increase in patients undergoing HSC mobilization with G-CSF or AMD3100, and that S1P plasma levels were not predictive for successful mobilization with G-CSF. Finally, we demonstrate that the S1P1 agonist SEW2871 enhances HSC/HPC mobilization after inhibition of CXCR4, raising the possibility that HSC mobilization of normal donors may be greatly simplified.
Methods
Patients
G-CSF and G-CSF/chemotherapy–mobilized PBSC donors were recruited through the Sydney Cellular Therapies Laboratories at Westmead Hospital. PB samples were collected before the commencement of G-CSF and on the morning of the first leukapheresis, with signed informed consent and approval by the Sydney West Area Health Service Human Research Ethics Committee in accordance with the Declaration of Helsinki. Details of autologous donors are provided in Table 1. Among the normal donors mobilized with G-CSF, there were 6 females and 16 males between the ages of 19 and 67, with a median age of 43 years. G-CSF–mobilized normal donors received 10 μg/kg/d of G-CSF in 2 divided doses for 5 days. AMD3100-mobilized donors were recruited through the Washington Medical School, with ethical approval through the Institutional Review Board (the Human Research Protection Office). These donors consisted of 7 males and 7 females between 37 and 65 years of age, with a median age of 49 years. They received 320 μg/kg of AMD3100 by IV infusion over 30 minutes. Blood was collected before AMD3100 administration and at the indicated time points thereafter.
Characteristics of autologous transplantation patients
| Patient ID . | Age/sex . | Diagnosis . | Mobilization protocol . | WBCs pre/post G-CSF, × 109/L . | CD34+ cells/μL . |
|---|---|---|---|---|---|
| 19 | 72/F | MM | Modified HD Cyclo 2 g/m2 | 7/38 | 7 |
| 30 | 55/M | NHL/DLCL relapse | ICE/PEG | 6/40 | NA |
| 31 | 48/F | NHL/DLCL | ICE/PEG | 5/13 | 27 |
| 32 | 56/M | MM | HD Cyclo | 10/11 | 33 |
| 43 | 53/M | Peripheral T-cell lymphoma relapse | ICE | 11/11 | 56 |
| 46 | 48/M | NHL/DLCL | RICE | 7/48 | 29 |
| 48 | 39/M | MM | HD Cyclo | 9/42 | 216 |
| 50 | 26/F | HD relapse | ICE | 7/8 | 7 |
| 59 | 56/M | MM | HD Cyclo | 12/13 | 64 |
| 66 | 61/F | MM | HD Cyclo | 7/16 | 101 |
| 67 | 53/F | MM | HD Cyclo | 8/32 | 198 |
| Patient ID . | Age/sex . | Diagnosis . | Mobilization protocol . | WBCs pre/post G-CSF, × 109/L . | CD34+ cells/μL . |
|---|---|---|---|---|---|
| 19 | 72/F | MM | Modified HD Cyclo 2 g/m2 | 7/38 | 7 |
| 30 | 55/M | NHL/DLCL relapse | ICE/PEG | 6/40 | NA |
| 31 | 48/F | NHL/DLCL | ICE/PEG | 5/13 | 27 |
| 32 | 56/M | MM | HD Cyclo | 10/11 | 33 |
| 43 | 53/M | Peripheral T-cell lymphoma relapse | ICE | 11/11 | 56 |
| 46 | 48/M | NHL/DLCL | RICE | 7/48 | 29 |
| 48 | 39/M | MM | HD Cyclo | 9/42 | 216 |
| 50 | 26/F | HD relapse | ICE | 7/8 | 7 |
| 59 | 56/M | MM | HD Cyclo | 12/13 | 64 |
| 66 | 61/F | MM | HD Cyclo | 7/16 | 101 |
| 67 | 53/F | MM | HD Cyclo | 8/32 | 198 |
MM indicates multiple myeloma; NHL, nonHodgkin lymphoma; DLC, diffuse large cell lymphoma; HD, Hodgkin disease; HD Cyclo, high-dose cyclophosphamide; ICE, ifosfamide, carboplatin, and etoposide; and RICE, rituximab and ICE.
Animals
C57BL/6, congenic B6.SJL-Ptprca Pep3b/BoyJ, DBA2, Balb/c mice were purchased from the Animal Resources Centre (Perth, Australia). Transgenic S1pr1 (S1P1fl/fl) mice16 were kindly provided by Prof R. Proia (National Institute for Diabetes and Digestive and Kidney Diseases, Bethesda, MD) and the SK1−/− mice17 by Prof J. Gamble (Centenary Institute, Sydney, Australia) with permission from Prof Proia. Mx1-Cre mice were obtained from the Walter and Eliza Hall Institute of Medical Research (Melbourne, Victoria, Australia) with permission from the Mouse Genetics Cologne Foundation (Munich, Germany). S1P1fl/fl mice were bred with Mx1-Cre mice to generate Mx1-Cre.S1P1fl/fl mice. S1pr1 was deleted in adult mice by administration of 15 mg/kg of poly I:poly C every second day for a total of 3 doses, and mice were then housed for a minimum of 2 weeks before use in experiments. Mice were used in accordance with guidelines of the Westmead Hospital Animal Ethics Committee.
Reagents
FTY720 was a gift from Novartis (Basel, Switzerland). 4′Deoxypyridoxine (DOP), SEW2871, and AMD3100 were purchased from Sigma-Aldrich and G-CSF (Filgrastim) from Amgen. TC14012 was synthesized by Auspep. A summary of reagents administered to mice is provided in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Anti-CD45.1–FITC, anti-CD45.2–PE, anti-B220 allophycocyanin cychrome 7 (APC-Cy7), anti–CD3 PE-Cy7, anti–Gr-1(Ly6G/C) (biotin), anti-CD4 (APC), anti-CD8 (APC-Cy7), and streptavidin-APC were obtained from BD Biosciences.
Mobilization and drug treatments
Mice were mobilized by subcutaneous injection of 10 mg/kg of AMD3100 or TC14012 1 hour before being killed. G-CSF (125 μg/kg) was administered twice daily by subcutaneous administration for 4 days, and mice were killed on day 5. When G-CSF and AMD3100 were used in combination, mice received G-CSF for 4 days and AMD3100 was administered on day 5, 1 hour before killing. FTY720 (1 mg/kg) was administered by IP injection 14 hours before mobilization, and DOP was added to glucose water at a final concentration of 30 mg/L for 3 days before mobilization. Mice mobilized with G-CSF were treated with 1 mg/kg of FTY720 14 hours before the first dose of G-CSF, and another 1 mg/kg/d for the 4 days of the mobilization.
Competitive repopulation assay and secondary transplantations
Donor PBMCs were obtained from indicated volumes of blood from mobilized or control animals (CD45.2+) by Ficoll density gradient centrifugation. Lethally (11 Gy) irradiated recipients (F1 mice from B6.SJL-Ptprca Pep3b/BoyJ × C57Bl/6 (CD45.1+CD45.2+) for the FTY720 experiment or B6.SJL-Ptprca Pep3b/BoyJ (CD45.1+) for the SEW2871 experiment) received PBMCs collected from 2 donor mice for the FTY720 experiment and 500 μL of donor blood for the SEW2871 experiment, mixed with 0.25 × 106 competitor BM mononuclear cells from untreated mice (CD45.1+ B6.SJL-Ptprca Pep3b/BoyJ for FTY720 experiment or RFP transgenic mice for SEW2871 experiment). Six recipients were used to measure the repopulation capacity of PB from PBS- or FTY720-treated mice, and 12 recipients were used to assess AMD3100-mobilized donors that had been pretreated with placebo or FTY720. Groups of 8-9 mice were recipients of AMD3100-, placebo-, or SEW2871-treated donors. Recipient mice were bled at the indicated times after transplantation to monitor donor engraftment. Mice were killed at week 16 and donor-derived B cells, T cells, and granulocytes were assessed by flow cytometry. To demonstrate the self-renewing capacity of the donor-derived long-term repopulating cells, 2.5 × 106 BM cells from primary recipients were transplanted into lethally irradiated CD45.1+ secondary recipients and donor-derived reconstitution was assessed.
CFU assays
Mononuclear cells were isolated from blood, BM, spleen, thymus, and lymph nodes of mice. Single-cell suspensions of spleen, thymus, and lymph nodes were obtained by pushing the tissue through a 70-μ nylon mesh. Mononuclear cells from tissues were assessed for CFU-C content by culturing cells in MethoCult medium supplemented with 50 ng/mL of recombinant mouse SCF, 10 ng/mL of recombinant mouse IL-3, 10 ng/mL of recombinant human IL-6, and 3 U/mL of recombinant human erythropoietin (StemCell Technologies). RBC lysis was performed on blood, BM, and spleen samples before plating in methylcellulose.
Assessment of S1P and CXCL12 concentrations in plasma
S1P levels in human and mouse plasma was determined using an S1P ELISA kit (Echelon Biosciences) according to the manufacturer's instructions. CXCL12 levels were assessed using an ELISA kit from Bioscientific. S1P in human plasma was also assessed using HPLC, as described previously.18
Chemotaxis assay
BM cells (5 × 105) were added to the upper chamber of a Transwell with 8-μm diameter pores (In Vitro Technologies). Test agents were plated in the bottom chamber and plates were incubated for 3 hours at 37°C. Cells recovered from the lower chamber were counted and plated in CFU assays, and the number of CFUs recovered from the lower chamber was calculated.
Assessment of S1P1 expression by immunohistochemistry
Thymuses were fixed in 10% neutral buffered formalin (Lomb Scientific) for 24 hours, and then embedded in paraffin. Sections were cut at a 4-μm thickness and mounted onto SuperFrost slides (Menzel-Glaser). After drying, sections were dewaxed with histolene, rehydrated in descending ethanol concentrations, and incubated for 30 minutes with 3% H2O2 (Sigma-Aldrich). The S1P1 antigen was unmasked by autoclaving in 10mM citrate, pH 6.0, at 120°C for 30 minutes. Sections were incubated in normal goat serum and then 2 μg/mL of S1P1 rabbit primary Ab (Santa Cruz Biotechnology) at 4°C overnight, followed by 1 hour at 37°C. An HRP-conjugated goat anti–rabbit secondary Ab (1:200; DakoCytomation) was used and then visualized using the diaminobenzidine chromogen (Envision Plus; DakoCytomation). All images were obtained using SPOT Version 4.6 software and a SPOT RTKE camera attached to an Olympus BX40 microscope in air at room temperature.
PCR and RT-PCR
Genomic DNA and total RNA were extracted from PB cells using TRIzol reagent (Invitrogen). For RNA, genomic DNA was removed by RNase-free DNase. RNA was reverse transcribed using MMLV reverse transcriptase and oligo-dT primers (all from Invitrogen) for 60 minutes at 42°C. PCR analysis was performed using Taq DNA polymerase (Promega) with the following cycles: 94°C for 30 seconds, 51°C for 30 seconds, and 72°C for 60 seconds, with a first cycle at 94°C for 3 minutes and a final cycle at 72°C for 2 minutes. Specific primers for GAPDH and the detection of exon 2 of genomic DNA have been reported previously,16,19 and those for the detection of S1P1 mRNA were: forward, GCTTCGTCCGGCTTGAGCGAG; reverse, GCCAGGTCAGCGAGCAATCCA. Amplified products were resolved in a 2% agarose gel, visualized with GelRed staining (Jomar Biosciences), and imaged using a Vilber Lourmat Gel Documentation System. Composite images compiled using Adobe Photoshop Version CS4.
Statistics
Comparisons between 2 groups were performed using Student t tests, and those between multiple groups using ANOVA analysis. Pairwise comparisons between groups were adjusted for multiple comparisons using the Bonferroni method. Linear regression was used to determine correlations between variables. Analysis of transplantation experiments was performed as follows. The level of engraftment was log transformed before analysis to stabilize the variance. Repeated-measures ANOVA was used to examine the joint effects of the within-mouse change over time and with treatment.
Results
FTY-720 inhibits HPC egress from the BM during mobilization by a CXCR4 antagonist but not by G-CSF
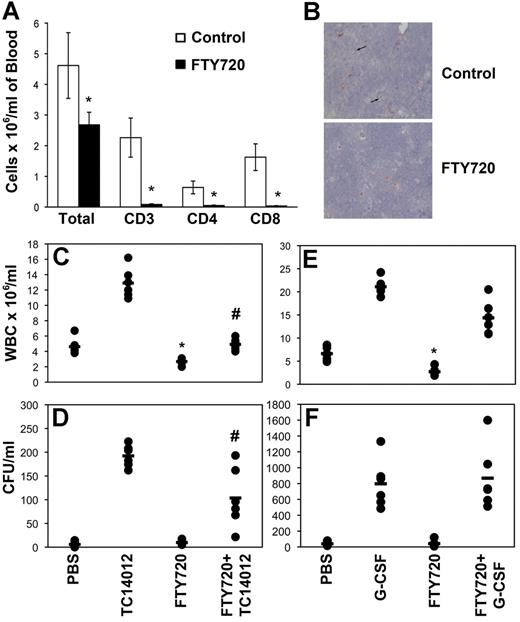
We used several pharmacologic agents known to disrupt the S1P/S1P1 axis to assess the contribution of S1P to the egress of HSCs/HPCs from the BM in a mouse model of mobilization. FTY720 is a potent immunosuppressive agent that induces internalization and degradation of the S1P receptors, including S1P1, rendering the cells unresponsive to S1P.20 HSCs express S1P1 and migrate in vitro and in vivo in response to S1P.10,21 Administration of 1 mg/kg of FTY720 to C57Bl6 mice resulted in an expected decline in the total WBC count, predominantly because of loss of circulating CD4+ and CD8+ T cells (Figure 1A).22 Loss of surface S1P1 expression in lymphoid cells in the thymus of treated animals demonstrated that these cells are no longer able to respond to S1P (Figure 1B). Despite the significant reduction in total WBCs in the initial cohort of 6 animals, no significant reduction in circulating CFUs was detected (Figure 1D and F). Examination of a larger cohort (n = 24) permitted the detection of a small but significant (27%, P = .03) reduction in PB CFUs after FTY720 treatment—considerably less than that reported by Massberg et al.10
FTY720 inhibits HPC mobilization by CXCR4 antagonists but not G-CSF. (A) Groups of 6 mice were treated with FTY720 or placebo 14 hours before being killed. PB was collected and analyzed for total WBCs and T cells by flow cytometry. The mean and SD of 6 animals is shown. *P < .05. (B) Thymi collected from mice treated as in panel A were stained for S1P1 using immunohistochemistry. Representative fields of view obtained using SPOT Version 4.6 software and a SPOT RTKE camera attached to an Olympus BX40 microscope with a UPlanFl 40×/0.75 lens are shown. Original magnification was 400×. (C-F) Groups of 6 mice were treated with FTY720 or placebo 14 hours before administration of TC14012 (C-D) or G-CSF (E-F). Mice mobilized with G-CSF also received FTY720 each day during the mobilization protocol. WBC counts (C and E) were performed on blood collected by cardiac puncture. CFUs (D and F) were enumerated using semisolid cultures and the number of CFUs/mL was calculated using total WBCs. Each dot represents data from a single animal and the bar indicates the mean for the cohort. Representative experiments are shown. n = 3 for panels C and D and n = 2 for panels E and F. *P < .05 compared with control; #P < .05 compared with TC14012 alone.
FTY720 inhibits HPC mobilization by CXCR4 antagonists but not G-CSF. (A) Groups of 6 mice were treated with FTY720 or placebo 14 hours before being killed. PB was collected and analyzed for total WBCs and T cells by flow cytometry. The mean and SD of 6 animals is shown. *P < .05. (B) Thymi collected from mice treated as in panel A were stained for S1P1 using immunohistochemistry. Representative fields of view obtained using SPOT Version 4.6 software and a SPOT RTKE camera attached to an Olympus BX40 microscope with a UPlanFl 40×/0.75 lens are shown. Original magnification was 400×. (C-F) Groups of 6 mice were treated with FTY720 or placebo 14 hours before administration of TC14012 (C-D) or G-CSF (E-F). Mice mobilized with G-CSF also received FTY720 each day during the mobilization protocol. WBC counts (C and E) were performed on blood collected by cardiac puncture. CFUs (D and F) were enumerated using semisolid cultures and the number of CFUs/mL was calculated using total WBCs. Each dot represents data from a single animal and the bar indicates the mean for the cohort. Representative experiments are shown. n = 3 for panels C and D and n = 2 for panels E and F. *P < .05 compared with control; #P < .05 compared with TC14012 alone.
We used the CXCR4 antagonists TC14012 and AMD3100 (both at 10 mg/kg) to induce HPC mobilization, a well-defined mobilizing strategy in which CXCL12 binding to CXCR4 is disrupted. Pretreatment of mice with FTY720 significantly inhibited the TC14012 antagonist-induced leukocytosis (Figure 1C, P < .0001). FTY720 also significantly decreased the number of circulating CFUs in the PB after administration of TC14012 (Figure 1D, P = .009). There was no measurable increase in the number of CFUs detected in the lymph nodes or spleens of FTY720-treated animals compared with placebo-treated animals (data not shown) regardless of coadministration of TC14012. This demonstrates that HPC mobilization into the PB by CXCR4 antagonists is inhibited by FTY720-mediated down-regulation of S1P1 receptor expression. In contrast, the leukocytosis and mobilization of HPCs by G-CSF were not significantly inhibited by FTY720-induced down-regulation of S1P1 (Figure 1E-F). Similar results were obtained using BALB/c mice (supplemental Figure 1). These results demonstrate that S1P1 is involved in the egress of HPCs from the BM during steady-state conditions and after CXCR4 antagonist–induced mobilization, but not after G-CSF-–induced mobilization. Inhibiting the S1P gradient or suppressing S1P1 expression had only minor effects on the makeup of colonies obtained, with a small but significant reduction in CFU-GEMM and a concomitant increase in the proportion of CFU-GM, suggesting that the more primitive progenitors are more sensitive to S1P (supplemental Table 2).
S1P1−/− mice demonstrate reduced HPC mobilization after administration of AMD3100
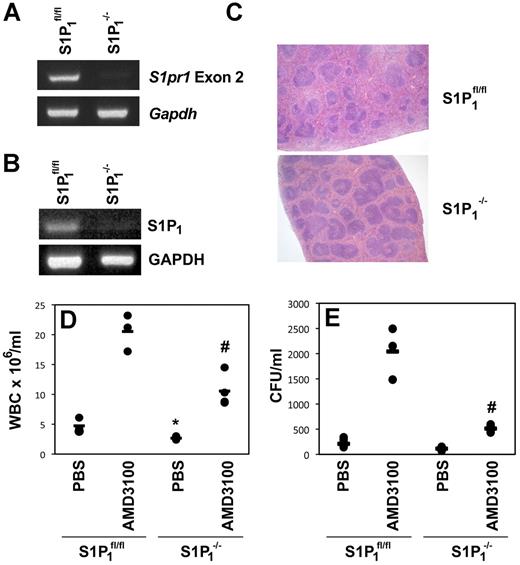
The results obtained from pharmacologic suppression of S1P1 by FTY720 were confirmed using conditional S1pr1 gene-deleted mice. Gene deletion of S1P1 results in embryonic lethality.16,23 To overcome this problem, we generated conditional S1ppr1-KO mice by crossing mice in which S1pr1 exon 2 was flanked by loxP sites with mice transgenic for Mx1-Cre. After poly I:poly C treatment, the S1pr1 gene was deleted in hematopoietic cells (Figure 2A), and no mRNA for S1P1 could be detected by RT-PCR (Figure 2B). Consistent with previous reports, deletion of the S1pr1 gene resulted in accumulation of lymphoid cells in the white pulp of the spleen (Figure 2C)6 and a reduction in the total WBCs (Figure 2D, P = .014). The leukocytosis induced by AMD3100 was also significantly inhibited (Figure 2D, P = .006). More importantly, the number of CFUs mobilized into the PB as a result of AMD3100 administration was significantly reduced in S1P1−/− mice (Figure 2E, P = .004), further supporting a role for S1P and S1P1 in AMD3100-mediated HPC mobilization. No difference was observed in the number of lineage-negative cells expressing Sca-1 and c-kit or in the colony-forming capacity of the BM after S1pr1 deletion (data not shown).
Mice deficient in S1P1 have an impaired response to AMD3100-induced HPC mobilization. (A) PCR amplification of exon 2 and GAPDH from DNA isolated from the blood of poly I:poly C–treated S1P1fl/fl or Mx1Cre.S1P1fl/fl (S1P1−/−) mice. (B) RT-PCR amplification of S1P1 and GAPDH mRNA isolated from the blood of poly I:poly C–treated S1P1fl/fl or Mx1Cre.S1P1fl/fl (S1P1−/−) mice. (C) H&E-stained sections of spleens isolated from S1P1fl/fl and S1P1−/− mice. Representative fields of view obtained using SPOT Version 4.6 software and a SPOT RTKE camera attached to an Olympus BX40 microscope with a PlanApo 2×/0.08 lens are shown. Original magnification was 20×. (D-E) S1P1−/− or S1P1fl/fl mice received AMD3100 or placebo. WBC counts (D) were performed on blood collected by cardiac puncture. CFUs (E) were enumerated using semisolid cultures, and the number of CFUs/mL was calculated using total WBCs. Each dot represents data from a single animal and the bar indicates the mean for the cohort. *P < .05 compared with controls in S1P1fl/fl mice; #P < .05 compared with AMD3100 in S1P1fl/fl mice. Pooled data collected as mice became available are shown.
Mice deficient in S1P1 have an impaired response to AMD3100-induced HPC mobilization. (A) PCR amplification of exon 2 and GAPDH from DNA isolated from the blood of poly I:poly C–treated S1P1fl/fl or Mx1Cre.S1P1fl/fl (S1P1−/−) mice. (B) RT-PCR amplification of S1P1 and GAPDH mRNA isolated from the blood of poly I:poly C–treated S1P1fl/fl or Mx1Cre.S1P1fl/fl (S1P1−/−) mice. (C) H&E-stained sections of spleens isolated from S1P1fl/fl and S1P1−/− mice. Representative fields of view obtained using SPOT Version 4.6 software and a SPOT RTKE camera attached to an Olympus BX40 microscope with a PlanApo 2×/0.08 lens are shown. Original magnification was 20×. (D-E) S1P1−/− or S1P1fl/fl mice received AMD3100 or placebo. WBC counts (D) were performed on blood collected by cardiac puncture. CFUs (E) were enumerated using semisolid cultures, and the number of CFUs/mL was calculated using total WBCs. Each dot represents data from a single animal and the bar indicates the mean for the cohort. *P < .05 compared with controls in S1P1fl/fl mice; #P < .05 compared with AMD3100 in S1P1fl/fl mice. Pooled data collected as mice became available are shown.
Reduction of the S1P gradient between BM and blood suppresses HPC egress from BM after mobilization with AMD3100
The experiments described in “Results” clearly demonstrate that S1P1 expression is required for optimal HPC mobilization. To assess the role of the S1P gradient between blood and BM on HPC mobilization directly, we used both pharmacologic and genetic approaches to disrupt the gradient. The S1P gradient between the PB and tissues is maintained by the S1P-degrading enzyme S1P lyase, which is abundant in tissue and maintains tissue S1P concentrations lower than those in the blood. S1P lyase activity can be inhibited with the vitamin-B6 antagonist DOP.8 The concentration of S1P in the BM of mice treated for 3 days with DOP was increased by 1.3- to 2.2-fold (data not shown), but did not result in a significant reduction in the PB WBC count or basal CFU levels (Figure 3A-B). Although the leukocytosis induced by 10 mg/kg of AMD3100 was reduced 22% by DOP, this did not reach statistical significance (Figure 3A). Similar to the effects of FTY720, DOP significantly suppressed the mobilization of CFUs into the PB (Figure 3B, P = .02).
Reducing the plasma S1P concentration or increasing the tissue S1P concentration inhibits AMD3100-mediated HPC mobilization. Groups of 6 mice treated with DOP or placebo for 3 days (A-B) or deficient in SK1 (C-D) were killed 1 hour after treatment with 10 mg/kg of AMD3100 or placebo. WBCs (A and C) and the number of CFUs present in cardiac blood (B and D) were determined as described in the “Methods. ” Representative data from 1 of 2 experiments are shown. #P < .05 compared with AMD3100 alone (B) or AMD3100-treated wild-type mice (D). ELISA was used to determine the level of S1P in the plasma of wild-type and SK1−/− mice (E) and the mean and SD of 3-4 animals for each group is shown. The ability of the plasma from wild-type and SK1−/− mice to induce the chemotaxis of HPCs was determined using a CFU assay to assess the population recovered from the lower chamber (F). *P < .05.
Reducing the plasma S1P concentration or increasing the tissue S1P concentration inhibits AMD3100-mediated HPC mobilization. Groups of 6 mice treated with DOP or placebo for 3 days (A-B) or deficient in SK1 (C-D) were killed 1 hour after treatment with 10 mg/kg of AMD3100 or placebo. WBCs (A and C) and the number of CFUs present in cardiac blood (B and D) were determined as described in the “Methods. ” Representative data from 1 of 2 experiments are shown. #P < .05 compared with AMD3100 alone (B) or AMD3100-treated wild-type mice (D). ELISA was used to determine the level of S1P in the plasma of wild-type and SK1−/− mice (E) and the mean and SD of 3-4 animals for each group is shown. The ability of the plasma from wild-type and SK1−/− mice to induce the chemotaxis of HPCs was determined using a CFU assay to assess the population recovered from the lower chamber (F). *P < .05.
To confirm the findings obtained with pharmacologic inhibition of S1P lyase, we examined SK1−/− mice, which lack Sphk1 but still express Sphk2. As a result, plasma concentrations of S1P are significantly reduced in SK1−/− mice (Figure 3E). This reduction in S1P concentrations in the plasma was reflected by a reduced capacity of the plasma from SK1−/− mice to attract HPCs into the lower well in a chemotaxis assay (Figure 3F). Despite reduced PB S1P concentrations, SK1−/− mice had normal WBCs compared with wild-type animals (Figure 3C). However, the mobilization of HPCs by AMD3100 was significantly reduced in these animals (Figure 3D). This demonstrates that the S1P gradient plays a significant role in HPC mobilization after blockade of CXCR4, and that basal S1P plasma concentrations are sufficient for this purpose.
The level of transplantable steady-state and AMD3100-mobilized HSCs is reduced after down-regulation of S1P1
To demonstrate the role of S1P1 in the mobilization of HSCs, we undertook competitive transplantation experiments in mice with or without FTY720-mediated suppression of S1P1. PB was collected from donor mice (CD45.2) treated with FTY720 or placebo with or without the addition of AMD3100, and injected into lethally irradiated recipients (CD45.1/CD45.2). To ensure the survival of all animals, BM-derived competitor (CD45.1) cells were also administered to all recipients. This system permitted the identification of donor (CD45.2), recipient (CD45.1/CD45.2), and competitor (CD45.1) cells by flow cytometry (Figure 4A). All recipient mice demonstrated multilineage engraftment (Figure 4B and E left panels), however, the recipients of AMD3100-mobilized blood from FTY720-treated animals had 2.1-fold (95% confidence interval [95% CI], 1.1-4.2, P = .034) lower donor chimerism (Figure 4C). Secondary transplantations into CD45.1+ recipients demonstrated that BM from all primary recipients receiving AMD3100-mobilized PBMCs were capable of producing multilineage engraftment of donor (CD45.2+) origin (Figure 4E right panel). More importantly, the contribution of donor-derived cells was 8.6-fold (95% CI, 1.9-38.1, P = .0009) less in mice receiving blood from donors treated with FTY720 (but not AMD3100, ie, under steady-state conditions) compared with mice receiving blood from placebo-treated controls (Figure 4D). The level of engraftment declined over the 16-week period in recipients receiving blood from FTY720-treated donors. This suggests that the cells transplanted had limited long-term repopulating ability. These data demonstrate that the S1P/ S1P1 axis plays a role in the egress of HSCs from the BM during steady-state conditions and after CXCR4 antagonist-induced mobilization.
Suppressing S1P1 using FTY720 inhibits the mobilization of transplantable HSCs. (A) Representative scatter plots showing the contribution of donor (CD45.2), recipient (CD45.1/CD45.2), and competitor (CD45.1) cells. (B) Dot plots showing T-cell (CD3), B-cell (B220) and granulocyte (Gr-1) lineage donor (CD45.2) cells in transplantation recipients. (C) The percentage contribution to hematopoiesis in recipient mice receiving PBMCs from placebo- or FTY720-treated donors mobilized with AMD3100 is shown. The mean and standard error of 12 recipient animals is shown. (D) The percentage contribution to hematopoiesis in recipient mice receiving PB from placebo or FTY720 treated donors that were not mobilized. The mean and standard error of 6 recipient animals is shown. (E) The proportion of B cells, T cells, and granulocytes within the donor (CD45.2+)–derived population in primary (left panel) or secondary (right panel) recipients. All primary donors had been mobilized with AMD3100 and treated with (FTY720) or without (Control) pretreatment with FTY720. Secondary recipients received 2.5 × 106 unfractionated BM from primary recipients. Data from 6 primary and 6 secondary recipients are shown for each treatment. Representative data from 1 of 2 experiments are shown.
Suppressing S1P1 using FTY720 inhibits the mobilization of transplantable HSCs. (A) Representative scatter plots showing the contribution of donor (CD45.2), recipient (CD45.1/CD45.2), and competitor (CD45.1) cells. (B) Dot plots showing T-cell (CD3), B-cell (B220) and granulocyte (Gr-1) lineage donor (CD45.2) cells in transplantation recipients. (C) The percentage contribution to hematopoiesis in recipient mice receiving PBMCs from placebo- or FTY720-treated donors mobilized with AMD3100 is shown. The mean and standard error of 12 recipient animals is shown. (D) The percentage contribution to hematopoiesis in recipient mice receiving PB from placebo or FTY720 treated donors that were not mobilized. The mean and standard error of 6 recipient animals is shown. (E) The proportion of B cells, T cells, and granulocytes within the donor (CD45.2+)–derived population in primary (left panel) or secondary (right panel) recipients. All primary donors had been mobilized with AMD3100 and treated with (FTY720) or without (Control) pretreatment with FTY720. Secondary recipients received 2.5 × 106 unfractionated BM from primary recipients. Data from 6 primary and 6 secondary recipients are shown for each treatment. Representative data from 1 of 2 experiments are shown.
Changes in plasma S1P levels are not involved in HSC mobilization
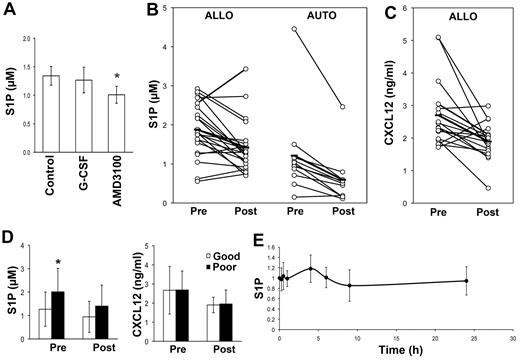
Our data demonstrate that S1P mediates the egress of HSCs/HPCs from the BM under steady-state conditions and after mobilization with CXCR4 antagonists, but not G-CSF mobilization. However, it has been suggested that HPC mobilization by AMD3100 or G-CSF is dependent on increases in plasma S1P levels.12 In an attempt to reconcile these findings with our data, we assessed S1P concentrations in the cardiac blood of mice treated with either AMD3100 or G-CSF. Surprisingly, both agents produced a slight decrease in plasma S1P concentrations, although only that induced by AMD3100 was statistically significant (Figure 5A).
Plasma CXCL12 and S1P concentrations decrease after AMD3100, G-CSF, or G-CSF plus chemotherapy–mediated mobilization. (A) Mice were treated twice daily with 250 mg/kg of G-CSF for 5 days or 10 mg/kg of AMD3100 1 hour before being killed. Plasma was collected and analyzed for S1P levels by ELISA. The mean and SD of data obtained from 6 mice is shown. *P < .05. (B-D) Donors for allogeneic transplantations were mobilized with G-CSF, whereas patients scheduled for autologous transplants were mobilized with G-CSF with or without chemotherapy. Premobilization samples (Pre) were collected before the commencement of the mobilization protocol. Postmobilization samples for G-CSF were collected just before the first leukapheresis. The concentration of S1P (B) and CXCL12 (C) is shown. Each dot represents data from a single patient and the horizontal line indicates the mean of the cohort. Connected dots indicate samples from the same patient. (D) The level of S1P (left panel) and CXCL12 (right panel) in autograft patients who mobilized well (Good, > 50 CD34+ cells/μL) or relatively poorly (Poor, < 50 CD34+ cells/μL). The mean and SD of the all samples assessed is shown for samples collected before (Pre) the commencement of the mobilization procedure or on the day of the first collection (Post). *P < .05. (E) Plasma S1P concentrations in 14 donors mobilized with AMD3100 collected at the indicated time points. The data for each patient were normalized to baseline S1P concentrations and the mean and SD of data from all 14 patients is shown.
Plasma CXCL12 and S1P concentrations decrease after AMD3100, G-CSF, or G-CSF plus chemotherapy–mediated mobilization. (A) Mice were treated twice daily with 250 mg/kg of G-CSF for 5 days or 10 mg/kg of AMD3100 1 hour before being killed. Plasma was collected and analyzed for S1P levels by ELISA. The mean and SD of data obtained from 6 mice is shown. *P < .05. (B-D) Donors for allogeneic transplantations were mobilized with G-CSF, whereas patients scheduled for autologous transplants were mobilized with G-CSF with or without chemotherapy. Premobilization samples (Pre) were collected before the commencement of the mobilization protocol. Postmobilization samples for G-CSF were collected just before the first leukapheresis. The concentration of S1P (B) and CXCL12 (C) is shown. Each dot represents data from a single patient and the horizontal line indicates the mean of the cohort. Connected dots indicate samples from the same patient. (D) The level of S1P (left panel) and CXCL12 (right panel) in autograft patients who mobilized well (Good, > 50 CD34+ cells/μL) or relatively poorly (Poor, < 50 CD34+ cells/μL). The mean and SD of the all samples assessed is shown for samples collected before (Pre) the commencement of the mobilization procedure or on the day of the first collection (Post). *P < .05. (E) Plasma S1P concentrations in 14 donors mobilized with AMD3100 collected at the indicated time points. The data for each patient were normalized to baseline S1P concentrations and the mean and SD of data from all 14 patients is shown.
To investigate these findings in a clinical setting, we examined plasma S1P concentrations in 22 normal donors undergoing G-CSF–mediated HSC mobilization for transplantation purposes. Blood samples were drawn before administration of the first G-CSF injection and on the morning of the first leukapheresis before establishing venous access. Plasma S1P concentrations, as determined by ELISA, declined from 1.9 ± 0.7 to 1.4 ± 0.7μM (P = .0003; Figure 5B). This finding was confirmed in a smaller group of donors using HPLC to determine the S1P concentration (1.1 ± 0.3μM before mobilization to 0.9 ± 0.3μM after mobilization, P = .01, n = 13). A slightly greater decline in the concentration of S1P in plasma (1.2 ± 1.1 vs 0.6 ± 0.7μM, P = .003) was observed in patients (n = 11) being mobilized with G-CSF and chemotherapy for autologous transplantation (Figure 5B). S1P plasma concentrations were not predictive of the number of CD34+ cells/μL obtained at the time of the first leukapheresis in allogeneic or autologous donors (data not shown). However, patients who mobilized poorly (ie, those with less than 50 CD34+ cells/μL) had significantly higher plasma levels of S1P before mobilization (2.01 ± 1.00 vs 1.27 ± 0.73μM, P = .03; Figure 5D left panel). This difference was no longer apparent at the time of collection (1.40 ± 0.89 vs 0.94 ± 0.66μM, P = .13). When the analysis was restricted to allogeneic donors, high S1P plasma concentrations remained predictive of a lower number of CD34+ cells/μL (data not shown). Plasma S1P is thought to originate from RBCs or possibly platelets,8 but neither the RBC nor platelet counts demonstrated any correlation with the concentration of S1P in steady-state or mobilized plasma. However, both RBC and platelet counts did significantly decrease as a result of G-CSF administration (supplemental Table 3).
AMD3100 is being increasingly used as a mobilizing agent,24 with alternative CXCR4 antagonists being developed for use as single agents.25,26 Therefore, we also examined plasma S1P concentrations in 14 donors mobilized with AMD3100 as a single agent. AMD3100 produced a median peak WBC count of 16 × 109/L (range 13-25, P < .001) and CD34+ cells/μL of 17 (range 4-46, P = .0002) 4 hours after AMD3100 administration. S1P concentrations remained stable over the 24 hours after AMD3100 administration (Figure 5E). Furthermore, there was no correlation between S1P plasma levels at baseline or after 4 hours and the number of CD34+ cells/μL.
CXCL12 signaling through CXCR4 has been considered to play a role in the egress of HSCs/HPCs from the BM during G-CSF–mediated mobilization,27 and plasma CXCL12 concentrations have been associated with mobilization efficiency.28 Therefore, we also assessed the CXCL12 levels in plasma from mobilized donors. In keeping with the findings of Gazitt et al,28 we observed a significant decrease in the concentration of CXCL12 in the plasma of normal PBSC donors on the day of collection compared with steady-state (Figure 5C). Unlike the findings by Gazitt et al in lymphoma patients undergoing autologous PBSC transplantation,28 the plasma concentration of CXCL12 was not associated with the number of CD34+ cells/μL, nor was there any difference in the CXCL12 concentration at steady-state or at the time of collection in donors that mobilized well (> 50 CD34+ cells/μL) compared with those with low CD34+ cells/μL (< 50 CD34+ cells/μL; Figure 5D right panel). These data demonstrate that HSC/HPC mobilization with G-CSF or AMD3100 in humans is not associated with elevations in plasma S1P, and strongly suggest that elevation in plasma S1P is not required for HSC/HPC mobilization with these agents.
IV administration of a S1P1 agonist enhances AMD3100-mediated mobilization
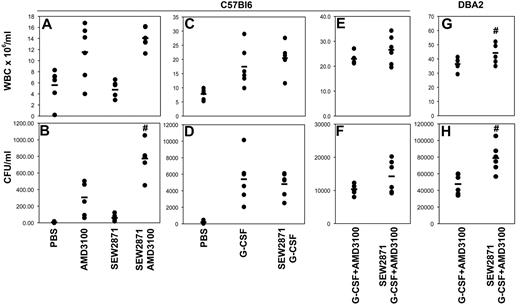
We have shown that steady-state S1P levels are sufficient to induce the egress of HSCs from the BM to blood, and that elevations in plasma S1P are not required for mobilization. We also sought to determine whether elevations in plasma S1P could promote greater mobilization. To this end, we used a chemical analog of S1P, SEW2871, because S1P itself can be rapidly degraded in vivo.29 IV administration of SEW2871 1 hour before AMD3100 administration resulted in a dose-dependent mobilization of HPCs, but only when AMD3100 was coadministered (data not shown). Although 25 mg/kg of SEW2871 had a minimal effect on total WBC counts (Figure 6A) when administered 1 hour before AMD3100, HPC mobilization was increased by 2.5-fold (Figure 6B, P = .002). As seen with other strategies for modulating the S1P1/S1P axis, SEW2871 did not significantly change G-CSF–mediated HPC mobilization (Figure 6D). However, when mice treated with the combination of G-CSF (for 4 days) and AMD3100 (on day 5) were given SEW2871 (1 hour before AMD3100), there was a further increase in HPC mobilization in DBA2 but not C57Bl6 mice (Figure 6F and 6H). An insignificant 1.4-fold (Figure 6F, P = nonsignificant) increase in HPCs in the blood was observed in C57Bl6 mice, and a significant 1.7-fold (Figure 6H, P = .004) increase was seen in DBA2 mice. The proportion of CFU-GEMM was also slightly but significantly increased with the addition of SEW2871 (supplemental Table 2) in DBA2 (P = .0002) but not C57Bl6 mice (P = nonsignificant). The concurrent addition of SEW2871 was associated with a small increase in the WBC count in DBA2 (good mobilizers), but not C57Bl6 (poor mobilizers), mice (Figure 6E and 6G).
The S1P1 agonist SEW2871 increases the mobilization of HPCs in response to AMD3100 and the combination of G-CSF and AMD3100. Mice received an IV injection of SEW2871 followed by AMD3100 1 hour later. Mice were killed after an additional hour. WBC counts (A, C, E, and G) and the number of CFUs present in cardiac blood (B, D, F, and H) of mice mobilized with AMD3100 (A-B), G-CSF (C-D), or G-CSF + AMD3100 (E-H) with or without the addition of SEW2871 as indicated were determined as described in “Methods.” #P < .05 compared with AMD3100 alone or G-CSF and AMD3100. Representative data from 1 of 2 experiments are shown.
The S1P1 agonist SEW2871 increases the mobilization of HPCs in response to AMD3100 and the combination of G-CSF and AMD3100. Mice received an IV injection of SEW2871 followed by AMD3100 1 hour later. Mice were killed after an additional hour. WBC counts (A, C, E, and G) and the number of CFUs present in cardiac blood (B, D, F, and H) of mice mobilized with AMD3100 (A-B), G-CSF (C-D), or G-CSF + AMD3100 (E-H) with or without the addition of SEW2871 as indicated were determined as described in “Methods.” #P < .05 compared with AMD3100 alone or G-CSF and AMD3100. Representative data from 1 of 2 experiments are shown.
To demonstrate that S1P agonists can enhance AMD3100-mediated HSC mobilization, we again used competitive transplantation experiments. PB was collected from donor mice (CD45.2) treated with SEW2871 or placebo, followed by AMD3100. Lethally irradiated recipients (CD45.1) received 500 μL of donor blood with the addition of 0.25 × 106 BM cells from RFP mice (Figure 7A). Mice were monitored by weekly tail-vein bleeds, and the percentage donor engraftment over time is shown in Figure 7B. The recipients of blood from donors treated with SEW2871 and AMD3100 had a 2.8-fold (95% CI, 1.7-4.7, P < .001) increase in donor chimerism compared with recipients of blood from animals mobilized with AMD3100 alone (Figure 7B). Analysis of the contribution of hematopoietic lineages revealed a similar distribution as that seen in the experiment using FTY720 to suppress S1P1 expression, which was not significantly altered by the addition of SEW2871 (Figure 7C). These data demonstrate that S1P1 agonists can significantly improve HSC mobilization induced by CXCR4 antagonists such as AMD3100.
The S1P1 agonist SEW2871 enhances AMD3100-mediated HSC mobilization. (A) Representative scatter plots showing the contribution of donor (CD45.2, RFP−), recipient (CD45.1+), and competitor (CD45.2, RFP+) cells. (B) The percentage contribution to hematopoiesis in recipient mice receiving PBMCs from placebo- or SEW2871-treated donors mobilized with AMD3100 is shown. The mean and standard error in recipient animals are shown. (C) Proportion of B cells, T cells, and granulocytes within the donor (CD45.2+, RFP−)–derived population. Data from a single transplantation experiment are shown.
The S1P1 agonist SEW2871 enhances AMD3100-mediated HSC mobilization. (A) Representative scatter plots showing the contribution of donor (CD45.2, RFP−), recipient (CD45.1+), and competitor (CD45.2, RFP+) cells. (B) The percentage contribution to hematopoiesis in recipient mice receiving PBMCs from placebo- or SEW2871-treated donors mobilized with AMD3100 is shown. The mean and standard error in recipient animals are shown. (C) Proportion of B cells, T cells, and granulocytes within the donor (CD45.2+, RFP−)–derived population. Data from a single transplantation experiment are shown.
Discussion
HSCs are retained in the HSC niche through chemokine gradients and adhesive interactions mediated by receptors expressed on HSCs, such as CXCR4 and VLA-4 and their respective ligands, CXCL12 and VCAM-1, which are expressed within the niche.30,31 These interactions are disrupted during HSC mobilization.32,33 The loss of retentive factors suggests 2 potential explanations for the egress of HSCs from the BM. The first is a stochastic model in which the HSCs randomly leave the niche as a result of reduced retention signals, with some entering the peripheral circulation. Alternatively, the cells may be directed by positive signals that attract them into the PB. This model requires the presence of a chemoattractant leading from the BM to the blood. Such a chemoattractant would need to be insufficient to overcome retentive forces during steady-state hematopoiesis to permit HSCs to largely remain in the BM. However, once the retentive factors were reduced, it would facilitate the migration of HSCs into the blood. S1P and its receptor, S1P1, control lymphocyte egress from primary and secondary lymphoid organs.7,34 In the present study, we demonstrate for the first time that S1P1 is important for the egress of HPCs and HSCs from the BM under steady-state conditions and after CXCR4 antagonist–mediated mobilization. Suppression of S1P1 expression by genetic or pharmacologic means significantly impaired the mobilization of HPCs into the blood in the presence of CXCR4 blockade and reduced the egress of HSCs in steady-state BM. In addition, the IV infusion of a S1P1 agonist, SEW2871, enhanced AMD3100-mediated HSC mobilization.
The S1P/S1P1 axis plays an important role in the trafficking of hematopoietic cells either directly or via regulation of endothelial cells.35 Because endothelial cells also express S1P1, the strategies used in this study to disrupt S1P1 would affect both the hematopoietic and endothelial compartment. We believe, however, that the results obtained were primarily because of effects on HSCs rather than on endothelial cells for the following reasons. First, the endothelial layer lining BM sinusoids is discontinuous, as opposed to the tight barrier imposed by lymph node endothelium.36 Second, according to the hypothesis proposed by Rosen et al,35 the agonist properties of FTY720 could result in increased vascular integrity and, as a result, decreased HSC mobilization. Deletion of the S1P1 receptor on vascular endothelium would therefore be expected to result in the opposite effect (ie, decreased vascular integrity and thus increased HPC mobilization). However, this increased HPC mobilization was not observed in the S1P1-KO mice.
In addition to a role for S1P1, we also demonstrated that the gradient of S1P between tissues and PB is also important for CXCR4 antagonist–mediated HPC mobilization. After both pharmacologic and genetic flattening of this gradient, HPC mobilization was significantly impaired. However, alterations in S1P levels, particularly in the BM, may have unintended effects on the health of HSCs/HPCs, which could in turn alter mobilization kinetics. S1P1 signaling can promote cell proliferation and maintain pluripotency in some systems,37 making this explanation seem unlikely in the case of the DOP experiments, in which increased S1P levels would have been expected to promote HSC/HPC numbers and thus enhance mobilization. Loss of S1P1 on HSCs by either genetic or pharmacologic means could result in decreased HSC viability and thus mobilization. However, we were unable to detect any effect of FTY720 exposure or S1P1 deletion on HPC viability as assessed by colony assays and BM HSC numbers (data not shown).
The failure to alter HPC mobilization in response to G-CSF by manipulating the S1P/S1P1 axis is consistent with previous studies10 and highlights the differences between G-CSF- and AMD3100-mediated mobilization. Mobilization with G-CSF produces multiple effects on the BM, including the disruption of the chemoattractant effects of CXCL12 and adhesive interactions such as those mediated by VCAM1 and c-kit.33,38,39 In contrast, mobilization with CXCR4 antagonists such as AMD3100 primarily functions by disrupting the effect of CXCL12.40 It is possible that remaining factors that retain HSCs in the BM impede movement into the blood even when the chemoattractant effects of CXCL12 have been blocked, increasing the importance of the S1P gradient when CXCR4 alone is blocked. The superiority of G-CSF over AMD3100 as a mobilizing agent would support this hypothesis. However, the ability of SEW2871 to further enhance HPC mobilization when added to the combination of G-CSF and AMD3100 suggests that more complex events are involved. One possibility is that the pools of HSCs/HPCs mobilized by G-CSF and AMD3100 are not identical. It is tempting to speculate that the rapid mobilization induced by AMD3100 preferentially targets HSCs/HPCs located close to the vasculature, whereas the slower-acting G-CSF mobilizes HSCs from a variety of niches. If this is the case, then the S1P/S1P1 axis may be important for the egress of cells from the vascular niche. This could explain why SEW2871 can enhance mobilization after G-CSF and AMD3100: the mobilization of the vascular pool of HPCs mobilized by AMD3100 could be enhanced independently of the actions of G-CSF.
Recently, Ratajczak et al demonstrated a role for S1P in HPC mobilization, although they identified mobilization-induced activation of the complement system and RBC lysis resulting in increased plasma S1P as being responsible.12 Consistent with that study, we observed a significant decline in both platelet and RBC numbers in response to G-CSF mobilization (supplemental Table 3). However, there was no correlation between RBC or platelet numbers (or the extent of the decrease in their levels) and plasma S1P concentrations before or after G-CSF administration. Indeed, S1P levels significantly declined during G-CSF–induced mobilization. Unlike the results of Ratajczak et al, our results show that basal plasma S1P concentrations are sufficient for mobilization, suggesting that it is the loss of the retentive CXCL12 gradient that is pivotal to the initiation of mobilization. Accordingly, we also showed that disruption of the S1P/S1P1 axis through FTY720-mediated S1P1 down-regulation significantly reduced steady-state transplantable HSCs in the blood of mice. This suggests that S1P1 is required for the normal trafficking of HSCs under steady-state conditions. Our results highlight the capacity of basal S1P concentrations to mediate the egress of HSCs from the BM during mobilization and during steady-state conditions.
The feasibility of using S1P1 agonists as adjunct mobilizing agents in the clinical setting remains to be determined. Although several S1P receptor agonists are available, few have been tested in clinical trials. FTY720, a nonselective S1P receptor agonist, has been approved for the treatment of multiple sclerosis and tested for renal transplant rejection, whereas the compound selective for S1P1, ACT128800, is in or has completed phase 2 trials for multiple sclerosis and psoriasis. Whereas there are suggestions that long-term exposure to these agents produces macular degeneration41 and conditions associated with increased vascular leak,42 there is little evidence of adverse events resulting from a single dose. FTY720 induces transient lymphopenia and bradycardia, with the latter thought to be because of interactions with S1P3.43 The doses of SEW2871 used in the present study are high, and careful evaluation of the safety of S1P1 agonists in this setting will need to be undertaken.
Our study found that expression of the S1P receptor S1P1 was important for both AMD3100-mediated mobilization and the trafficking of HSCs under steady-state conditions. Possibly the most important finding was that AMD3100-mediated HSC mobilization could be enhanced by IV infusion of the S1P1 agonist SEW2871. The addition of IV SEW2871 was unable to enhance G-CSF–mediated HPC mobilization. However, mobilization after the combination of G-CSF and AMD3100 was improved by the addition of SEW2871, although this was most evident in good rather than poor mobilizers. This suggests that this may not be an appropriate strategy for improving outcomes for donors who are poor mobilizers. However, enhanced AMD3100-mediated HSC mobilization by SEW2871 raises the possibility that combining CXCR4 antagonists with a S1P agonist may provide a rapid and less toxic mobilization strategy for normal allogeneic donors.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Novartis (Basel, Switzerland) for the supply of FTY720; Prof Richard Proia for providing the S1P1fl/fl and SK1−/− mice; Virginia James of the Histology Service of the Westmead Millennium Institute for assistance with the preparation of slides; Tamra Cox and Jacky Wong for the collection of patient samples at Westmead; Dr Sandra Lopez for assistance in coordinating patient samples through the Department of Medicine at Washington University; and Dr Karen Byth for assistance with the statistical analysis of the transplantation data.
This study was funded by Cancer Council New South Wales (grant 632890), by the National Health and Medical Research Council (grant 512431), and by the Anthony Rothe Foundation.
Authorship
Contribution: J.G.J. designed and performed the research and assisted in writing the manuscript; N.H. and M.T. performed the research, analyzed the data, and assisted in writing the manuscript; R.W., R.B., and A.D.P. performed the research and analyzed the data; S.M.P. performed the research, analyzed the data, and contributed analytical tools; M.R. collected and contributed the patient material; J.F.D. contributed patient material and information; K.F.B. interpreted the data, provided patient information, and wrote manuscript; and L.J.B. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: M.R. has received honoraria from and has been a consultant for Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Dr Linda Bendall, Westmead Institute for Cancer Research, Westmead Millennium Institute, Westmead, NSW 2145, Australia; e-mail: linda_bendall@wmi.usyd.edu.au.
References
Author notes
J.G.J. and N.H. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal