T cell–engaging CD19/CD3-bispecific BiTE Ab blinatumomab has shown an 80% complete molecular response rate and prolonged leukemia-free survival in patients with minimal residual B-lineage acute lymphoblastic leukemia (MRD+ B-ALL). Here, we report that lymphocytes in all patients of a phase 2 study responded to continuous infusion of blinatumomab in a strikingly similar fashion. After start of infusion, B-cell counts dropped to < 1 B cell/μL within an average of 2 days and remained essentially undetectable for the entire treatment period. By contrast, T-cell counts in all patients declined to a nadir within < 1 day and recovered to baseline within a few days. T cells then expanded and on average more than doubled over baseline within 2-3 weeks under continued infusion of blinatumomab. A significant percentage of reappearing CD8+ and CD4+ T cells newly expressed activation marker CD69. Shortly after start of infusion, a transient release of cytokines dominated by IL-10, IL-6, and IFN-γ was observed, which no longer occurred on start of a second treatment cycle. The response of lymphocytes in leukemic patients to continuous infusion of blinatumomab helps to better understand the mode of action of this and other globally T cell–engaging Abs. The trial is registered with www.clinicaltrials.gov identifier NCT00560794.
Introduction
Among all immune cells, cytotoxic T cells appear to have the highest potential for treatment of malignant diseases.1 This typically requires specific T-cell clones recognizing tumor cell–associated peptide Ags in the context of MHC class I molecules. Multiple vaccination strategies,2,–4 anti-CTLA4 Abs boosting T-cell responses,5,6 and adoptive transfer of ex vivo–expanded, tumor-infiltrating lymphocytes7,–9 have in many cases demonstrated the ability of tumor-specific T cells to treat late-stage cancers, albeit often with low response rates. Likewise, numerous mouse models have shown eradication of solid tumors by cytotoxic T-cell responses and long-term protection from recurrence.10,11 Recently, 2 novel therapeutic modalities have gained clinical proof-of-concept for the treatment of lymphoma and leukemia in which cytotoxic T cells are globally engaged irrespective of their TCR specificity. One used the ectopic expression of a CD19-specific chimeric Ag receptor construct in transfected autologous T cells of patients.12 Patients with chronic lymphocytic leukemia (CLL) showed complete hematologic responses after transfer of gene-modified, CD19-specific T cells.13 The other approach uses a CD19/CD3-bispecific T cell–engaging BiTE Ab called blinatumomab, which can transiently tether any cytotoxic T cell to CD19+ target B cells.14 Blinatumomab has shown high response rates in patients with non-Hodgkin lymphoma (NHL)15 and acute lymphoblastic leukemia (ALL).16 This BiTE Ab has preclinically been characterized in great detail,17,–19 and is currently tested in pivotal trials in ALL patients. Two other BiTE Abs for treatment of solid tumors are currently in phase 1 clinical trials.20,21
A recently published phase 2 study has shown a complete molecular response rate of 80% to monotherapy with blinatumomab in 20 evaluable minimal residual disease–positive (MRD+) ALL patients.16 Recurrence or persistence of ALL in BM—as detected by patient-specific PCR-based assays with a sensitivity down to one tumor cell in 104 BM cells—is among the most important prognostic predictors for this disease.22,–24 Conversion of patients into an MRD-negative status by blinatumomab predicted a positive outcome on long-term leukemia-free survival.16 In this trial, blinatumomab was continuously infused to patients by a portable mini-pump and port system at a dose level of 15 μg/m2/d for 4 weeks. Subsequent treatment cycles were spaced by 2-week treatment-free intervals. The 15-μg dose was found earlier to induce clearance of BM infiltrations in NHL patients, whereas a dose of 60 μg/m2/d was required for achieving a high response rate in lymphoma tissue in these diseases.15 Here, we analyzed all evaluable samples from the phase 2 study to understand the consequences of continuous and repeated exposure of MRD+ ALL patients to T cell–engaging BiTE Ab blinatumomab on (1) T-cell kinetics, redistribution, and behavior; (2) kinetics and durability of target B-cell depletion; (3) kinetics and release of cytokines; and (4) serum concentrations of blinatumomab and pharmacokinetic (PK) parameters. We also assessed whether 4 nonresponding patients had immunologic parameters deviating from those of 16 responding patients.
We here report that T and B lymphocytes in a defined population of MRD+ ALL patients showed a highly predictable behavior on start and during continuous IV infusion of blinatumomab. The observed immunopharmacologic reactions are consistent with the mode of action of a T cell–engaging Ab with the potential to globally activate T cells in a target cell-dependent fashion. The data also explain why the continuous infusion of blinatumomab is well tolerated, and allows for a tight control over T-cell activation. Lastly, the lymphocyte response to blinatumomab of 16 responding patients did not significantly differ from that of 4 nonresponding patients.
Methods
Patient characteristics
Detailed patient characteristics have been published elsewhere.16 Adult patients with B-lineage ALL in hematologic complete remission (CR) were eligible if they expressed the precursor B-phenotype and were either molecularly refractory or had a molecular relapse with quantifiable MRD load of ≥ 1 × 10−4 starting at any time point after consolidation I of frontline therapy within German Multicenter Study Group on Adult Acute Lymphoblastic Leukemia (GMALL) protocols. Of 21 patients enrolled into this study, 14 had individual TCR/Ig gene rearrangements, 5 presented with a BCR-ABL, and 2 with an MLL-AF4 translocation. Twenty patients completed at least 1 treatment cycle and were assessable for pharmacokinetic and pharmacodynamic parameters. All patients enrolled into this study gave written informed consent. The clinical study was approved by the local ethics committees of the participating university hospitals and the responsible authority of the Federal Republic of Germany, and was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Study design and drug administration
The study was designed as an open-label, multicenter, single-arm, phase 2 clinical trial that investigated the efficacy, safety, and tolerability, and pharmacokinetics and pharmacodynamics of blinatumomab.16 Using a portable mini-pump and port system, patients received blinatumomab as continuous IV infusion at a dose of 15 μg/m2/d over a 4-week cycle followed by a treatment-free period of 2 weeks. Patients were treated as outpatients and only hospitalized for the first 2 to 3 days of each treatment cycle. Responders were permitted to receive up to 3 additional consolidation cycles of treatment with blinatumomab.16
Measurement of response
The primary end point of this study was defined by the incidence of MRD negativity (ie, < 1 × 10−4) within 7 treatment cycles with blinatumomab. Quantitative PCR for individual TCR/Ig gene rearrangements, and BCR-ABL and MLL-AF4 translocations, was performed as described.16 MRD levels were quantified and reassessed after each treatment cycle. Detection of MRD negativity was confirmed by a second BM biopsy taken within 2 weeks after the first one.
PK assay and analysis
Blood samples for the determination of serum concentrations of blinatumomab were taken at different time points during infusion and after infusion stop to allow determination of PK parameters. Quantification of blinatumomab in human serum samples was performed as described previously.15 Briefly, the assay is based on CD69 up-regulation on the surface of newly activated T cells upon dual binding of blinatumomab to T cells and Raji lymphoma cells. Expression of CD69 is monitored by FACS analysis and is increased in a drug dose-dependent manner. The lower limit of quantification (LLOQ) of the assay was 100 pg/mL. The average steady-state serum concentration (Css) in an individual patient was calculated from available and reliable data points at plateau in each cycle. The systemic clearance (CL) was calculated according to the equation CL = Ro/Css, where Ro is the dosing rate in μg/m2/d. Other PK parameters such as elimination serum half-life (T1/2, β), volume of distribution (VZ), and area under the serum concentration-time curve to 28 days and to infinity (AUC 0-28 and AUC 0-inf, respectively) were estimated by applying standard noncompartmental methods using WinNonlin software (Pharsight).
Cytokine assay
Serum concentrations of 6 different cytokines (IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α) were assessed using a commercially available FACS-based cytometric bead array (CBA) kit (BD Biosciences). The assay was used according to the manufacturer's protocol and was internally validated for the intended use. The limit of detection (LOD) for the cytokine determination was 20 pg/mL, the LLOQ was 125 pg/mL.
Analysis of lymphocyte subpopulations
Lymphocytes in patients' peripheral blood were analyzed by FACS and quantified as described previously.15 Briefly, blood samples were drawn at 0.75, 2, 6, 12, 24, 30, and 48 hours after the start of treatment as well as on treatment days 7, 14, 21, and 28 plus 1 week after the end of treatment. PBMCs were isolated by an adapted Ficoll density gradient separation and subsequently stained with fluorescence-labeled Abs against cell surface or intracellular markers as follows: B cells: CD19; B-cell apoptosis: annexin V; T cells: γδ TCR, CD3, CD4, CD8, CD56; T-cell activation: CD69, CD25, HLA-DR; CD8+ T-cell subsets: CD45RA, CD28, CD197; and CD4+ regulatory T cells: CD25, Foxp3. FACS data collection was conducted on a FACSCalibur or FACSCanto II instrument (BD Biosciences) and statistical analysis was performed either with the software CellQuest Pro (BD Biosciences) or FCS Express (De Novo Software). Finally, percentage values of lymphocyte subpopulations were correlated with the absolute lymphocyte number of a differential blood count to calculate the respective absolute subpopulation numbers. The overall AUC was determined as the area under the αβ T-cell curve of the entire 4-week treatment period; and the baseline AUC as the corresponding area above the respective baseline αβ T-cell count.
Statistical analysis
Depending on whether means or medians were compared, a paired t test or a Wilcoxon matched-pairs signed rank test/Mann-Whitney test was used for statistical analysis in Prism (GraphPad Software). A 2-tailed P value < .05 was considered statistically significant.
Results
Steady-state serum concentrations of continuously infused blinatumomab are very stable during repeated 4-week treatment cycles
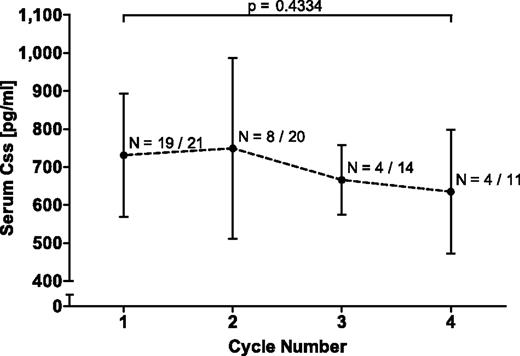
Blinatumomab was continuously intravenously administered for 4-week infusion periods to MRD+ ALL patients via an implanted port system using a portable mini-pump. One of 21 patients enrolled into this study was not evaluable for response because of early dropout. Patient characteristics have been reported elsewhere (see Table 1 in Topp et al16 ). To determine levels of biologically active BiTE Ab in serum samples of patients, a sensitive PK bioassay was developed with a lower limit of quantification of 100 pg/mL in human serum. PK parameters from the first 4-week treatment cycle are shown in Table 1. The steady-state serum concentration (Css) across all assessable patients ranged from 492-1050 pg/mL with a mean (± SD) of 731 ± 163 pg/mL. The elimination serum half-life of blinatumomab (T1/2, β) was on average (± SD) 1.25 ± 0.63 hours. The clearance (CL) was on average (± SD) 22.3 ± 5.0 L/d/m2, and the volume of distribution (VZ) 1.61 ± 0.74 L/m2. The average Css of evaluable patients over four 4-week treatment cycles, each followed by a 2-week treatment-free interval, are shown in Figure 1. Mean Css for cycles 2-4 were statistically not different from the average of cycle 1. The 2-tailed P value for cycle 1 relative to cycle 4 was .4334. No human anti–mouse Ab (HAMA) response to blinatumomab was detected in any of the patients.
Pharmacokinetic parameters of continuously IV-infused blinatumomab in the first treatment cycle of MRD+ ALL patients
| . | AUC0-28, d × pg/mL . | AUC0-inf, d × pg/mL . | Css, pg/mL . | T1/2, β, h . | CL, L/d/m2 . | VZ, L/m2 . |
|---|---|---|---|---|---|---|
| Mean ± SD | 19 659 ± 4413 | 19 764 ±4474 | 731 ± 163 | 1.250 ± 0.634 | 22.3 ± 5.0 | 1.61 ±0.74 |
| Range | 12 864-27 979 | 12 914-28 135 | 492-1050 | 0.52-2.76 | 15.2-32.5 | 0.87-3.27 |
| . | AUC0-28, d × pg/mL . | AUC0-inf, d × pg/mL . | Css, pg/mL . | T1/2, β, h . | CL, L/d/m2 . | VZ, L/m2 . |
|---|---|---|---|---|---|---|
| Mean ± SD | 19 659 ± 4413 | 19 764 ±4474 | 731 ± 163 | 1.250 ± 0.634 | 22.3 ± 5.0 | 1.61 ±0.74 |
| Range | 12 864-27 979 | 12 914-28 135 | 492-1050 | 0.52-2.76 | 15.2-32.5 | 0.87-3.27 |
MRD indicates minimal residual disease; AUC, area under the curve; Css, steady-state serum concentration; T1/2,β, elimination serum half-life; CL, systemic clearance; and VZ, distribution volume.
Mean steady-state serum concentrations of continuously intravenously infused blinatumomab in up to 4 consecutive treatment cycles. Css were calculated for the indicated number of evaluable patients per cycle and the mean Css ± SD is shown for each cycle. Denominators give the total number of treated patients in each cycle. The P value for the nonsignificant difference between cycle 1 and 4 is depicted by a bracket. Likewise, all other cycles did not differ significantly among each other. Css indicates steady-state serum concentration.
Mean steady-state serum concentrations of continuously intravenously infused blinatumomab in up to 4 consecutive treatment cycles. Css were calculated for the indicated number of evaluable patients per cycle and the mean Css ± SD is shown for each cycle. Denominators give the total number of treated patients in each cycle. The P value for the nonsignificant difference between cycle 1 and 4 is depicted by a bracket. Likewise, all other cycles did not differ significantly among each other. Css indicates steady-state serum concentration.
Blinatumomab causes sustained depletion of peripheral B cells
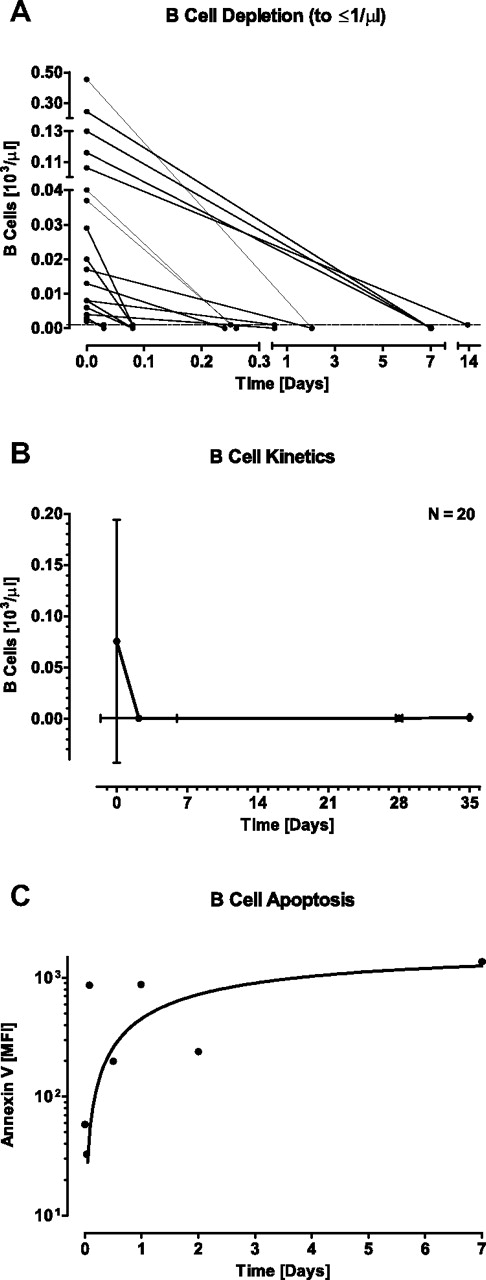
We next studied, in blood samples from MRD+ ALL patients, the effect of blinatumomab on counts of peripheral B cells, which are targets for BiTE-engaged T cells. In vitro coculture experiments have shown that blinatumomab can induce redirected lysis of CD19+ B lymphocytes and malignant B-cell lines by previously resting peripheral T cells at sub-pM concentrations.25 Patients presented with a wide range of B-cell counts at baseline ranging from 2 to 454 B cells/μL (mean ± SD was 75 ± 119 B cells/μL; Table 2). One-half of the patients had low B-cell counts ≤ 20 cells/μL at baseline, which most likely resulted from previous treatment with standard chemotherapy (Figure 2A). On start of continuous infusion of blinatumomab, the majority of patients showed a decline of B cells to the limit of detection of 1 cell/μL within < 1 day. B-cell counts dropped below the detection limit within a mean (± SD) of 2.18 ± 3.80 days (range from 0.03 to 13.94 days; Figure 2B). B-cell counts remained below the detection limit for the entire treatment period as shown for cycle 1 (Figure 2B, supplemental Figure 1A) and cycle 2 (supplemental Figure 1D). Apoptotic B-cell death and not B-cell redistribution was the most likely reason for a sustained absence of B cells during continuous blinatumomab infusion. This is suggested by increased binding of annexin V, an early apoptosis marker,26 to B cells as exemplified for one patient (Figure 2C). Annexin V staining of B cells was only feasible in those patients with high initial and slowly declining B-cell counts. Of note, peripheral B-cell counts did not detectably recover during or in between up to 7 analyzed treatment cycles (supplemental Figure 1A,D; data not shown for cycles 3-7).
Pharmacodynamic effects of continuously intravenously infused blinatumomab on T- and B-cell kinetics in the first treatment cycle of MRD+ ALL patients
| . | Mean ± SD . | Range . |
|---|---|---|
| T cells | ||
| Time, d | ||
| Time to T-cell nadir | 0.36 ± 0.24 | 0.06-1.09 |
| Time to 50% T-cell recovery | 3.13 ± 1.85 | 0.82-9.10 |
| Time to maximal T-cell increase | 17.57 ± 9.00 | 2.06-28.06 |
| T cells/μL | ||
| T-cell count at baseline | 532 ± 283 | 112-1364 |
| T-cell count at nadir | 36 ± 49 | 2-197 |
| Maximal T-cell count during treatment | 1013 ± 430 | 376-1954 |
| T-cell count at end of treatment | 737 ± 317 | 152-1410 |
| T-cell count after 1-wk recovery period | 825 ± 432 | 287-1599 |
| T cells, % | ||
| Maximal T-cell increase relative to baseline | 220 ± 123 | 93-547 |
| B cells | ||
| Time, d | ||
| Time to B-cell depletion | 2.18 ± 3.80 | 0.03-13.94 |
| B cells/μL | ||
| B-cell count at baseline | 75 ± 119 | 2-454 |
| B-cell count at end of treatment | 1 ± 1 | 0-4 |
| B-cell count after 1-wk recovery period | 1 ± 3 | 0-13 |
| . | Mean ± SD . | Range . |
|---|---|---|
| T cells | ||
| Time, d | ||
| Time to T-cell nadir | 0.36 ± 0.24 | 0.06-1.09 |
| Time to 50% T-cell recovery | 3.13 ± 1.85 | 0.82-9.10 |
| Time to maximal T-cell increase | 17.57 ± 9.00 | 2.06-28.06 |
| T cells/μL | ||
| T-cell count at baseline | 532 ± 283 | 112-1364 |
| T-cell count at nadir | 36 ± 49 | 2-197 |
| Maximal T-cell count during treatment | 1013 ± 430 | 376-1954 |
| T-cell count at end of treatment | 737 ± 317 | 152-1410 |
| T-cell count after 1-wk recovery period | 825 ± 432 | 287-1599 |
| T cells, % | ||
| Maximal T-cell increase relative to baseline | 220 ± 123 | 93-547 |
| B cells | ||
| Time, d | ||
| Time to B-cell depletion | 2.18 ± 3.80 | 0.03-13.94 |
| B cells/μL | ||
| B-cell count at baseline | 75 ± 119 | 2-454 |
| B-cell count at end of treatment | 1 ± 1 | 0-4 |
| B-cell count after 1-wk recovery period | 1 ± 3 | 0-13 |
Mean values ± SD and ranges for 20 evaluable patients are shown.
MRD indicates minimal residual disease; and ALL, acute lymphoblastic leukemia.
Effect of continuously intravenously infused blinatumomab on peripheral B-cell counts and apoptosis during the first treatment cycle of MRD+ ALL patients. (A) Individual B-cell counts of 20 evaluable patients are shown at baseline and at first occurrence of B-cell depletion ≤ 1 cell/μL. (B) Mean B-cell counts are shown at baseline, at the mean nadir of ≤ 1 cell/μL, at the end of the 4-week treatment period, and at 1 week after the end of infusion. Error bars indicate SD for the respective cell counts and time points. (C) Binding of annexin V to B cells of one patient with slowly declining peripheral B-cell counts. MFI indicates median fluorescence intensity as determined by FACS staining.
Effect of continuously intravenously infused blinatumomab on peripheral B-cell counts and apoptosis during the first treatment cycle of MRD+ ALL patients. (A) Individual B-cell counts of 20 evaluable patients are shown at baseline and at first occurrence of B-cell depletion ≤ 1 cell/μL. (B) Mean B-cell counts are shown at baseline, at the mean nadir of ≤ 1 cell/μL, at the end of the 4-week treatment period, and at 1 week after the end of infusion. Error bars indicate SD for the respective cell counts and time points. (C) Binding of annexin V to B cells of one patient with slowly declining peripheral B-cell counts. MFI indicates median fluorescence intensity as determined by FACS staining.
Blinatumomab induces a swift decline of peripheral T-cell counts on each infusion start followed by recovery
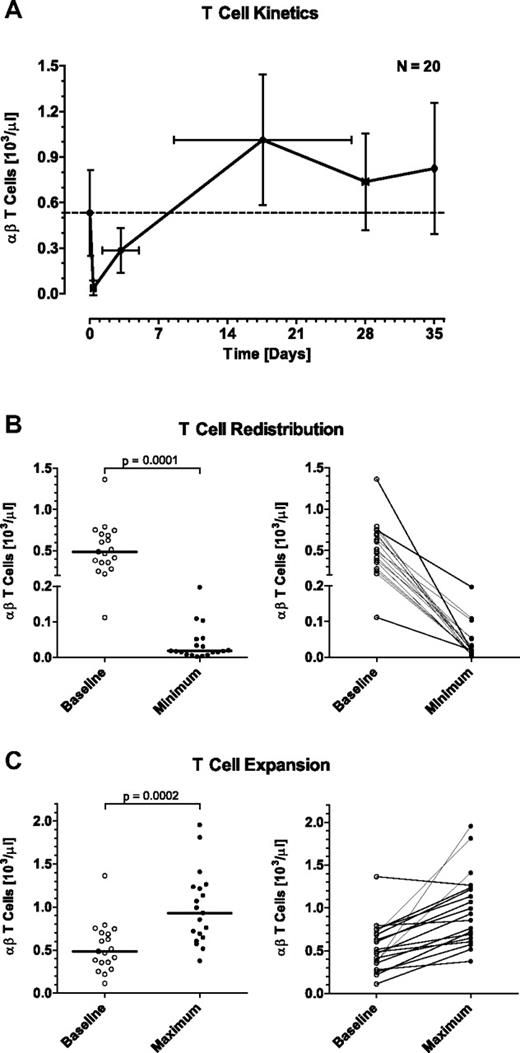
At baseline, MRD+ ALL patients presented with a mean (± SD) of 532 ± 283 T cells/μL (range from 112 to 1364 T cells/μL; Table 2). A peculiar reaction of peripheral T cells in response to the start of blinatumomab infusion was a fast drop of their counts within the first hours of exposure to the BiTE Ab (Figure 3A-B). A nadir with a mean count (± SD) of 36 ± 49 T cells/μL (range from 2 to 197 T cells/μL) was reached within 0.36 ± 0.24 days (range from 0.06 to 1.09 days). In support of a redistribution phenomenon, T-cell counts recovered and reached 50% of baseline counts after a mean (± SD) of 3.13 ± 1.85 days (range from 0.82 to 9.10 days), and were back to baseline levels after 8 to 9 days (Figure 3A). The initial eclipse of T cells was observed for every patient and was of high statistical significance (P = .0001; Figure 3B). In the second treatment cycle, every patient again showed a temporary eclipse of T cells on start of blinatumomab infusion with an accelerated recovery to baseline compared with cycle 1 (supplemental Figure 1A,D). This redistribution phenomenon was also observed in all subsequent treatment cycles with similar kinetics to those in cycle 2 (data not shown).
Effect of continuously intravenously infused blinatumomab on peripheral T-cell counts during the first treatment cycle of MRD+ ALL patients. (A) Mean T-cell counts of 20 evaluable patients are shown at baseline, at the mean nadir of the initial redistribution, at the mean time point of 50% recovery to baseline, at the mean time point of maximal expansion, at the end of the 4-week treatment period, and at 1 week after the end of infusion. Error bars indicate SD for the respective cell counts and time points. Numbers of this graph are given in Table 2. (B) Statistical analysis of the initial T-cell redistribution observed during the first day, and (C) of the maximal T-cell expansion during blinatumomab infusion. Bars in the respective left subpanels give median values; brackets indicate P values. Individual patient correlations are shown in the respective right subpanels.
Effect of continuously intravenously infused blinatumomab on peripheral T-cell counts during the first treatment cycle of MRD+ ALL patients. (A) Mean T-cell counts of 20 evaluable patients are shown at baseline, at the mean nadir of the initial redistribution, at the mean time point of 50% recovery to baseline, at the mean time point of maximal expansion, at the end of the 4-week treatment period, and at 1 week after the end of infusion. Error bars indicate SD for the respective cell counts and time points. Numbers of this graph are given in Table 2. (B) Statistical analysis of the initial T-cell redistribution observed during the first day, and (C) of the maximal T-cell expansion during blinatumomab infusion. Bars in the respective left subpanels give median values; brackets indicate P values. Individual patient correlations are shown in the respective right subpanels.
Blinatumomab induces an expansion of the peripheral T-cell compartment during the first treatment cycle
After the initial eclipse, T-cell counts not only returned to baseline in all patients under continued treatment with blinatumomab but, in all but one patient, exceeded baseline levels (Figure 3A,C). A maximal expansion of T cells during the 4-week infusion period was reached after a mean (± SD) of 17.57 ± 9.00 days (range from 2.06 to 28.06 days; Table 2) with T cells increasing with high significance (P = .0002) relative to baseline (= 100%) to a mean (± SD) of 220 ± 123% (range from 93% to 547%).
Individual αβ T-cell responses for all evaluable patients are depicted in supplemental Figure 1A. Analysis of T-cell subpopulations showed that in 9 of 20 patients, CD4+ T cells expanded stronger than CD8+ T cells, in 4 of 20 patients CD8+ T cells more than CD4+ T cells, and in 7 of 20 patients more or less equally. In only one patient (a responder), the CD4+ T-cell expansion also involved an increase of CD4+ regulatory T cells while otherwise CD4+ regulatory T cells stayed at very low counts throughout blinatumomab treatment (supplemental Figure 1B). A major contribution to the number of expanding T cells was made by effector memory T (TEM) cells of the CD45RA−/CD197− phenotype as shown for CD8+ T cells (supplemental Figure 1C). TEM cells expressing CD45RA (TEMRA cells) also detectably contributed to expanding CD8+ T cells.
Eleven of 14 patients who were available for pharmacodynamic analysis of their second treatment cycle after a 2-week break still had T-cell counts higher than at baseline of the first cycle. However, in 11 of these 14 patients, T cells did not further expand above baseline of the second cycle after recovery from the initial eclipse (supplemental Figure 1D). No further T-cell expansion was observed in all subsequent treatment cycles (data not shown).
Blinatumomab increases the percentage of activated peripheral CD8+ and CD4+ T cells
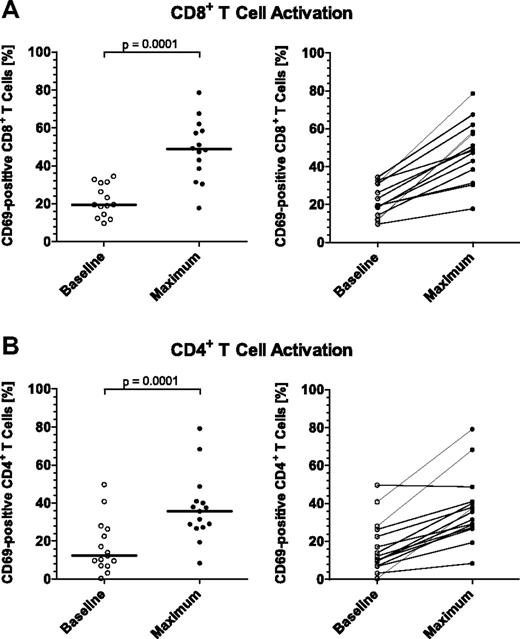
To explore causes for a transient redistribution of a large proportion of peripheral T cells in response to blinatumomab (Figure 3), we investigated the activation state of reappearing T cells by FACS-based analysis of the surface expression of immediate early activation marker CD69, and of late T-cell activation markers CD25 and HLA-DR. Most consistent data were obtained with CD69, which are here shown for both CD8+ and CD4+ T cells (Figure 4A and B, respectively). In all assessable patients, the percentage of CD69+ peripheral T cells did increase with peak activation occurring within the first 48 hours after start of infusion. The percentage of CD8+/CD69+ T cells maximally increased from a median of 19.47% (range from 9.66% to 34.43%) to 48.78% (range from 17.77% to 78.61%), and for CD4+/CD69+ T cells from a median of 12.32% (range from 0.40% to 49.71%) to 35.63% (range from 8.42% to 79.15%). Both increases were statistically highly significant (P = .0001). In conclusion, blinatumomab induced an increase of the percentage of activated circulating T cells consistent with a polyclonal T-cell activation by the CD3-engaging BiTE Ab.
Effect of continuously intravenously infused blinatumomab on the activation of peripheral CD8+ and CD4+ T cells during the first treatment cycle of MRD+ ALL patients. The expression of the immediate early activation marker CD69 on the surface of gated (A) CD8+ and (B) CD4+ T-cell subpopulations was determined by FACS at baseline and throughout treatment, and the percentages of activated, CD69+ T-cell subpopulations were calculated. Values of (A) 14 and (B) 15 assessable patients are shown at baseline and at the time point of maximal T-cell activation which occurred within the first 2 days after infusion start for all patients. Bars in the respective left subpanels give median percentages; brackets indicate P values. Individual patient correlations are shown in the respective right subpanels.
Effect of continuously intravenously infused blinatumomab on the activation of peripheral CD8+ and CD4+ T cells during the first treatment cycle of MRD+ ALL patients. The expression of the immediate early activation marker CD69 on the surface of gated (A) CD8+ and (B) CD4+ T-cell subpopulations was determined by FACS at baseline and throughout treatment, and the percentages of activated, CD69+ T-cell subpopulations were calculated. Values of (A) 14 and (B) 15 assessable patients are shown at baseline and at the time point of maximal T-cell activation which occurred within the first 2 days after infusion start for all patients. Bars in the respective left subpanels give median percentages; brackets indicate P values. Individual patient correlations are shown in the respective right subpanels.
T-cell parameters of responding patients are not different from those of nonresponding patients
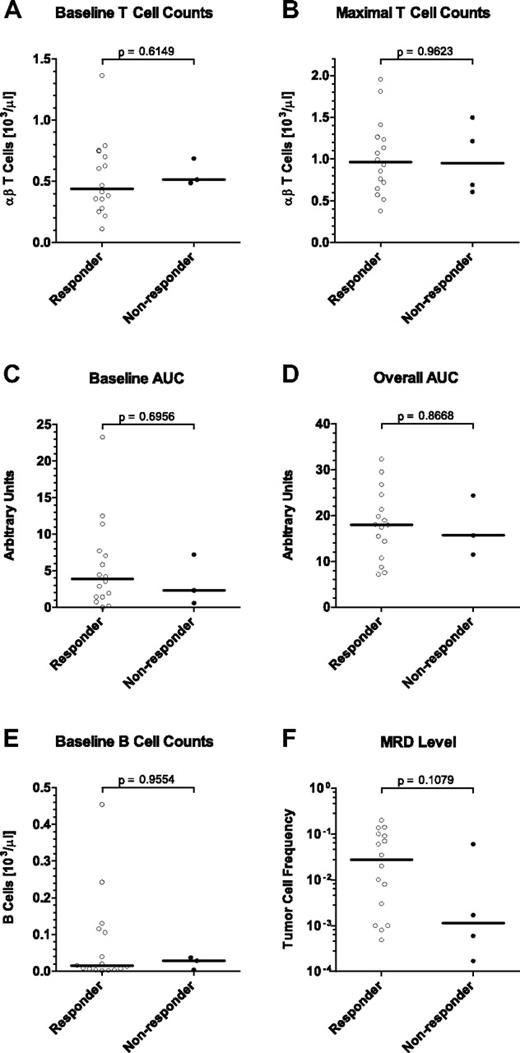
In this phase 2 study, 4 of 20 evaluable MRD+ ALL patients did not respond by a complete molecular response to treatment with blinatumomab monotherapy.16 Here, we assessed whether T-cell parameters of nonresponding patients were different from those of responding patients. Retrospective statistical analysis of baseline T-cell counts (Figure 5A), maximally reached T-cell counts (Figure 5B), or T-cell exposure by baseline (Figure 5C), or overall area under the αβ T-cell curve (Figure 5D) did not significantly correlate with response (P = .6149, P = .9623, P = .6956, and P = .8668, respectively). Obviously, nonresponding patients had similar levels, exposure to and reactivity of T cells as responders. The load of tumor cells in BM and counts of peripheral target B cells did likewise not correlate with response (Figure 5E-F; P = .1079 and P = .9554, respectively). From these analyses, it did not appear that T- or B-cell parameters, or tumor load in BM can provide an explanation for the nonresponsiveness of 4 patients to blinatumomab in this phase 2 study.
Correlation of T- and B-cell parameters and MRD level with clinical response to blinatumomab treatment in MRD+ ALL patients. Sixteen patients responding with a complete molecular response in BM after the first treatment cycle are compared with 4 nonresponders. Statistical analyses are shown for (A) T-cell counts at baseline, (B) maximal T-cell counts reached during expansion, (C) the area under the T-cell curve above the respective baseline T-cell count, (D) the overall area under the T-cell curve regardless of the respective baseline T-cell count, (E) target B-cell counts at baseline, and (F) the tumor load in the BM before treatment as determined by patient-specific PCR. Bars give median values; brackets indicate P values. AUC indicates area under the curve and is used as a measure of T-cell exposure.
Correlation of T- and B-cell parameters and MRD level with clinical response to blinatumomab treatment in MRD+ ALL patients. Sixteen patients responding with a complete molecular response in BM after the first treatment cycle are compared with 4 nonresponders. Statistical analyses are shown for (A) T-cell counts at baseline, (B) maximal T-cell counts reached during expansion, (C) the area under the T-cell curve above the respective baseline T-cell count, (D) the overall area under the T-cell curve regardless of the respective baseline T-cell count, (E) target B-cell counts at baseline, and (F) the tumor load in the BM before treatment as determined by patient-specific PCR. Bars give median values; brackets indicate P values. AUC indicates area under the curve and is used as a measure of T-cell exposure.
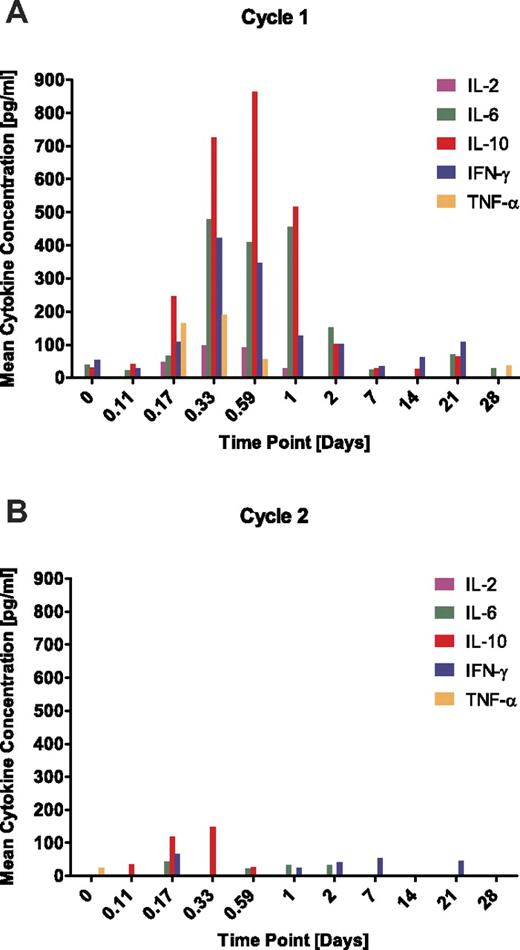
Start of blinatumomab infusion causes a transient release of cytokines which does not recur on start of a second treatment cycle
Concentrations of selected cytokines known to be released by activated T cells were measured in serum samples from MRD+ ALL patients all of whom received glucocorticoid coverage on start of each treatment cycle. IL-2, IL-6, IL-10, IFN-γ, and TNF-α were detectable during the first 2 days after start of infusion with maximal levels reached during the first day (Figure 6A). IL-4 was not detectable. The highest cytokine levels were observed for IL-10, followed by IL-6 and IFN-γ with average serum peak concentrations (± SD) of 864 ± 1201 pg/mL, 479 ± 735 pg/mL, and 422 ± 599 pg/mL, respectively. TNF-α and IL-2 reached lower peak concentrations which were on average (± SD) at 190 ± 213 pg/mL and 99 ± 79 pg/mL, respectively. After 2 days, cytokine levels declined quickly and were barely detectable in sera of patients later during treatment. On start of a second treatment cycle, a robust cytokine release was no longer detectable (Figure 6B). Of note, cytokine responses showed a high interpatient variability, and approximately one-half of the patients showed hardly any release of cytokines in cycle 1 (Figure 7). The magnitude of the cytokine response did not significantly correlate with target B-cell frequency in blood or BM (data not shown).
Effect of continuously intravenously infused blinatumomab on cytokine release into sera of MRD+ ALL patients. Serum samples of assessable patients were analyzed for concentrations of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α at the indicated time points. Mean values are shown for the (A) first and (B) second treatment cycle. IL-4 concentrations were below the limit of detection throughout all patients and are not depicted.
Effect of continuously intravenously infused blinatumomab on cytokine release into sera of MRD+ ALL patients. Serum samples of assessable patients were analyzed for concentrations of IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α at the indicated time points. Mean values are shown for the (A) first and (B) second treatment cycle. IL-4 concentrations were below the limit of detection throughout all patients and are not depicted.
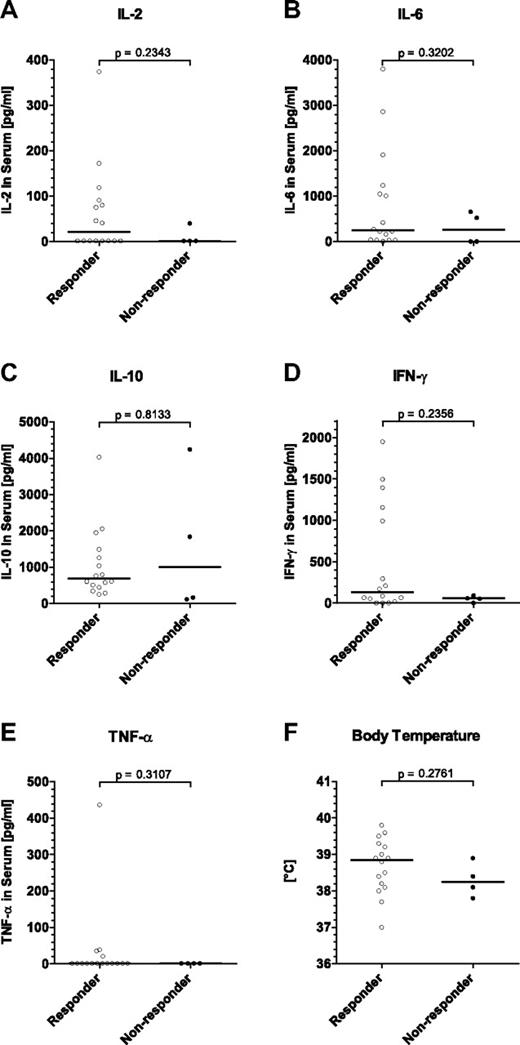
Correlation of peak cytokine concentrations and body temperature with clinical response to blinatumomab treatment in MRD+ ALL patients. Sixteen patients responding with a complete molecular response in BM after the first treatment cycle are compared with 4 nonresponders. All depicted values represent individual (A-E) peak cytokine concentrations or (F) peak body temperatures within the first 3 days after infusion start. Statistical analyses are shown for (A) IL-2, (B) IL-6, (C) IL-10, (D) IFN-γ, (E) TNF-α, and (F) body temperature. IL-4 concentrations were below the limit of detection throughout all patients and were not analyzed. Bars give median values; brackets indicate P values.
Correlation of peak cytokine concentrations and body temperature with clinical response to blinatumomab treatment in MRD+ ALL patients. Sixteen patients responding with a complete molecular response in BM after the first treatment cycle are compared with 4 nonresponders. All depicted values represent individual (A-E) peak cytokine concentrations or (F) peak body temperatures within the first 3 days after infusion start. Statistical analyses are shown for (A) IL-2, (B) IL-6, (C) IL-10, (D) IFN-γ, (E) TNF-α, and (F) body temperature. IL-4 concentrations were below the limit of detection throughout all patients and were not analyzed. Bars give median values; brackets indicate P values.
The peak levels of transiently released cytokines IL-2, IL-6, IL-10, IFN-γ, or TNF-α did not correlate with a clinical response to blinatumomab monotherapy (Figure 7A-E; P = .2343, P = .3202, P = .8133, P = .2356, and P = .3107, respectively). Likewise, a transient increase in body temperature observed at treatment start showed no correlation with response (Figure 7F; P = .2761).
Discussion
This is the first study investigating in detail in leukemic patients the pharmacologic and pharmacodynamic effects of continuous IV infusion of the globally T cell–activating CD3/CD19-bispecific BiTE Ab blinatumomab. The continuous IV dosing regimen has produced a high response rate of 80% in a clinical phase 2 study in MRD+ ALL patients.16 At the time of revision of this manuscript, 4 of 10 nontransplanted MRD-negative responders are still in remission corresponding to a progression-free survival of up to 38 months. Here, we analyzed the set of 20 response-evaluable patients from this study for pharmacokinetics, response of B and T cells, and cytokine release during blinatumomab administration.
Key observations were the following: (1) Continuous IV infusion of blinatumomab gave rise to a mean steady-state serum concentration in patients of 0.73 ng/mL (13pM) that did not change in up to 4 treatment cycles. (2) As seen in in vitro coculture experiments,17,25 blinatumomab caused a durable depletion of CD19-expressing target B cells in patients by redirected lysis. (3) On start of infusion, peripheral T-cell counts swiftly dropped but recovered within a few days, and then expanded above baseline counts under treatment. (4) A large proportion of recovering T cells up-regulated the activation marker CD69. (5) Cytokines were transiently released only on start of the first infusion with a high interpatient variability, and were no longer detected on start of a second treatment cycle. Of note, most of the pharmacokinetic and pharmacodynamic effects of blinatumomab showed very little variation in the MRD+ ALL patient population, which was characterized by a low load of target B cells in blood and BM. Months of constant exposure of patients to the globally T cell–activating BiTE Ab blinatumomab did neither lead to signs of uncontrolled T-cell activation (eg, a cytokine storm) nor to signs of T-cell anergy, as could have been evident from the recovery of target B-cell counts under continued treatment.
Continuous IV infusion of blinatumomab led to stable and comparable steady-state levels in MRD+ ALL patients within each 4-week treatment period. This indicates that blinatumomab was not significantly sequestered, for instance, by binding to CD19 on target B cells or to CD3 on T cells. At mean serum concentrations of only 0.013nM and equilibrium binding constants of blinatumomab for the CD19 and CD3 Ags of ca 1 and 100nM, respectively,17 significant cell binding of the BiTE Ab is also not expected from a theoretical position. Comparable steady-state levels also indicate that blinatumomab was not sequestered by a neutralizing Ab response to the BiTE Ab, which is consistent with a low immunogenicity rate of < 1% observed across all trials with blinatumomab.15,16 Steady-state levels were rapidly achieved after start of infusion and—because of the short serum half-life of the drug—serum concentrations of blinatumomab declined quickly after stop of infusion. In case of severe adverse events, the latter can provide for an effective means to control drug exposure by health care practitioners. The short serum half-life and relatively high clearance of blinatumomab are consistent with a renal elimination of the 55-kDa Ab construct lacking an Fc domain, which mediates the long serum half-life of regular IgG Abs.27
The decline of peripheral B cells on start of blinatumomab infusion below the limit of detection was most likely the consequence of their redirected lysis. This is not only consistent with observations in in vitro cocultures,17,25 where comprehensive redirected lysis of CD19+ target cells by BiTE-engaged T cells is detected at the same blinatumomab concentrations reached in patients, but also with binding of annexin V to declining B cells of MRD+ ALL patients, an effect that had also been noticed earlier in NHL patients.15 Induction of apoptosis after BiTE-mediated synapse formation by activation of caspases 3/7 in target cells was also demonstrated for other BiTE Abs to be relevant for efficient cytotoxicity.28 The observation that B-cell counts did not detectably recover under continued BiTE treatment for up to seven 4-week cycles suggests that T cells in the presence of blinatumomab were continuously active in depleting new B cells derived from CD19− pluripotent stem cells.
The majority of adverse events of blinatumomab in MRD+ ALL patients was recorded during the first days following start of blinatumomab infusion.16 Most frequent were flu-like symptoms such as pyrexia, headache, chills, and fatigue, which predominantly were below Common Toxicity Criteria grade 3. As reported here, these side effects coincided with the maximal increase of activated CD8+ and CD4+ T cells within 2 days after start of infusion, and the maximal release of cytokines during the first day of infusion. Likewise, the eclipse of T cells on treatment start fell into this early phase of T-cell activity. The observation that T-cell activation, redistribution, and cytokine release were all transient under continued treatment with blinatumomab suggests an adaptive reaction of newly activated T cells, which is also known as “first-dose effect.” In vitro coculture experiments have also shown that cytokine release and CD69 up-regulation by BiTE-activated T cells are transient.17,20,25 Of note, both are not necessary for redirected lysis of target B cells as it continues for days after cytokine release and CD69 expression have ceased. The expansion of peripheral T cells above baseline counts may be explained by either proliferation or redistribution of T cells from tissues to blood, or both. The increase of T-cell counts during treatment week 2 and 3 was dominated by an expansion of the TEM and TEMRA subsets in most patients. This selective response of memory T cells to BiTE stimulus may be because of less stringent requirements or lower thresholds for full activation because of differences in used signaling pathways compared with naive T cells.29,30 The expansion of T cells in itself may be considered beneficial for these heavily immunocompromised patients.
While T-cell redistribution was observed with the start of each treatment cycle, a release of cytokines and an expansion of peripheral T cells were confined to the first cycle. This indicates that the extent of cytokine release and T-cell expansion may correlate with target B-cell load while the initial T-cell redistribution is independent thereof. This redistribution is likely because of an increased adhesion of T cells to the blood endothelium which appears to be triggered by monovalent binding of blinatumomab to CD3. However, monovalent binding of blinatumomab does not activate T cells which is strictly dependent on the presence of target cells.31 In vitro coculture experiments with endothelial cells are ongoing to investigate in more detail the redistribution phenomenon of T cells on start of BiTE treatment.
Four of 20 evaluable MRD+ ALL patients did not respond to blinatumomab treatment with a complete molecular response.16 These 4 patients had a rather low tumor cell load in their BM, showed with the exception of IL-10 rather low cytokine responses, but had average T-cell counts and an average responsiveness of T cells to the BiTE Ab. Moreover, many responders had no detectable proinflammatory cytokine release, high IL-10 levels as well, and much lower T-cell counts and exposure than the 4 nonresponders. Based on the present dataset, we could not identify an immunologic or pharmacologic parameter that would correlate with the response of MRD+ ALL patients to blinatumomab treatment. This nonresponsiveness could be caused by yet-to-be defined differences in the aggressiveness of the underlying leukemia. Hampered T-cell activity by tyrosine kinase inhibitor comedication or reduced accessibility to target cells might also be of importance. Lastly, inherent resistance mechanisms of target cells may involve protection from perforin/granzyme B, alterations of the caspase pathway, or outgrowth of CD19− leukemic cell clones. Future studies will have to investigate in more detail the cellular mechanisms underlying a potential induction of resistance to blinatumomab treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Elke Burghart, Sandra Wissing, and Christiane Simmich for excellent technical support, and Metronomia Clinical Research GmbH for data compilation.
Authorship
Contribution: M.K. designed and performed research, analyzed and interpreted data, and edited the manuscript; C.B. performed research; G.Z., R.C.B., M.S.T., N.G., D.H., E.D., and D.N. were involved in trial design and study conduct; Y.H. performed pharmacokinetic analysis; N.G., S.N., M.G., A.V., and M.S. procured patient samples and collected clinical data; M.B. analyzed MRD status; P.A.B. wrote the manuscript; and A.W. and P.K. designed research and analyzed and interpreted data.
Conflict-of-interest disclosure: M.K., C.B., G.Z., Y.H., E.D., D.N., P.A.B., A.W., and P.K. are employees of Amgen, and have ownership interests. R.C.B., M.S.T., N.G., S.N., M.G., A.V., M.S., and M.B. received research funding from Amgen. R.C.B., M.S.T., and D.H. have consultancy agreements with Amgen and are members of its advisory committees.
Correspondence: Patrick A. Baeuerle, Amgen Research (Munich) GmbH, Staffelseestr 2, 81477 Munich, Germany; e-mail: baeuerle@amgen.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal