To prospectively evaluate allogeneic stem cell transplantation (allo-SCT) for myeloma as part of first-line therapy, a donor versus no-donor analysis was performed of patients treated in the HOVON-50 study, a study that was originally designed to examine thalidomide combined with intensive therapy. Two hundred sixty patients having received an autologous-SCT fulfilled the criteria to be included, 138 patients without an HLA-identical sibling donor and 122 patients with a donor. After a median follow-up of 77 months, complete remission, progression-free survival (PFS), and overall survival were not significantly different between the 2 groups. PFS at 6 years was 28% for patients with a donor versus 22% for patients without a donor (P = .19) and overall survival at 6 years from high-dose melphalan was 55%, irrespective of having a donor (P = .68). Cumulative incidence of nonrelapse mortality at 6 years after autologous-SCT was 16% in the donor group versus 3% in the no-donor group (P < .001). However, PFS was significantly prolonged in the 99 patients who actually proceeded to allo-SCT compared with the 115 patients who continued maintenance or received a second high-dose melphalan, but the difference did not translate into a prolonged survival benefit. These results do not support a general application of allo-SCT in all myeloma patients as part of first-line therapy.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 6399.
Disclosures
Henk M. Lokhorst serves as a consultant for Celgene and Genmab. Monique C. Minnema has received speaker's fees from Janssen and Novartis. Sonja Zweegman serves as a consultant for Janssen. Pieter Sonneveld serves as a consultant for Janssen and Millenium-Takeda and has received honoraria and research funding from Janssen. The remaining authors, the Associate Editor A. Keith Stewart, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Compare complete remission and overall survival in patients with multiple myeloma who did and did not have an HLA-identical sibling donor for allogeneic stem cell transplantation (allo-SCT).
Compare progression-free survival in patients with multiple myeloma who had or did not have a donor, and in those with a doctor who underwent allo-SCT, with those who continued maintenance or received a second treatment with high-dose melphalan in both the donor and no-donor groups.
Compare nonrelapse mortality in the donor group versus the no-donor group, and describe overall clinical implications.
Release date: June 28, 2012; Expiration date: June 28, 2013
Introduction
The role of allogeneic stem cell transplantation (allo-SCT) for multiple myeloma (MM) is still disputed. Although the existence of a curative graft versus myeloma effect is beyond doubt as demonstrated by the sustained molecular remissions after donor lymphocyte infusions, it is questionable whether and when this treatment option should be offered to all myeloma patients as part of first-line therapy.1 High-dose chemotherapy and autologous stem cell transplantation combined with novel antimyeloma agents has become standard therapy for the younger myeloma patient. In the current era, median survival for patients being eligible for stem cell transplantation has been found to be more than 5 years in 3 large phase 3 trials. Moreover, a substantial proportion of patients live 10 years or more with excellent quality of life.2,,,,,–8 A biologic randomization approach for allo-SCT based on the availability of an HLA-identical sibling donor is accepted as a reliable surrogate for true randomized phase 3 trials.9,10 We have performed a donor versus no-donor (DvND) analysis of patients included in the phase 3 Hematologie Oncologie Volwassenen Nederland-50 MM trial (HOVON-50). In this study, the effect of thalidomide combined with autologous stem cell transplantation (auto-SCT) was evaluated.8 In addition, the study allowed patients with an HLA-identical sibling donor to proceed to the HOVON-54 study of allo-SCT after reduced intensity conditioning (allo-RIC) between 2 and 6 months after auto-SCT, a policy that was strictly followed by center preference.
Methods
Patients
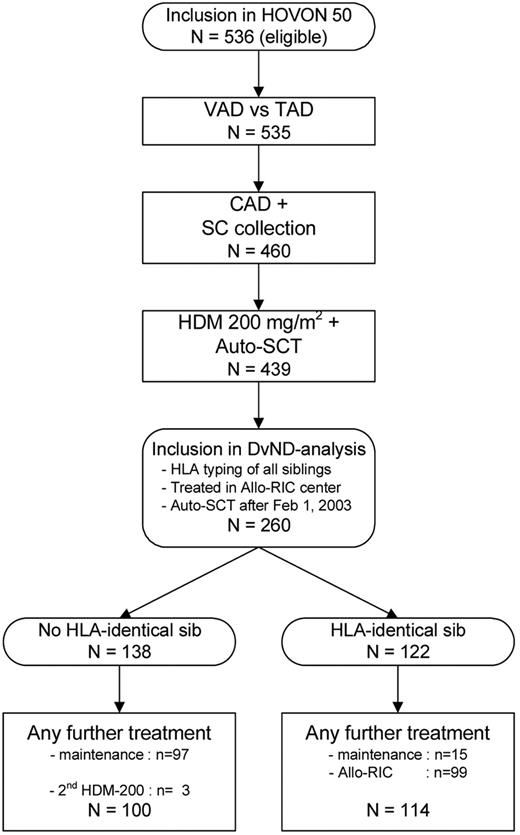
Patients included in the HOVON-50 study, with a fully matched (10/10) HLA-identical sibling were eligible for inclusion in the separate HOVON-54 allo-RIC study. To approximate a real randomized study that would have been performed after auto-SCT, inclusion criteria for the DvND analysis were as follows: (1) center policy to include allo-RIC as part of first-line therapy; (2) HLA typing of patient and all sibs, before auto-SCT; (3) having received auto-SCT after February 1, 2003; and (4) fulfilling general eligibility criteria to receive an allo-RIC.
Thirty-one centers with an allo-RIC policy included patients in the study of which 25 centers referred their patients for the allo-RIC to 6 Dutch allo-SCT centers. Among the 536 eligible patients randomized in the HOVON-50 trial, ultimately 260 patients were eligible to be included into the DvND analysis, 122 patients with a donor and 138 patients without a donor. Reasons for exclusion of the other 276 patients were as follows: hospital had no allo-RIC policy (n = 123), no high-dose melphalan (HDM) + auto-SCT (n = 67) performed, donor availability unknown (n = 42) and auto-SCT before February 1, 2003 (n = 44), which was the date inclusion in the HOVON-54 study became possible. The high percentage of patients with a sibling donor can be explained by the high large families that were founded in the decade after the end of World War II.
Among the 138 no-donor patients, 97 (70%) patients started with maintenance and 3 patients (2%) received a second HDM 200 (Figure 1). Forty-one patients in the no-donor group did not start with maintenance after HDM, because of ineligibility for IFN (n = 5), no partial response (PR; n = 1), toxicity (n = 9), progression/relapse (n = 5), intercurrent death (n = 1), refusal (n = 8), or other reasons (n = 12). Among the 122 donor patients, 99 patients (81%) actually received an allo-RIC and 15 patients (14%) started with maintenance.
Patient characteristics, summarized in Table 1, were in general equally distributed between the groups; however, 45% of the donor patients had been randomized to receive thalidomide induction therapy versus 58% of the no-donor patients (P = .04). The data were analyzed as available on August 16, 2011. The median time of follow-up after auto-SCT for the 132 patients still alive was 77 months (range, 56-96 months). The design of the study is illustrated in Figure 1.
Patient characteristics
| . | No donor, 138 (%) . | Donor, 122 (%) . | P* . |
|---|---|---|---|
| Treatment arm, VAD/HDM/IFN | 58 (42) | 67 (55) | .04 |
| Treatment arm, TAD/HDM/thalidomide | 80 (58) | 55 (45) | |
| Sex and age | .13 | ||
| Male | 93 (67) | 71 (58) | |
| Female | 45 (33) | 51 (42) | |
| Median age, y (range) | 54 (30-65) | 54 (32-65) | |
| Salmon-Durie stage | .61 | ||
| IIA | 29 (21) | 23 (19) | |
| IIB | 1 (1) | 2 (2) | |
| IIIA | 90 (65) | 86 (70) | |
| IIIB | 18 (13) | 11 (9) | |
| β-2 microglobulin at diagnosis, mg/L | .17 | ||
| > 3 | 61 (44) | 44 (36) | |
| ≤ 3 | 53 (38) | 56 (46) | |
| Unknown | 24 (17) | 22 (18) | |
| M-protein | 1.00 | ||
| IgA | 28 (20) | 25 (20) | |
| IgG | 80 (58) | 73 (60) | |
| IgD | 2 (1) | 2 (2) | |
| Light chain disease | 25 (18) | 22 (18) | |
| Unknown | 3 (2) | 0 | |
| ISS | .81 | ||
| I | 64 (46) | 51 (42) | |
| II | 24 (17) | 22 (18) | |
| III | 19 (14) | 19 (16) | |
| Unknown | 31 (22) | 30 (25) | |
| Del(13) karyotype | .23 | ||
| Yes | 20 (14) | 12 (10) | |
| No | 93 (67) | 89 (73) | |
| Unknown | 25 (18) | 21 (17) | |
| Del (13) FISH | .20 | ||
| Yes | 18 (13) | 26 (21) | |
| No | 54 (39) | 49 (40) | |
| Unknown | 66 (48) | 47 (39) | |
| Response at inclusion | .82 | ||
| CR | 10 (7) | 6 (5) | |
| Very good PR | 46 (33) | 38 (31) | |
| PR | 51 (37) | 50 (41) | |
| Less than PR | 31 (22) | 28 (23) |
| . | No donor, 138 (%) . | Donor, 122 (%) . | P* . |
|---|---|---|---|
| Treatment arm, VAD/HDM/IFN | 58 (42) | 67 (55) | .04 |
| Treatment arm, TAD/HDM/thalidomide | 80 (58) | 55 (45) | |
| Sex and age | .13 | ||
| Male | 93 (67) | 71 (58) | |
| Female | 45 (33) | 51 (42) | |
| Median age, y (range) | 54 (30-65) | 54 (32-65) | |
| Salmon-Durie stage | .61 | ||
| IIA | 29 (21) | 23 (19) | |
| IIB | 1 (1) | 2 (2) | |
| IIIA | 90 (65) | 86 (70) | |
| IIIB | 18 (13) | 11 (9) | |
| β-2 microglobulin at diagnosis, mg/L | .17 | ||
| > 3 | 61 (44) | 44 (36) | |
| ≤ 3 | 53 (38) | 56 (46) | |
| Unknown | 24 (17) | 22 (18) | |
| M-protein | 1.00 | ||
| IgA | 28 (20) | 25 (20) | |
| IgG | 80 (58) | 73 (60) | |
| IgD | 2 (1) | 2 (2) | |
| Light chain disease | 25 (18) | 22 (18) | |
| Unknown | 3 (2) | 0 | |
| ISS | .81 | ||
| I | 64 (46) | 51 (42) | |
| II | 24 (17) | 22 (18) | |
| III | 19 (14) | 19 (16) | |
| Unknown | 31 (22) | 30 (25) | |
| Del(13) karyotype | .23 | ||
| Yes | 20 (14) | 12 (10) | |
| No | 93 (67) | 89 (73) | |
| Unknown | 25 (18) | 21 (17) | |
| Del (13) FISH | .20 | ||
| Yes | 18 (13) | 26 (21) | |
| No | 54 (39) | 49 (40) | |
| Unknown | 66 (48) | 47 (39) | |
| Response at inclusion | .82 | ||
| CR | 10 (7) | 6 (5) | |
| Very good PR | 46 (33) | 38 (31) | |
| PR | 51 (37) | 50 (41) | |
| Less than PR | 31 (22) | 28 (23) |
Restricted to patients with data.
Treatment
In the HOVON-50 study, patients with newly diagnosed MM, Salmon-Durie stage II or III, aged 18 to 65 years inclusive, were randomly assigned to 3 cycles of standard vincristine, adriamycin, dexamethasone (VAD) or thalidomide (200-400 mg daily), adriamycin, dexamethasone (TAD). After stem cell harvest with cyclophosphamide, adriamycin, dexamethasone (CAD), patients received HDM-200 with autologous stem cell rescue. Patients randomized to arm VAD received maintenance therapy with α-interferon (3 × 106 IU thrice weekly) starting between 2 and 3 months after HDM, and patients randomized to arm B received 50 mg of thalidomide daily starting between 2 and 3 months after HDM-200 until progression (for details, see the design of the HOVON-50 study in Lokhorst et al.8 ) Patients with a fully matched (10/10) HLA-identical sibling donor were eligible for the separate HOVON-54 study and could proceed to allo-RIC between 2 and 6 months after auto-SCT. Main exclusion criteria for the HOVON-54 study were WHO performance status greater than 3, glomerular filtration rate less than 30 mL/minute, or other substantial organ failure. Conditioning was low-dose total body irradiation (TBI; 2 Gy). In the allo-RIC–transplanted patients, median time between auto- and allograft was 3.9 months. GVHD prophylaxis consisted of cyclosporine (6.5 mg/kg twice daily) and mycophenolate mophetil (MMF) 15 mycophenolate mophetil (MMF) mg/kg twice daily. Cyclosporin was tapered from day 90 to day 180 and MMF from day 28 to 56. The HOVON-50 and HOVON-54 studies were approved by the ethics committees of the participating centers and were conducted in accordance with the Declaration of Helsinki. All study patients gave their written informed consent.
Chromosomal and FISH analysis
Classic karyotyping was done in 82% of patients at diagnosis, in 82% of patients in the no-donor group, and in 83% of patients in the donor group. Fluorescence in situ hybridization (FISH) analysis for chromosome 13-deletion only—del (13q14)—was performed in 147 patients (57%) in 72 (52%) of the no-donor patients and in 73 (61%) of patients with a donor.
Response criteria
Response was evaluated according to the European Group for Blood and Marrow Transplant criteria.
Definition of end points
All patients who were classified in the donor and no-donor groups were evaluated as from the day of auto-SCT after HDM. The primary end points were PFS and overall survival (OS) from auto-SCT. PFS was assessed from the date of auto-SCT until progression, relapse, or death, whichever came first. OS was measured from auto-SCT until death from any cause. Patients still alive at the date of last contact were censored. Causes of death were classified as relapse mortality (RM) if patients died because of MM or after previous progression or relapse, or as nonrelapse mortality (NRM) otherwise.
Secondary endpoints were prognostic factor analysis and PFS and OS from last treatment actually received for the patient who received their allocated therapy, that is, allo-RIC, a second HDM-200, or maintenance therapy with thalidomide or α-interferon (denoted as PFS2 and OS2).
Statistical analyses
The cumulative risks of RM and NRM over time were calculated as competing risks with actuarial methods where patients alive without progression or relapse were censored at the date of last contact.10 Cox regression analysis for PFS, OS, RM, and NRM was applied on an intention-to-treat basis to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the donor group versus the no-donor group, or according to last treatment for PFS2 and OS2. Because patients had either been randomized to VAD or TAD, the primary Cox regression analyses were adjusted for treatment arm. All P values for tests that compare the outcomes in the donor and no-donor group were based on log likelihood ratio tests, except when explicitly stated otherwise. Log likelihood ratio tests also were used to test for interactions (ie, to test for differences in the donor effect between subgroups for each of the end points). The following subgroups were evaluated: induction treatment arm in HOVON-50 (VAD vs TAD); β2-microglobulin (≤ 3 vs > 3 mg/L); International Staging System (ISS) stage (I vs II vs III); and del(13/13q) determined by conventional cytogenetics and FISH (no vs yes), measured at entry into the HOVON-50 study. To compare our results with those previously published, we also evaluated the prognostic value of donor availability for these subgroups separately. As reported previously, only if the statistical interaction test supported subgroup analysis, conclusions could be influenced. P values of these tests for interaction are only mentioned in “Prognostic factor analysis” when less than or equal to .10.11,12 Kaplan-Meier curves were generated to illustrate differences between subgroups and compared using the log-rank test, stratified by arm. All reported P values are 2-sided, and a significance level α = .05 was used.
Results
Donor versus no-donor comparison
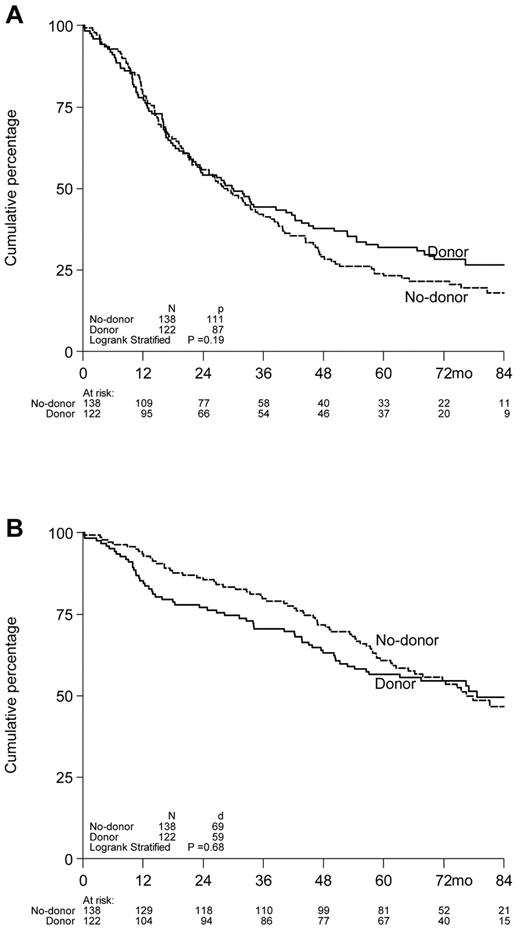
PFS.
Six-year PFS was 28% for patients with a sibling donor versus 22% for patients without a donor. No statistically significant benefit was found for donor availability with respect to PFS (with adjusted for HOVON-50 treatment arm): HR = 0.82, 95% CI = 0.62-1.09, P = .17 (Figure 2A). No interaction was found between donor availability and randomization arm of the HOVON-50 study (P = .68), indicating that there was no benefit of having a donor when treated with thalidomide or not, and subgroup analyses were therefore not warranted.
Kaplan-Meier survival curves of 260 myeloma patients included in the HOVON-50 study by donor availability. Actuarial rates of PFS (A) and OS (B) according to availability of an HLA-identical sibling, that is, donor versus no-donor. PFS and OS are presented as from the date of autologous SCT.
Kaplan-Meier survival curves of 260 myeloma patients included in the HOVON-50 study by donor availability. Actuarial rates of PFS (A) and OS (B) according to availability of an HLA-identical sibling, that is, donor versus no-donor. PFS and OS are presented as from the date of autologous SCT.
OS.
OS at 6 years from auto-SCT was 55% in patients with a donor as well as without a donor (Figure 2B): HR = 1.07, 95% CI = 0.75-1.52, P = .72. The test for interaction between donor availability and randomization arm of the HOVON-50 study also showed no difference between the 2 groups (P = .96). The best response as measured by CR was 43% in the donor group versus 37% in the no-donor group (P = .67).
Best response.
In the no-donor group, best response was achieved in 45 patients (33%) before HDM, in 33 patients (24%) after HDM, in 34 (25%) after maintenance, in 6 patients (4%) during follow-up, in 18 patients (13%) after reinduction, and in 2 patients (1%) unknown.
In the donor group, best response was achieved in 35 patients (29%) before HDM, in 31 patients (25%) after HDM, in 7 patients (5%) after maintenance, in 36 patients (30%) after all-SCT, in 5 patients (4%) during follow-up, in 6 patients 6 (5%) after reinduction, and in 2 patients (1%) unknown.
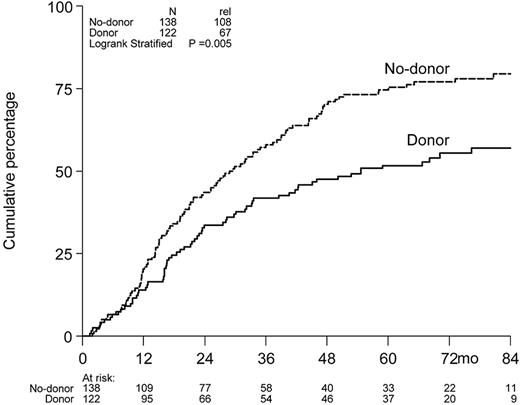
Cumulative incidence of relapse.
The cumulative incidence of relapse at 6 years was 77% in the no-donor arm versus 55% in the donor arm (Figure 3).
Cumulative incidence of relapse. Cumulative incidence of relapse at 6 years was 77% in the no-donor arm versus 55% in the donor arm.
Cumulative incidence of relapse. Cumulative incidence of relapse at 6 years was 77% in the no-donor arm versus 55% in the donor arm.
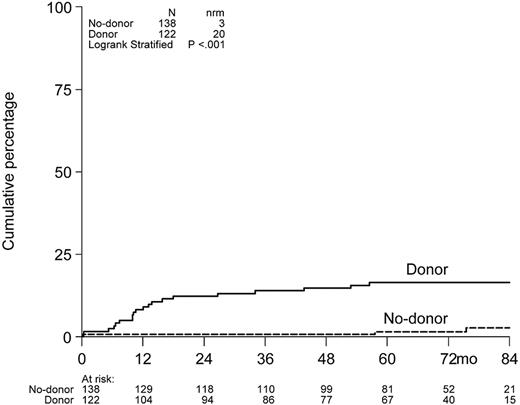
Cumulative incidence of NRM.
Cumulative incidence of NRM at 6 years after auto-SCT was 16% in the donor group versus 3% in the no-donor group (P < .001; Figure 4).
Nonrelapse mortality. NRM for patients having or not having a donor included in the HOVON-50 study.
Nonrelapse mortality. NRM for patients having or not having a donor included in the HOVON-50 study.
Prognostic factor analysis
Higher ISS stage (I vs II vs III) and also β-2 microglobulin more than 3 mg independently impacted on PFS and OS in the whole group of patients, whereas treatment arm in the HOVON-50 study (VAD vs TAD) or chromosome 13 abnormalities as determined by classic cytogenetics or by FISH had no influence. We found no significant prognostic value of donor availability for these high-risk subgroups separately, which also may be because of the relatively small number of patients. For example, among the 32 patients with del(13/13q) as determined by conventional cytogenetics, PFS at 6 years was 33% in the donor group (n = 12) versus 13% in the no-donor group (n = 20), but P = .72.
There were only 33 patients with ISS stage III: 17 received maintenance of second HDM, whereas 17 patients received allo-SCT. The estimated 5-year PFS (41% vs 13%; P = .17) and 5-year OS (65% vs 42%; P = .55) values were higher in the allo patients, but because of the very small numbers these values were not significant.
The number of patients with B2M great than 3 mg/L was substantially higher: 47 received other treatment and 46 allo-SCT; 5-year PFS was 35% after allo-SCT versus 15% after other treatment (P = .13), whereas 5-year OS was 59% (allo) versus 42% (other; P = .31).
In the subgroup of donor-patients who actually received an allo-SCT, higher age was significantly associated with worse PFS (HR = 1.04, 95% CI = 1.01-1.07, P = .02) and OS (HR = 1.05, 95% CI = 1.01-1.09, P = .01). Data on CMV status (patient and donor) and donor sex have not been collected in the HOVON-50 study.
Comparison of auto- and allo-RIC versus automaintenance
Among the 99 patients who received an allo-SCT, 16 (16%) patients were in CR, 42 (42%) were in very good PR, 36 (36%) were in PR, and in 5 (5%) of the patients the response was MR or unknown. Among the 115 patients who started with maintenance, 16 (16%) patients were in CR, 42 (42%) were in very good PR, 36 (36%) were in PR, and in 5 (5%) of the patients the response was MR or unknown.
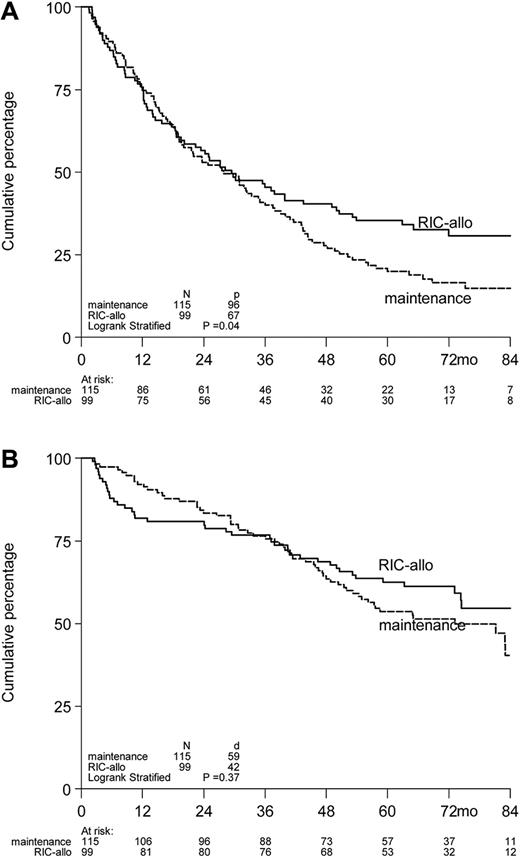
The outcome as regards to PFS was superior for the 99 patients who actually received an allo-RIC from fully matched donors compared with the patients who received a second auto-SCT (n = 3) or started with maintenance treatment after the first HDM200 (n = 112; HR = 0.75, 95% CI = 0.55-1.03, P = .07; Figure 5A).
Kaplan-Meier survival curves of 214 myeloma patients included in the HOVON-50/54 studies by treatment according to protocol initiated after auto-SCT. Actuarial rates of PFS2 (A) and OS2 (B) according to treatment started after auto-SCT, ie, allo-RIC versus maintenance with thalidomide or α-interferon (including 3 patients who received a second HDM-200). PFS2 and OS2 are presented as from the date of allo-RIC or second HDM, or start maintenance, whichever applicable.
Kaplan-Meier survival curves of 214 myeloma patients included in the HOVON-50/54 studies by treatment according to protocol initiated after auto-SCT. Actuarial rates of PFS2 (A) and OS2 (B) according to treatment started after auto-SCT, ie, allo-RIC versus maintenance with thalidomide or α-interferon (including 3 patients who received a second HDM-200). PFS2 and OS2 are presented as from the date of allo-RIC or second HDM, or start maintenance, whichever applicable.
However, improved PFS in patients actually having received allo-SCT did not translate into a statistically significant OS benefit. OS estimated 61% in patients having proceeded to allo-SCT compared with 51% in patients who received a second HDM or started with maintenance (HR = 0.84, 95% CI = 0.56-1.24, P = .38; Figure 5B). Again, there was no interaction between donor availability and randomization arm of the HOVON-50 study (P = .50 for PFS and P = .64 for OS), indicating that the benefit of allo-RIC was similar in patients treated with thalidomide or not. Accordingly, similar results were observed for subgroups according to ISS, B2M, or del(13). NRM at 6 years after last treatment was 17% in the allo-RIC patients versus only 1% in the non–allo-patients (P < .001).
The impact of well-known unfavorable prognostic factors could not be determined in the patients treated with allo-SCT: among the 260 patients included in this analysis, there were 224 (86%) with conventional karyotyping data available. However, only 23 patients had del(13/13q), of whom only 10 received an allo-SCT. These numbers are too small to draw any conclusion. FISH for t(4;14) or 17P was performed in a few patients. Among the99 patients who received an allo-SCT, 49 had progression/relapse afterward, of whom 30 (also) have received donor lymphocyte infusion (DLI). The outcome of DLI has not been recorded.
PFS and OS after HDM of no-donor patients who did not start with maintenance were lower during the first months after HDM compared with those with maintenance, because 6 no-donor patients did not start with maintenance because of progression or death. However, at 5 years, PFS (no-donor 27% vs 23%) and OS (no-donor 66% vs 59%) were very similar.
Treatment from relapse after maintenance or allo-SCT
From the 115 patients treated with maintenance, 95 patients progressed, of which 75 (82%) received further treatment; and from the 99 allo-SCT patients, 50 patients progressed, of which 40 (80%) received additional therapy. The percentages of patients treated with bortezomib and thalidomide was equal in both groups (70% and 54%, respectively), whereas lenidomide was given to 46% of patients relapsed from maintenance and to 78% of patients relapsed after allo-SCT. DLI after progression or relapse was given to 75% of the allo-SCT patients. The outcome of the different treatment regimens was not analyzed.
Comparison of HOVON-50 patients included or not included in the donor versus no-donor analysis
To exclude selection bias of the patients included in the donor versus no-donor analysis, their outcomes with regard to PFS and OS were compared with those of the HOVON-50 patients who also had received HDM but were not included in the DvND study. PFS and OS were exactly identical for both groups and were at 60 months 28% (P = .81) and 59% (P = .82), respectively.
GVHD and treatment-related mortality
Detailed information on GVHD was available for 80 patients. Acute GVHD occurred in 48% of the patients, including 3 patients (4%) with grade 3 and 4 patients (5%) with grade 4. Seven patients (9%) developed limited GVHD, and 44 patients (55%) developed extensive GVHD.
Among the 17 allo-patients who died from treatment-related mortality, 11 died within 1 year and 6 between 13 and 53 months. Only 3 patients never had chronic GVHD (but they died from acute GVHD or graft failure), and 2 had limited and 12 had had extensive GVHD at some time after allo-SCT. Among the 6 patients who died more than 1 year after allo-SCT, 2 had had limited GVHD and 4 had extensive GVHD.
Discussion
The present donor versus no-donor analysis was designed to prospectively evaluate allo-RIC as part of first-line therapy in MM. In accordance with center policy, patients included in the HOVON-50 study with an HLA-identical sibling were candidate for allo-RIC, which should be performed then within 6 months after preceding auto-SCT. No significant differences with respect to CR, PFS, and OS were found after mature follow-up of more than 6 years. PFS curves started to diverge after 36 months (Figure 2A) in favor of the donor group and the cumulative incidence of relapse at 6 years was significantly higher in the no-donor arm (77% vs 55%; P = .005; Figure 3), which, however, did not translate into a statistically significant benefit in PFS nor in OS.
Some previous studies also showed no benefit for allo-RIC as part of first-line therapy. The studies by the Pethema group and by French IFM,13,–15 however, differed significantly from our study with respect to patient selection and conditioning regimen. In the Pethema study,13 only patients were included not achieving a CR after a first autologous transplant and melphalan 140 mg/m2 was part of the conditioning regimen. In the IFM studies,14,15 only risk patients were included and dose antithymoglobulin was part of the conditioning regimen.
Three prospective studies with a comparable design were published by the Italian study group led by B. Bruno, by the European Group for Blood and Marrow Transplantation (EBMT), and recently by Blood and Marrow Transplant Clinical Trials Network (BMT CTN).16,,–19 Differences with our design were the use of double autologous SCT in the Italian, EBMT, and BMT CTN study studies, whereas only a single auto-SCT followed by maintenance was applied in non–allo-SCT patients in the HOVON study. Double auto-SCT also was allowed in the HOVON study but was only applied in 3 patients. Low-dose TBI only was used as conditioning regimen in the Italian, BMT-CTN, and HOVON studies, whereas fludarabine plus low-dose TBI was used in the EBMT study. Both the Italian and the EBMT studies reported an improved PFS and OS for patients with a sibling donor. The absence of a benefit in the present study may be explained by a much better outcome for the no-donor group in our study compared with the Italian and EBMT studies, even though those patients received a single autologous transplant followed by maintenance with thalidomide or with α-interferon. Remarkably, outcome of patients with a donor seemed rather similar in the 3 studies. The 5-year PFS for the donor patients was 36% and 32% in the EBMT and HOVON studies, respectively, whereas the 6-year PFS in the updated Italian study was ∼ 30%.17 In addition,survival seemed rather similar, with 5-year OS rates of 65, 60, and 57% in the EBMT, Italian, and HOVON studies, respectively. The large US multicenter trial from BMT-CTN comparing tandem auto-SCT with auto/allo-SCT completed the targeted accrual in March 2007 with 226 patients biologically randomized to the auto/allo-SCT group and 484 patients to double auto-SCT. In the double auto-SCT arm 233 patients were randomly allocated to thalidomide plus dexamethasone and 234 patients to observation. The results showed no difference in the 3-year PFS and OS. The 3-year PFS and OS were 43% and 77%, respectively, for the auto/allo-SCT group versus 46% and 80% for the double auto-SCT group. However the follow-up of this study is probably too short to draw definite conclusions.
It is known that the possible favorable effect of allo-SCT may become apparent only after a long follow-up. The US Intergroup trial (S9321) demonstrated a PFS plateau of ∼ 22% at 7 years in the 36 patients undergoing allo-SCT, which was superior to the 7-year PFS of patients in the trial who received auto-SCT.20 PFS and OS curves in the EBMT registry study plateaus and became apparent after prolonged follow-up.21
Our data were analyzed after a median follow-up of 77 months, which is longer than the first published analysis of the Italian study, but also significant longer than the EBMT study and the BMT-CTN study. The use of low-dose TBI only cannot explain the differences in outcome because this conditioning regimen also was used in the EBMT, Italian, and BMT-CTN studies. It may be that low dose TBI with or without fludarabine as used in the 4 prospective trials is a suboptimal conditioning regimen even in the tandem auto-allo setting. However, data on a more intensive (semiablative) regimen have to be awaited, such as is now explored prospectively in a German multicenter trial of upfront allo-RIC in newly diagnosed patients with high-risk features.22
One explanation for the difference in survival might be that our study was initiated later and included 1 of the novel agents, thalidomide, and that bortezomib and lenalidomide could be routinely given to patients with relapsed disease. In the HOVON-50 study, the PFS for patients randomized to thalidomide was significantly prolonged,8 Because patients receiving thalidomide had no significantly better outcome, it is more likely that the availability of the new antimyeloma agents for relapse may explain the comparable survival of the patients who did receive their allocated therapy, although the PFS curves from that analysis (Figure 4A) diverge significantly after 30 months in favor of the allo-RIC group. It should be noted that survival of both the allo-RIC patients and the patients whostarted maintenance is remarkably good: the median overall survival of the allo-RIC–transplanted patients has not even reached (Figure 4B). Importantly we could exclude that this favorable outcome was because of selection bias of the patients whowere included in the donor versus no-donor analysis PFS and OS were very similar for the HOVON-50 patients, who actually received HDM, whether they were included in DvND analysis.
Collectively, 4 DvND studies presently available fail to show a survival benefit, whereas in the 2 studies that do show a benefit, the survival of the patients included in the double auto-SCT groups was shorter than expected. These findings do evoke the question whether allo-RIC should routinely be applied as part of first-line therapy in all patients with stage II-III MM in the current era of novel agents.23 The answer seems to be no because bortezomib induction and lenalidomide maintenance as part of first-line therapy are likely to further improve the prognosis of younger myeloma patients. However, an important unanswered question is whether allo-RIC may be of benefit for patients with prognostic unfavorable features. Nowadays, high-risk subgroups of MM with poor survival can be identified with cytogenetics and gene expression profiling, even when these patients are treated upfront with novel antimyeloma agents.24 Molecular diagnostic techniques were not routinely available in the present study. However, we did look at risk factors more commonly applied, including higher ISS stage and elevated β-microglobulin. However, subgroup analysis did not reveal a beneficial effect of allo-SCT in these bad-risk patients, although the study was clearly not powered to address that question.
In conclusion, we found no benefit for allo-RIC as general part of first-line therapy for patients presenting with stage II-III MM. Therefore, it is suggested that allo-RIC should only be performed as part of a clinical trial, preferably including patients with a poor prognosis such as those with a rapid relapse after first-line therapy or having (very) unfavorable features such as the presence of 17P.25
There is an Inside Blood commentary on this article in this issue.
Presented in abstract form at the 50th annual meeting of American Society of Hematology, San Francisco, CA, December 8, 2008; the 15th Congress of the European Hematology Association, Barcelona, Spain, June 12, 2010; and the XIIIth Myeloma Workshop, Paris, France, May 5, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the Dutch Cancer Society.
Authorship
Contribution: H.M.L., M.Z., and P.S. contributed to study conception and design; H.M.L., B.v.d.H., J.J.C., M.-J.K., M.v.O., R.R., M.C.M., S.Z., J.J.J., M.Z., G.B., N.S., S.W., O.d.W., R.A., and P.S. collected and assembled the data; H.M.L., B.v.d.H., R.A., and P.S. conducted data analysis and interpretation; and all authors were involved in manuscript writing and approved the final version of the manuscript.
Conflict-of-interest disclosure: H.M.L. serves as a consultant for Celgene and Genmab; M.C.M. has received speaker's fees from Janssen and Novartis; S.Z. serves as a consultant for Janssen; P.S. serves as a consultant for Janssen and Millenium-Takeda and has received honoraria and research funding from Janssen. The remaining authors declare no competing financial interests.
Correspondence: Henk M. Lokhorst, Department of Hematology, University Medical Center Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail: h.lokhorst@umcutrecht.nl.