Abstract
To determine whether in vivo T-cell depletion, which lowers GVHD, abrogates the antileukemic benefits of myeloablative total body irradiation–based conditioning and unrelated donor transplantation, in the present study, we analyzed 715 children with acute lymphoblastic leukemia. Patients were grouped for analysis according to whether conditioning included antithymocyte globulin (ATG; n = 191) or alemtuzumab (n = 132) and no in vivo T-cell depletion (n = 392). The median follow-up time was 3.5 years for the ATG group and 5 years for the alemtuzumab and T cell–replete groups. Using Cox regression analysis, we compared transplantation outcomes between groups. Compared with no T-cell depletion, grade 2-4 acute and chronic GVHD rates were significantly lower after in vivo T-cell depletion with ATG (relative risk [RR] = 0.66; P = .005 and RR = 0.55; P < .0001, respectively) or alemtuzumab (RR = 0.09; P < .003 and RR = 0.21; P < .0001, respectively). Despite lower GVHD rates after in vivo T-cell depletion, nonrelapse mortality, relapse, overall survival, and leukemia-free survival (LFS) did not differ significantly among the treatment groups. The 3-year probabilities of LFS after ATG-containing, alemtuzumab-containing, and T cell–replete transplantations were 43%, 49%, and 46%, respectively. These data suggest that in vivo T-cell depletion lowers GVHD without compromising LFS among children with acute lymphoblastic leukemia who are undergoing unrelated donor transplantation with myeloablative total body irradiation–based regimens.
Introduction
The use of total body irradiation (TBI) transplantation conditioning regimens for acute lymphoblastic leukemia (ALL) is standard. Whereas some conditioning strategies incorporate in vivo T-cell depletion using infusions of alemtuzumab or antithymocyte globulin (ATG) preparations to diminish GVHD, others opt for a T cell–replete strategy.1 GVHD rates are high after unrelated donor transplantation: in children, the rate of grade 2-4 acute GVHD is approximately 45% and that of chronic GVHD is 30%.2,3 GVHD, and in particular chronic GVHD, has a significant impact on long-term morbidity and mortality. In adults, a recently completed phase 3 trial involving randomized use of ATG (Fresenius) showed lower rates of acute and chronic GVHD in the ATG arm without an increase in relapse, resulting in comparable overall survival (OS) between the 2 arms.4,5 In the present study, all participants received a myeloablative conditioning regimen followed by hematopoietic stem cell grafts from an HLA-matched unrelated donor and GVHD prophylaxis with cyclosporine and methotrexate. Alternatively, in vivo T-cell depletion with alemtuzumab is used widely in the setting of reduced intensity transplantation. In a recent report from the Center for International Blood and Marrow Transplant Research (CIBMTR), an alemtuzumab-containing alkylating agent plus fludarabine in reduced-intensity conditioning regimens in adults led to lower GVHD but increased the likelihood of disease relapse, which had a negative impact on disease-free survival.6 Alemtuzumab has been less commonly used in adults undergoing myeloablative transplantation except in the United Kingdom.7
Several groups have adopted in vivo T-cell depletion as a strategy to reduce the risk of acute and chronic GVHD after unrelated donor transplantation for ALL in children and adolescents.8 Because outcomes in adults do not necessarily correspond to those in children, in the present study, we explored whether in vivo T-cell depletion would result in a lower risk of GVHD, in particular chronic GVHD, and potentially lower nonrelapse mortality without a consequent increase in leukemia relapse. In the absence of a randomized clinical trial, we examined the effect of in vivo T-cell depletion versus none on the various transplantation outcomes retrospectively in more than 700 children and adolescents with ALL using data reported to 2 registries.
Methods
Patients
The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a statistical center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program Coordinating Center in Minneapolis. Participating centers are required to report all transplantations consecutively and compliance is monitored by on-site audits. Patients are followed longitudinally. The CIBMTR collects data on more than 80% of unrelated donor transplantations in the United States. Data were also collected from consecutive transplantations performed at 3 major transplantation centers in the United Kingdom performing more than 70% of all unrelated donor transplantations for pediatric leukemia in the United Kingdom. All patients or their guardians provided written informed consent in accordance with the Declaration of Helsinki. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Inclusion criteria
Eligible were patients with pre-B or T-ALL who were < 18 years of age at the time of transplantation. All patients received a myeloablative TBI-based conditioning regimen and BM or peripheral blood progenitor cells (PBPCs) from HLA-matched or mismatched unrelated adult donors. Transplantations were performed from 1998-2007. Excluded were patients who received ex vivo T cell–depleted BM or CD34-selected PBPC grafts. Also excluded were recipients of prior allogeneic transplantation.
End points
Neutrophil recovery was defined as achieving an absolute neutrophil count of ≥ 0.5 × 109/L for 3 consecutive measurements and platelet recovery as achieving platelet levels ≥ 20 × 109/L unsupported by transfusion for 7 days. Incidences of grade 2-4 acute and chronic GVHD were based on reports from each transplantation center using standard criteria.9,10 Nonrelapse mortality was defined as death not related to leukemia recurrence or progression and relapse was defined as disease recurrence based on morphological evaluation with or without reappearance of pretransplantation abnormalities in cytogenetic or molecular analyses. Leukemia-free survival (LFS) was defined as survival in a state of continuous complete remission. Overall mortality was defined as death from any cause.
Statistical analysis
Patients were considered in 3 groups: (1) those receiving ATG, (2) those receiving alemtuzumab, and (3) those receiving no anti–T-cell therapy. Variables related to patients, disease, and transplantation (Table 1) were compared among the 3 groups using χ2 analyses. The probabilities of LFS and OS were calculated with the Kaplan-Meier estimator.11 Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, and nonrelapse mortality and relapse were calculated with the cumulative incidence estimator to accommodate competing risks.11 For neutrophil and platelet recovery and acute and chronic GVHD, death without the event was the competing risk; for nonrelapse mortality, relapse was the competing risk; and for relapse, nonrelapse mortality was the competing risk. For analysis of LFS, relapse or death from any cause (ie, treatment failure) was considered an event. For OS, death from any cause was considered an event. In all analyses, data on patients without an event were censored at last follow-up.
Patient, disease, and transplantation-related characteristics
| . | ATG . | Alemtuzumab . | T cell–replete . | P . |
|---|---|---|---|---|
| No. of patients | 191 | 132 | 392 | |
| Age at transplantation, y | ||||
| Median (range) | 10 (1-18) | 9 (< 1-18) | 10 (< 1-18) | .10 |
| 1-5, n (%) | 18 (9) | 19 (14) | 65 (17) | |
| 6-10, n (%) | 78 (41) | 58 (44) | 141 (36) | |
| 11-18, n (%) | 95 (50) | 55 (42) | 186 (47) | |
| Male sex, n (%) | 128 (67) | 74 (56) | 247 (63) | .13 |
| FAB subtype, n (%) | ||||
| Pre-B | 144 (75) | 115 (87) | 336 (86) | .001 |
| B cell | 3 ( 2) | 1 ( 1) | 6 ( 2) | |
| T cell | 36 (19) | 13 (10) | 32 ( 8) | |
| Not reported | 8 ( 4) | 3 ( 2) | 18 ( 5) | |
| Cytogenetic risk group, n (%)* | ||||
| Standard-risk | 111(58) | 81 (62) | 209 (53) | |
| Poor-risk | 33 (17) | 37 (28) | 72 (18) | |
| Not reported | 47 (25) | 14 (11) | 111 (28) | |
| Disease status at transplantation, n (%) | ||||
| 1st CR | 53 (28) | 31 (23) | 91 (23) | .67 |
| 2nd CR | 87 (46) | 72 (55) | 200 (51) | |
| 3rd and beyond CR | 34 (18) | 22 (17) | 68 (17) | |
| Primary induction failure/relapse | 17 (9) | 7 (5) | 33 (8) | |
| Conditioning regimen, n (%) | ||||
| TBI + cyclophosphamide + other agents† | 70 (37) | 3 (2) | 54 (14) | < .001 |
| TBI + cyclophosphamide‡ | 85 (45) | 122 (92) | 311 (79) | |
| TBI + other agents§ | 36 (19) | 7 (5) | 27 (7) | |
| TBI dose, cGy, n (%) | ||||
| 1000 | 4 ( 2) | 31 ( 7) | < .001 | |
| 1200 | 162 (85) | 17 (13) | 155 (40) | |
| 1320 | 11 ( 6) | 90 (23) | ||
| 1400 | 13 ( 7) | 44 (33) | 93 (24) | |
| 1440 | 1 (< 1) | 71 (54) | 23 ( 6) | |
| Graft type, n (%) | ||||
| BM | 148 (77) | 111 (84) | 329 (84) | .13 |
| Peripheral blood | 43 (23) | 21 (16) | 63 (16) | |
| GVHD prophylaxis, n (%) | ||||
| Tacrolimus-containing regimen | 36 (19) | 53 (40) | 103 (27) | < .001 |
| Cyclosporine-containing regimen | 155 (81) | 79 (60) | 289 (73) | |
| Donor-recipient HLA match, n (%) | ||||
| 8 of 8 HLA matched | 122 (64) | 80 (61) | 225 (57) | .31 |
| 1 or 2 loci mismatched | 69 (36) | 52 (39) | 167 (43) | |
| Donor-recipient sex match, n (%) | ||||
| Male donor/male recipient | 89 (47) | 47 (36) | 157 (40) | .24 |
| Male donor/female recipient | 39 (20) | 29 (22) | 83 (21) | |
| Female donor/male recipient | 36 (19) | 27 (20) | 90 (23) | |
| Female donor/female recipient | 24 (13) | 29 (22) | 62 (16) | |
| Not reported | 3 (2) | |||
| Donor-recipient CMV status, n (%) | ||||
| Donor seronegative/recipient seronegative | 57 (30) | 49 (37) | 142 (36) | .01 |
| Donor seropositive/recipient seronegative | 29 (15) | 31 (23) | 83 (21) | |
| Donor seronegative/recipient seropositive | 72 (38) | 27 (20) | 95 (24) | |
| Donor seropositive /recipient seropositive | 28 (15) | 21 (16) | 65 (17) | |
| Not reported | 5 (3) | 4 (3) | 7 (2) | |
| Year of transplantation, n (%) | ||||
| 1998-2003 | 100 (52) | 56 (42) | 246 (63) | < .001 |
| 2004-2007 | 91 (48) | 76 (58) | 146 (37) | |
| Median follow-up of survivors, mo (range) | 42 (3-132) | 58 (8-125) | 60 (4-131) | |
| . | ATG . | Alemtuzumab . | T cell–replete . | P . |
|---|---|---|---|---|
| No. of patients | 191 | 132 | 392 | |
| Age at transplantation, y | ||||
| Median (range) | 10 (1-18) | 9 (< 1-18) | 10 (< 1-18) | .10 |
| 1-5, n (%) | 18 (9) | 19 (14) | 65 (17) | |
| 6-10, n (%) | 78 (41) | 58 (44) | 141 (36) | |
| 11-18, n (%) | 95 (50) | 55 (42) | 186 (47) | |
| Male sex, n (%) | 128 (67) | 74 (56) | 247 (63) | .13 |
| FAB subtype, n (%) | ||||
| Pre-B | 144 (75) | 115 (87) | 336 (86) | .001 |
| B cell | 3 ( 2) | 1 ( 1) | 6 ( 2) | |
| T cell | 36 (19) | 13 (10) | 32 ( 8) | |
| Not reported | 8 ( 4) | 3 ( 2) | 18 ( 5) | |
| Cytogenetic risk group, n (%)* | ||||
| Standard-risk | 111(58) | 81 (62) | 209 (53) | |
| Poor-risk | 33 (17) | 37 (28) | 72 (18) | |
| Not reported | 47 (25) | 14 (11) | 111 (28) | |
| Disease status at transplantation, n (%) | ||||
| 1st CR | 53 (28) | 31 (23) | 91 (23) | .67 |
| 2nd CR | 87 (46) | 72 (55) | 200 (51) | |
| 3rd and beyond CR | 34 (18) | 22 (17) | 68 (17) | |
| Primary induction failure/relapse | 17 (9) | 7 (5) | 33 (8) | |
| Conditioning regimen, n (%) | ||||
| TBI + cyclophosphamide + other agents† | 70 (37) | 3 (2) | 54 (14) | < .001 |
| TBI + cyclophosphamide‡ | 85 (45) | 122 (92) | 311 (79) | |
| TBI + other agents§ | 36 (19) | 7 (5) | 27 (7) | |
| TBI dose, cGy, n (%) | ||||
| 1000 | 4 ( 2) | 31 ( 7) | < .001 | |
| 1200 | 162 (85) | 17 (13) | 155 (40) | |
| 1320 | 11 ( 6) | 90 (23) | ||
| 1400 | 13 ( 7) | 44 (33) | 93 (24) | |
| 1440 | 1 (< 1) | 71 (54) | 23 ( 6) | |
| Graft type, n (%) | ||||
| BM | 148 (77) | 111 (84) | 329 (84) | .13 |
| Peripheral blood | 43 (23) | 21 (16) | 63 (16) | |
| GVHD prophylaxis, n (%) | ||||
| Tacrolimus-containing regimen | 36 (19) | 53 (40) | 103 (27) | < .001 |
| Cyclosporine-containing regimen | 155 (81) | 79 (60) | 289 (73) | |
| Donor-recipient HLA match, n (%) | ||||
| 8 of 8 HLA matched | 122 (64) | 80 (61) | 225 (57) | .31 |
| 1 or 2 loci mismatched | 69 (36) | 52 (39) | 167 (43) | |
| Donor-recipient sex match, n (%) | ||||
| Male donor/male recipient | 89 (47) | 47 (36) | 157 (40) | .24 |
| Male donor/female recipient | 39 (20) | 29 (22) | 83 (21) | |
| Female donor/male recipient | 36 (19) | 27 (20) | 90 (23) | |
| Female donor/female recipient | 24 (13) | 29 (22) | 62 (16) | |
| Not reported | 3 (2) | |||
| Donor-recipient CMV status, n (%) | ||||
| Donor seronegative/recipient seronegative | 57 (30) | 49 (37) | 142 (36) | .01 |
| Donor seropositive/recipient seronegative | 29 (15) | 31 (23) | 83 (21) | |
| Donor seronegative/recipient seropositive | 72 (38) | 27 (20) | 95 (24) | |
| Donor seropositive /recipient seropositive | 28 (15) | 21 (16) | 65 (17) | |
| Not reported | 5 (3) | 4 (3) | 7 (2) | |
| Year of transplantation, n (%) | ||||
| 1998-2003 | 100 (52) | 56 (42) | 246 (63) | < .001 |
| 2004-2007 | 91 (48) | 76 (58) | 146 (37) | |
| Median follow-up of survivors, mo (range) | 42 (3-132) | 58 (8-125) | 60 (4-131) | |
FAB indicates French-American-British.
Poor risk was defined as presence of Philadelphia chromosome, translocation (4;11), hypodiploid (< 44), and iAMP21; standard risk was defined as all other abnormalities and normal cytogenetics.
Cyclophosphamide dose, 120 mg/kg; 35 of 70 patients in the ATG group received thiotepa (150-350 mg/m2) and 35 patients received etoposide (1-2 G/m2). One patient in the alemtuzumab group received thiotepa (500 mg/m2) and 2 patients received etoposide (1.5 G/m2). Five patients in the T cell–replete group received thiotepa (300 mg/m2) and 49 patients received etoposide (1-2 G/m2).
Cyclophosphamide dose was 120 mg/kg.
Thirty-one of 36 patients in the ATG group received etoposide (1-2.5 G/m2), 3 patients received melphalan (125 or 140 mg/m2), and 2 patients received thiotepa (300 mg/m2). All 7 patients in the alemtuzumab group received etoposide (1-2.5 G/m2). Thirteen patients in the T cell–replete group received etoposide (1-2.5 G/m2), 8 patients received cytosine arabinoside (3.5 G/m2), 5 patients received melphalan (140 mg/m2), and 1 patient received thiotepa (300 mg/m2).
Cox proportional hazard regression models were constructed for acute and chronic GVHD, nonrelapse mortality, relapse, LFS, and OS.12 Multivariate models were built with the use of forward stepwise selection procedures and confirmed with use of backward stepwise selection procedures. All variables significant at P ≤ .05 were included in the final models. Proportional-hazards assumption was tested for each variable individually and all variables met this assumption.
The primary objective of the present study was to describe the overall impact of in vivo T-cell depletion (alemtuzumab or ATG) on the outcome of myeloablative transplantation for ALL in children and adolescents. Therefore, we compared transplantation outcomes after in vivo T cell–depleted and T cell–replete transplantations. Results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). The variable for in vivo T-cell depletion (alemtuzumab vs ATG vs T-cell replete) was held in all steps of model building. Other variables considered were patient age (1-5 vs 6-10 vs 11-18 years), performance score (90-100 vs < 90), disease status at transplantation (first complete remission [CR] vs subsequent CR vs not in remission at transplantation), graft type (BM vs PBPC), donor-recipient CMV serostatus (donor/recipient seronegative vs donor seropositive/recipient seronegative vs donor seronegative/recipient seropositive vs donor/recipient seropositive), donor-recipient sex match (female donor/male recipient vs others), and transplantation year (1998-2003 vs 2004-2007). Individual covariates were entered as categorical variables. There were no significant interactions between the variable for in vivo T-cell depletion and other significant variables in the final model. There were no significant center effects detected using the random-effects model.13 All P values are 2-sided. Analyses were done with SAS Version 9.2 software.
Results
The characteristics of patients, their disease, and transplantations are shown in Table 1. A total of 191 patients received ATG, 132 patients received alemtuzumab, and 392 patients received T cell–replete PBPC or BM grafts. Transplantations were performed at 113 centers; 31 centers used ATG only, 4 centers alemtuzumab only, and 56 centers performed T cell–replete transplantations only; 3 centers used ATG or alemtuzumab, 1 center used alemtuzumab or performed T cell–replete transplantations, and 18 centers used ATG or performed T cell–replete transplantations. The 3 treatment groups were similar with respect to age at transplantation, disease status, donor-recipient HLA-match, donor-recipient sex match, and graft type. Patients who received ATG were more likely to have T-cell ALL and those who received alemtuzumab were more likely to have poor-risk cytogenetics. Patients who received ATG were more likely to be CMV seropositive. All patients received a myeloablative TBI (≥ 1000 cGy) conditioning regimen. The predominant conditioning regimen for recipients of alemtuzumab or T cell–replete transplantations was TBI and cyclophosphamide. However, the TBI dose varied: 87% of alemtuzumab recipients received a TBI dose of 1400 or 1440 cGy, compared with 7% of ATG recipients and 30% of recipients of T cell–replete grafts. The TBI dose of 1200 cGy was the predominant dose in the ATG group. GVHD prophylaxis included either cyclosporine or tacrolimus; cyclosporine regimens were more commonly used with ATG-containing and T cell–replete transplantations. Recipients of ATG were more likely to receive TBI and cyclophosphamide or TBI, cyclophosphamide with etoposide, or thiotepa. Whereas the use of ATG remained constant during transplantation periods 1998-2003 and 2004-2007 (25% vs 29%), the use of alemtuzumab was more frequent in 2004-2007 (25%) than in the earlier period (14%). Consequently, the use of T cell–replete grafts decreased to 47% of transplantations during the period 2004-2007 compared with 61% in the earlier period. The median total dose of alemtuzumab was 1 mg/kg (range, 0.8-1.2). Among patients who received ATG, 47% of recipients received rabbit ATG (median dose, 7.5 mg/kg; range, 4-12) and 44% received horse ATG (median dose, 85 mg/kg; range, 30-120). The type of ATG or dose was not reported for 8% of patients. The median follow-up time of surviving patients is 3.5 years for the group that received ATG and 5 years for the groups that received alemtuzumab or T cell–replete grafts.
Hematopoietic recovery
Almost all patients achieved neutrophil recovery: 186 of 191 patients in the ATG group, 60 of 62 patients in the alemtuzumab group, and 383 of 392 patients in the T cell–replete group. The median times to neutrophil and platelet recoveries were 18 days and 26 days, respectively. The day-28 probabilities of neutrophil recovery after ATG-containing, alemtuzumab-containing, and T cell–replete regimens were not significantly different in the 3 groups: 92% (95% CI, 86-95), 94% (95% CI, 88-97), and 94% (95% CI, 91-96), respectively (P = .63). Similarly, the day 60 probabilities of platelet recovery after ATG-containing, alemtuzumab-containing, and T cell–replete regimens were not significantly different: 73% (95% CI, 66-79), 75% (95% CI, 64-83), and 76% (95% CI, 71-80), respectively (P = .77). Secondary graft failure was uncommon and occurred in 2 of 186 (1%) patients in the ATG group, 2 of 60 (3%) patients in the alemtuzumab group, and 12 of 629 (2%) patients in the T cell–replete group.
Acute and chronic GVHD
Compared with T cell–replete transplantations, risks of grade 2-4 acute GVHD were lower after in vivo T-cell depletion mediated by ATG (HR = 0.59; 95% CI, 0.46-0.77; P < .0001) or alemtuzumab (HR = 0.24; 95% CI, 0.16-0.36; P < .0001) regimens. Acute GVHD risks were also lower after conditioning that included alemtuzumab compared with ATG (HR = 0.40; 95% CI, 0.26-0.63; P < .0001). The only other factor associated with grade 2-4 acute GVHD was transplantation of allografts from mismatched unrelated donors, for which the risk of grade 2-4 acute GVHD was higher compared with fully matched donors (HR = 1.35; 95% CI, 1.09-1.68; P = .007). The day-100 cumulative probabilities of grade 2-4 acute GVHD among the ATG, alemtuzumab, and T cell–replete groups were 39% (95% CI, 32-46), 18% (95% CI, 12-25), and 58% (95% CI, 53-63), respectively (P < .001). The risk of chronic GVHD was also significantly lower for the ATG (HR = 0.55; 95% CI, 0.41-0.74; P < .001) and alemtuzumab (HR = 0.21; 95% CI, 0.13-0.34; P < .001) groups compared with the T cell–replete group. Chronic GVHD risk was lower when conditioning included alemtuzumab rather than ATG (HR = 0.39; 95% CI, 0.23-0.64; P < .0001). Other factors associated with chronic GVHD included age and graft type. The risk of chronic GVHD was higher among patients 11-18 years of age compared with younger patients (HR = 1.29; 95% CI, 1.02-1.66; P = .037) and among recipients of PBPCs compared with those who received BM (HR = 2.08; 95% CI, 1.57-2.76; P < .0001). The observed higher risk of chronic GVHD in older patients and transplantation of PBPCs was independent of in vivo T-cell depletion. The 3-year cumulative probabilities of chronic GVHD among the ATG, alemtuzumab, and T cell–replete groups were 32% (95% CI, 25-39), 15% (95% CI, 9-22), and 48% (95% CI, 43-53), respectively (P < .001).
We also looked for differences in GVHD risks by type of ATG (rabbit vs horse). The day-100 probability of grade 2-4 acute GVHD was lower at 26% (95% CI, 17-35) in patients who received rabbit ATG compared with those receiving horse ATG (52%; 95% CI, 41-62), respectively (P = .0003). There was no difference in chronic GVHD; the 5-year probabilities of chronic GVHD in recipients of rabbit and horse ATG were 29% (95% CI, 18-40) and 38% (95% CI, 27-49), respectively (P = .23).
Nonrelapse mortality and relapse
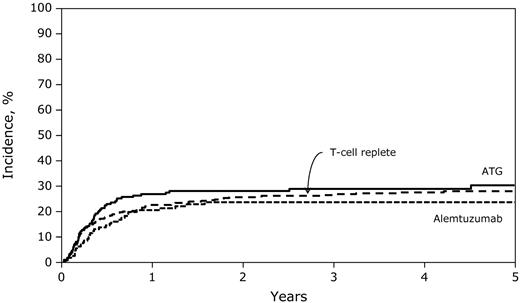
The 3-year rates of nonrelapse mortality were similar after conditioning that included ATG (29%; 95% CI, 22-36) or alemtuzumab (24%; 95% CI, 17-31) compared with the T cell–replete group (26%; 95% CI, 22-31; Figure 1 and Table 2; P = .57). We did not observe significant differences in nonrelapse mortality risks between the ATG and alemtuzumab groups (HR = 1.32; 95% CI, 0.85-2.05; P = .22). Nonrelapse mortality risks were higher among patients 11-18 years of age compared with younger patients (HR = 2.11; 95% CI, 1.58-2.82; P < .0001), recipients of mismatched transplantations compared with fully matched transplantations (HR = 1.68; 95% CI, 1.27-2.23; P = .0003), and recipients of PBPCs compared with those who received BM (HR = 3.33; 95% CI, 2.00-5.56; P < .0001). EBV posttransplantation lymphoproliferative disease (EBV-PTLD) developed in 9 patients. Two patients in the T cell–replete group developed EBV-PTLD compared with 4 patients who received alemtuzumab and 3 patients who received ATG. The 3-year cumulative incidences of EBV-PTLD in the 3 treatment groups were 0.5%, 3%, and 1.6%, respectively (P = .17).
Five-year probabilities of transplantation-related mortality after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations.
Five-year probabilities of transplantation-related mortality after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations.
Multivariate analysis of nonrelapse mortality, relapse, treatment failure, and overall mortality
| . | HR (95% CI) . | P . | |
|---|---|---|---|
| Nonrelapse mortality | T cell–replete | 1.00 | |
| ATG | 1.12 (0.81-1.55) | .50 | |
| Alemtuzumab | 0.85 (0.57-1.26) | .42 | |
| Relapse | T cell–replete | 1.00 | |
| ATG | 1.02 (0.74-1.42) | .89 | |
| Alemtuzumab | 0.85 (0.57-1.27) | .43 | |
| Treatment failure | T cell–replete | 1.00 | |
| ATG | 1.08 (0.86-1.36) | .53 | |
| Alemtuzumab | 0.92 (0.69-1.22) | .56 | |
| Overall mortality | T cell–replete | 1.00 | |
| ATG | 1.07 (0.84-1.37) | .58 | |
| Alemtuzumab | 0.83 (0.62-1.11) | .20 | |
| . | HR (95% CI) . | P . | |
|---|---|---|---|
| Nonrelapse mortality | T cell–replete | 1.00 | |
| ATG | 1.12 (0.81-1.55) | .50 | |
| Alemtuzumab | 0.85 (0.57-1.26) | .42 | |
| Relapse | T cell–replete | 1.00 | |
| ATG | 1.02 (0.74-1.42) | .89 | |
| Alemtuzumab | 0.85 (0.57-1.27) | .43 | |
| Treatment failure | T cell–replete | 1.00 | |
| ATG | 1.08 (0.86-1.36) | .53 | |
| Alemtuzumab | 0.92 (0.69-1.22) | .56 | |
| Overall mortality | T cell–replete | 1.00 | |
| ATG | 1.07 (0.84-1.37) | .58 | |
| Alemtuzumab | 0.83 (0.62-1.11) | .20 | |
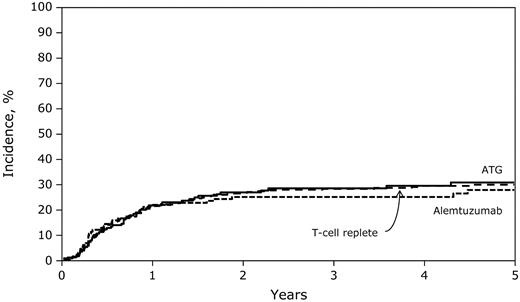
Relapse risks were not significantly different among the ATG, alemtuzumab, and T cell–replete groups (Table 2). Similarly, relapse risks were not significantly different among the ATG and alemtuzumab groups (HR = 1.20; 95% CI, 0.77-1.86; P = .41). The 3-year probabilities of relapse among the ATG, alemtuzumab, and T cell–replete groups were 29% (95% CI, 22-35), 25% (95% CI, 18-33), and 28% (95% CI, 24-33), respectively (Figure 2; P = .74). Cytogenetic risk, disease status at transplantation, and transplantation period were associated with leukemia relapse. Compared with patients with standard-risk cytogenetics, relapse risks were higher for those with poor-risk cytogenetics (HR = 1.73; 95% CI, 1.15-2.59; P = .008). Compared with patients given transplantations in relapse, those given transplantations in first CR (HR = 0.32; 95% CI, 0.23-0.63; P = .0001) and in second or third CR (HR = 0.41; 95% CI, 0.29-0.68; P = .0002) experienced significantly lower relapse risks. Relapse risks were higher in recipients given transplantations in 1998-2003 compared with the later period (HR = 1.47; 95% CI, 1.09-1.97; P = .011).
Five-year probabilities of relapse after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations.
Five-year probabilities of relapse after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations.
There were differences in nonrelapse mortality and relapse risks by type of ATG. The 5-year probability of nonrelapse mortality was lower at 18% (95% CI, 11-27) in patients who received rabbit ATG compared with those who received horse ATG, 37% (95% CI, 26-47); P = .01. In contrast, the 5-year probability of leukemia recurrence was higher in recipients of rabbit ATG at 42% (95% CI, 29-54) compared with 24% (95% CI, 15-33) in recipients of horse ATG (P = .03).
LFS and OS
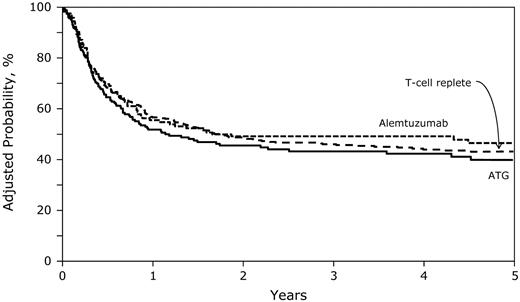
Compared with the T cell–replete group (45%; 95% CI, 40-50), 3-year rates of LFS were similar for the ATG (43%; 95% CI, 35-50) and alemtuzumab (51%; 95% CI, 42-59) groups (Figure 3 and Table 2; P = .31). We did not observe differences in treatment failure (relapse or death; inverse of LFS) after conditioning that included ATG or alemtuzumab (HR = 1.17; 95% CI, 0.86-1.59; P = .32). Age, disease status at transplantation, graft type, and transplantation period were associated with treatment failure. Compared with patients given transplantations in relapse, those given transplantations in first CR (HR = 0.58; 95% CI, 0.42-0.85; P = .005) and in second or third CR (HR = 0.65; 95% CI, 0.46-0.91; P = .011) experienced significantly lower treatment failure (ie, higher LFS). Treatment failure was higher among patients 11-18 years of age (HR = 1.43; 95% CI, 1.17-1.75; P = .0004) compared with younger patients, those who received PBPCs compared with those who received BM (HR = 1.90; 95% CI, 1.35-2.69; P = .0002), and those given transplantations before 2004 (HR = 1.36; 95% CI, 1.10-1.67; P = .004) compared with those given transplantations in the later period.
Five-year probabilities of LFS after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations adjusted for age, disease status at transplantation, graft type, and transplantation period.
Five-year probabilities of LFS after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations adjusted for age, disease status at transplantation, graft type, and transplantation period.
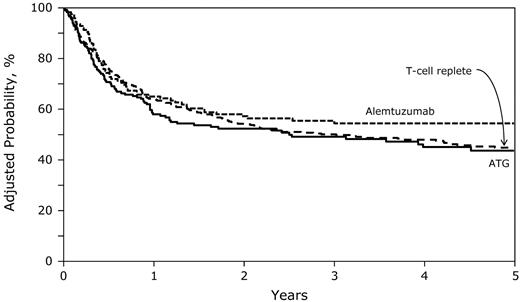
Similarly, 3-year rates of OS were similar for the ATG (49%; 95% CI, 41-56) and alemtuzumab (56%; 95% CI, 47-64) groups compared with the T cell–replete group (50%; 95% CI, 45-55; Figure 4 and Table 2; P = .36). We did not observe differences in overall mortality risks between the ATG and alemtuzumab groups (HR = 1.29; 95% CI, 0.93-1.79; P = .12). Age, disease status at transplantation, graft type, and donor-recipient HLA match were associated with overall mortality. Compared with patients given transplantations in relapse, those given transplantations in first CR (HR = 0.59; 95% CI, 0.40-0.87; P = .008) experienced lower overall mortality. For patients given transplantations in second or third CR, the observed lower risk for overall mortality did not reach statistical significance (HR = 0.72; 95% CI, 0.51-1.02; P = .069). Overall mortality was higher in patients 11-18 years of age (HR = 1.51; 95% CI, 1.23-1.86; P < .0001) compared with younger patients, those who received PBPCs compared with those who received BM (HR = 1.70; 95% CI, 1.23-2.36; P = .002), and those who received allografts from mismatched donors compared with those who received allografts from fully matched donors (HR = 1.31; 95% CI, 1.06-1.61; P = .011).
Five-year probabilities of OS after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations adjusted for age at transplantation, disease status at transplantation, graft type, and transplantation period.
Five-year probabilities of OS after T cell–replete, ATG-containing, and alemtuzumab-containing transplantations adjusted for age at transplantation, disease status at transplantation, graft type, and transplantation period.
We observed no differences in the probabilities of LFS or OS by ATG type. The 5-year probabilities of LFS in recipients of rabbit and horse ATG were 40% (95% CI, 27-53) and 40% (95% CI, 29-50), respectively (P = .96). The corresponding probabilities of OS were 49% (95% 36-60) and 40% (95% CI, 29-51), respectively (P = .30).
The causes of death for the 3 treatment groups are shown in Table 3. In all treatment groups, recurrent disease was the most common cause of death and accounted for approximately 40% of deaths. The numbers of patients dying from organ failure and GVHD were similar across the treatment groups. The numbers of patients dying from infectious complications in the alemtuzumab group demonstrated a higher rate at 22% compared with 13% and 10% in the ATG and T cell–replete groups, respectively, although this was not statistically significant. The number of patients dying of interstitial pneumonitis or adult respiratory distress syndrome was higher after in vivo T-cell depletion. There were no deaths attributed to EBV-PTLD.
Causes of death
| . | ATG . | Alemtuzumab . | T cell–replete . | P . |
|---|---|---|---|---|
| No. of deaths | 98 | 59 | 210 | |
| Primary disease, n (%) | 41 (42) | 25 (42) | 91 (43) | .97 |
| GVHD, n (%) | 10 (10) | 6 (10) | 23 (11) | .97 |
| Infection, n (%) | 10 (10) | 13 (22) | 27 (13) | .10 |
| IPN/ARDS, n (%) | 17 (17) | 7 (12) | 14 (7) | .02 |
| Organ failure, n (%) | 15 (15) | 7 (12) | 34 (16) | .71 |
| Other causes, n (%) | 5 (5) | 1 (2) | 21 (10) | .06 |
| . | ATG . | Alemtuzumab . | T cell–replete . | P . |
|---|---|---|---|---|
| No. of deaths | 98 | 59 | 210 | |
| Primary disease, n (%) | 41 (42) | 25 (42) | 91 (43) | .97 |
| GVHD, n (%) | 10 (10) | 6 (10) | 23 (11) | .97 |
| Infection, n (%) | 10 (10) | 13 (22) | 27 (13) | .10 |
| IPN/ARDS, n (%) | 17 (17) | 7 (12) | 14 (7) | .02 |
| Organ failure, n (%) | 15 (15) | 7 (12) | 34 (16) | .71 |
| Other causes, n (%) | 5 (5) | 1 (2) | 21 (10) | .06 |
IPN indicates interstitial pneumonitis; and ARDS, adult respiratory distress syndrome.
Discussion
The results of this analysis of 715 children and adolescents with ALL confirm and extend those reported previously for adults.14 In vivo T-cell depletion lowers the risk of acute and chronic GVHD in children and adolescents with ALL without negatively affecting LFS or OS after unrelated donor transplantation with myeloablative TBI-containing conditioning. TBI dose was not associated with relapse, LFS, or OS. Lower GVHD after in vivo T-cell depletion with horse ATG or alemtuzumab was not associated with concomitant lower nonrelapse mortality, because patients were more likely to succumb to infections, interstitial pneumonitis, and adult respiratory distress syndrome. In vivo T-cell depletion with rabbit ATG was associated higher risks of leukemia recurrence, which in turn negated any advantages with respect to LFS or OS. To our knowledge, there are no ongoing or reported randomized trials that have examined the role of alemtuzumab in the setting of myeloablative conditioning. The present analysis is the largest retrospective study to document transplantation outcomes after in vivo T-cell depletion (ATG or alemtuzumab) for ALL in children and adolescents compared with T cell–replete transplantations. Whereas in vivo T-cell depletion diminishes GVHD risks, nonrelapse mortality and relapse risks ultimately resulted in comparable LFS and OS rates across the transplantation strategies in the present study.
Chronic GVHD is a major cause of morbidity and mortality in long-term ALL survivors. Although not the focus of the present analysis, others have reported significant impairments in health-related quality of life in patients with chronic GVHD.15,16 The present study used data reported to an observational database, which prevented us from studying health-related quality of life for patients in the 3 treatment groups. Therefore, we are unable to describe a detailed account of the burden morbidity in these patients. However, we examined performance scores in survivors, and the number of patients across the 3 treatment groups who reported scores of 90 or 100 were similar: 12% of ATG, 10% of alemtuzumab, and 14% of T cell–replete transplantation recipients reported scores less than 90. Another important question is whether T-cell depletion reduces length of stay and hospital costs. In a single-center study involving adult patients, T-cell depletion did significantly reduce length of stay or hospital costs despite having no effect on acute or chronic GVHD or altering quality of life before and after transplantation.17 Data on length of hospital stay or hospital costs were not available for patients included in the present study.
An important observation of the present study is that the use of PBPC grafts with or without in vivo T-cell depletion led to significantly higher risks of chronic GVHD, higher nonrelapse mortality, and lower LFS and OS. This is in contrast to the finding of a largely adult study using in vivo alemtuzumab in the myeloablative setting, in which the incidence of chronic GVHD, either overall (BM, 47%; PBSCs, 49%) or extensive (BM, 15%; PBSCs, 13%), was not increased in patients receiving PBPCs.7 A recently completed phase 3 randomized clinical trial of BM and PBPCs for hematologic malignancies in adults reported higher chronic GVHD after PBPC transplantations, but without an adverse effect on mortality.18 The data thus far suggest that there are differences in outcome between adult and pediatric recipients of PBPC grafts. In an earlier study performed after HLA-matched sibling transplantation for acute leukemia in children, higher chronic GVHD after transplantation of PBPCs resulted in higher mortality.19 The present study and the earlier study on HLA-matched sibling transplantations for acute leukemia support avoiding transplantation of PBPCs in children and adolescents, even in the setting of in vivo T-cell depletion.
Both the extent and specificity of T-cell depletion affect GVHD, infectious complications, relapse, and survival. In the present study, any benefit derived from lower nonrelapse mortality rates after rabbit ATG was negated by higher relapse rates. Horse ATG and alemtuzumab resulted in toxicities and therefore nonrelapse mortality rates comparable to that after non–T cell–depleted transplantations. Single-center studies in pediatric patients have shown less GVHD with alemtuzumab, but slower immune reconstitution20 and more adenovirus infections,21 compared with ATG. Particular and prolonged vigilance with regard to viral infections is essential in transplantations in which alemtuzumab is included in the conditioning therapy. Cytotoxic T-lymphocyte therapy with donor T cells against CMV, EBV, and adenovirus has been used successfully to prevent and treat these infections.22,23
The major limitation of the present observational study is that choice of treatment strategy, including whether to use in vivo T-cell depletion, was at the discretion of each transplantation center and was therefore subject to bias. We also lack data on ATG or alemtuzumab pharmacokinetics, which might more accurately determine the efficacy of T-cell depletion. Although we performed a carefully controlled comparison of T cell–replete and in vivo T cell–depleted transplantations that considered known prognostic factors, there may be several unknown or unmeasured factors that also contributed to the observed outcomes. Nevertheless, our data strongly suggest that in vivo T-cell depletion with anti–T-cell Ab preparations lowers the risk of acute and chronic GVHD without the survival advantage seen with myeloablative TBI regimens. These data also support BM as the preferred graft type for children and adolescents undergoing unrelated donor myeloablative transplantation for ALL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases (National Institutes of Health, Bethesda, MD), and by the Heath Resources and Services Administration (HHSH234200637015C).
National Institutes of Health
Authorship
Contribution: P.V., R.F.W., S.S., and M.E. designed the study and interpreted the data; P.V. and M.E. wrote the manuscript; K.W.A. performed the statistical analysis; S.S. and W.H. prepared the dataset; R.F.W., K.W.A., S.S., W.H., D.B., J. Craddock, J. Cornish, S.M.D., C.C.D., R.E.D., T.G.G., N.K., C.K., R.A.K., W.L., V.A.L., C.S., J.E.W., and P.A.C. interpreted the data and critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Paul Veys, Great Ormond Street Hospital for Children NHS Trust, London WC1N3JH, United Kingdom; e-mail: paul.veys@gosh.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal