Abstract
B cells are involved in the pathogenesis of chronic GVHD (cGVHD). We hypothesized that prophylactic anti–B-cell therapy delivered 2 months after transplantation would decrease allogeneic donor B-cell immunity and possibly the incidence of cGVHD. Therefore, in the present study, patients with high-risk chronic lymphocytic leukemia (n = 22) and mantle-cell lymphoma (n = 13) received a total lymphoid irradiation of 80 cGy for 10 days and antithymocyte globulin 1.5 mg/kg/d for 5 days. Rituximab (375 mg/m2) was infused weekly on days 56, 63, 70, and 77 after transplantation. The incidence of acute GVHD was 6%. The cumulative incidence of cGVHD was 20%. Nonrelapse mortality was 3%. Rituximab treatment after allogeneic transplantation significantly reduced B-cell allogeneic immunity, with complete prevention of alloreactive H-Y Ab development in male patients with female donors (P = .01). Overall survival and freedom from progression at 4 years for chronic lymphocytic leukemia patients were 73% and 47%, respectively; for mantle-cell lymphoma patients, they were 69% and 53%, respectively. This study is registered at www.clinicaltrials.gov as NCT00186628.
Introduction
Chronic GVHD (cGVHD) remains a significant cause of late morbidity and mortality after allogeneic hematopoietic cell transplantation (alloHCT). However, strategies to prevent cGVHD have been largely disappointing.1-4 Although traditionally thought to be mediated by alloreactive T lymphocytes,5,6 increasing evidence supports a role for B cells in the pathogenesis of cGVHD.7 Autoantibody and alloantibody associations with cGVHD have also been reported.8 Specifically, alloreactive Abs against H-Y antigens9,10 and coordinated B- and T-cell responses11 were found to be strongly associated with the occurrence of cGVHD in sex-mismatched alloSCT. A murine study demonstrated that allogeneic Abs deposit in cGVHD-affected tissues, and cGVHD was prevented when the donor graft was genetically prevented from secreting IgG.12 Other evidence comes from studies showing dysregulated B-cell reconstitution13 and increased B-cell activating factor levels in cGVHD patients.14 B cells collected from cGVHD patients were more responsive to TLR-9 signaling and exhibited increased CD86 expression.15 Furthermore, established steroid-refractory cGVHD patients have reduced numbers of naive B cells and increased activated CD27+ B cells,13,14 further supporting a role for B cells in cGVHD pathogenesis. Clinically, anti–B-cell–directed therapy with rituximab has been shown to be an effective treatment for established cGVHD, with several studies reporting clinical response rates of 40%-70% in steroid-refractory cases.16-20
Evidence for the potential use of rituximab as cGVHD prophylaxis comes from clinical observations that rituximab added to fludarabine and cyclophosphamide conditioning resulted in a low rate of cGVHD in 10 chronic lymphocytic leukemia (CLL) patients.21 Others have shown a decrease in acute GVHD (aGVHD) and/or cGVHD in patients with B-cell malignancies treated with rituximab within 6 months before alloHCT.22,23
These findings suggest that rituximab depletion of donor B cells after alloHCT may reduce cGVHD. We hypothesized that prophylactic anti–B-cell therapy with rituximab after alloHCT would deplete adoptively transferred alloreactive donor B cells and thus decrease cGVHD. The present study investigated the effect of rituximab treatment infused 2 months after alloHCT, focusing on safety, feasibility, B-cell immune reconstitution, and overall clinical outcomes. Cognizant that allogeneic B-cell responses may also have antitumor benefits, the present study piloted in vivo B cell–depletion strategy in patients with CD20-expressing B-cell malignancies. We modified our institution's total lymphoid irradiation-antithymocyte globulin (TLI-ATG) alloHCT regimen to study in vivo B-cell depletion after rituximab treatment, and show decreased allogeneic H-Y Ab development with promising low chronic GVHD incidence.
Methods
Patient selection
Between July 15, 2005 and November 30, 2007, 35 patients with high-risk CLL (n = 22) and mantle cell lymphoma (MCL; n = 13) were enrolled in the protocol, which was approved by the Stanford institutional review board (Table 1). High-risk CLL eligibility included: (1) FISH with 17p deletion or 11q deletion, (2) unmutated heavy chain immunoglobulin (VH-IgG; < 2% nucleotide change compared with germline sequence), or (3) fludarabine-refractory disease.24 MCL patients with a complete response (CR) or partial response (PR) were eligible.
Treatment plan
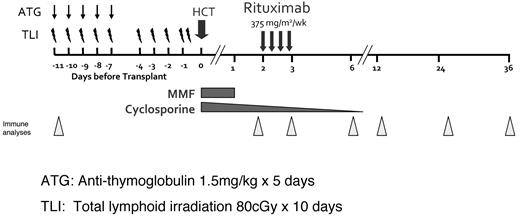
The reduced-intensity conditioning (RIC) regimen of TLI-ATG was adapted for this trial.25,26 As shown schematically in Figure 1, TLI was administered at 80 cGy for 10 days and rabbit ATG (thymoglobulin; Genzyme) at 1.5 mg/kg for 5 days, followed by infusion of unfractionated G-CSF–mobilized peripheral blood progenitor cells on day 0. The experimental treatment of this study was the infusion of rituximab 375 mg/m2 weekly for 4 weeks on days 56, 63, 70, and 77. The infusion of rituximab 2 months after transplantation was timed to coincide with peripheral blood donor B-cell reconstitution after TLI-ATG conditioning.25 Primary GVHD prophylaxis included cyclosporine and mycophenolate mofetil. Figure 1 shows the immunosuppression tapering schedule and timing of the research sample collection.
Trial schema. RIC using 80 cGy TLI for 10 days and ATG 1.5 mg/kg on days 1-5, followed by peripheral blood progenitor cell infusion on day 0. Rituximab infusion (375 mg/m2) was infused on days 56, 63, 70, 77. Cyclosporine and mycophenolate mofetil were used as primary GVHD prophylaxis. Triangles indicate time points for peripheral blood immune analyses.
Trial schema. RIC using 80 cGy TLI for 10 days and ATG 1.5 mg/kg on days 1-5, followed by peripheral blood progenitor cell infusion on day 0. Rituximab infusion (375 mg/m2) was infused on days 56, 63, 70, 77. Cyclosporine and mycophenolate mofetil were used as primary GVHD prophylaxis. Triangles indicate time points for peripheral blood immune analyses.
HLA typing and matching
Patients and donors were HLA-typed by high-resolution techniques.27 All matched related recipients were matched with their donors for 10 of 10 HLA alleles. Of the 16 unrelated recipients, 3 had single HLA allele level (9 of 10) mismatches (Tables 1–2).
CLL patient characteristics (n = 22)
| Patient characteristics . | CLL disease features . | CLL transplantation features . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPN . | Age, y . | Sex . | Cytogenetics by FISH . | Fludarabine refractory* . | CLL VH-IgG . | No. of prior regimens . | Mos from prior R . | Alemtuzumab before HCT . | CLL Status at HCT . | LN > 5cm before HCT . | BM CLL, %CD45 . | Time from diagnosis to HCT, mo . | Donor sex/age, y . | RD/URD . |
| 3399 | 65 | M | 11q−13q− | N | Unmutated† | 3 | 17 | N | PR | N | 15 | 67 | F/55 | RD |
| 3409 | 55 | M | NA | Y | Unmutated | 3 | 1 | N | PR | N | 50 | 9 | M/50 | RD |
| 3431 | 46 | F | 11q 13q− 17p− | N | Unmutated | 4 | 1 | Y | PD | Y | 10 | 30 | F/53 | RD |
| 3455 | 57 | F | complex cyto | Y | Unmutated | 5 | 23 | Y | PR | N | 2 | 66 | F/21 | URD |
| 3515 | 64 | M | 11q− 13q− | Y | ND | 2 | 15 | Y | PR | N | 4 | 29 | M/62 | RD |
| 3632 | 46 | M | 17p− | Y | Unmutated | 3 | 12 | Y | PR | N | 1 | 51 | F/38 | URD-DQ allele MM |
| 3697 | 54 | F | NA | N | Unmutated | 1 | 7 | N | PR | N | 5 | 9 | F/55 | RD |
| 3703 | 64 | M | NA | N | Unmutated | 4 | 2 | N | PR | N | 10 | 57 | M/25 | URD |
| 3719 | 61 | M | 11q− 17p− | N | ND | 1 | 8 | Y | CR | N | 0 | 15 | F/22 | URD |
| 3723 | 44 | F | 17p− complex cyto | Y | Unmutated | 2 | 2 | N | PD | Y | 50 | 48 | F/22 | URD |
| 3732 | 51 | M | 6q− 13q− +11 | Y | Unmutated | 3 | 3 | N | PR | Y | 30 | 29 | F/50 | RD |
| 3740 | 58 | F | 17p− | Y | Unmutated | 5 | 1 | N | PR | Y | 80 | 128 | M/56 | RD |
| 3751 | 56 | M | 17p− | Y | Unmutated | 6 | 2 | Y | PR | N | < 1 | 43 | M/26 | URD |
| 3835 | 64 | M | 13q− 17p− | Y | Unmutated | 2 | 1 | N | CR | N | 0 | 10 | M/36 | URD |
| 3855 | 31 | M | NA | Y | Unmutated | 2 | 30 | N | PD | N | 35 | 79 | F/55 | URD-DQ,DP MM |
| 3870 | 50 | M | NA | N | Unmutated | 2 | 6 | N | CR | N | 0 | 27 | M/46 | RD |
| 3873 | 59 | F | 13q− | N | Unmutated | 4 | 2 | Y | PR | N | 50 | 82 | M/53 | RD |
| 3879 | 43 | F | NA | Y | Mutated | 5 | 1 | N | PR | N | 20 | 195 | F/36 | RD |
| 3903 | 50 | M | 13q− | Y | Unmutated | 3 | 10 | Y | PR | N | 2 | 16 | M/22 | URD |
| 3926 | 54 | M | 13q− | Y | Mutated | 3 | 2 | N | PR | N | 0 | 55 | F/57 | URD |
| 3934 | 60 | M | 11q− 13q− | Y | Unmutated | 7 | 1 | Y | PR | N | 10 | 110 | M/57 | RD |
| 3975 | 48 | M | 17p− | Y | Unmutated | 3 | 5 | Y | CR | N | 0 | 60 | F/41 | RD |
| Patient characteristics . | CLL disease features . | CLL transplantation features . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPN . | Age, y . | Sex . | Cytogenetics by FISH . | Fludarabine refractory* . | CLL VH-IgG . | No. of prior regimens . | Mos from prior R . | Alemtuzumab before HCT . | CLL Status at HCT . | LN > 5cm before HCT . | BM CLL, %CD45 . | Time from diagnosis to HCT, mo . | Donor sex/age, y . | RD/URD . |
| 3399 | 65 | M | 11q−13q− | N | Unmutated† | 3 | 17 | N | PR | N | 15 | 67 | F/55 | RD |
| 3409 | 55 | M | NA | Y | Unmutated | 3 | 1 | N | PR | N | 50 | 9 | M/50 | RD |
| 3431 | 46 | F | 11q 13q− 17p− | N | Unmutated | 4 | 1 | Y | PD | Y | 10 | 30 | F/53 | RD |
| 3455 | 57 | F | complex cyto | Y | Unmutated | 5 | 23 | Y | PR | N | 2 | 66 | F/21 | URD |
| 3515 | 64 | M | 11q− 13q− | Y | ND | 2 | 15 | Y | PR | N | 4 | 29 | M/62 | RD |
| 3632 | 46 | M | 17p− | Y | Unmutated | 3 | 12 | Y | PR | N | 1 | 51 | F/38 | URD-DQ allele MM |
| 3697 | 54 | F | NA | N | Unmutated | 1 | 7 | N | PR | N | 5 | 9 | F/55 | RD |
| 3703 | 64 | M | NA | N | Unmutated | 4 | 2 | N | PR | N | 10 | 57 | M/25 | URD |
| 3719 | 61 | M | 11q− 17p− | N | ND | 1 | 8 | Y | CR | N | 0 | 15 | F/22 | URD |
| 3723 | 44 | F | 17p− complex cyto | Y | Unmutated | 2 | 2 | N | PD | Y | 50 | 48 | F/22 | URD |
| 3732 | 51 | M | 6q− 13q− +11 | Y | Unmutated | 3 | 3 | N | PR | Y | 30 | 29 | F/50 | RD |
| 3740 | 58 | F | 17p− | Y | Unmutated | 5 | 1 | N | PR | Y | 80 | 128 | M/56 | RD |
| 3751 | 56 | M | 17p− | Y | Unmutated | 6 | 2 | Y | PR | N | < 1 | 43 | M/26 | URD |
| 3835 | 64 | M | 13q− 17p− | Y | Unmutated | 2 | 1 | N | CR | N | 0 | 10 | M/36 | URD |
| 3855 | 31 | M | NA | Y | Unmutated | 2 | 30 | N | PD | N | 35 | 79 | F/55 | URD-DQ,DP MM |
| 3870 | 50 | M | NA | N | Unmutated | 2 | 6 | N | CR | N | 0 | 27 | M/46 | RD |
| 3873 | 59 | F | 13q− | N | Unmutated | 4 | 2 | Y | PR | N | 50 | 82 | M/53 | RD |
| 3879 | 43 | F | NA | Y | Mutated | 5 | 1 | N | PR | N | 20 | 195 | F/36 | RD |
| 3903 | 50 | M | 13q− | Y | Unmutated | 3 | 10 | Y | PR | N | 2 | 16 | M/22 | URD |
| 3926 | 54 | M | 13q− | Y | Mutated | 3 | 2 | N | PR | N | 0 | 55 | F/57 | URD |
| 3934 | 60 | M | 11q− 13q− | Y | Unmutated | 7 | 1 | Y | PR | N | 10 | 110 | M/57 | RD |
| 3975 | 48 | M | 17p− | Y | Unmutated | 3 | 5 | Y | CR | N | 0 | 60 | F/41 | RD |
R indicates rituximab; LN, lymph node; RD, matched related donor; URD, unrelated donor; MM, mismatch; NA, not available; ND, not determined; and PD, progressive disease.
Defined as failure to achieve a PR or CR to at least 1 fludarabine-containing regimen, disease progression while on fludarabine treatment, or disease progression within 6 months of the last dose of fludarabine.
Unmutated VH-IgG CLL clone (CLL VH-IgG sequence varies by < 2% from germline).
MCL patient characteristics (n = 13)
| Patient characteristics . | MCL disease features . | MCL transplantation features . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPN . | Age, y . | Sex . | BM involved . | Ki67 index . | No. of prior regimens . | Mo from prior R . | PET status at HCT . | MCL status at HCT . | Time from diagnosis to HCT, mo . | Donor sex/age, y . | RD/URD . |
| 3411 | 55 | M | Y | 30% | 2 | 2 | + | PR | 16 | M/35 | URD |
| 3439 | 64 | M | N | 80% | 5 | 2 | No PET | PR | 36 | F/55 | RD |
| 3489 | 57 | F | Y | NA | 3 | 2 | + | PR | 44 | M/55 | RD |
| 3547 | 63 | M | Y | NA | 4 | 3 | − | PR | 72 | F/65 | RD |
| 3567 | 55 | F | Y | 40% | 1 | 2 | − | CR | 6 | M/48 | RD |
| 3580 | 63 | M | Y | NA | 2 | 2 | − | CR | 18 | M/47 | RD |
| 3635 | 63 | M | Y | 75% | 1 | 2 | − | CR | 6 | M/37 | URD |
| 3636 | 50 | F | Y | NA | 1 | 4 | − | CR | 7 | F/53 | RD |
| 3807 | 62 | M | Y | NA | 1 | 3 | − | CR | 8 | M/27 | URD-A allele MM |
| 3814 | 60 | F | N | 30% | 2 | 1 | − | CR | 38 | F/25 | URD |
| 3842 | 58 | M | Y | > 90% | 3 | 1 | − | CR | 28 | F/26 | URD |
| 3928 | 66 | F | Y | 90% | 1 | 3 | − | CR | 7 | M/21 | URD |
| 3969 | 56 | M | Y | 50% | 1 | 1 | − | CR | 6 | M/53 | RD |
| Patient characteristics . | MCL disease features . | MCL transplantation features . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPN . | Age, y . | Sex . | BM involved . | Ki67 index . | No. of prior regimens . | Mo from prior R . | PET status at HCT . | MCL status at HCT . | Time from diagnosis to HCT, mo . | Donor sex/age, y . | RD/URD . |
| 3411 | 55 | M | Y | 30% | 2 | 2 | + | PR | 16 | M/35 | URD |
| 3439 | 64 | M | N | 80% | 5 | 2 | No PET | PR | 36 | F/55 | RD |
| 3489 | 57 | F | Y | NA | 3 | 2 | + | PR | 44 | M/55 | RD |
| 3547 | 63 | M | Y | NA | 4 | 3 | − | PR | 72 | F/65 | RD |
| 3567 | 55 | F | Y | 40% | 1 | 2 | − | CR | 6 | M/48 | RD |
| 3580 | 63 | M | Y | NA | 2 | 2 | − | CR | 18 | M/47 | RD |
| 3635 | 63 | M | Y | 75% | 1 | 2 | − | CR | 6 | M/37 | URD |
| 3636 | 50 | F | Y | NA | 1 | 4 | − | CR | 7 | F/53 | RD |
| 3807 | 62 | M | Y | NA | 1 | 3 | − | CR | 8 | M/27 | URD-A allele MM |
| 3814 | 60 | F | N | 30% | 2 | 1 | − | CR | 38 | F/25 | URD |
| 3842 | 58 | M | Y | > 90% | 3 | 1 | − | CR | 28 | F/26 | URD |
| 3928 | 66 | F | Y | 90% | 1 | 3 | − | CR | 7 | M/21 | URD |
| 3969 | 56 | M | Y | 50% | 1 | 1 | − | CR | 6 | M/53 | RD |
R indicates rituximab; RD, matched related donor; URD, unrelated donor; MM, mismatch; NA, not available; and PET, positron emission tomography.
Supportive care
Patients were hospitalized for the ATG infusion for the first 5 days only. Antimicrobial, antiviral, and antifungal prophylaxis was administered as described previously.25 Monitoring for CMV and EBV by PCR assay was performed starting on day 7 after transplantation and continued weekly for at least the first 90 days after transplantation. IgG levels were assessed pre-rituximab and at day 90, day 180, and 1 year.
FACS analyses
B-cell reconstitution was assessed in donors and patients pre-rituximab and at days 56, 90, and 180. Subsequent analyses of B-cell recovery after rituximab will be reported separately (B.S., A. C. Logan, B.N., S.A., J. Wilhelmy, J.R., P. Wadia, K. Heydari, W. Xiao, M. Mindrinos, D.B.M.; B-cell phenotype in chronic graft-versus-host disease patients; manuscript in preparation). For comparison, we measured B-cell recovery in 19 TLI-ATG patients who had never received rituximab. Cryopreserved PBMCs were thawed and washed.28 Cells were stained with a cocktail of fluorochrome-conjugated Abs against cell-surface markers: CD3 Qdot605 (Invitrogen), CD19-PE, CD5-APC, CD23-APC-Cy7, and CD20 CY5-PerCP (BD BioSciences). Staining proceeded for 15 minutes on ice.29 Cells were then washed and resuspended in staining medium containing 0.4% formaldehyde before analysis on an LSRII flow cytometer (BD Biosciences). Gates were set using the fluorescence-minus-one samples as negative controls. Data were analyzed using FlowJo Version 8.8.6 software (TreeStar). Significance was calculated using the nonparametric Wilcoxon/Kruskal-Wallis test.
Rituximab quantitation
Rituximab levels were measured by ELISA30 on pretransplantation and day 56 serum samples.
GVHD grading and therapy
Analysis of donor chimerism and disease responses
Chimerism analyses were performed on whole blood and PBMCs separated into CD3, CD19, CD15, and CD56 populations using Dynal-coated immunomagnetic beads. Donor engraftment used DNA genotyping of simple sequence-length polymorphic markers that encode short tandem repeats, as described previously.33 Chimerism analyses were performed at 30, 56, and 90 days after transplantation. Full donor chimerism was defined as ≥ 95% donor peripheral blood CD3+ T cells.
Disease responses for CLL were assessed using the updated National Cancer Institute Working Group Criteria.34 Minimal residual disease (MRD) was monitored by quantitative allele-specific oligonucleotide-IgH PCR35,36 for those CLL patients in whom a clone was detected before transplantation. For MCL patients, disease response was assessed using the Revised Response Criteria for Malignant Lymphoma.37
Disease progression was managed with immunosuppression withdrawal and donor lymphocyte infusion (DLI).
H-Y Ab assays
Plasma samples from the 10 male patients with female grafts treated on the trial were tested by ELISA for Abs to 5 H-Y antigens.10,11 Samples were diluted 1:50 and quantified for H-Y–specific IgG by ELISA with absorption at 550-450 nm in optical density units. An optical density of 0.1 was established as the cutoff value for positive Ab reactivity for all antigens.10,11 For comparison, plasma samples were similarly tested for H-Y Abs from 25 male patients with female donors undergoing concurrent TLI-ATG alloHCT who had never received rituximab and who had a 1-year posttransplantation blood sample collected.
Statistical analysis
Overall survival and freedom from progression were estimated by the Kaplan-Meier method.38 Cumulative incidence estimates were calculated for aGVHD and cGVHD, relapse, and nonrelapse mortality. Death and relapse were treated as competing events in analyses of GVHD. Factors considered in the univariate analyses of relapse/progression and cGVHD included pretransplantation rituximab level, graft CD34 dose, graft CD3 and CD19 composition, absolute CD19 B-cell count at day 56, donor T-cell (CD3) chimerism at 30 and 90 days, MCL versus CLL, matched related donor versus unrelated donor, and prior aGVHD. All P values were derived from log-rank statistics.
Results
Patient characteristics
The high-risk features of the 22 CLL patients are described in Table 1. Fifteen of 22 patients (68%) were fludarabine refractory, and 18 of 20 (90%) had an unmutated VH-IgG. Eight of 16 CLL patients (50%) had a 17p deletion and 5 of 16 (31%) had an 11q deletion. Only 4 CLL patients were in clinical remission before transplantation.34 The MCL group characteristics are shown in Table 2.
Hematopoietic recovery
The median CD34-cell dose for the 35 patients on study was 7.5 × 106/kg (range, 2.3-19.2) and the median CD3-cell dose was 2.7 × 108/kg (range, 1.6-6.1). All patients had hematopoietic recovery except for 1 patient with primary graft failure who had autologous recovery 30 days after transplantation and remains alive with CLL. Ten patients (7 with CLL and 3 with MCL) had neutropenia (< 500/μL) before graft infusion (day 0). Twenty-five patients (71%) never reached a platelet nadir below 20 000/μL.
B-cell reconstitution and rituximab infusion
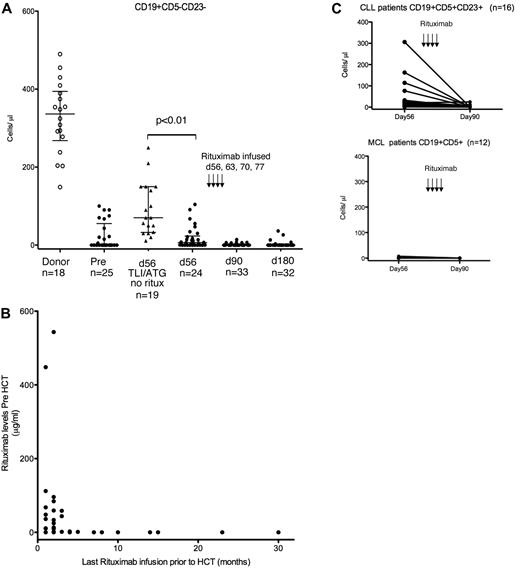
Our prior studies showed that donor B-cell engraftment by donor chimerism analysis occurred approximately 60 days after TLI-ATG transplantation.25 Therefore, in the present study, rituximab infusion on days 56, 63, 70, and 77 was timed to coincide with this donor B-cell recovery. We quantified blood CD19+ B cells present before rituximab infusion on day 56, and confirmed rituximab-mediated B-cell depletion at 90 and 180 days after alloHCT by FACS analysis. CD19+CD5− cells were reported to exclude persistent CD5+ malignant B cells from donor B-cell reconstitution. Figure 2A shows that whereas normal donors have 175-500 CD19+CD5− cells/μL of blood, CLL and MCL patients had significantly fewer absolute donor B cells at day 56 (median, 7 cells/μL), with 13 patients having no detectable CD19+CD5− B cells. In comparison, the B-cell recovery at day 56 in 19 TLI-ATG patients who had never received rituximab (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) was significantly higher (median, 70 CD19+CD5− cells/μL; P < .01). We presumed that this reduced donor B-cell recovery at day 56 and the similarly low number of B cells observed before alloHCT in the CLL/MCL group resulted from extensive rituximab therapy before transplantation that persisted in the patient's blood after transplantation, depleting donor B cells. To test this directly, pretransplantation and day-56 serum samples were measured for rituximab by ELISA.30 All patients who received rituximab within 6 months of transplantation had detectable drug before transplantation. The patients with detectable rituximab before transplantation had fewer day-56 donor B cells than those who had no detectable rituximab at the time of transplantation (Figure 2B). Alemtuzumab treatment before transplantation also appeared to impair day-56 donor B-cell engraftment (supplemental Figure 1).
B-cell reconstitution and rituximab quantification. (A) Blood CD19+CD5−CD23− B-cell quantification. Blood samples collected from HLA identical donors (○), and study subjects (●) before TLI-ATG conditioning and 56 days after alloHCT were FACS analyzed to quantify CD19+ B cells. Recipient MCL and CLL cancer cells were excluded from this donor B-cell quantification by excluding CD5+ or CD23+ cells. Blood CD19+CD5−CD23− B-cell quantification performed 90 and 180 days after alloHCT showed limited donor B-cell recovery after rituximab. For comparison, donor B-cell recovery 56 days after TLI-ATG alloHCT was determined in 19 patients who never received rituximab (▴) to control for passive transmission of rituximab infused before HCT. (B) Rituximab infused 6 months or less before HCT is detected by ELISA at transplantation. Blood collected immediately before conditioning was measured by ELISA for rituximab concentration (μg/mL; y-axis). Each pre-HCT rituximab level was related to the number of months since their last rituximab infusion (x-axis). (C) CLL decreases following rituximab infusion 56 days after alloHCT. Immunophenotyping detected persistent CD19+CD5+CD23+ CLL cells 56 days after HCT in most CLL patients. Ten of 16 (63%) patients had > 10 CD19+CD5+CD23+ cells/μL of peripheral blood. After rituximab infusion, only 4 of 20 had CLL detected by flow cytometry on day 90. Twelve MCL patients had negligible CD19+CD5+CD23− cells in the blood measured on both days 56 and 90.
B-cell reconstitution and rituximab quantification. (A) Blood CD19+CD5−CD23− B-cell quantification. Blood samples collected from HLA identical donors (○), and study subjects (●) before TLI-ATG conditioning and 56 days after alloHCT were FACS analyzed to quantify CD19+ B cells. Recipient MCL and CLL cancer cells were excluded from this donor B-cell quantification by excluding CD5+ or CD23+ cells. Blood CD19+CD5−CD23− B-cell quantification performed 90 and 180 days after alloHCT showed limited donor B-cell recovery after rituximab. For comparison, donor B-cell recovery 56 days after TLI-ATG alloHCT was determined in 19 patients who never received rituximab (▴) to control for passive transmission of rituximab infused before HCT. (B) Rituximab infused 6 months or less before HCT is detected by ELISA at transplantation. Blood collected immediately before conditioning was measured by ELISA for rituximab concentration (μg/mL; y-axis). Each pre-HCT rituximab level was related to the number of months since their last rituximab infusion (x-axis). (C) CLL decreases following rituximab infusion 56 days after alloHCT. Immunophenotyping detected persistent CD19+CD5+CD23+ CLL cells 56 days after HCT in most CLL patients. Ten of 16 (63%) patients had > 10 CD19+CD5+CD23+ cells/μL of peripheral blood. After rituximab infusion, only 4 of 20 had CLL detected by flow cytometry on day 90. Twelve MCL patients had negligible CD19+CD5+CD23− cells in the blood measured on both days 56 and 90.
CLL decreased after day-56 rituximab
Immunophenotyping detected persistent CLL 56 days after transplantation in most CLL patients; 10 of 16 (63%) patients had > 10 CD19+CD5+CD23+ cells/μL in the peripheral blood (Figure 2C). However, after the completion of 4 weekly rituximab doses, only 6 of 20 (30%) CLL patients had any detectable CLL cells by flow cytometry, suggesting that rituximab provided an antitumor benefit. There was negligible MCL detected by flow cytometry 56 days after HCT or on day 90 after rituximab (Figure 2C).
Prophylactic rituximab infusion 2 months after transplantation is associated with low cGVHD
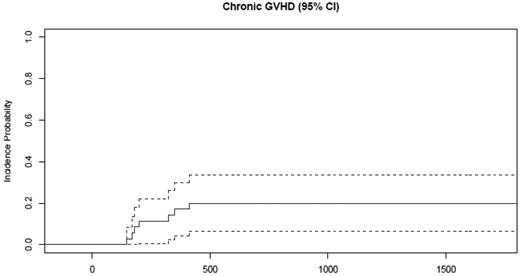
Grade II-IV aGVHD was observed in 2 of 35 patients (6%; Table 3). The 2 affected patients had grade 2 skin involvement that resolved with a short course of prednisone. cGVHD developed in 7 of the 35 patients, including the 2 patients with prior aGVHD (Table 3). The cumulative incidence of cGVHD at 4 years was 20% (95% confidence interval [CI], 634%; Figure 3).
aGVHD and cGVHD manifestations
| SPN . | RD/URD . | CD34 cell dose/kg, × 106 . | Full donor chimerism* . | aGVHD onset . | aGVHD grade . | aGVHD organs involved . | cGVHD onset . | cGVHD severity . | cGVHD organs involved . | Days on prednisone . |
|---|---|---|---|---|---|---|---|---|---|---|
| 3431 | RD | 7.5 | D28 | D322 | Moderate | Mouth, liver, fasciitis | 241 | |||
| 3489 | RD | 10.4 | D90 | D70 | 2 | Skin | D350 | Mild | Skin, liver | 133 |
| 3567 | RD | 4.9 | D180 | D180 | Moderate | Mouth, liver, joints | 378 | |||
| 3723 | URD | 4.8 | D28 | D25 | 2 | Skin | D146 | Severe | Skin, gut, liver | Never stopped, death (562 days on steroids) |
| 3879 | RD | 6.2 | D90 | D413 | Moderate | Skin, fasciitis | Never stopped, death (425 days on steroids) | |||
| 3934 | RD | 5.6 | D28 | D168 | Moderate | Mouth, gut, skin | 644 | |||
| 3969 | RD | 6.6 | D180 | D200 | Mild | Skin, gut | 471 |
| SPN . | RD/URD . | CD34 cell dose/kg, × 106 . | Full donor chimerism* . | aGVHD onset . | aGVHD grade . | aGVHD organs involved . | cGVHD onset . | cGVHD severity . | cGVHD organs involved . | Days on prednisone . |
|---|---|---|---|---|---|---|---|---|---|---|
| 3431 | RD | 7.5 | D28 | D322 | Moderate | Mouth, liver, fasciitis | 241 | |||
| 3489 | RD | 10.4 | D90 | D70 | 2 | Skin | D350 | Mild | Skin, liver | 133 |
| 3567 | RD | 4.9 | D180 | D180 | Moderate | Mouth, liver, joints | 378 | |||
| 3723 | URD | 4.8 | D28 | D25 | 2 | Skin | D146 | Severe | Skin, gut, liver | Never stopped, death (562 days on steroids) |
| 3879 | RD | 6.2 | D90 | D413 | Moderate | Skin, fasciitis | Never stopped, death (425 days on steroids) | |||
| 3934 | RD | 5.6 | D28 | D168 | Moderate | Mouth, gut, skin | 644 | |||
| 3969 | RD | 6.6 | D180 | D200 | Mild | Skin, gut | 471 |
RD indicates matched related donor; URD, unrelated donor.
Peripheral blood CD3 > 95% first achieved.
Only 20% of patients receiving rituximab prophylaxis developed cGVHD. The cumulative incidence of cGVHD at 4 years was 20% (95% CI, 6%-34%).
Only 20% of patients receiving rituximab prophylaxis developed cGVHD. The cumulative incidence of cGVHD at 4 years was 20% (95% CI, 6%-34%).
The median time to onset of cGVHD was 200 days (range, 146-413). Using National Institutes of Health consensus guidelines for scoring cGVHD severity,32 2 patients had mild, 4 had moderate, and 1 had severe cGVHD. All 5 surviving patients were successfully tapered off of prednisone and their disease remains quiescent. Two patients (Stanford patient number [SPN] 3723 and SPN 3879) died from complications of infection after cGVHD, 1 with H1N1 infection.
Rituximab prophylaxis prevents allogeneic Ab development
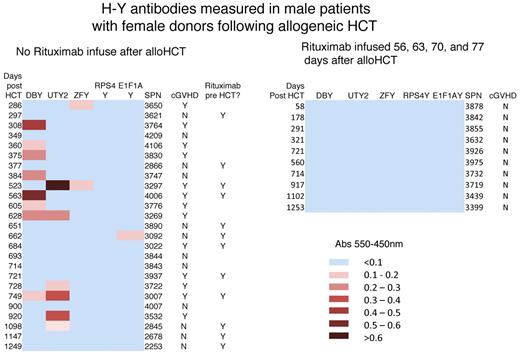
We hypothesized that prophylactic rituximab infusion after alloHCT would deplete alloreactive B cells and alloantibodies. To measure B-cell alloimmunity, we evaluated H-Y Ab development in male HCT patients with female donors (F → M HCT). The frequency and intensity of Ab development to 5 H-Y antigens are shown by heat-map presentation (Figure 4). None of the 10 F → M HCT patients who received prophylactic rituximab in the present study developed H-Y Abs and none developed cGVHD. As a comparison, 25 F → M HCT patients who underwent the same TLI-ATG conditioning during this time period without posttransplantation rituximab were evaluated, and 14 of the 25 (56%) developed H-Y Abs, with 13 of the 25 (52%) patients developing cGVHD. Supplemental Table 2 shows the concordance of H-Y Ab development in association with cGVHD (P < .005), which is consistent with what we have reported previously.11 Whereas rituximab infusion 2 months after F → M HCT prevented H-Y Ab development (P = .01), rituximab infusion before F → M HCT did not. Twelve of the 25 comparison patients had received rituximab within 6 months before alloHCT; 5 of 12 (42%) developed H-Y Abs and 3 of 5 developed cGVHD. The results of the present study confirm that H-Y Ab develops in association with cGVHD after TLI-ATG conditioning, and rituximab infusion after this RIC regimen has achieved our goal of reducing B-cell alloimmunity with no H-Y Ab development. We believe, but have not yet proven, that reduced B-cell alloimmunity may decrease cGVHD incidence.
Rituximab prophylaxis prevents H-Y allogeneic Ab development. Blood IgGs against 5 H-Y antigens were determined by ELISA in 25 F → M HCT patients who never received rituximab after TLI-ATG (left panel) and 10 study patients treated with rituximab on days 56, 63, 70, and 77. These heat maps show that no alloreactive H-Y Abs developed in study patients receiving rituximab 2 months after TLI-ATG alloHCT, whereas 1 or more H-Y Abs developed in 56% (14 of 25) of patients receiving TLI-ATG without posttransplantation rituximab. Considering all patients who survived 9 months, rituximab prophylaxis prevents H-Y Ab development (P = .01).
Rituximab prophylaxis prevents H-Y allogeneic Ab development. Blood IgGs against 5 H-Y antigens were determined by ELISA in 25 F → M HCT patients who never received rituximab after TLI-ATG (left panel) and 10 study patients treated with rituximab on days 56, 63, 70, and 77. These heat maps show that no alloreactive H-Y Abs developed in study patients receiving rituximab 2 months after TLI-ATG alloHCT, whereas 1 or more H-Y Abs developed in 56% (14 of 25) of patients receiving TLI-ATG without posttransplantation rituximab. Considering all patients who survived 9 months, rituximab prophylaxis prevents H-Y Ab development (P = .01).
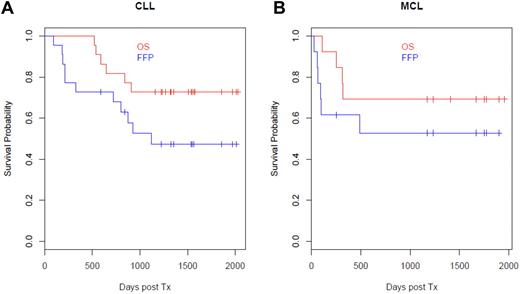
Allogeneic HCT with posttransplantation rituximab maintains disease control
The median clinical follow-up time for our patients is 4 years. For the CLL patients, the 4-year overall survival was 73% (95% CI, 57%-94%) and 4-year freedom from progression was 47% (95% CI, 30%-75%). For the MCL patients, the 4-year overall survival was 69% (95% CI, 48%-99%) and 4-year freedom from progression was 53% (95% CI, 31%-89%; Figure 5A-B). We further assessed CLL disease by measuring MRD using allele-specific oligonucleotide quantitative PCR assays in 19 of the 22 patients who had measurable clones. Ten of 19 (53%) of the VH-IgG–unmutated CLL patients achieved MRD negativity by 1 year after HCT. Of the 10 patients who were MRD− at 1 year after transplantation, 8 (80%) are still alive and in remission.
Overall survival after rituximab prophylaxis exceeds 70%. For the CLL patients, the 4-year overall survival was 73% (95% CI, 57%-94%) and freedom from progression was 47% (95% CI, 30%-75%). For the MCL patients, the 4-year overall survival was 69% (95% CI, 48%-99%) and freedom from progression was 53% (95% CI, 31%-89%).
Overall survival after rituximab prophylaxis exceeds 70%. For the CLL patients, the 4-year overall survival was 73% (95% CI, 57%-94%) and freedom from progression was 47% (95% CI, 30%-75%). For the MCL patients, the 4-year overall survival was 69% (95% CI, 48%-99%) and freedom from progression was 53% (95% CI, 31%-89%).
Twelve patients (11 CLL and 1 MCL) received DLI after disease progression. Six of the CLL patients achieved MRD negativity and remain in clinical remission. The 1 MCL patient who has received 3 DLIs is alive with persistent mixed CD3 chimerism and no GVHD. Ten trial patients have died: 7 from relapse, 2 from infection and cGVHD, and 1 from DLI-related GVHD.
Predictors for cGVHD, disease progression, and overall survival: univariate analysis
Graft CD34 cell dose, CD3 and CD19 cell composition, absolute CD19 B-cell count at day 56, donor T-cell (CD3) chimerism at days 30 and 90, disease type (MCL vs CLL), matched related versus unrelated donor, prior aGVHD, and pretransplantation rituximab level were explored in univariate analysis. Although none of the variables was significant for the development of cGVHD, relapse/progression, or overall survival, there was a trend toward an association of relapse with CD3 chimerism at day 90 after transplantation (P = .07) and a trend toward more cGVHD (P = .056) in patients with no detectable rituximab at the time of transplantation.
Rituximab infusion after transplantation is well tolerated with a low infection rate
Nonrelapse mortality at day 100 and 1 year was 0% and 3%, respectively. No rituximab-related infusional toxicities occurred. However, rituximab-related neutropenia (defined as any absolute neutrophil count < 500/μL detected after the day-56 rituximab infusion) developed in 14 (8 CLL and 6 MCL) of the 35 patients (40%). Supplemental Figure 2 illustrates when neutropenia was detected in patients on the trial. Fourteen patients were neutropenic 1 or more times after the day-56 rituximab infusion, but 10 of these 14 were neutropenic before day-56 rituximab as well. Rituximab-related neutropenia was treated with G-CSF 5 μg/kg/d for an average of 5 doses under the discretion of the treating physician. In general, the neutropenia resolved without infectious complications. Only 1 patient was hospitalized with infection and neutropenia after day-56 rituximab. The average duration of neutropenia was 2 weeks to 2 months. One patient (SPN 3489) had persistent neutropenia and required intermittent G-CSF support for 1.5 years until resolution. This patient's MCL disease remains in remission with normal blood counts 5 years after HCT. Univariate analysis of rituximab-related neutropenia was not significant for survival outcome.
Documented infections
CMV reactivation occurred in 12 of 21 patients at risk (57%) and is detailed in supplemental Figure 3. The median time to CMV reactivation was 10 days after transplantation (range, −4 to 83), which was before rituximab infusion, suggesting that TLI-ATG conditioning alone leads to early CMV reactivation, as has been found previously.39 Only 1 patient (SPN 3975) developed pulmonary CMV disease which resolved with intravenous ganciclovir and immunoglobulin. Overall, 15 of the 35 patients (43%) had severe grade 3 infectious complications in the first year after transplantation, none of which were fatal. Among the 15 patients, there were 5 bacterial, 9 viral, and 5 fungal infections, with 3 patients having a combination. IgG concentrations remained unchanged during the first year.
Discussion
In this first prospective study of rituximab as cGVHD prophylaxis after alloSCT, we show that rituximab infusion 56, 63, 70, and 77 days after alloHCT is well tolerated and reduces B-cell allogeneic immunity. The results of the present study confirmed that H-Y Abs develop in association with cGVHD after TLI-ATG conditioning, and rituximab infusion after this RIC regimen has achieved our goal of reducing B-cell alloimmunity with no H-Y Ab development. We believe, but have not yet proven, that reduced B-cell alloimmunity may decrease cGVHD incidence. Our institution's standard TLI-ATG RIC regimen has already reported a low incidence of aGVHD of 2%-10%,25,26 and the results of the present study support this finding. The addition of rituximab to the TLI-ATG regimen was associated with a low cGVHD cumulative incidence of 20% (95% CI, 6%-34%). We recognize that our group has previously reported a low cGVHD cumulative incidence of 27% for TLI-ATG without rituximab,26 however, it should be noted that 60% of the patients reported on previously had a diagnosis of non-Hodgkin lymphoma and had received prior rituximab. The reduction of allogeneic Ab against H-Y antigens in the F → M HCT patients was correlated with the clinical reduction in cGVHD, providing biologic support for rituximab prophylaxis decreasing B-cell alloimmunity with low cGVHD incidence.
Investigators at the Dana-Farber Cancer Institute are studying an alternative rituximab prophylaxis dose schedule infusing 375 mg/m2 at 3, 6, 9, and 12 months.40 The 1-year cumulative incidence of cGVHD was 40% compared with 65% for their historical controls, adding support to the hypothesis that B-cell depletion with rituximab may be effective in preventing or controlling cGVHD.40 Reduction of extensive cGVHD has also been observed in patients treated with rituximab within 6 months before reduced intensity transplantation in a retrospective series.23 Our prospective study took this observation a step further by quantifying rituximab levels before transplantation. Patients with no detectable rituximab at the time of transplantation appeared to have a trend toward more cGVHD (P = .056); however, these results must be interpreted with caution because of the small numbers of patients.
The design of our study developed from our hypothesis that prophylactic anti–B-cell therapy delivered 2 months after transplantation would decrease allogeneic donor B-cell immunity, and possibly the incidence of cGVHD. In normal B-cell development, CD20, the target of rituximab, is first expressed after heavy- and light-chain gene rearrangement, and CD20 is no longer expressed on the majority of mature plasma cells. Therefore, B-cell depletion 2 months after alloSCT was expected to: (1) deplete donor-derived alloreactive B cells, (2) permit reconstitution of newly generated tolerant B cells derived from donor hematopoietic stem cells, and (3) maintain CD20− plasma cells and immunoglobulin levels to protect against infection. Using H-Y Ab development as a biomarker for alloreactivity, we demonstrated directly that rituximab infusion 2 months after alloSCT depleted B cells that are alloreactive for the recipient. None of the F → M HCT patients receiving rituximab 2 months after transplantation developed H-Y Abs or cGVHD, In contrast, 14 of 25 (56%) F → M HCT patients who never received rituximab after alloHCT did develop H-Y Ab in strong association with cGVHD (P < .005), further supporting our hypothesis that alloreactive B cells play a central role in cGVHD pathogenesis. The prevention of H-Y Ab development and cGVHD is in agreement with a recent murine study showing that donor B-cell alloantibody deposition and germinal center B-cell infiltration affected cGVHD liver and lung tissue.12 Further, Srinivasan et al demonstrated that BM grafts obtained from mice genetically incapable of somatic hypermutation or undergoing IgG isotype switching significantly decreases cGVHD development.12 Consistent with this murine study, our low cGVHD incidence may result from rituximab prophylaxis 2 months after alloHCT decreasing/preventing allogeneic Ab development.
Another interesting observation of our study was that rituximab within 6 months before alloHCT did not prevent H-Y Ab development, although our rituximab prophylaxis 2 months after alloHCT did. Patients receiving rituximab within 6 months before alloHCT did have decreased early donor B-cell engraftment, but this pre-HCT rituximab did not prevent allogeneic H-Y Ab development. In contrast, rituximab prophylaxis 2 months after alloHCT decreased, and possibly prevented, allogeneic H-Y Ab development with low cGVHD incidence. With the recent suggestion that allogeneic Abs are pathogenic for cGVHD development,12 the prevention of allogeneic Ab development may be a worthwhile pharmacodynamic goal for cGVHD prevention.
Extensive BM immunophenotyping studies of patients in the present study confirmed that rituximab treatment depleted adoptively transferred immunoglobulin-expressing mature donor B cells. After rituximab prophylaxis, B cells developed from donor hematopoietic stem cells and lymphoid progenitor cells by 1 year after transplantation (B.S., manuscript in preparation). Long-lived CD20− plasma cells were unaffected by rituximab in the TLI-ATG transplantation, thereby explaining the relatively unchanged IgG concentrations in our study patients.
Our study of prophylactic rituximab was restricted to patients with CD20+ malignancies, so any loss of potential graft-versus-tumor (GVT) effects by allogeneic B-cell depletion would be offset by the direct antitumor effects of the mAb. We recognize that donor B-cell reconstitution could be affected by a patient's pretransplantation rituximab therapy. Compared with the patients with myeloid malignancies undergoing the same TLI-ATG conditioning but never receiving rituximab, the CLL and MCL patients reconstituted fewer CD19+ B cells at day 56 after HCT (Figure 2A). Therefore, future studies of posttransplantation rituximab in patients with non–B-cell malignancies will be required to further elucidate the potential impact of prophylactic anti–B cell–directed therapy on cGVHD incidence and severity.
The survival and freedom from progression at 4 years for related and unrelated donors in high-risk CLL patients with RIC is comparable to other published studies from Sorror et al41 and Khouri et al,21 albeit with low transplantation-related risk. Moreover, MRD durability was achieved with associated GVT responses in 53% of patients, with low incidence and severity of cGVHD. Only 4 of the 10 MRD− patients developed cGVHD, and all surviving patients have been tapered off of immune-suppression medications. The present results compare favorably with those of the CLL3X trial performed by the German CLL Study Group.42 With MCL, the patients were more varied between CR and PR status, but overall survival and freedom from progression were comparable to prior reports.43 The nonrelapse mortality of only 3% at 1 year and the low incidence of aGVHD (6%) and cGVHD (20%) compare favorably.
Neutropenia after rituximab infusion was observed in 40% of our patients and should be a caution when using rituximab after transplantation. Neutropenia has been reported in other rituximab studies, including some from our own institution.44-46 Often, neutropenia will resolve with short-course G-CSF support without serious infectious complications. Whereas infections were generally not increased in our cohort, one important consideration in using posttransplantation rituximab is the impaired ability to mount a humoral immune response against neoantigens such as H1N1, as was observed in 1 of the fatalities among the cGVHD patients in the present study. Long-term clinical follow-up and immunologic reconstitution studies are critical to fully evaluate rituximab prophylaxis after alloHCT.
Because this trial used a nonmyeloablative regimen, mixed chimerism was expected, and provision was made for patients with progressive disease after transplantation to receive dose-escalated DLI. Thirteen patients received DLI for disease progression and 8 of the patients are alive. Mixed chimerism at day 90 had a trend of association with an increased relapse rate in univariate analysis (P = .07) Rituximab was not felt to have an effect on mixed chimerism in this study because the rate was comparable to that seen in our standard TLI-ATG regimen.26 There was also the observation that 53% of the CLL patients achieved CR to the level of molecular remission from PR without DLI, which must be attributed to potent GVT effects, even with the minimal-intensity conditioning of TLI-ATG.
In conclusion, the results of the present study show that rituximab prophylaxis after RIC transplantation is feasible and associated with a low cGVHD incidence while maintaining disease control. Furthermore, posttransplantation rituximab depletes alloreactive B cells, as shown by H-Y Ab testing. In this first prophylactic study of rituximab after alloHCT, we provide important insights into donor B-cell depletion by applying rituximab pharmacokinetic measurements, B-cell phenotyping, and allogeneic Ab assessment of 35 patients receiving day 56, 63, 70, and 77 rituximab. The optimal timing of rituximab prophylaxis may be suggested by comparing clinical outcomes using 3 dosing schedules: (1) rituximab-included conditioning,21 (2) rituximab depletion of alloreactive B cells 2 months after alloHCT, or (3) sustained and repeated B-cell depletion infusing rituximab every 3 months through 1 year after alloHCT.40 Correlative laboratory studies of rituximab pharmacokinetics, donor B-cell reconstitution, and both allogeneic and protective anti-infection Abs will aid the design of a well-powered and informative randomized trial to test the efficacy and safety of rituximab prophylaxis for cGVHD prevention.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this study and all of the BM transplantation nurses, patient coordinators, and staff at Stanford University Medical Center who made this work possible; David Maloney for providing quantitative rituximab measurements; and Renee Letsinger and Linda Elder for data management assistance.
This work was supported by a Leukemia & Lymphoma Society Translational Research Award (R618-09), the National Cancer Institute (P01 CA049605), and the Stanford University Cancer Institute.
National Institutes of Health
Authorship
Contribution: S.A., B.S., and D.B.M. designed the experiments, analyzed the data, and wrote the manuscript; B.N. analyzed the data; G.L.C. and J.R. performed the experiments; C.D.J. and J.L.Z. designed the experiments and analyzed the data; S.A., R.L., J.A.S., L.J.J., G.G.L., W.-K.W., J.E.B., J.S., J.B., R.S.N., and D.B.M. treated patients on protocol; and all authors revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David B. Miklos, MD, PhD, Department of Medicine, Division of Blood and Marrow Transplantation, Stanford University School of Medicine, 269 West Campus Dr, CCSR 2205, MS5642, Stanford, CA 94305; e-mail: dmiklos@stanford.edu.