Abstract
POEMS syndrome is a paraneoplastic syndrome whose acronym stands for less than half of the defining features of the disease, that is, polyradiculoneuropathy, organomegaly, potentially including coexisting Castleman disease, endocrinopathy, monoclonal plasma cell neoplasm, and skin changes. The other important features include papilledema, extravascular volume overload, sclerotic bone lesions, thrombocytosis, elevated VEGF, and abnormal pulmonary function. The diagnosis is based on having both the polyradiculoneuropathy and the monoclonal plasma cell disorder, and at least 1 of the other 3 major criteria (Castleman disease, sclerotic bone lesions, or elevated VEGF) and at least one minor criterion. The diagnosis is often delayed with intervening incorrect diagnoses of chronic inflammatory demyelinating polyradiculoneuropathy, myeloproliferative disorder, and monoclonal gammopathy of undetermined significance. Prompt treatment directed at the underlying plasma cell clone produces dramatic responses in the majority of patients. Although there are no randomized clinical trial data to direct best therapy, for patients with disseminated disease, high-dose chemotherapy with peripheral blood transplantation has yielded durable benefit, whereas radiation therapy is typically effective for patients with a more localized presentation. More universal recognition of and more scientific inquiry into the underpinnings of the disease will provide direction toward the best treatment strategies in the future.
Introduction
POEMS syndrome,1 also known as osteosclerotic myeloma, Takatsuki syndrome,2 and Crow-Fukase syndrome,3,4 is a rare paraneoplastic syndrome resulting from an underlying plasma cell disorder. There acronym POEMS refers to several, but not all, of the features of the syndrome: polyradiculoneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes. There are 3 important points that relate to this memorable acronym. First, not all of the features within the acronym are required to make the diagnosis (Table 1). Second, there are other important features not included in the POEMS acronym, including papilledema, extravascular volume overload, sclerotic bone lesions, thrombocytosis/erythrocytosis, elevated VEGF levels, abnormal pulmonary function tests, and a predisposition toward thrombosis. Lastly, there is a Castleman disease variant of POEMS syndrome that may not be associated with a clonal plasma cell disorder.5,6 Table 1 outlines the range of expected frequencies of each of the features based on the largest published series.2,7-11
The pathogenesis of the syndrome is not well understood. To date, VEGF is the cytokine that correlates best with disease activity,12-20 although it may not be the driving force of the disease based on the mixed results seen with anti-VEGF therapy.5,21-29 VEGF, which is expressed by osteoblasts, macrophages, tumor cells30 (including plasma cells),31,32 and megakaryocytes/platelets,33 is known to target endothelial cells, induce a rapid and reversible increase in vascular permeability, and be important in angiogenesis. Both IL-1β and IL-6 have been shown to stimulate VEGF production.30 Little is known about the plasma cells in POEMS syndrome, except that more than 95% of the time they are λ light chain restricted with restricted immunoglobulin light chain variable gene usage (IGLV1).5
Diagnosis
The diagnosis is made based on a composite of clinical and laboratory features. Most notably, the constellation of peripheral neuropathy (especially demyelinating) and any of the following should elicit an in-depth search for POEMS syndrome: monoclonal protein (especially when λ restricted), thrombocytosis, anasarca, or papilledema. The requirements set forth in Table 1 are designed to retain both sensitivity and specificity, potentially erring on the side of specificity. Making the diagnosis can be a challenge, but a good history and physical examination followed by appropriate testing (most notably radiographic assessment of bones,34 measurement of VEGF,14,18,35,36 and careful analysis of a bone marrow biopsy37 ) can differentiate this syndrome from other conditions, such as chronic inflammatory polyradiculoneuropathy (CIDP), monoclonal gammopathy of undetermined significance, neuropathy, and immunoglobulin light chain amyloid neuropathy. Other important baseline tests include: complete blood count, creatinine, creatinine clearance, serum and urine protein electrophoresis with immunofixation, serum immunoglobulin free light chains, thyroid-stimulating hormone, prolactin, parathyroid hormone, testosterone (or estradiol), luteinizing hormone, follicle-stimulating hormone, plasma VEGF, bone marrow aspirate, and biopsy with immunohistochemical stains to document λ-restricted plasma cells, pulmonary function tests, electromyelogram with nerve conduction studies, and PET/CT, with special attention to the bone windows of the CT. A biopsy of a sclerotic lesion is not imperative in the proper clinical context.
Criteria for the diagnosis of POEMS syndrome
| . | Criteria/other symptoms and signs . | Affected, %* . |
|---|---|---|
| Mandatory major criteria (both required) | 1. Polyradiculoneuropathy (typically demyelinating) | 100 |
| 2. Monoclonal plasma cell disorder (almost always λ) | 100† | |
| Other major criteria (1 required) | 3. Castleman disease‡ | 11-25 |
| 4. Sclerotic bone lesions | 27-97 | |
| 5. VEGF elevation§ | ||
| Minor criteria (1 required) | 6. Organomegaly (splenomegaly, hepatomegaly, or lymphadenopathy) | 45-85 |
| 7. Extravascular volume overload (edema, pleural effusion, or ascites) | 29-87 | |
| 8. Endocrinopathy (adrenal, thyroid,‖ pituitary, gonadal, parathyroid, pancreatic‖) | 67-84 | |
| 9. Skin changes (hyperpigmentation, hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, white nails) | 68-89 | |
| 10. Papilledema | 29-64 | |
| 11. Thrombocytosis/polycythemia¶ | 54-88 | |
| Other symptoms and signs | Clubbing, weight loss, hyperhidrosis, pulmonary hypertension/restrictive lung disease, thrombotic diatheses, diarrhea, low vitamin B12 values |
| . | Criteria/other symptoms and signs . | Affected, %* . |
|---|---|---|
| Mandatory major criteria (both required) | 1. Polyradiculoneuropathy (typically demyelinating) | 100 |
| 2. Monoclonal plasma cell disorder (almost always λ) | 100† | |
| Other major criteria (1 required) | 3. Castleman disease‡ | 11-25 |
| 4. Sclerotic bone lesions | 27-97 | |
| 5. VEGF elevation§ | ||
| Minor criteria (1 required) | 6. Organomegaly (splenomegaly, hepatomegaly, or lymphadenopathy) | 45-85 |
| 7. Extravascular volume overload (edema, pleural effusion, or ascites) | 29-87 | |
| 8. Endocrinopathy (adrenal, thyroid,‖ pituitary, gonadal, parathyroid, pancreatic‖) | 67-84 | |
| 9. Skin changes (hyperpigmentation, hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, white nails) | 68-89 | |
| 10. Papilledema | 29-64 | |
| 11. Thrombocytosis/polycythemia¶ | 54-88 | |
| Other symptoms and signs | Clubbing, weight loss, hyperhidrosis, pulmonary hypertension/restrictive lung disease, thrombotic diatheses, diarrhea, low vitamin B12 values |
The diagnosis of POEMS syndrome is confirmed when both of the mandatory major criteria, 1 of the 3 other major criteria, and 1 of the 6 minor criteria are present.
Takasuki and Nakanishi series are included, even though only 75% of patients had a documented plasma cell disorder. Because these are among the earliest series describing the syndrome, they are included.
There is a Castleman disease variant of POEMS syndrome that occurs without evidence of a clonal plasma cell disorder that is not accounted for in this table. This entity should be considered separately.
A plasma VEGF level of 200 pg/mL is 95% specific and 68% sensitive for a POEMS syndrome.28
Because of the high prevalence of diabetes mellitus and thyroid abnormalities, this diagnosis alone is not sufficient to meet this minor criterion.
Approximately 50% of patients will have bone marrow changes that distinguish it from a typical monoclonal gammopathy of undetermined significance or myeloma bone marrow.37
How I treat POEMS syndrome
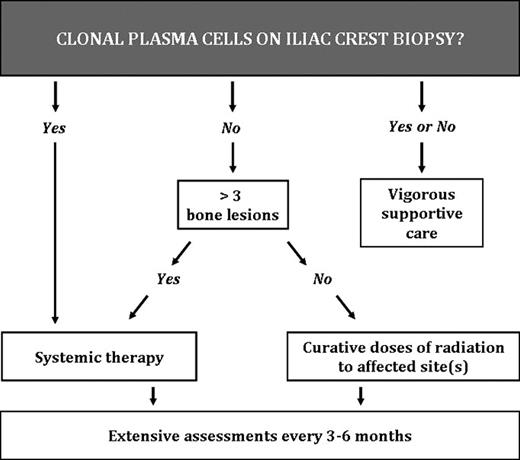
Treatment of the POEMS syndrome can be broken down into 2 major categories: targeting the underlying clone and targeting the rest of the syndrome. Both are important to achieve the best outcomes. An algorithm for choosing therapy is shown in Figure 1. Monitoring for hematologic response is a challenge because the serum M-protein is typically small making standard multiple myeloma response criteria inapplicable in most cases. In addition, patients can derive substantial clinical benefit, even in the absence of an M-protein response.38,39 In addition, although the immunoglobulin free light chains are elevated in 90% of POEMS patients, the ratio is normal in all but 18%,40 making the test of limited value for patients with POEMS syndrome. Following VEGF is rather straightforward, but one must be cognizant that spurious VEGF levels do occur. Following the other features of the syndrome is also challenging because there are more than 2 dozen parameters that could be assessed in a given patient with POEMS syndrome, given the multisystem nature of the disease.41 I focus on features that were present at baseline and follow each abnormality at each visit or at every other visit.
Targeting the clone
If there is no involvement of the bone marrow on iliac crest biopsy as documented with immunohistochemical stains, but only 1 to 3 bone lesions, radiation is the preferred strategy. One-third of patients do not have clonal plasma cells on their iliac crest biopsy. These are the patients who present with a solitary or “multiple solitary plasmacytomas.” The other two-thirds of patients have a low burden of clonal plasma cells in their bone marrow, often akin to a “plasmacytoma plus.”42 For this first group, radiation doses of 40 Gy are most standard because the goal is potential cure rather than mere palliation.9,43-46 If a patient is rapidly deteriorating, simultaneous use of corticosteroids (eg, dexamethasone 40 mg days 1-4 every 2 weeks or daily prednisone at ∼ 1 mg/kg) is reasonable as adjuvant therapy. The corticosteroids can be tapered over the ensuing months, but be vigilant looking for signs of adrenal insufficiency, especially if they had decreased adrenal reserve at diagnosis. It is important to note that assessing the bone marrow for clonality using flow cytometry alone is insufficient given the typical pattern of a small λ-clone in an increased polyclonal background. Using κ and λ immunohistochemistry, these small clones are seen in the bone marrow both in the interstitium and more importantly rimming lymphoid aggregates, the latter of which is a pathognomonic finding.37 Not only does radiation to an isolated (or even 2 or 3) lesion(s) improve the symptoms of POEMS syndrome over the course of 3 to 36 months, but it can be curative.
If there is bone marrow involvement on iliac crest sampling, as is the case for two-thirds of cases, 91% of which are clonal λ with a median of less than 5%,37 radiation alone is less effective in these patients with disseminated bone marrow disease. The sooner this plasma cell neoplasm is addressed with systemic chemotherapy, the better will be the recovery of the patient's peripheral neuropathy. Because there are no randomized clinical trials among patients with POEMS syndrome, recommendations for systemic therapy are based on case series and anecdotes. Therapeutic strategies are borrowed from other plasma cell disorders, most notably multiple myeloma and light chain amyloidosis. Tables 2 to 5 demonstrate a summary of observed outcomes. Corticosteroids may provide symptomatic improvement, but response duration is limited.5 The most experience has been with alkylator-based therapy, either high dose with peripheral blood stem cell transplant or low dose with corticosteroids (Table 2).
Activity of radiation or alkylator-based therapies for the treatment of POEMS syndrome
| Regimen . | Outcome . |
|---|---|
| Radiation9,43-46 | ≥ 50% of patients have significant clinical improvement |
| Mel-Dex38 | 81% hematologic response rate; 100% with some neurologic improvement |
| Corticosteroids5 | ≥ 15% of patients have significant clinical improvement |
| ASCT5 | 100% of surviving patients have significant clinical improvement |
| Regimen . | Outcome . |
|---|---|
| Radiation9,43-46 | ≥ 50% of patients have significant clinical improvement |
| Mel-Dex38 | 81% hematologic response rate; 100% with some neurologic improvement |
| Corticosteroids5 | ≥ 15% of patients have significant clinical improvement |
| ASCT5 | 100% of surviving patients have significant clinical improvement |
For those patients well enough to tolerate high-dose melphalan (140 mg/m2 to 200 mg/m2) as conditioning for autologous peripheral blood stem cell transplantation (ASCT), ASCT is my first choice based on our own experience and reports from others.19,39,47-59 Because these patients have low tumor burden and their plasma cell clone is not rapidly proliferating, I do not typically recommend induction chemotherapy unless a patient is too sick to undergo ASCT immediately or if there are anticipated delays to bring him/her to ASCT. In the former instance, I typically use either cyclical cyclophosphamide (750 mg/m2 intravenously every 3 weeks) with 4 to 5 days of corticosteroid or lenalidomide (15-25 mg orally days 1-21 every 28 days) with weekly dexamethasone. Cyclophosphamide is often more expedient because there is no associated wait period or insurance hassle that is associated with lenalidomide acquisition. The competing risks of thrombosis and bleeding in a patient who is a fall risk must also be weighed when making the decision about using the lenalidomide-dexamethasone combination and when choosing whether to use either aspirin or full anticoagulation with the lenalidomide-dexamethasone combination.
With ASCT, responses are durable, but relapses have been reported.27,60 We recently reviewed our series of 59 patients with POEMS syndrome who were treated with ASCT.61 With a median follow-up of 45 months, 14 patients have relapsed or progressed. The progression-free survival was 98% and 75% at 1 and 5 years, respectively. Overall survival was 98% and 94% at 1 and 5 years, respectively. Risk factors for progression included an immunoglobulin G-λ monoclonal component and FDG-avid lesions on baseline PET scan. Tandem ASCT has been used to treat patients with POEMS,54,62 but I have not pursued this approach given the excellent results with a single ASCT. Treatment-related morbidity and mortality can be minimized by recognizing and promptly treating an engraftment-type syndrome characterized by fevers, rash, diarrhea, weight gain, and respiratory symptoms and signs that occur anytime between days 7 to 15 after stem cell infusion.39 A starting dose of prednisone ranging between 20 and 1500 mg/day has been used, but personal experience would place the daily starting dose anywhere between 1 to 2 mg/kg and 500 mg. The taper can typically start within 2 days and should be completed no sooner than 10 days. Splenomegaly was the baseline factor that best predicted for a complicated peritransplant course.39 Patients with POEMS typically have a higher than expected transfusion need, with median numbers of platelet and erythrocyte transfusions being 5 apheresis units and 6 units, respectively, and delayed neutrophil engraftment (absolute neutrophils of 500/μL at a median of 16 days, with only 10% engrafting by day 13).
In the first reported prospective clinical trial to treat POEMS syndrome,38 31 patients were treated with 12 cycles of low-dose oral melphalan and dexamethasone and found that 81% of patients had hematologic response, 100% had VEGF response, and 100% with at least some improvement in neurologic status. A limitation of this study is that follow-up is only 21 months.
Other promising treatments include lenalidomide (Table 3),63-66 thalidomide,25,67-69 and bortezomib (see Table 4),70-74 drugs all of which can have a direct antiplasma cell effect as well as anti-VEGF and anti-TNF effects. Enthusiasm for the latter 2 therapies should be tempered by the high risk of peripheral neuropathy induced by these drugs. The limited experience with lenalidomide so far has been positive. We observed dramatic improvements in a patient treated with the lenalidomide-dexamethasone combination.63 In France, 9 patients, 1 of whom was newly diagnosed, were treated with lenalidomide and dexamethasone.64 Serious side effects were noted in 3 patients with 2 hematologic toxicities and a cutaneous allergy. All evaluable for hematologic response had at least a partial hematologic response, and clinical responses, including improvement in performance status and neurologic symptoms, were documented among the 8 who had sufficient follow-up. One patient relapsed 5 months after discontinuing therapy but responded to reintroduction of the drug. In a retrospective review of 10 patients with previously treated POEMS syndrome who were treated with lenalidomide with or without dexamethasone in Spain, all patients improved.65 Median time from last therapy to lenalidomide was 4 months (range, 1-36 months). Because the prior therapy in 7 patients was intravenous immunoglobulin (IVIG) with or without prednisone, this is a relatively ineffective regimen and the benefit observed in these patients most certainly would have been the result of the lenalidomide with or without dexamethasone. Yet another case report combining lenalidomide with cyclophosphamide and dexamethasone produced dramatic improvements lasting more than 1 year after completing therapy.66 Thalidomide with dexamethasone has been reported to be effective in 12 patients,25,67-69 but it is not my first choice given the risk of introducing thalidomide-induced small fiber neuropathy on top of the demyelinating peripheral neuropathy that is the dominant symptom of the POEMS syndrome.
Activity of immune modulatory drug therapies for the treatment of POEMS syndrome
| Drug therapy . | Outcome . |
|---|---|
| Thal after MP67 | No hematologic response but improved ascites; stabilized PN, splenomegaly, pulmonary hypertension |
| Thal + Dex after CAD68 | CD/POEMS: improved ascites, effusions, pulmonary hypertension, PN, renal function, IL-6 level |
| Thal + Dex69 | 9 patients: VEGF improved in all; PN improved in 66%; stable in 33%; improved edema; no HCR |
| Thal after VAD, CTX, Bev25 | Improved cardiopulmonary status, but no improved PN and rising VEGF |
| Len + Dex63 | Improved ascites, PS, PN, VEGF, testosterone, pulmonary function tests |
| Len + Dex64 | 9 patients: all had hematologic response; clinical responses in all evaluable patients, including PS, neurologic syndrome, edema, and VEGF |
| Len ± Dex65 | 10 patients: all had prior therapy a median of 4 mo (range, 1-36 mo) before starting Len; for 7, only Pred and IVIG were used as prior therapy, making it improbable that the salutatory effect was related to anything other than Len; after a median of 7.5 cycles of Len, all had clinical improvement despite the fact that only half achieved CR; 5 were consolidated with ASCT |
| Len + CTX + Dex66 | After 4 cycles of therapy, patient was able to walk without support; and after 6 cycles, papilledema and IgA disappeared; 1 y after 9 cycles, she remains in remission |
| Drug therapy . | Outcome . |
|---|---|
| Thal after MP67 | No hematologic response but improved ascites; stabilized PN, splenomegaly, pulmonary hypertension |
| Thal + Dex after CAD68 | CD/POEMS: improved ascites, effusions, pulmonary hypertension, PN, renal function, IL-6 level |
| Thal + Dex69 | 9 patients: VEGF improved in all; PN improved in 66%; stable in 33%; improved edema; no HCR |
| Thal after VAD, CTX, Bev25 | Improved cardiopulmonary status, but no improved PN and rising VEGF |
| Len + Dex63 | Improved ascites, PS, PN, VEGF, testosterone, pulmonary function tests |
| Len + Dex64 | 9 patients: all had hematologic response; clinical responses in all evaluable patients, including PS, neurologic syndrome, edema, and VEGF |
| Len ± Dex65 | 10 patients: all had prior therapy a median of 4 mo (range, 1-36 mo) before starting Len; for 7, only Pred and IVIG were used as prior therapy, making it improbable that the salutatory effect was related to anything other than Len; after a median of 7.5 cycles of Len, all had clinical improvement despite the fact that only half achieved CR; 5 were consolidated with ASCT |
| Len + CTX + Dex66 | After 4 cycles of therapy, patient was able to walk without support; and after 6 cycles, papilledema and IgA disappeared; 1 y after 9 cycles, she remains in remission |
Thal indicates thalidomide; MP, PN, polyneuropathy; Dex, dexamethasone; CAD, cyclophosphamide, doxorubicin, and dexamethasone; CD, Castleman disease; HCR, hematologic complete response; VAD, vincristine, doxorubicin, dexamethasone; CTX, cyclophosphamide; Bev, bevacizumab; Len, lenalidomide; PS, performance status; and Pred, prednisone.
Bortezomib use has been reported in 3 patients (Table 4).70,71,74 The first report is difficult to interpret because the patient had several chemotherapies before receiving a bortezomib, doxorubicin, and dexamethasone combination.70 There was early evidence of improvement, even before starting the bortezomib regimen. The second report, using 7 cycles of bortezomib and dexamethasone, resulted in patient improvement and was more convincing.71 We recently reported an astounding clinical and biochemical response in a patient with relapsed POEMS syndrome using the combination of cyclophosphamide, bortezomib, and dexamethasone.74 Whether that response might have been achieved with cyclophosphamide and dexamethasone alone is unknown, but the patient was progressing on dexamethasone, and his anasarca began to resolve within weeks of initiating the combination.
Activity of proteosome inhibitors for the treatment of POEMS syndrome
| Treatment . | Outcome . |
|---|---|
| Bortez + AD after VAD, CTX, Mel-Pred, + AD70 | Improved M-protein, VEGF, paresthesias, splenomegaly, effusions, muscle strength, gynecomastia, and skin changes |
| Bortez + Dex71 | Improved M-protein, polyneuropathy, hepatomegaly, testosterone; no change in electromyelography |
| Bortez × 5 cycles + Thal added at cycle 6 (prior Dex and Mel-Pred)72 | Improvement of anasarca, PN, VEGF, and PET scan with Bortez alone, but Thal added because of persistent edema, M-protein, PN, and barely elevated VEGF; with Thal, disappearance of pleural effusion, ascites, and M-protein and normalization of VEGF |
| Bortez Dex*73 | Improvement by 3 cycles, but continued for 6; complete remission 4 y after completing therapy; improvement in adenopathy, pleural effusion and ascites, hepatosplenomegaly, and IL-6 |
| Bortez, CTX, Dex74 | Clinical response of anasarca within 6 wks and tolerated therapy for 18 mo achieving a nCR and a VEGF response; PN, hyperpigmentation, pulmonary hypertension improved significantly |
| Treatment . | Outcome . |
|---|---|
| Bortez + AD after VAD, CTX, Mel-Pred, + AD70 | Improved M-protein, VEGF, paresthesias, splenomegaly, effusions, muscle strength, gynecomastia, and skin changes |
| Bortez + Dex71 | Improved M-protein, polyneuropathy, hepatomegaly, testosterone; no change in electromyelography |
| Bortez × 5 cycles + Thal added at cycle 6 (prior Dex and Mel-Pred)72 | Improvement of anasarca, PN, VEGF, and PET scan with Bortez alone, but Thal added because of persistent edema, M-protein, PN, and barely elevated VEGF; with Thal, disappearance of pleural effusion, ascites, and M-protein and normalization of VEGF |
| Bortez Dex*73 | Improvement by 3 cycles, but continued for 6; complete remission 4 y after completing therapy; improvement in adenopathy, pleural effusion and ascites, hepatosplenomegaly, and IL-6 |
| Bortez, CTX, Dex74 | Clinical response of anasarca within 6 wks and tolerated therapy for 18 mo achieving a nCR and a VEGF response; PN, hyperpigmentation, pulmonary hypertension improved significantly |
Bortez indicates bortezomib; AD, doxorubicin and dexamethasone; VAD, vincristine, doxorubicin, dexamethasone; CTX, cyclophosphamide; Mel, mephalan; Pred, prednisone; Dex, dexamethasone; PN, polyneuropathy; Thal, thalidomide; and nCR, near complete response.
Castleman variant of POEMS syndrome.
Although an anti-VEGF strategy is theoretically appealing, the results with bevacizumab have been mixed (Table 5).22-29 Five patients who had also received alkylator during and/or predating the bevacizumab had benefit,24-26,29 including one who had improvement, but was then consolidated with high-dose chemotherapy with ASCT,25 and another 2 who were treated with radiation and cyclophosphamide with initial clinical and VEGF response within approximately 6 months of therapy, but were given bevacizumab, and had “impressive improvement of neurologic symptoms.”29 These data are difficult to interpret because dramatic neurologic improvement does not typically occur in this syndrome until approximately 6 to 12 months after definitive treatment, which is precisely the time after radiation and cyclophosphamide that the bevacizumab was given in most of these cases. In 4 other case reports, patients receiving bevacizumab died very shortly thereafter.21,22,27,28
Activity of VEGF inhibition for the treatment of POEMS syndrome
| Treatment . | Outcome . |
|---|---|
| Bev alone21 | Death within 6 wks |
| Bev + mycophenolate + Dex97 | One mo after starting therapy, patient deteriorated further with worsening ascites and shortness of breath; Bev and Dex were discontinued; Mel and Pred were begun; patient died 1 mo later |
| Bev alone22 | Worsening PN, anasarca, multiorgan failure; died of pneumonia 5 wks after therapy |
| Bev alone23 | Improved pain, breathing, and walking |
| Bev + Mel-Dex24 | Improved effusions/ascites |
| Prior VAD/CTX25 | Improved edema, pain, weakness, and VEGF |
| Bev + CTX-Dex26 | Initial worsening; repeat with Bev → improved pulmonary HTN, anasarca, skin changes |
| Bev + CTX-CS27 | Initial improvement, but multiorgan failure and death |
| Bev + CTX radiation29 | Two patients: (1) first patient treated with radiation and CTX and then Bev; clinical improvement started before Bev; at radiologic relapse, Bev no use, so Len plus Dex used with benefit; and (2) second patient treated with same sequence, but course complicated by sepsis; biochemical and early neurologic response before Bev started |
| Bev + CTX28 | Clinical and biochemical relapse; no response to CTX, so bevacizumab added; death |
| Treatment . | Outcome . |
|---|---|
| Bev alone21 | Death within 6 wks |
| Bev + mycophenolate + Dex97 | One mo after starting therapy, patient deteriorated further with worsening ascites and shortness of breath; Bev and Dex were discontinued; Mel and Pred were begun; patient died 1 mo later |
| Bev alone22 | Worsening PN, anasarca, multiorgan failure; died of pneumonia 5 wks after therapy |
| Bev alone23 | Improved pain, breathing, and walking |
| Bev + Mel-Dex24 | Improved effusions/ascites |
| Prior VAD/CTX25 | Improved edema, pain, weakness, and VEGF |
| Bev + CTX-Dex26 | Initial worsening; repeat with Bev → improved pulmonary HTN, anasarca, skin changes |
| Bev + CTX-CS27 | Initial improvement, but multiorgan failure and death |
| Bev + CTX radiation29 | Two patients: (1) first patient treated with radiation and CTX and then Bev; clinical improvement started before Bev; at radiologic relapse, Bev no use, so Len plus Dex used with benefit; and (2) second patient treated with same sequence, but course complicated by sepsis; biochemical and early neurologic response before Bev started |
| Bev + CTX28 | Clinical and biochemical relapse; no response to CTX, so bevacizumab added; death |
Bev indicates bevacizumab; Dex, dexamethasone; Mel, mephalan; Pred, prednisone; PN, polyneuropathy; VAD, vincristine, doxorubicin, dexamethasone; CTX, cyclophosphamide; HTN, hypertension; CS, corticosteroids; and Len, lenalidomide.
Although IVIG and plasmapheresis are very effective for CIDP, neither of these therapies is helpful for patients with POEMS syndrome.75 A recent report, however, describes reduction in serum VEGF and clinical improvement with single-agent IVIG. The response was not durable, which prompted another course of IVIG with radiation to a solitary plasmacytoma.76 Other treatments, such as interferon-α, tamoxifen, trans-retinoic acid, ticlopidine, argatroban, and strontium-89, have been reported as having activity mostly as single case reports.75
Targeting other features of the disease
Targeting the peripheral neuropathy.
The neuropathy is usually the dominant characteristic. The quality and extent of the neuropathy, which is typically peripheral, ascending, symmetric, and affecting both sensation and motor function, should be elicited. Pain may be a dominant feature in approximately 10% to 15% of patients, seemingly more commonly in reports from Japan with reported rates of hyperesthesia or pain in 50% to 79% of their subjects.77,78 The most common misdiagnosis made in patients with POEMS syndrome before the correct diagnosis is established is CIDP because both disorders are predominantly demyelinating neuropathies. In a series comparing 51 patients with POEMS and 46 patients with CIDP, patients with POEMS syndrome were significantly more likely to have muscle atrophy and distal dominant muscle weakness and to report severe leg pain.78 Electrophysiologically, there is growing evidence that demyelination is predominant in the nerve trunk rather than in the distal nerve terminals. Axonal loss is also often seen in the lower limb nerves.78,79
The 2 best ways to approach the peripheral neuropathy are to target the clone and to direct the patient to work intensively with physical therapy and occupational therapy. I encourage stretching, strengthening, and balance exercises. Ankle braces, canes, walkers, and wheelchairs should be used as needed. I treat the painful peripheral neuropathy if present with drugs, such as gabapentin, pregabalin, amitriptyline, nortriptyline, duloxetine, topical lidocaine patches, and topical ketamine, lidocaine, and amitriptyline compounds.
Targeting VEGF.
Plasma and serum levels of VEGF are markedly elevated in patients with POEMS12,30,80 and correlate with the activity of the disease, even better than the serum M-spike.14,18,28,30 We found that a plasma VEGF level of 200 pg/mL has a specificity of 95% and a sensitivity of 68% for POEMS syndrome.28 Although VEGF is the best measure of disease activity for the majority of patients, reduction of levels using bevacizumab is not clearly effective therapy (see “Targeting the clone”), even though it drops VEGF levels to undetectable levels. This paradox would suggest that VEGF is not the primary driver of the disease, but rather that it is a surrogate. There are clinical assays available to measure both serum and plasma VEGF; the former levels are 10 to 50 times higher than the latter.81 There is a debate as to which test is better, but it is imperative that one select a laboratory assay and continue to use throughout the course of the patient's disease. I prefer the plasma VEGF because the higher level observed in serum is attributable to the release of VEGF from platelets in vitro during serum processing. I measure levels every 3 to 6 months to track a patient's progress. If I observe a rise in a patient's VEGF without any evidence of clinical deterioration, I will repeat the testing in one to 3 months before considering a new therapy.
Targeting extravascular volume overload.
Signs of volume overload are present in the majority of patients in the form of peripheral edema; however, ascites, pleural effusions, and pericardial effusions may be present in as many as 50% of patients depending on the series.2,7-11 After the peripheral neuropathy, refractory ascites and anasarca cause the most morbidity (Figure 2A). The mechanism of this feature of the syndrome is not well understood, but it has been speculated that in part VEGF contributes to the capillary leak. Although this manifestation may be present at presentation or at relapse, it is one of the most common preterminal events. In extreme cases, the third spacing is not controllable with diuretics and patients become prerenal and even develop renal failure through this mechanism. I have found that the use of serial paracentesis and albumin-forced diuresis may provide benefit, but results can also be disappointing. In a recent personal case, neither bevacizumab nor cyclophosphamide-dexamethasone alleviated the third spacing, and the patient died approximately 4.5 years after his original diagnosis.28 In another recent personal case, the combination of cyclophosphamide, bortezomib, and dexamethasone brought a patient from paracentesis dependence (Figure 2A) to a diuretic-independent normal dry weight.74
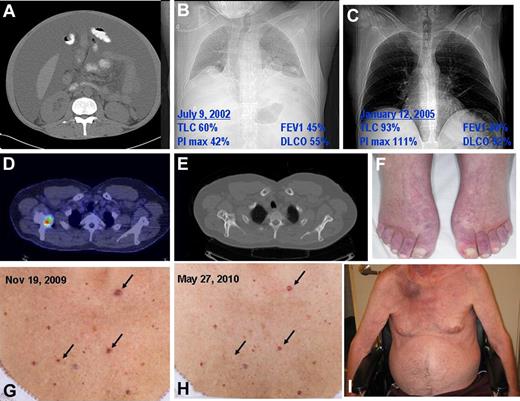
Classic findings of POEMS syndromes. (A) Massive ascites and lipodystrophy. (B) Chest radiograph and pulmonary function test results demonstrating reduced lung volumes because of neuromuscular weakness, small effusions, and reduced diffusing capacity of carbon monoxide. (C) Improved chest radiograph and pulmonary function tests 2.5 years after ASCT (same patient as in panel H). (D) Fusion CT PET of mixed lytic/sclerotic lesion in right scapula. (E) Bone windows of CT of mixed lytic/sclerotic lesion in right scapula. (F) Hyperemia of extremities and white nails. (G) Outcropping of cherry angiomata at diagnosis. (H) Shrinkage and disappearance of cherry angiomata after radiation to solitary osteosclerotic lesion in right femur. (I) Plasmacytoma right scapula with overlying erythema as well as gynecomastia, muscle wasting, and ascites. Also present but unrelated is florid tinea corpis resulting from chronic steroid used for the incorrect diagnosis of CIDP.
Classic findings of POEMS syndromes. (A) Massive ascites and lipodystrophy. (B) Chest radiograph and pulmonary function test results demonstrating reduced lung volumes because of neuromuscular weakness, small effusions, and reduced diffusing capacity of carbon monoxide. (C) Improved chest radiograph and pulmonary function tests 2.5 years after ASCT (same patient as in panel H). (D) Fusion CT PET of mixed lytic/sclerotic lesion in right scapula. (E) Bone windows of CT of mixed lytic/sclerotic lesion in right scapula. (F) Hyperemia of extremities and white nails. (G) Outcropping of cherry angiomata at diagnosis. (H) Shrinkage and disappearance of cherry angiomata after radiation to solitary osteosclerotic lesion in right femur. (I) Plasmacytoma right scapula with overlying erythema as well as gynecomastia, muscle wasting, and ascites. Also present but unrelated is florid tinea corpis resulting from chronic steroid used for the incorrect diagnosis of CIDP.
Targeting renal disease.
Serum creatinine levels are normal in most cases, but serum cystatin C, a surrogate marker for renal function, is high in 71% of patients.40 In our experience, at presentation, fewer than 10% of patients have proteinuria exceeding 0.5 g/24 hours, and only 6% have a serum creatinine greater than or equal to 1.5 mg/dL.9 In another series from China, at diagnosis 37% of patients had a creatinine clearance of less than 60 mL/min, 9% had a creatinine clearance of less than 30 mL/min, and 15% had microhematuria.10 I find that overt renal disease is more likely to occur in patients who have coexisting Castleman disease and also as a preterminal event in association with uncontrollable ascites and anasarca. In the rare cases where there is significant kidney pathology warranting biopsy, the renal histologic findings are diverse, with membranoproliferative features and evidence of endothelial injury being most common.48 There is no known specific therapy to treat these instances of renal disease other than targeting the underlying plasma cell clone.
Targeting abnormal pulmonary function.
Respiratory complaints are usually limited given patients' neurologic status impairing their ability to induce cardiovascular challenges, but abnormal pulmonary function tests are present in the majority.50,82 The pulmonary manifestations include pulmonary hypertension, restrictive lung disease, impaired neuromuscular respiratory function, and impaired diffusion capacity of carbon monoxide.82,83 Patients with significant neuromuscular weakness should be screened for sleep apnea so either continuous positive airways pressure or bilevel positive airway pressure can be prescribed as necessary. All of these abnormalities can improve with effective therapy targeting the plasma cell clone (Figure 2B-C).75,82,83
Targeting organomegaly.
The hepatosplenomegaly and lymphadenopathy do not require specific therapy. The enlargement is typically not sufficient to cause localized discomfort. These tissues are often biopsied during the course of establishing a diagnosis. Except when there is coexisting Castleman disease, biopsies of these tissues are uninformative. The organomegaly and adenopathy resolve with effective treatment of the underlying plasma cell disorder. When a patient has POEMS with coexisting Castleman disease as is the case in up to 30% of cases,5 the approach is not different, except that in these cases the IL-6 should also be followed. In contrast, if the patient has what I call the POEMS variant of Castleman disease (ie, no plasma cell clone documented and potentially a less apparent or even absent peripheral neuropathy, but many of the other features of POEMS syndrome), the treatment strategy is different.6 In these patients, anti–IL-6 antibodies, anti–IL-6 receptor antibodies, and rituximab are therapies that should be considered in addition to alkylator and steroid-based therapy.84,85 Patients with Castleman variant of POEMS should also be tested for HIV and HHV-8.
Targeting endocrinopathy.
Endocrinopathy is a central but poorly understood feature of POEMS. In a recent series,11 approximately 84% of patients had a recognized endocrinopathy, with hypogonadism as the most common endocrine abnormality, followed by thyroid abnormalities, glucose metabolism abnormalities, and lastly by adrenal insufficiency. In my experience, endocrine abnormalities can improve after chemotherapy, including successful tapering off of thyroid replacement, androgen replacement, and corticosteroid replacement in at least one-third of the patients. The clinically silent, but biochemically evident, rises in prolactin typically improves within the first year as well.
Targeting papilledema.
Papilledema (optic disc edema) is present in at least one-third of patients and may be associated with increased intracranial pressure. Of the 33 patients at our institution referred for a formal ophthalmologic examination during a 10-year period, 67% had ocular signs and symptoms, the most common of which was papilledema in 52% of those examined.86 In most cases, the optic disc edema is asymptomatic, but when it is not and when pressures are high, treatment with acetazolamide and corticosteroids may control symptoms until definitive chemotherapy or radiation therapy directed at the underlying clone can control the disease. In rare cases, serial therapeutic lumbar puncture may be required. Ventriculoperitoneal shunts are typically not required. Response of the optic disc edema is typically rapid with clinical improvements noted within 3 months after ASCT.
Targeting sclerotic bone lesions.
Osteosclerotic lesions occur in approximately 95% of patients and can be confused with benign bone islands, aneurysmal bone cysts, nonossifying fibromas, and fibrous dysplasia.7,9,87,88 Some lesions are densely sclerotic, whereas others are lytic with a sclerotic rim (Figure 2D) and still others have a mixed soap-bubble appearance. FDG-PET/CT is a useful tool for screening for POEMS syndrome34 as is (99m)Tc-HMDP bone scintigraphy.89 Bone windows of CT body images (Figure 2E) are often more informative than the scintigraphy at diagnosis, especially if there is no lytic component to the bone lesion, but after treatment FDG uptake is a useful tool to monitor response.90 Bone lesions in POEMS syndrome do not typically cause bone pain or threaten skeletal integrity and therefore do not require any specific therapy other than using radiation to target the underlying clone. Radiating these lesions as primary therapy among those patients without bone marrow involvement is appropriate. Applying adjuvant radiation 12 months after ASCT to those FDG-avid lesions, which have not had reduction in their SUV, may also be appropriate on a case-to-case basis.
Targeting skin changes.
A whole skin examination should be performed looking for hyperpigmentation, hypertrichosis, acrocyanosis, dependent rubor (Figure 2F), white nails, a recent outcropping of hemangioma (Figure 2G), and sclerodermoid changes, flushing or clubbing. Also seen is facial lipoatrophy58 and very rarely calciphylaxis.91 Even more rarely can a violaceous skin patch overlying a solitary plasmacytoma of bone (Figure 2I), associated with enlarged regional lymph node, be seen.92 With the exception of calciphylaxis, none of the skin changes requires any specific therapy, and they all gradually improve after definitive therapy. In contrast, calciphylaxis can be devastating. There are 4 reports in the literature,91,93-95 and I have seen 2 additional cases. Of these 6, 3 patients died, 2 had resolution, and 1 did not have outcome described. Yoshikawa et al treated their patient with etidronate with improvement in skin, but sudden death within 3 months of calciphylaxis.91 Of the 2 cases I have seen, 1 died, and I am currently treating another who is improving over the course of the past 8 months on cyclophosphamide and prednisone along with meticulous wound care and acetic acid gauze changes along with topical potassium permanganate for topical antimicrobial effect. Skin lesions, including hemangiomata, improve with therapy (Figure 2H).
Targeting thrombocytosis and polycythemia.
Approximately 50% of patients with POEMS have thrombocytosis. Unlike multiple myeloma, anemia is rare unless there is coexisting Castleman disease or renal insufficiency. Many patients are thought to have a JAK2-negative myeloproliferative disorder before the diagnosis of POEMS syndrome is made because megakaryocyte hyperplasia and megakaryocyte clustering are seen in 54% and 93% of cases, respectively.37 The question of whether to treat these patients with hydroxyurea to lower their platelet count arises not infrequently. There are no data to guide whether lowering the platelet count is necessary, with the exception of indirect data from our series on cerebrovascular events among patients with POEMS syndrome.96 A high platelet count was a risk factor for developing a cerebral infarction. With that in mind, I will use hydroxyurea in those patients with significant thrombocytosis who presented with a cerebral event until I can start therapy directed at the underlying clone. I am also less inclined to use lenalidomide-dexamethasone in this same high-risk patient population unless I am considering full anticoagulation. Once plasma cell-directed therapy has been commenced, I typically taper the hydroxyurea. The erythrocytosis observed in approximately 10% to 15% of patients is typically modest, and treating the underlying plasma cell disorder is sufficient. Both thrombocytosis and erythrocytosis improve after therapy.
Conclusion
Patients with POEMS syndrome present with a complex conglomerate of symptoms, signs, and objective abnormalities, making the diagnosis, management, and follow-up a challenge. Early diagnosis and a prompt multidisciplinary approach increase the likelihood of reduced long-term irreversible morbidity. Parameters associated with the poorest outcomes include fingernail clubbing, respiratory symptoms, and extravascular volume overload.5 The number of POEMS-specific features is not prognostic. The best choice of therapy has not been derived through clinical trials, but rather through case series, and ASCT has become a favored therapy. Other therapies that are effective in myeloma also appear to be effective in patients with POEMS syndrome as well. Both therapies directed at other features of the disease as well as emotional support should be a major part of the care plan. Follow-up and measurement of response are difficult at best because no one measurement is reliable enough to direct therapy. VEGF response appears to correlate with disease activity better than serum M-spike or PET scan as long as anti-VEGF antibodies have not been used. It is my practice to consider plasma cell-directed therapy to be effective as long as the VEGF normalizes, even if there is a residual M-spike. If there is still FDG avidity on PET scan 1 year after completing therapy, I consider adjuvant radiation. If primary therapy was radiation and at 1 year there is still FDG avidity on PET scan but the VEGF is normal and the patient is otherwise continuing to improve clinically, I have not been administering adjuvant chemotherapy. Serial assessments of clinical stigmata (peripheral neuropathy, volume status, eyes, skin, and organomegaly) of blood (M-spike, VEGF, affected endocrine parameters) should be done every 3 months for at least the first several years. Pulmonary function tests and bone assessments should be done annually. I follow all patients at least once or twice a year indefinitely because patients do relapse and these patients can be salvaged. Once the underlying pathogenesis of the disease is better understood, more targeted therapy will be possible.
Acknowledgments
This work is supported in part by the National Institutes of Health (grants CA125614, CA107476, and CA111345), the Predolin Foundation, and the JABBS Foundation.
National Institutes of Health
Authorship
Contribution: A.D. wrote the manuscript.
Conflict-of-interest disclosure: A.D. received research dollars from Celgene and travel compensation from Binding Site and Celgene, and was on the unpaid advisory board to Onyx and Millenium.
Correspondence: Angela Dispenzieri, Department of Medicine and Laboratory Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.