Human herpes virus 8 (HHV-8) or Kaposi sarcoma-associated herpes virus is the etiologic agent of Kaposi sarcoma, primary effusion lymphoma, and plasma cell-type multicentric Castleman disease (MCD). HHV-8 encodes a viral homolog of human IL-6, called viral IL-6 (vIL-6), which does not require the cellular IL-6 receptor for binding to the ubiquitously expressed gp130 receptor subunit and subsequent JAK-STAT signaling. Thus, in contrast to IL-6, vIL-6 can stimulate virtually all cells in the body. To elucidate the mechanism by which vIL-6 drives human diseases, we generated transgenic mice that constitutively express vIL-6 under control of the MHC class I promoter. The mice were found to exhibit vIL-6 serum levels comparable with those observed in HHV-8–infected patients, to contain elevated amounts of phosphorylated STAT3 in spleen and lymph nodes, where vIL-6 was produced, and to spontaneously develop key features of human plasma cell-type MCD, including splenomegaly, multifocal lymphadenopathy, hypergammaglobulin-emia, and plasmacytosis. Transfer of the vIL-6 transgene onto an IL-6–deficient genetic background abrogated MCD-like phenotypes, indicating that endogenous mouse IL-6 is a crucial cofactor in the natural history of the disease. Our results in mice suggest that human IL-6 plays an important role in the pathogenesis of HHV-8–associated MCD.
Introduction
Human herpes virus 8 (HHV-8), also known as Kaposi sarcoma-associated herpes virus (KSHV), is found in 100% of patients with Kaposi sarcoma (KS)1,2 and is predominantly associated with disease in HIV-positive immunocompromised patients.3,4 KS is a tumor of endothelial origin,5 and a causative role for HHV-8 in tumorigenesis was demonstrated by the introduction of an HHV-8 bacterial artificial chromosome (KSHVBac36) into mouse bone marrow endothelial-lineage cells that formed KS-like tumors in mice.6 HHV-8 is also associated with B-lymphoproliferative disorders, such as primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD).2,4,7,8 PEL is a monoclonal B-cell malignancy.7 MCD is a rare condition characterized by abnormal populations of polyclonal plasmablasts in multiple lymph nodes with a propensity toward development of lymphoma.9 All HIV-associated MCD patients are infected with HHV-8, whereas approximately 50% of HIV-negative MCD patients harbor HHV-8.8
The HHV-8 genome encodes at least 10 genes with significant homology to human genes involved in inflammation, angiogenesis, cell cycle control, and modulation of the immune system, including a viral G protein-coupled receptor (vGPCR, ORF74), viral Flice inhibitory protein (vFLIP), and viral IL-6 (vIL-6).10 Transgenic mice have been important to understand the function of vGPCR and vFLIP in tumorigenesis and hyperproliferative disease, but until now a vIL-6 transgenic mouse has not been reported. vGPCR is structurally related to cellular chemokine receptors, and mice harboring a vGPCR transgene developed tumors resembling KS lesions,11,12 the formation of which is accelerated by HIV type 1 Tat.13 Expression of vFLIP leads to activation of NF-κB, and vFLIP transgenic mice develop a disease that resembles PEL14,15 and exhibit B-cell trans-differentiation and MCD-like abnormalities.16 vIL-6 was shown to be capable of inducing B lymphoproliferative disease when vIL-6–expressing NIH3T3 cells were injected into athymic mice, which developed tumors at the site of injection and also manifested characteristics of plasma cell-type MCD, such as splenomegaly, hepatomegaly, and plasmacytosis in spleen and lymph nodes.17 NIH3T3 cells are not normally infected by HHV-8. The significance of vIL-6 expression from cells that are naturally targeted by HHV-8 in immune-competent mice has remained unexplored.
The vIL-6 protein is detectable in serum of patients with KS, PEL, and HHV-8–associated plasma cell-type MCD.18,19 A prominent role for vIL-6 in MCD is suggested by the finding that cells from MCD patients express a higher level of the viral cytokine compared with cells from either KS or PEL patients.2 That vIL-6 plays a significant role in the pathology of MCD is widely accepted, although the molecular basis of its function in the disease remains obscure.20 Interestingly, it has been reported that vIL-6 can induce expression of its human IL-6 homolog (hIL-6) in MCD patient-derived cells.21 Elevated hIL-6 has long been associated with Castleman disease pathology,22,,,–26 and antibody-based therapies targeting the IL-6 receptor (IL-6R) or IL-6 itself are entering clinical practice for the management of MCD.27,28 Despite these advancing treatment options, the biologic significance of the interplay between vIL-6 and IL-6 remains unknown.
Although vIL-6 and its hIL-6 homolog are functionally related cytokines, there is little amino acid homology between them (∼ 25%), and they have distinctive ways by which they initiate downstream signaling.29 Classic IL-6 signaling is characterized by the binding of mammalian forms of IL-6 to the membrane bound IL-6R. The IL-6/IL-6R complex binds and activates gp130, leading to downstream activation of signaling pathways, such as JAK-STAT (Janus kinase/signal transducer and activator of transcription). IL-6 has no measurable affinity for gp130, so cells lacking the IL-6R fail to respond to the cytokine. Thus, this mode of signaling is limited because the IL-6R is only expressed on hepatocytes, some leukocytes, and some epithelial cells.30 We have shown that, in contrast to human IL-6, vIL-6 does not require expression of the nonsignaling IL-6R for stimulation of cells but instead binds directly to and activates the ubiquitously expressed signal-transducing receptor subunit gp130.31,–33 Cells expressing only gp130 but no IL-6R can still be stimulated by vIL-6, and also to IL-6 bound to a naturally occurring soluble form of the IL-6R (sIL-6R), in a process called trans-signaling.34 Because all cells in the body express gp130, vIL-6, like trans-signaling, has the potential to elicit broader effects than classic IL-6 signaling.10 Overall, IL-6-based signaling is complex and requires definitive genetic systems in which to tease out the individual contributions of the molecules involved, including vIL-6.
We generated transgenic mice expressing vIL-6 from an MHC class I promoter (H2K) that is predominantly active in hematopoietic organs and lymphocytes,35,36 corresponding to sites of natural infection by HHV-8. vIL-6 transgenic mice developed symptoms strongly reminiscent of plasma cell-type MCD, including hyperplasia of spleen and lymph nodes, hypergammaglobulinemia, plasmacytosis in peripheral lymph nodes (PLNs), and splenic extramedullary hematopoiesis. Remarkably, these symptoms disappeared when the vIL-6 transgene was crossed onto an IL-6–deficient background. Our findings provide evidence that vIL-6–mediated MCD requires the help of endogenous IL-6.
Methods
Cloning
The cDNA encoding for codon optimized vIL-6 was synthesized by Geneart and delivered in pPCR-script plasmid (pPCR-script-vIL-6opt). We used the plasmid p163/7, which contains the MHC class I H2 promoter,35 to subclone the cDNA for codon optimized vIL-6 into the XhoI site. The resulting plasmid was named p163/7-vIL-6opt. The plasmid p163/7 was a kind gift of Dr Ulrich Rüther (Düsseldorf, Germany).
Generation of vIL-6tg and vIL-6tg/IL-6–deficient mice
The vIL-6 coding HindIII-SacI fragment of p163/7-vIL-6opt was gel purified and microinjected into the pronuclei of fertilized eggs of B6D2 mice (IBF). After screening by Southern blotting and PCR, sera of vIL-6–positive mice were screened by ELISA for vIL-6 expression. vIL-6 founder mice were backcrossed to C57/Bl6 background to for 6 and more generations (Charles River Laboratories). C57/Bl6 IL-6–deficient mice (IL-6KO)37 were obtained from Charles River Laboratories. To obtain vIL-6 transgenic (vIL-6tg)/IL-6–deficient mice, mice from the vIL-6 line vIL-6tg2 were crossed with IL-6–deficient mice. vIL-6tg mice, which were heterozygous for the IL-6 knockout allele, were crossed again with IL-6–deficient mice to obtain vIL-6 transgenic mice on an IL-6–deficient background.
Mouse treatment
Mice were maintained in a 12-hour light-dark cycle under standard conditions and were provided with food and water ad libitum. Procedures involving mice and their care were conducted in conformity with national and international laws and policies. Mice were killed by cervical dislocation. Blood was drawn from mice by cardiac puncture after cervical dislocation. The blood of founder mice was drawn retrobulbarly under general anesthesia. All mice used for the experiments were between 12 and 21 weeks of age. All mice experiments were carried out with approval from the University of Kiel.
Southern blotting
A total of 10 μg of genomic DNA was digested by XhoI, separated on 1% agarose gels, and transferred to a nylon membrane (GE Healthcare), which was hybridized at 65°C overnight with the α-32P-dATP–labeled XhoI restriction fragment obtained by digestion of pPCR-script-vIL-6opt containing the vIL-6 cDNA. The membrane was washed at 65°C, and signals were detected by phosphoimaging.
Genotyping PCR
Genomic DNA was screened for the vIL-6 transgene using the following primers: 5′vIL-6, 5′-GAACCAGAGGCAAGCTGCCTGACG-3′; and 3′vIL-6, 5′-AGGGATGCTGTCCAGCACTCTCAC-3′. As a control, β-globin was amplified using the following primers: 5′-β-globin, 5′-CCAATCTGCTCACACAGGATAGAGAGGGCAGG-3′; and 3′-β-globin, 5′-CCTTGAG GCTGTCCAAGTGATTCAGGCCATCG-3′. To amplify DNA sequences, DreamTaq DNA polymerases (Fermentas) were used for detection of specific DNA sequences. Therefore, DNA was isolated using Gentra Puregene Mouse Tail Kit (QIAGEN) following the manufacturer's instructions, and DNA concentration was determined by UV spectrophotometry. A total of 100 ng of isolated DNA was mixed with 10μM primer pairs, 10mM dNTPs, and 1 U polymerase. The PCR was performed with 35 cycles of 95°C for 60 seconds, 55°C for 60 seconds, and 72°C for 60 seconds.
ELISAs
ELISA for vIL-6 was performed as described.18 In brief, monoclonal mouse anti–vIL-6 antibody (v6m12.1.1) was coated at 4 μg/mL in carbonate buffer (pH 9.0) overnight at 4°C. After washing the plates with PBS-T (PBS with 0.05% Tween-20) and blocking with 5% BSA in PBS for 2 hours at room temperature, the samples were added to the wells (1:20 dilution in PBS-T containing 0.5% BSA) overnight. After washing, rabbit polyclonal anti–vIL-6 antibody (0.5 μg/mL) was diluted in PBS-T containing 0.5% BSA and added to the wells for 2 hours at room temperature. The wells were washed again, and goat anti–rabbit HRP-conjugated antibody (1:1000; Thermo Scientific) was added to the wells for 2 hours at room temperature. After washing, the enzymatic reaction was performed with peroxidase substrate BM blue POD (Roche Diagnostics) and stopped by adding 1.8M H2SO4. The absorbance was read at 450 nm. Purified recombinant vIL-6 was used as a standard.32 Murine IgG levels were measured by commercial available ELISA (Bethyl Laboratories). Samples were diluted 1:50 000.
RT-PCR
RNA was isolated using Nucleospin Extract II Kit (Macherey-Nagel) following the manufacturer's instructions, and RNA concentration was determined by UV spectrophotometry. A total of 2 μg of RNA from different organs was reverse-transcribed into cDNA using 200 U of RevertAid M-MuLV Reverse Transcriptase (Fermentas); 1 μL of this RT reaction was used for subsequent PCR (see “Genotyping PCR”). vIL-6 transcripts were amplified using the primers 5′-vIL-6 and 3′vIL-6. In addition, β-actin was amplified using the primers: 5′-β-actin, 5′-GTGGGGCGCCCCAGGCACCA-3′; and 3′-β-actin, 5′-CTCCTTAAT-GTCACGCACGATTTC-3′.
Western blotting
PLNs (axillary, brachial, and inguinal) and spleens were homogenized in lysis buffer (150mM NaCl, 2mM EDTA, 50mM Tris-HCl, pH 7.4, 1% Triton X-100, and 1% NP-40; 1mM NaF, 1mM Na3VO4) containing complete protease inhibitor (Roche Diagnostics), and equal amounts of homogenates were used for SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes (GE Healthcare). The membranes were first probed with a primary antibody against P-STAT3 at a dilution of 1:2000 (Cell Signaling), washed and incubated with an HRP-conjugated anti–rabbit antibody (1:10 000; Thermo Scientific). Proteins were visualized by ECL detection system (GE Healthcare). After detection, the membranes were stripped with stripping buffer (62.5mM Tris-HCl, pH 6.8, 2% SDS, 0.1% β-mercaptoethanol), and probed with an antibody against STAT3 (Cell Signaling) at a dilution of 1:1000 and HRP-conjugated anti–mouse antibody (1:10 000; Thermo Scientific).
Densitometric analysis
Densitometric analysis from P-STAT3 and STAT3 Western blots was performed using ImageJ Version 1.45 software (http://rsbweb.nih.gov/ij/index.html).
Tissue processing, IHC, and histopathology
PLNs (axillary, brachial, and inguinal) and spleen tissue were fixed in 4% formalin, processed, and embedded in paraffin. Sections of 5μM were stained with hematoxylin and eosin or Giemsa using standard procedures. Staining for P-STAT3 was performed using a rabbit anti–P-STAT3 antibody (Cell Signaling) diluted 1:300 in sample diluent (Dako Denmark). After incubation with EnVision+ System-HRP–labeled polymer anti–rabbit antibody (Dako Denmark), the signal was developed with DAB substrate (3,3′ diaminobenzidine, Dako Denmark) and counterstained with Gill 3 hematoxylin (Thermo Scientific). Staining for Ki67 was performed using a rabbit anti-Ki67 antibody (Epitomics), diluted 1:100. After incubation with Histofine simple stain MAX PO (Multi; Medac), the signal was developed with DAB substrate (Dako Denmark) and counterstained with Gill 3 hematoxylin (Thermo Scientific). Staining for mIL-6 was performed using a rat anti–mIL-6 antibody (MP5-20F3) diluted 1:100 in sample diluents (Dako Denmark). After incubation with biotinylated anti–rat antibody and HRP-conjugated streptavidin (Dako Denmark), the signal was enhanced using the TSA plus Biotin kit (PerkinElmer Life and Analytical Sciences), developed with DAB substrate (Dako Denmark), and counterstained with Gill 3 hematoxylin (Thermo Scientific). Pictures were obtained with an Axiophot microscope (Zeiss) and the camera ProgRes CF Jenoptik Laser Systeme GmbH). Picture processing was done using the software ProgRes Rapture Pro 2.7 (Jenoptik Laser Systeme GmbH). Staining for κ- and λ-light chains was performed using anti–κ-light chains and anti–λ-light chain antibodies (Dako Denmark), diluted (anti–κ-light chains: 1:100 000; anti–λ-light chains: 1:50 000) in sample diluents (Dako Denmark). To visualize κ-light chains and λ-light chains, the Bond Polymer Refine Red Detection kit (Leica Microsystems) was used. Staining for κ- and λ-light chains was performed on a Bond Max System (Leica Microsystems).
Scoring of MCD in transgenic mice
There are 3 histopathologic signs for plasma cell-type MCD, 2 major signs being plasmacytosis and follicular hyperplasia, and 1 minor sign, which is vascular proliferation. To classify the lymph nodes for plasma cell-type MCD-like phenotype, the following criteria were defined. Plasmacytosis was defined as the appearance of clusters of mature plasma cells (20 cells/HPF) in contrast to discrete plasmacytosis, which was defined as a diffuse increase of mature plasma cells without forming clusters. To specify follicular hyperplasia, the appearance of > 1 florid follicle/lymph node was stated in contrast to discrete follicular hyperplasia, which was defined as an increase of naive follicles. Moderate or increased vessel proliferation was assessed in the paracortex and the lymph node pulp compared with wild-type mice by eyeballing. Lymph nodes, which showed plasmacytosis, follicular hyperplasia, and vascular proliferation, were determined as plasma cell-type MCD-like phenotype. If 1 or both of the major signs were only stated as “discrete,” the term ambiguous plasma cell-type MCD-like phenotype was used. However, in case of doubt, neither of the 2 terms was used. To define increased or discrete increased hematopoiesis in spleen sections, the following definition was used: increased number of megakaryocytes and overt clusters of erythropoiesis and granulopoiesis were defined as increased hematopoiesis, whereas discrete increased hematopoiesis was defined as discrete increase of megakaryocytes, no overt increase in erythropoiesis and granulopoiesis. All slices (H&E- and Giemsa-stained) were blinded and examined by 2 pathologists.
Statistical analysis
All results are shown as arithmetic mean ± SD. Data from 2 groups were analyzed for significance using the Student t test. Statistical significance was set at P ≤ .01 and P ≤ .001.
Results
Generation of vIL-6 transgenic mice
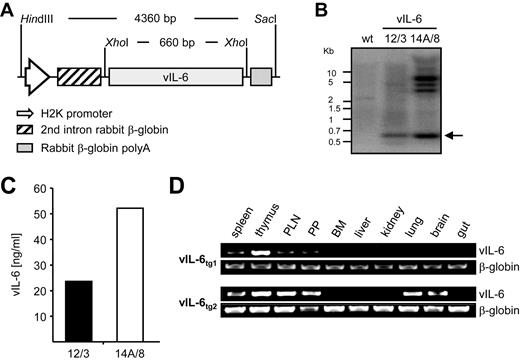
Because our earlier attempts to generate vIL-6 transgenic mice that harbor the native HHV-8–encoded gene were unsuccessful (results not shown), we here codon-optimized the DNA sequence of the viral gene for improved expression in the mouse. In addition, to facilitate biochemical studies, we added a short stretch of DNA that encodes a C-terminal MYC tag to the vIL-6 protein, which was previously shown not to interfere with gp130 receptor activation and subsequent JAK-STAT signal transduction.31 The MYC-tagged vIL-6 cDNA was subcloned into the H2K MHC class I promoter expression cassette35 to generate vIL-6 transgenic mice (Figure 1A). Of 29 vIL-6 transgenic founder mice identified by Southern blotting (Figure 1B) and genomic PCR (not shown), 8 founders exhibited serum vIL-6 levels that were measurable using ELISA and found to range from 20 to 500 ng/mL (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All founders with serum vIL-6 levels greater than 130 ng/mL died (5 of 8) or had to be killed because of poor health by 20 weeks of age (1 of 8), indicating that high expression of vIL-6 is not well tolerated in strain B6D2 mice. We therefore selected the 2 founders with the lowest serum levels of vIL-6 (12/3, 20 ng/mL; 14A/8, 50 ng/mL; Figure 1C) for breeding and transfer of transgenes from strain B6D2 to C57BL/6 (B6) mice. Introgressive backcrossing to N6 established 2 independent strains of mice that we designated vIL-6tg1 and vIL-6tg2. RT-PCR analysis verified transgene expression in both strains (Figure 1D), although the levels of expression were somewhat higher and involved more tissues in strain vIL-6tg2 than strain vIL-6tg1. ELISA showed that transfer of transgenes onto B6 was accompanied by increasing serum levels of vIL-6, averaging approximately 75 ng/mL in case of vIL-6tg2 N6 mice.
Generation of vIL-6 transgenic mice. (A) Schematic illustration showing the genetic organization of the vIL-6–expression cassette used for the pronuclei injection of fertilized mouse eggs (p167/2-H2-vIL-6-myc-opt). (B) Southern blot of DNA from 2 founder vIL-6 transgenic mice, 12/3 and 14A/8, using a radiolabeled probe to the complete vIL-6 cDNA. Arrow indicates expected size. (C) Determination of vIL-6 serum concentrations of transgenic founder mice by ELISA. (D) RT-PCR analysis of vIL-6 and β-actin (loading control) expression in various tissues from 2 independent vIL-6 transgenic mouse strains fully backcrossed onto the C57BL/6 genetic background. PP indicates Peyer patches; and BM, bone marrow.
Generation of vIL-6 transgenic mice. (A) Schematic illustration showing the genetic organization of the vIL-6–expression cassette used for the pronuclei injection of fertilized mouse eggs (p167/2-H2-vIL-6-myc-opt). (B) Southern blot of DNA from 2 founder vIL-6 transgenic mice, 12/3 and 14A/8, using a radiolabeled probe to the complete vIL-6 cDNA. Arrow indicates expected size. (C) Determination of vIL-6 serum concentrations of transgenic founder mice by ELISA. (D) RT-PCR analysis of vIL-6 and β-actin (loading control) expression in various tissues from 2 independent vIL-6 transgenic mouse strains fully backcrossed onto the C57BL/6 genetic background. PP indicates Peyer patches; and BM, bone marrow.
vIL-6 TG mice are prone to changes resembling human plasma cell-type MCD
Because preliminary comparison of vIL-6tg1 and vIL-6tg2 mice demonstrated that the latter exhibited more severe and earlier phenotypic changes than the former (compare Figure 2A-B with supplemental Figure 1, and Table 1 with supplemental Table 2), we concentrated the efforts described in the following on strain vIL-6tg2. We found that mice approximately 15 weeks of age harbored enlarged spleens (Figure 2A) and lymph nodes (Figure 2B) relative to age- and sex-matched nontransgenic (non-TG) littermates. Histopathologic analysis of peripheral and deep abdominal lymph nodes demonstrated a number of changes in 15-week-old vIL-6tg2 mice that were not present in non-TG controls (Figure 2; supplemental Figure 2). These included enlarged B-cell follicles that contained hyperplastic germinal centers and vascular proliferations (Figure 2C) and massive accumulations of plasma cells (Figure 2D), which led to 4-fold elevated serum levels of IgG in vIL-6tg2 mice compared with controls (Figure 2E). Hyperplastic germinal centers and plasmacytosis with concomitant hypergammaglobulinemia are hallmarks of plasma-cell type MCD in human beings.9,38
vIL-6 mice display characteristics of plasma-cell type MCD, including splenomegaly, lymphadenopathy, lymph node vascular proliferation and plasmacytosis, and hypergammaglobulinemia. (A) Splenomegaly in vIL-6tg2 mice. Top panel: Photograph of a representative wild-type and vIL-6tg2 mouse spleen. Bottom panel: Average ratio of spleen to body weight determined from 15-week-old wild-type (n = 7) and vIL-6tg2 (n = 10) mice. (B) Lymphadenopathy in vIL-6tg2 mice. Top panel: Photographs of PLNs from 3 independent wild-type and vIL-6tg2 mice. Bottom panel: Averaged weights of inguinal lymph nodes from 15-week-old wild-type (n = 7) and vIL-6tg2 (n = 10) mice (based on the sum of the weight of both nodes per mouse). (C) H&E-stained lymph node of a representative vIL-6tg2 mouse demonstrating interfollicular vascular proliferation (20-fold magnification). Blood vessels are marked by arrows. (D) Giemsa staining from the same vIL-6tg2 lymph node revealing plasmacytosis (20-fold magnification). A cluster of mature plasma cells (encircled) is filling the sinusoids. (E) Hypergammaglobulinemia in vIL-6tg2 mice. Average relative serum IgG levels are shown from 15-week-old wild-type (n = 7) and vIL-6tg2 (n = 10) mice, as measured by ELISA. Error bars represent SD. *P ≤ .01. **P ≤ .001.
vIL-6 mice display characteristics of plasma-cell type MCD, including splenomegaly, lymphadenopathy, lymph node vascular proliferation and plasmacytosis, and hypergammaglobulinemia. (A) Splenomegaly in vIL-6tg2 mice. Top panel: Photograph of a representative wild-type and vIL-6tg2 mouse spleen. Bottom panel: Average ratio of spleen to body weight determined from 15-week-old wild-type (n = 7) and vIL-6tg2 (n = 10) mice. (B) Lymphadenopathy in vIL-6tg2 mice. Top panel: Photographs of PLNs from 3 independent wild-type and vIL-6tg2 mice. Bottom panel: Averaged weights of inguinal lymph nodes from 15-week-old wild-type (n = 7) and vIL-6tg2 (n = 10) mice (based on the sum of the weight of both nodes per mouse). (C) H&E-stained lymph node of a representative vIL-6tg2 mouse demonstrating interfollicular vascular proliferation (20-fold magnification). Blood vessels are marked by arrows. (D) Giemsa staining from the same vIL-6tg2 lymph node revealing plasmacytosis (20-fold magnification). A cluster of mature plasma cells (encircled) is filling the sinusoids. (E) Hypergammaglobulinemia in vIL-6tg2 mice. Average relative serum IgG levels are shown from 15-week-old wild-type (n = 7) and vIL-6tg2 (n = 10) mice, as measured by ELISA. Error bars represent SD. *P ≤ .01. **P ≤ .001.
Summary of histopathologic analysis of peripheral lymph nodes from wild-type, vIL-6tg2, IL-6–deficient (IL-6KO), and vIL-6tg2/IL-6–deficient (IL-6tg2/IL-6KO) mice with respect to plasma cell-type MCD-like phenotype, extramedullary hematopoiesis, and plasmacytosis
| . | Wild-type . | vIL-6tg2 . | IL-6KO . | vIL-6tg2/IL-6KO . |
|---|---|---|---|---|
| MCD-like phenotype | 0/10 | 7/25 (5/25)* | 0/17 (0/17)* | 1/17 (0/17)* |
| Extramedullary hematopoiesis | 0/10 | 8/25 (11/25)† | 0/14 (1/14)† | 4/15 (0/15)† |
| Plasmacytosis | 0/10 | 11/25 (7/25)† | 0/14 (0/14)† | 1/15 (4/15)† |
| . | Wild-type . | vIL-6tg2 . | IL-6KO . | vIL-6tg2/IL-6KO . |
|---|---|---|---|---|
| MCD-like phenotype | 0/10 | 7/25 (5/25)* | 0/17 (0/17)* | 1/17 (0/17)* |
| Extramedullary hematopoiesis | 0/10 | 8/25 (11/25)† | 0/14 (1/14)† | 4/15 (0/15)† |
| Plasmacytosis | 0/10 | 11/25 (7/25)† | 0/14 (0/14)† | 1/15 (4/15)† |
Values in parentheses are additional border line cases.
Values in parentheses are additional discrete cases.
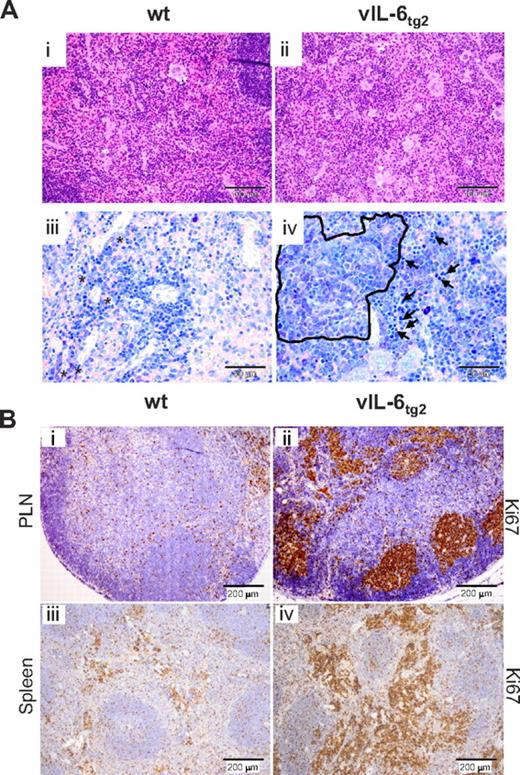
Histologic abnormalities in spleens from 15-week-old vIL-6tg2 mice included elevated numbers of megakaryocytes and increased granulocytopoiesis (Figure 3Ai-ii), as well as extensive clusters of plasma cells and numerous foci of erythropoiesis (Figure 3Aiii-iv, the latter indicated by arrows). Increased extramedullary hematopoiesis and plasma cell hyperplasia in spleen are well-established characteristics of human plasma-cell type MCD.38 Further characterization included immunostaining of tissue sections for Ki67 to evaluate the proliferative activity of spleen and lymph node cells in vIL-6tg2 mice (Figure 3B). Germinal centers in lymph nodes exhibited striking reactivity for the proliferation marker Ki67 (Figure 3Bii) compared with background reactivity seen in non-TG mice (Figure 3Bi). Similarly, vIL-6tg2 mice showed strong expression of Ki67 in the red pulp of spleen, the main site of extramedullary hematopoiesis in mouse (Figure 3Biv), whereas non-TG littermates exhibited weak expression levels (Figure 3Biii). To screen for evidence of clonal outgrowth of plasmablasts and plasma cells, a clue for incipient plasma cell neoplasia such as plasmacytoma, secondary lymphoid tissues were also analyzed for Ig κ- and λ-light chain production by immunolabeling. Lymph nodes and spleens of vIL-6tg2 contained abundant κ+ and λ+ plasma cells at approximately normal ratios and were intermingled with each other (supplemental Figure 3), consistent with plasmacytosis but not neoplastic plasma cell development.
vIL-6tg2 mice exhibit increased splenic extramedullary hematopoiesis and plasmacytosis, and increased cell proliferation in lymph nodes and spleen. (A) Representative hematoxylin and eosin-stained sections of spleen from 21-week-old wt (Ai) and vIL-6tg2 (Aii) mice, showing an increased number of megakaryocytes and granulocytes in vIL-6tg2 only (20-fold magnification). (Aiii-iv) Giemsa staining of serial sections from the same spleen shown in subpanels i and ii at 40-fold magnification. vIL-6tg2 display a cluster of mature plasma cells (encircled) and several foci of erythropoiesis as marked by arrows (iv) that are not observed in wt (iii). (B) Ki67 staining of PLNs (Bi-ii) and spleen (Biii-iv) of a 15-week-old wild-type (i,iii) and vIL-6tg2 (ii,iv; 10-fold magnifications). Increased proliferation was observed in hyperplastic germinal centers of lymph nodes from transgenic mice (ii). vIL-6tg2 spleen tissue (iv) showed increased proliferation in the red pulp of vIL-6tg2 spleen, indicating hematopoiesis. Representative images are shown.
vIL-6tg2 mice exhibit increased splenic extramedullary hematopoiesis and plasmacytosis, and increased cell proliferation in lymph nodes and spleen. (A) Representative hematoxylin and eosin-stained sections of spleen from 21-week-old wt (Ai) and vIL-6tg2 (Aii) mice, showing an increased number of megakaryocytes and granulocytes in vIL-6tg2 only (20-fold magnification). (Aiii-iv) Giemsa staining of serial sections from the same spleen shown in subpanels i and ii at 40-fold magnification. vIL-6tg2 display a cluster of mature plasma cells (encircled) and several foci of erythropoiesis as marked by arrows (iv) that are not observed in wt (iii). (B) Ki67 staining of PLNs (Bi-ii) and spleen (Biii-iv) of a 15-week-old wild-type (i,iii) and vIL-6tg2 (ii,iv; 10-fold magnifications). Increased proliferation was observed in hyperplastic germinal centers of lymph nodes from transgenic mice (ii). vIL-6tg2 spleen tissue (iv) showed increased proliferation in the red pulp of vIL-6tg2 spleen, indicating hematopoiesis. Representative images are shown.
Table 1 summarizes the findings in 25 mice. It shows that 7 (28%), 8 (32%), and 11 (44%) of 25 vIL-6tg2 mice exhibited MCD-like changes, increased extramedullary hematopoiesis, and plasmacytosis in the red pulp of spleens. Five, 11, and 7 additional vIL-6tg2 mice exhibited abnormalities that were categorized by a hematopathologist as borderline or discrete. None of the age-matched control mice developed these phenotypes. Taken together, the results support the contention that vIL-6 drives an MCD-like process in mouse, which mimics the polyclonal plasma-cell variant of the disease more closely than the monoclonal plasmablastic variant, which is less common and usually associated with HIV infection.38
Viral IL-6 activates STAT3 and increases production of endogenous mouse IL-6
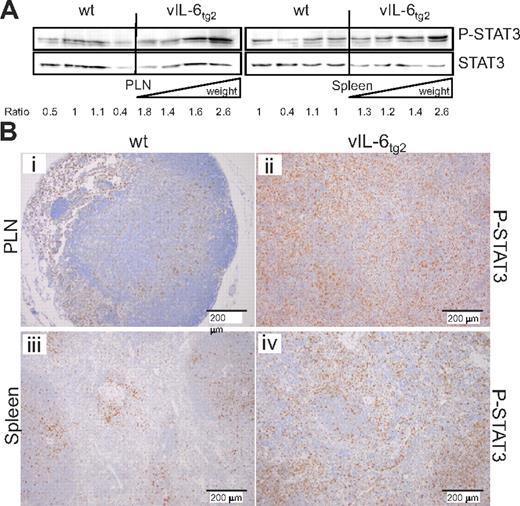
Western blotting and immunolabeling of tissue sections were used to evaluate whether transgenic production of vIL-6 led to phosphorylation of STAT3 at tyrosine 705 (P-STAT3), an activating posttranslational modification. P-STAT3 increased proportionally with increasing tissue mass in both spleen and PLNs by immunoblotting (Figure 4A). Correspondingly, lymph nodes and spleens of vIL-6tg2 mice harbored higher P-STAT3 reactivity by IHC than corresponding tissues from non-TG mice (Figure 4B). The results support the interpretation that vIL-6 induced gp130-dependent STAT3 signaling in lymphoid tissues in the mouse.
Increased STAT-3 phosphorylation in PLNs and spleen of vIL-6tg2 mice. (A) Western blot for STAT3 tyrosine-705 phosphorylation (P-STAT3) and total STAT3 in PLN and spleen tissue from 4 wild-type and 4 vIL-6tg2 mice (15 weeks old). Lysates from vIL-6tg2 tissues were loaded according to increasing weight for the PLN (inguinal nodes combined; wt, 5.3-7.1 mg; vIL-6tg2, 9.2-38.9 mg) and spleen (wt, 60.9-78.8 mg; vIL-6tg2, 76.8-166.2 mg). Ratio of P-STAT3 to total STAT3 band intensities is given. (B) IHC for P-STAT3 of 21-week-old wild-type (i,iii) and vIL-6tg2 (ii,iv) lymph node (i-ii) and spleen (iii-iv; 10-fold magnification).
Increased STAT-3 phosphorylation in PLNs and spleen of vIL-6tg2 mice. (A) Western blot for STAT3 tyrosine-705 phosphorylation (P-STAT3) and total STAT3 in PLN and spleen tissue from 4 wild-type and 4 vIL-6tg2 mice (15 weeks old). Lysates from vIL-6tg2 tissues were loaded according to increasing weight for the PLN (inguinal nodes combined; wt, 5.3-7.1 mg; vIL-6tg2, 9.2-38.9 mg) and spleen (wt, 60.9-78.8 mg; vIL-6tg2, 76.8-166.2 mg). Ratio of P-STAT3 to total STAT3 band intensities is given. (B) IHC for P-STAT3 of 21-week-old wild-type (i,iii) and vIL-6tg2 (ii,iv) lymph node (i-ii) and spleen (iii-iv; 10-fold magnification).
It has been reported that expression of vIL-6 induces endogenous IL-6 in various cell lines and, importantly, in patients with MCD.21 We asked whether increased mouse IL-6 (mIL-6) expression could be observed in vIL-6tg2 mice. As shown in Figure 5, little or no mIL-6 was detected by IHC in lymph nodes and spleens from wild-type mice (Figure 5A,C). In contrast, robust expression was seen in both these tissues from vIL-6tg2 mice (Figure 5B,D), demonstrating that enforced expression of vIL-6 results in increased production of mIL-6 in lymphoid tissues of the mouse.
Increased mIL-6 expression in PLNs and spleen of vIL-6tg2 mice. IHC for mIL-6 in lymph node (PLN; A-B) and spleen (C-D) from 21-week-old wild-type (A,C; 20-fold magnification) and vIL-6tg2 (B,D; 40-fold magnification) mice. A subset of sinusoidal cells in PLN tissue expressed mIL-6 only in transgenic mice (B). A subset of cells in the red pulp of spleens from transgenic mice expressed mIL-6 (D).
Increased mIL-6 expression in PLNs and spleen of vIL-6tg2 mice. IHC for mIL-6 in lymph node (PLN; A-B) and spleen (C-D) from 21-week-old wild-type (A,C; 20-fold magnification) and vIL-6tg2 (B,D; 40-fold magnification) mice. A subset of sinusoidal cells in PLN tissue expressed mIL-6 only in transgenic mice (B). A subset of cells in the red pulp of spleens from transgenic mice expressed mIL-6 (D).
STAT3 phosphorylation can result from vIL-6 and mIL-6 stimulation. To gain insight into STAT3 phosphorylation/activation by vIL-6, B-lymphocytes, the principal target cells in the pathophysiology of plasma cell-type MCD in patients, were isolated from wild-type or IL-6–deficient mice37 and treated with exogenous vIL-6 or mIL-6 in the presence or absence of sgp130Fc, a well-established inhibitor of vIL-6 and IL-6/sIL-6R–induced trans-signaling.34 Figure 6 shows that normal, MACS-fractionated B220+ splenocytes treated with 500 ng/mL vIL-6 in vitro contained a significantly higher level of P-STAT3 (lane 5) than untreated (lane 1) IL-6–deficient or –proficient B cells (compare upper and lower panels). Splenocytes treated with 5 ng/mL mIL-6 showed considerably greater STAT3 phosphorylation than cells treated with vIL-6 (compare lanes 3 and 4 with lanes 5 and 6). In keeping with the function of vIL-6 in trans-signaling, cotreatment with 5 μg/mL sgp130Fc significantly diminished the level of P-STAT3 in B cells from IL-6−/− mice (lower panel, lanes 5 and 6). IL-6–proficient B cells showed an increase in the level of P-STAT3 compared with the level of total STAT3 and the loading control HPRT1 (upper panel, lanes 5 and 6). These findings confirm that vIL-6 alone is less potent than mIL-6 and suggest that the B-cell activation brought about by vIL-6 alone is not sufficient for the massive STAT3 activation required for the establishment of the MCD phenotype.
vIL-6–mediated signaling in mouse splenic B cells can only be inhibited in the absence of endogenous IL-6. Western blot for STAT3 tyrosine-705 phosphorylation (P-STAT3), total STAT3, and HPRT1 (loading control) from primary B cells isolated from normal (wt) or IL-6–deficient mice before treatment with or without mIL-6 (5 ng/mL), vIL-6 (500 ng/mL), or the trans-signaling inhibitor sgp130Fc (5 μg/mL) as indicated, for 18 hours. Ratio of P-STAT3 to total STAT3 to HPRT1 band intensities is given. *Nonspecific bands.
vIL-6–mediated signaling in mouse splenic B cells can only be inhibited in the absence of endogenous IL-6. Western blot for STAT3 tyrosine-705 phosphorylation (P-STAT3), total STAT3, and HPRT1 (loading control) from primary B cells isolated from normal (wt) or IL-6–deficient mice before treatment with or without mIL-6 (5 ng/mL), vIL-6 (500 ng/mL), or the trans-signaling inhibitor sgp130Fc (5 μg/mL) as indicated, for 18 hours. Ratio of P-STAT3 to total STAT3 to HPRT1 band intensities is given. *Nonspecific bands.
mIL-6 is an essential cofactor for MCD-like disease in vIL-6 TG mice
The results presented in the preceding paragraph raised the possibility that vIL-6 collaborates with mIL-6 to drive MCD-like changes in the mouse. To test this hypothesis with the help of a definitive, genetic approach, we transferred the vIL-6 transgene onto the B6 background of mIL-6 deficiency37 (IL-6KO), thus generating strain vIL-6tg2/IL-6KO mice in which mIL-6 cannot contribute to whatever change vIL-6 might induce. These mice showed no difference in spleen and lymph node size (Figure 7A-B) or an increase in serum IgG levels (Figure 7C) compared with age-matched, normal B6 and B6.IL-6KO mice. The absence of vIL-6-dependent changes could not have been caused by a drop in vIL-6 production because serum levels of vIL-6 were essentially the same in vIL-6tg2/IL-6KO mice and their IL-6–proficient counterparts (Figure 7D).
vIL-6tg2 mice deficient for mIL-6 do not display MCD-like phenotypes. (A) Average ratio of spleen to body weight determined from wild-type (n = 7), IL-6–deficient (IL-6KO; n = 6), and vIL-6tg2/IL-6–deficient mice (vIL-6tg2/IL-6KO; n = 6). (B) The averaged sum of inguinal lymph nodes per mouse determined from the same wild-type, IL-6KO, and vIL-6tg2/IL-6KO mice as in Figure 2B. (C) Average serum IgG levels as measured by ELISA from wild-type (n = 7), IL-6KO (n = 7), and vIL-6tg2/IL-6KO mice (n = 8). (D) Determination of vIL-6 serum concentrations of vIL-6tg2 mice with (n = 7) or without (n = 6) MCD and vIL-6tg2/IL-6KO mice (n = 11) by ELISA. (E) The percentage of mice with MCD (filled) or borderline MCD (open) in vIL-6tg2 (n = 25) and vIL-6tg2/IL-6KO (n = 17) mice. Error bars represent SD. *P ≤ .01. All mice were 13 to 20 weeks old.
vIL-6tg2 mice deficient for mIL-6 do not display MCD-like phenotypes. (A) Average ratio of spleen to body weight determined from wild-type (n = 7), IL-6–deficient (IL-6KO; n = 6), and vIL-6tg2/IL-6–deficient mice (vIL-6tg2/IL-6KO; n = 6). (B) The averaged sum of inguinal lymph nodes per mouse determined from the same wild-type, IL-6KO, and vIL-6tg2/IL-6KO mice as in Figure 2B. (C) Average serum IgG levels as measured by ELISA from wild-type (n = 7), IL-6KO (n = 7), and vIL-6tg2/IL-6KO mice (n = 8). (D) Determination of vIL-6 serum concentrations of vIL-6tg2 mice with (n = 7) or without (n = 6) MCD and vIL-6tg2/IL-6KO mice (n = 11) by ELISA. (E) The percentage of mice with MCD (filled) or borderline MCD (open) in vIL-6tg2 (n = 25) and vIL-6tg2/IL-6KO (n = 17) mice. Error bars represent SD. *P ≤ .01. All mice were 13 to 20 weeks old.
Histologic investigations of vIL-6tg2/IL-6KO mice, identical to those presented in Figures 2 to 4, revealed that loss of IL-6 effectively abrogates MCD-like disease in mouse. Thus, in striking contrast to age-matched IL-6–proficient vIL-6tg2 mice, hyperplastic germinal centers, extramedullary hematopoiesis, and plasmacytosis were largely absent in lymphoid tissues of vIL-6tg2/IL-6KO mice 4 to 5 months of age (Figure 7E; Table 1). These results indicate that mIL-6 is an essential cofactor for vIL-6–induced MCD-like phenotypes in mouse. They also suggest that IL-6 trans-signaling plays a pathophysiologic role of great relevance for the massive accumulation of plasma cells seen in patients with plasma cell-type MCD.
Discussion
There are 2 major findings in this study. First, we show that transgenic expression of vIL-6 is sufficient to induce a MCD-like phenotype as characterized by hyperplastic germinal centers in the lymph nodes and elevated levels of extramedullary hematopoiesis or plasmacytosis in the spleen. These features together with hypergammaglobulinemia are hallmarks of plasma cell-type MCD in humans. Although not a perfect model for MCD because of incomplete penetrance by the time of analysis (12-21 weeks of age) and an absence of liver abnormalities, vIL-6 mice faithfully develop a majority of the prominent features of MCD. Second, we demonstrate that endogenous mammalian IL-6 is essential to the development of this phenotype because the abnormalities are absent in vIL-6 transgenic mice that are IL-6–deficient.
The association of HHV-8 with KS, PEL, and MCD is widely accepted.10 Besides vIL-6, HHV-8 encodes several genes suspected to have pathologic roles, including vGPCR, a transmembrane glycoprotein encoded by the K1 gene, and vFLIP.11,15,39 Other HHV-8 genes, such as ORFs K9 and K12, are also thought to possess transforming potential, whereas others are probably involved in proliferation or persistence of virus-infected cells.10 With the exception of vFLIP, none of these viral gene products has been shown to cause a MCD-like phenotype as did vIL-6 transgenic mice. Interestingly, vFLIP has been shown to induce expression of mammalian IL-6.40,41 This may indicate that, as is the case for vIL-6 transgenic mice, mIL-6 is required for the MCD-like manifestations in vFLIP transgenic mice. Signs of KS or PEL were not observed in vIL-6 transgenic mice, suggesting that vIL-6 alone plays little, if any, causative role in the development of these diseases.
There is no standard treatment for patients with MCD. The monoclonal antibody rituximab, which binds to the B-cell antigen CD20, has shown promising results in HHV-8-infected patients.10 An emerging line of treatment for MCD are IL-6 neutralization-based therapies.27,42 In 2005, Nishimoto et al28 published results from a clinical trial in which the neutralizing anti–IL-6R antibody tocilizumab was administered to 28 patients with MCD. In this study, 2 of the 28 patients were seropositive for HHV-8. Within 16 weeks, patients receiving tocilizumab showed improvement of both lymphadenopathy and the inflammatory condition.28 At first, it was counterintuitive that the 2 HHV-8 positive patients also improved because vIL-6 does not bind to or requires the IL-6R for gp130 stimulation.31,–33 Nishimoto et al speculated that human IL-6 might be involved in the development of the disease in these 2 patients.28 Our study now, for the first time, provides an explanation of this phenomenon. In the mouse, the presence of vIL-6 leads to local production of mIL-6, which is largely required for the development of the MCD-like phenotype. It is not a stretch to presume that the presence of vIL-6 leads to the elevated level of human IL-6 in MCD patients, especially given that vIL-6 has been shown to induce human IL-6 expression in cell lines from patients with MCD.21 Thus, treatment of HHV-8 seropositive patients with agents that neutralize IL-6 or IL-6R should be effective, even though vIL-6 itself is not directly neutralized by these agents.
Our findings with vIL-6 mice reinforce the notion that inflammatory mediators, especially IL-6, are important for the pathogenesis of MCD.38 It is also consistent with the observation that, even though abnormalities are common in the B-cell compartment, not all different IL-6 transgenic mice are equal. Transgenic mice that express human IL-6 under the transcriptional control of a human Ig heavy chain enhancer displayed polyclonal hypergammaglobulinemia and massive plasmacytosis.43 Transgenic mice in which the human IL-6 gene was controlled by the MHC-1 H-2Ld promoter on a BALB/c genetic background developed transplantable plasmacytomas with chromosomal translocations,44 a finding in line with the observation that IL-6 was required for plasmacytoma formation in BALB/c mice.45 We previously generated mice that were transgenic for human IL-6 and human sIL-6R under the control of liver specific promoters.46 These mice exhibited an abnormally high level of trans-signaling that corresponded with hyperplastic germinal centers in lymph nodes and extramedullary hematopoiesis or plasmacytosis in the spleen, as well as spontaneous hepatocellular proliferation.47 Particularly pertinent to the present study is the report that mice transgenic for mIL-6 under the control of the same promoter used to drive transgenic vIL-6 did not develop MCD-like characteristics.35 These mice, instead, developed lymphomas at an advanced age (∼ 18 months). When considered with our finding that the MCD-like phenotype in vIL-6 TG mice depends largely on endogenous mIL-6, the Woodroofe et al35 finding suggests that elevations of mIL-6 alone are not sufficient for manifestation of MCD. The reasons for this are unclear at present but may, in part, be related to the complexity of IL-6 signaling, perhaps involving a necessary combination of classic IL-6R signaling by mammalian IL-6 and direct stimulation of gp130 by vIL-6.
A very recent publication showed that vIL-6 interacts with a splice variant of vitamin K epoxide reductase complex subunit 1 (VKORC1v2) within the ER of vIL-6 expressing cells. VKORC1v2 itself showed growth stimulatory and antiapoptotic activity, which was independent of gp130 signaling. The authors speculate that there exists an alternative pathway of vIL-6 activity via VKORC1v2.48 If confirmed, these data help to provide an explanation for the unusual biologic activity of vIL-6.
We have previously shown that a soluble form of gp130 (sgp130) is the natural inhibitor of IL-6 trans-signaling and that this protein does not interfere with classic IL-6 signaling via the membrane bound IL-6R.49 It is now understood that the proinflammatory properties of IL-6 are mostly mediated by the IL-6/sIL-6R complex that mediates trans-signaling.34 It has been suggested that the sgp130 protein recombinantly expressed as a dimerized fusion protein with the constant portion of human IgG1 (sgp130Fc) could be used as a therapeutic protein in the treatment of inflammatory diseases and inflammation-associated cancer.50 Because sgp130Fc inhibits not only the IL-6/sIL-6R complex but also directly blocks vIL-6,31,32,49 this protein may be an ideal candidate to add to treatment of patients with HHV-8–associated MCD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Elsbeth Schulz for most skillful and excellent technical assistance with histology.
This work was supported in part by the Multiple Myeloma Research and International Waldenström's Macroglobulinemia foundations (research awards; S.J.), the National Institutes of Health (R01CA151354, S.J.; T32-HL07734, V.S.T.; and 5T32AI007485-17, T.R.R.), the Deutsche Forschungsgemeinschaft, Bonn, Germany (J. Scheller and S.R.-J.; SFB415, projects B5, C6; SFB877, projects A1, A2), and the Cluster of Excellence Inflammation at Interfaces.
National Institutes of Health
Authorship
Contribution: J. Suthaus performed the experiments and analyzed data; V.S.T. performed research, analyzed data, and revised the manuscript; C.S.-L., W.K., and T.R.R. performed research and analyzed data; G.T. contributed vital new reagents and analyzed data; S.J. generated supplemental results and provided editorial assistance; and J. Scheller and S.R.-J. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: G.T. is a co-inventor on a patent describing the measurement of KSHV vIL-6. This invention was made when G.T. was an employee of the US government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the US Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing finanicial interests.
Correspondence: Stefan Rose-John, Institute of Biochemistry, Christian-Albrechts-University, Olshausenstr 40, Kiel, Germany; e-mail: rosejohn@biochem.uni-kiel.de; and Jürgen Scheller, Institute of Biochemistry and Molecular Biology II, Medical Faculty, Heinrich-Heine-University, Universitätsstr 1, Düsseldorf, Germany; e-mail: jscheller@uni-duesseldorf.de.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal