Abstract
Invariant natural killer T (iNKT) cells are powerful immunomodulatory cells that in mice regulate a variety of immune responses, including acute GVHD (aGVHD). However, their clinical relevance and in particular their role in clinical aGVHD are not known. We studied whether peripheral blood stem cell (PBSC) graft iNKT-cell dose affects on the occurrence of clinically significant grade II-IV aGVHD in patients (n = 57) undergoing sibling, HLA-identical allogeneic HSCT. In multivariate analysis, CD4− iNKT-cell dose was the only graft parameter to predict clinically significant aGVHD. The cumulative incidence of grade II-IV aGVHD in patients receiving CD4− iNKT-cell doses above and below the median were 24.2% and 71.4%, respectively (P = .0008); low CD4− iNKT-cell dose was associated with a relative risk of grade II-IV aGVHD of 4.27 (P = .0023; 95% CI, 1.68-10.85). Consistent with a role of iNKT cells in regulating aGVHD, in mixed lymphocyte reaction assays, CD4− iNKT cells effectively suppressed T-cell proliferation and IFN-γ secretion in a contact-dependent manner. In conclusion, higher doses of CD4− iNKT cells in PBSC grafts are associated with protection from aGVHD. This effect could be harnessed for prevention of aGVHD.
Introduction
Invariant natural killer T (iNKT) cells are a subset of rare but powerful immunomodulatory T cells that are highly conserved between humans and mice.1 They are selectively activated by glycolipids such as the prototypic ligand α-galactosylceramide (αGC) presented by CD1d and are characterized by an invariant TCRα pairing with a diverse TCRβ chain (TCRVα24Jα18 and TCRVβ11 in humans).1 iNKT cells comprise 2 main subsets, CD4+ and CD4− cells, which in humans have distinct cytokine secretion profiles.2 Although the ability of murine iNKT cells to modulate immune responses against pathogens, in autoimmunity and in alloreactivity, including experimental acute GVHD (aGVHD), is firmly established,3 and the functional role, if any, of human iNKT cells in physiology and disease is ill-defined.4
Acute GVHD is the main source of treatment-related morbidity and mortality in patients receiving a T cell–replete allogeneic HSCT. It is caused by alloreactive donor T cells that are activated by host APCs as a result of minor or major histocompatibility Ag disparity between donor and recipient5,6 and subsequently target recipient tissues such as the skin, liver, and gut.6 The critical role of T cells as effectors of aGVHD is highlighted by the dramatic reduction in the incidence and severity of aGVHD in patients receiving T cell–depleted or syngeneic allografts.7 Because T-cell depletion of the graft is also associated with an increased risk of leukemia relapse and infections, research has also focused on identifying other cellular components of the graft that affect the incidence and severity of aGVHD. Indeed, the effect of the graft content of several immune effectors such as T, NK8 and more recently B cells9 as well as of the CD4+CD25hiFoxP3+ T regulatory cells (Tregs)10,11 on the risk of aGVHD has been studied extensively.
The role of graft iNKT cells on the risk of aGVHD has not been investigated in humans. By contrast, in murine models, both recipient and donor iNKT cells were shown to effectively protect against experimental aGVHD. In a model that involved lymphoablation with total lymphoid irradiation and antithymocyte globulin (ATG), recipient iNKT cells preferentially survive because of radioresistance, secrete IL-4, and thus inhibit aGVHD.12 In line with these findings, ATG/lymphoablation with total lymphoid irradiation conditioning was shown to be associated with reduced incidence of aGVHD in humans.13 Remarkably, iNKT cells in G-CSF–mobilized grafts were shown to protect from experimental aGVHD while enhancing the GVL effect,14 and in vitro–expanded donor iNKT cells alleviate aGVHD in a MHC haploidentical setting.15,16 Recent work also found the ability of unmanipulated, adoptively transferred donor iNKT cells to protect from experimental aGVHD without prior in vitro expansion.17
To directly test whether the protective role of donor iNKT cells shown in preclinical models also holds true in clinical allogeneic HSCT, we studied the effect of the dose of graft iNKT cells on the incidence and severity of aGVHD after a T cell–replete allogeneic HSCT from HLA-identical sibling donors.
Methods
Donors
The research protocol was approved by the Imperial College Healthcare NHS Trust Research Ethics Committee, and all participants gave written informed consent in accordance with the Declaration of Helsinki. We analyzed the frequency of effector and regulatory lymphocytes in cryopreserved samples of 78 sibling donor, G-CSF–mobilized peripheral blood stem cell (PBSC) grafts used for allogeneic HSCT between 1998 and 2011. In all cases, stem cell mobilization, collection, and storage of the graft were performed according to the same, standard operating procedures approved by the Joint Accreditation Committee of International Society for Cellular Therapy Europe and European Group for Blood and Marrow Transplantation. aGVHD diagnosis and grading criteria were as described.18
Flow cytometry and flow sorting
Staining of PBSC graft cells was performed at 4°C for 20 minutes in the presence of directly fluorescently conjugated mAbs. Cells were resuspended in PBS plus 0.5% BSA. Fc blocking reagent (Miltenyi Biotec) was used to reduce nonspecific staining, and 4′,6-diamidino-2-phenylindole was used for dead cell exclusion. The mAbs used were anti-TCRVα24–PE, TCRVβ11-FITC (Beckman Coulter), CD4-PE–Cy7, CD161-Allophycocyanin, CD34-PerCP, CD16-FITC, CD8-Allophycocyanin (BD Biosciences), CD3-eFluor450, and CD56-PE, CD19-Allophycocyanin–eFlour780 (eBioscience). For intracellular FoxP3 staining, after surface staining, cells were fixed, permeabilized, and stained with the use of buffers and an anti–human FoxP3-FITC mAb (clone PCH101) from eBioscience, according to the manufacturer's instructions. In parallel, samples were stained with appropriate isotypic controls. Samples were analyzed in FACSCalibur and LSR Fortessa analyzers, whereas FACS was performed with a FACSDiva sorter (BD Biosciences). Data analysis was performed with FlowJo software (TreeStar). Because iNKT cells are a rare cell population, either a minimum of 0.5 × 106 CD3+ cells or 200 iNKT cells were recorded to facilitate accurate calculation of the total, CD4−, and CD4+ iNKT-cell frequencies.
Graft cellular component frequency and dose
The frequencies of CD34+ cells, total CD3+ T cells, CD4+ and CD8+ T cells, CD19+ B cells, and CD3−CD56+CD16+/− NK cells were determined by flow cytometry as a percentage of the total live cells. CD3+CD4+CD25hiFoxP3+ Tregs, CD3+CD161+ T cells, total, CD4+, and CD4− CD3+TCRVα24+TCRVβ11+ iNKT-cell frequencies are presented as percentage of the total T cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Infused cell doses of cellular components were calculated on the basis of the CD34+ cell dose infused to the patient.
CD3+ T-cell selection
Buffy coats from healthy blood donors were obtained from the North London Blood Transfusion Service and PBMCs were isolated after layering over Ficoll (Axis Shield Diagnostics). For negative selection of CD3+ cells the StemSep Human T Cell Enrichment Cocktail (StemCell Technologies) was used according to the manufacturer's instructions. Purity of CD3+ cells was always > 95% (not shown).
iNKT-cell expansion
PBMCs were placed in 24 well plates at 1-2 × 106 cells/mL in complete T-cell medium (TCM) consisting of RPMI 1640 without l-glutamine (Invitrogen) supplemented with 5% heat-inactivated human serum obtained from the North London Blood Transfusion Service, penicillin + streptomycin (Sigma-Aldrich), and l-glutamine (Sigma-Aldrich). αGC was added at 100 ng/mL; IL-2 was added on day 3 at 10 U/mL and every 3-4 days thereafter. Cells were harvested on day 10 for further experiments without testing for cytokine or perforin secretion.
MLRs and proliferation assays
For mixed lymphocyte reactions (MLRs), 3-5 × 104 negatively selected CD3+ cells (responders) were placed in triplicates against equal numbers of autologous or allogeneic (stimulators) irradiated (30 Gy [3000 rad]) PBMCs in 96-well plates for 96 hours. 3H-thymidine (Amersham Biosciences) was added at 1 μCi (0.037 Bq)/well for the last 16 hours of the MLR. Proliferation was measured with the use of a liquid scintillation counter (Wallac β counter).
Cytokine ELISAs
The Quantikine kit (all from R&D Systems) was used according to the manufacturer's instructions for the measurement of IFN-γ in the supernatant fluids of the MLRs.
Transwell experiments
To determine whether the suppressive effect was contact dependent, MLRs were set up in a transwell system (transwells of pore size 0.4 μm; Costar) such that 3 × 104/well responder CD4+ or CD4− iNKT cells that had undergone FACS were either placed in the lower chamber along with 3 × 105/well responder CD3+ cells and 6 × 105/well irradiated stimulator cells, or they were segregated from the responder cells by being placed in the top chamber. An MLR in which responders and stimulators were mixed in the lower chambers without iNKT cells served as the baseline. After 96 hours equal volumes of cells were removed and set up in triplicate in 96-well plates for a primary proliferation assay. 3H-thymidine was added at 1 μCi (0.037 Bq)/well for the final 16 hours when proliferation was measured in a liquid scintillation counter.
DC generation
For myeloid dendritic cell (DC) generation, CD14-selected cells were initially plated at 1 × 106/mL in a 24-well plate in medium consisting of RPMI 1640 (Invitrogen), 10% heat inactivated human serum (normal screened donors; North London Blood Center) supplemented with 1% penicillin/streptomycin (Sigma-Aldrich) and l-glutamine (Sigma-Aldrich). GM-CSF (Sigma-Aldrich) at 50 ng/mL and IL-4 (Sigma-Aldrich) at 1000 U/mL were added to each well to generate immature DC. After 4 days in culture DCs were matured with TNFα (Sigma-Aldrich) at 50 ng/mL and cultured for a further 40 hours. Maturity was checked by flow cytometry with the use of the following Abs: CD14-FITC, CD80-PE, CD83-PE, CD86-PE, and HLA-DR-Allophycocyanin (BD Biosciences). Mature DCs were identified by the increased CD80, CD83, CD86, and HLA-DR and decreased CD14 expression (not shown).
Cr51 release assay
Target cells were resuspended in TCM, labeled with Cr51 (100 μCi [3.7 Bq]/107 cells) for 1 hour at 37°C, 5% CO2; after 3 washes with PBS they were seeded into V-shaped 96-well plates at a concentration of 104 cells/well. Effector CD4− iNKT cells were added at different effector-to-target cell (E/T) ratios in triplicates. For measurement of the spontaneous release of Cr,51 TCM was added to target cells instead of iNKT cells and for the measurement of total release, Triton-X (1% final concentration) was added to target cells instead of iNKT cells. After 4 hours of incubation at 37°C, 5% CO2, Cr51 release was measured with the use of a β counter. The Cr51-specific release was calculated with the following formula: percentage of specific Cr51release = release of test-spontaneous release/total release-spontaneous release.
Statistical analysis
Cellular subset variables were divided into low- and high-dose groups with the use of the median value. Probabilities of grades 0-I and II-IV aGVHD were calculated with the cumulative incidence procedure, with relapse and death without aGVHD within 180 days (n = 4) treated as competing events. Univariate comparisons for both cellular subset and clinical variables were made with Gray test.19 Variables significant at the P < .1 level were entered into a regression model by Jason and Gray,20 and a backward stepping procedure was used to find the best model. Graft cell subset doses infused were compared between the aGVHD groups with the use of the Mann-Whitney test. Statistical analyses were performed with the software packages SPSS Version 19 (SPSS) and R (The R Project). P values < .05 were deemed statistically significant.
Results
Frequency distribution of effector and regulatory lymphocytes in G-CSF–mobilized PBSC donor grafts
The frequencies of total, CD4+, and CD4− iNKT cells; CD4+, CD8+, and total T cells; B cells; NK cells; and Tregs were analyzed by multicolor flow cytometry in 78 healthy donor G-CSF–mobilized PBSC grafts (supplemental Figure 1). In addition, the frequency of CD3+CD161+ Tregs previously shown to be enriched in noninvariant, CD1d-restricted T cells that can suppress in vitro alloreactivity was determined.21 The frequencies and the absolute doses of the graft cell populations infused to patients at the time of PBSC transplantation are shown in Table 1. A striking feature of iNKT cells that distinguishes them from the other cellular components is their distinct skewed distribution pattern and dramatic (up to a 1000-fold) variation of their frequency (supplemental Figure 2; Table 1). This variation is independent of sex and age (supplemental Figure 3). Finally, there was no correlation between frequency of iNKT cells with that of Tregs or any of the other T-cell subsets (data not shown).
PBSC graft cell subset frequencies and cell doses from 78 healthy donors
| . | Frequencies of graft cell subsets, (%) . | Doses of graft cell subsets infused, 106 cells/kg of body weight . | ||
|---|---|---|---|---|
| Mean ± SD . | Median (min-max) . | Mean ± SD . | Median (min-max) . | |
| CD34+ | 2.1 ± 1.7 | 1.6 (0.4-12.6) | 5.9 ± 2.8 | 5.7 (1.1-16.8) |
| NK cells | 3.6 ± 2.5 | 3.2 (0.5-18.4) | 13.2 ± 12.3 | 10.5 (0.46-80.8) |
| B cells | 11.9 ± 6.5 | 10.5 (0.9-34.8) | 46.1 ± 52.1 | 33.9 (5.1-162.3) |
| T cells | 45.6 ± 11.4 | 45.30 (21.8-75.4) | 170.5 ± 119 | 145 (16.8-684.1) |
| CD4+ T cells | 30.4 ± 8.6 | 29.8 (12.6-56.5) | 115 ± 86.9 | 89.9 (8.8-511) |
| CD8+ T cells | 13.1 ± 5.8 | 11.8 (4.1-29.2) | 47.7 ± 34.6 | 36.8 (5.4-154) |
| Tregs | 3.8 ± 1.6 | 3.5 (0.9-8.2) | 6.68 ± 6.5 | 4.8 (0.4-41.3) |
| CD3+CD161+ | 12.7 ± 6.9 | 12.6 (1.6-32.6) | 10.1 ± 11.3 | 6.4 (0.6-62.1) |
| iNKT cells | 0.139 ± 0.23 | 0.043 (0.001-1.07) | 0.2 ± 0.312 | 0.052 (0.002-1.4) |
| iNKT CD4− | 0.091 ± 0.175 | 0.019 (0.001-0.76) | 0.113 ± 0.2 | 0.031 (0.001-1.04) |
| iNKT CD4+ | 0.061 ± 0.12 | 0.015 (0.001-0.68) | 0.091 ± 0.175 | 0.025 (0.001-1.09) |
| . | Frequencies of graft cell subsets, (%) . | Doses of graft cell subsets infused, 106 cells/kg of body weight . | ||
|---|---|---|---|---|
| Mean ± SD . | Median (min-max) . | Mean ± SD . | Median (min-max) . | |
| CD34+ | 2.1 ± 1.7 | 1.6 (0.4-12.6) | 5.9 ± 2.8 | 5.7 (1.1-16.8) |
| NK cells | 3.6 ± 2.5 | 3.2 (0.5-18.4) | 13.2 ± 12.3 | 10.5 (0.46-80.8) |
| B cells | 11.9 ± 6.5 | 10.5 (0.9-34.8) | 46.1 ± 52.1 | 33.9 (5.1-162.3) |
| T cells | 45.6 ± 11.4 | 45.30 (21.8-75.4) | 170.5 ± 119 | 145 (16.8-684.1) |
| CD4+ T cells | 30.4 ± 8.6 | 29.8 (12.6-56.5) | 115 ± 86.9 | 89.9 (8.8-511) |
| CD8+ T cells | 13.1 ± 5.8 | 11.8 (4.1-29.2) | 47.7 ± 34.6 | 36.8 (5.4-154) |
| Tregs | 3.8 ± 1.6 | 3.5 (0.9-8.2) | 6.68 ± 6.5 | 4.8 (0.4-41.3) |
| CD3+CD161+ | 12.7 ± 6.9 | 12.6 (1.6-32.6) | 10.1 ± 11.3 | 6.4 (0.6-62.1) |
| iNKT cells | 0.139 ± 0.23 | 0.043 (0.001-1.07) | 0.2 ± 0.312 | 0.052 (0.002-1.4) |
| iNKT CD4− | 0.091 ± 0.175 | 0.019 (0.001-0.76) | 0.113 ± 0.2 | 0.031 (0.001-1.04) |
| iNKT CD4+ | 0.061 ± 0.12 | 0.015 (0.001-0.68) | 0.091 ± 0.175 | 0.025 (0.001-1.09) |
CD34+, NK, B, T, CD4+, and CD8+ T-cell frequencies are shown as percentage of the total live cells. Tregs, CD3+CD161+, and iNKT-cell frequencies are shown as percentage of the T cells.
Patient characteristics
To analyze the effect of clinical variables and graft cellular components on aGVHD, of the 78 studied donors we selected a cohort of 57 consecutive donor-recipient pairs in whom a G-CSF–mobilized PBSC graft was used for a T cell–replete, sibling HLA-identical, either myeloablative (n = 41) or reduced intensity conditioning (n = 16) allogeneic HSCT for hematologic malignancies. These included chronic myeloid leukemia (CML; n = 18), acute myeloid leukemia (n = 23), acute lymphoblastic leukemia (n = 6), myelodysplastic syndrome (n = 5), multiple myeloma (n = 2), myelofibrosis (n = 2), and Sezary syndrome (n = 1; supplemental Table 1). None of the 57 patients received either ATG or alemtuzumab in the preparative regimen. aGVHD prophylaxis was cyclosporine-A plus methotrexate. The inclusion criteria aimed at minimizing confounding factors that would mask the effect of graft iNKT cells on the incidence and severity of aGVHD. Therefore, T cell–depleted HSCTs were not included.
PBSC graft content and aGVHD
Because iNKT cells uniquely among the lymphocyte subsets showed a distinct skewed distribution pattern, we predicted that, in conjunction with the marked variability of their frequency in PBSC grafts, iNKT-cell graft dose could be an important determinant of the incidence and severity of aGVHD.
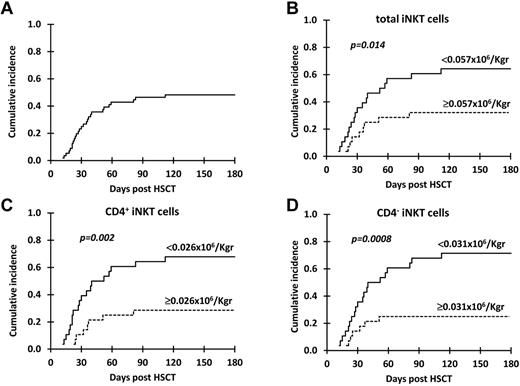
The cumulative incidence of grade II-IV aGVHD for the 57 patients receiving a G-CSF–mobilized PBSC, T cell–replete, HLA-identical, sibling donor allograft was 47.3% at 180 days after transplantation (Figure 1A). The effect of the dose of the principal cellular components of the PBSC graft and of clinical variables (Table 2) previously shown to affect the incidence of grade II-IV aGVHD22-26 were analyzed.
Cumulative incidence of grade II-IV aGVHD. Cumulative incidence of grade II-IV aGVHD in all patients (A) and in patients receiving below versus above the median dose of (B) total iNKT, (C) CD4+ iNKT, and (D) CD4− iNKT cells.
Cumulative incidence of grade II-IV aGVHD. Cumulative incidence of grade II-IV aGVHD in all patients (A) and in patients receiving below versus above the median dose of (B) total iNKT, (C) CD4+ iNKT, and (D) CD4− iNKT cells.
Clinical variables and PBSC graft cell subset doses tested in univariate analysis
| Variables* . | Cumulative incidence of grade II-IV aGVHD, % (95% CI) . | P . |
|---|---|---|
| TBI | .26 | |
| TBI/Cy (n = 39) | 51 (34-66) | |
| Non-TBI (n = 18) | 39 (16-61) | |
| Recipient age | .91 | |
| < 47.2 y | 46 (27-63) | |
| ≥ 47.2 y | 48 (29-65) | |
| Donor age | .85 | |
| < 47.5 y | 46 (27-64) | |
| ≥ 47.5 y | 48 (29-65) | |
| Sex mismatch | .46 | |
| Yes (n = 19) | 50 (33-65) | |
| No (n = 38) | 42 (20-63) | |
| Disease risk† | .74 | |
| Standard (n = 16) | 44 (19-66) | |
| High (n = 41) | 49 (36-63) | |
| Conditioning | .53 | |
| Myeloablative (n = 41) | 44 (19-67) | |
| RIC (n = 16) | 49 (33-63) | |
| Diagnosis | .038 | |
| CML (n = 18) | 61 (34-80) | |
| Other (n = 39) | 41 (25-56) | |
| CD34+ cells | .52 | |
| < 5.66 × 106 cells/kg of body weight | 54 (33-70) | |
| ≥ 5.66 × 106 cells/kg of body weight | 41 (23-59) | |
| NK cells | .28 | |
| < 11.7 × 106 cells/kg of body weight | 39 (21-57) | |
| ≥ 11.7 × 106 cells/kg of body weight | 55 (35-71) | |
| B cells | .12 | |
| < 33.5 × 106 cells/kg of body weight | 57 (36-73) | |
| ≥ 33.5 × 106 cells/kg of body weight | 38 (20-55) | |
| T cells | .24 | |
| < 154 × 106 cells/kg of body weight | 54 (32-70) | |
| ≥ 154 × 106 cells/kg of body weight | 41 (23-58) | |
| CD4+ T cells | .71 | |
| < 91.2 × 106 cells/kg of body weight | 46 (27-64) | |
| ≥ 91.2 × 106 cells/kg of body weight | 48 (29-65) | |
| CD8+ T cells | .09 | |
| < 40.3 × 106 cells/kg of body weight | 57 (36-73) | |
| ≥ 40.3 × 106 cells/kg of body weight | 38 (20-55) | |
| Tregs | .6 | |
| < 4.9 × 106 cells/kg of body weight | 50 (30-67) | |
| ≥ 4.9 × 106 cells/kg of body weight | 45 (26-61) | |
| CD3+CD161+ cells | .62 | |
| < 7.07 × 106 cells/kg of body weight | 46 (27-64) | |
| ≥ 7.07 × 106 cells/kg of body weight | 48 (28-65) | |
| iNKT cells | .014 | |
| < 0.057 × 106 cells/kg of body weight | 64 (43-79) | |
| ≥ 0.057 × 106 cells/kg of body weight | 31 (15-48) | |
| CD4+ iNKT cells | .002 | |
| < 0.026 × 106 cells/kg of body weight | 68 (46-82) | |
| ≥ 0.026 × 106 cells/kg of body weight | 26 (13-45) | |
| CD4− iNKT cells | .0008 | |
| < 0.031 × 106 cells/kg of body weight | 71 (50-85) | |
| ≥ 0.031 × 106 cells/kg of body weight | 24 (10-41) |
| Variables* . | Cumulative incidence of grade II-IV aGVHD, % (95% CI) . | P . |
|---|---|---|
| TBI | .26 | |
| TBI/Cy (n = 39) | 51 (34-66) | |
| Non-TBI (n = 18) | 39 (16-61) | |
| Recipient age | .91 | |
| < 47.2 y | 46 (27-63) | |
| ≥ 47.2 y | 48 (29-65) | |
| Donor age | .85 | |
| < 47.5 y | 46 (27-64) | |
| ≥ 47.5 y | 48 (29-65) | |
| Sex mismatch | .46 | |
| Yes (n = 19) | 50 (33-65) | |
| No (n = 38) | 42 (20-63) | |
| Disease risk† | .74 | |
| Standard (n = 16) | 44 (19-66) | |
| High (n = 41) | 49 (36-63) | |
| Conditioning | .53 | |
| Myeloablative (n = 41) | 44 (19-67) | |
| RIC (n = 16) | 49 (33-63) | |
| Diagnosis | .038 | |
| CML (n = 18) | 61 (34-80) | |
| Other (n = 39) | 41 (25-56) | |
| CD34+ cells | .52 | |
| < 5.66 × 106 cells/kg of body weight | 54 (33-70) | |
| ≥ 5.66 × 106 cells/kg of body weight | 41 (23-59) | |
| NK cells | .28 | |
| < 11.7 × 106 cells/kg of body weight | 39 (21-57) | |
| ≥ 11.7 × 106 cells/kg of body weight | 55 (35-71) | |
| B cells | .12 | |
| < 33.5 × 106 cells/kg of body weight | 57 (36-73) | |
| ≥ 33.5 × 106 cells/kg of body weight | 38 (20-55) | |
| T cells | .24 | |
| < 154 × 106 cells/kg of body weight | 54 (32-70) | |
| ≥ 154 × 106 cells/kg of body weight | 41 (23-58) | |
| CD4+ T cells | .71 | |
| < 91.2 × 106 cells/kg of body weight | 46 (27-64) | |
| ≥ 91.2 × 106 cells/kg of body weight | 48 (29-65) | |
| CD8+ T cells | .09 | |
| < 40.3 × 106 cells/kg of body weight | 57 (36-73) | |
| ≥ 40.3 × 106 cells/kg of body weight | 38 (20-55) | |
| Tregs | .6 | |
| < 4.9 × 106 cells/kg of body weight | 50 (30-67) | |
| ≥ 4.9 × 106 cells/kg of body weight | 45 (26-61) | |
| CD3+CD161+ cells | .62 | |
| < 7.07 × 106 cells/kg of body weight | 46 (27-64) | |
| ≥ 7.07 × 106 cells/kg of body weight | 48 (28-65) | |
| iNKT cells | .014 | |
| < 0.057 × 106 cells/kg of body weight | 64 (43-79) | |
| ≥ 0.057 × 106 cells/kg of body weight | 31 (15-48) | |
| CD4+ iNKT cells | .002 | |
| < 0.026 × 106 cells/kg of body weight | 68 (46-82) | |
| ≥ 0.026 × 106 cells/kg of body weight | 26 (13-45) | |
| CD4− iNKT cells | .0008 | |
| < 0.031 × 106 cells/kg of body weight | 71 (50-85) | |
| ≥ 0.031 × 106 cells/kg of body weight | 24 (10-41) |
TBI indicates total body irradiation; Cyclo, cyclophosphamide; and RIC, reduced intensity conditioning.
Median values are shown.
Standard risk indicates CML chronic phase 1, acute myeloid leukemia/acute lymphoblastic leukemia standard risk in first complete remission; and high risk, all other.
In univariate analyses, total iNKT-, CD4+ iNKT-, and CD4− iNKT-cell doses and the diagnosis of CML were significantly (P < .05) associated with grade II-IV aGVHD (Figure 1B-D; Table 2). The cumulative incidence of grade II-IV aGVHD at 180 days after HSCT for recipients receiving cell doses below and above the median for total iNKT cells was 64.3% versus 31% (P = .014), for CD4+ iNKT cells it was 67.5% versus 27.6% (P = .002), and for CD4− iNKT cells it was 71.4% versus 24.2% (P = .0008). In line with these results, iNKT-cell doses were the only graft variables that were statistically different between patients who subsequently developed grade II-IV and those with grade 0-I aGVHD (supplemental Table 2). Unlike iNKT cells, graft Treg dose did not predict grade II-IV aGVHD; a similar cumulative incidence of aGVHD was found in patients receiving cell doses below and above the median (50% vs 45%; P = .6; Table 2).
Several clinical variables that affect the risk of aGVHD such as sibling versus unrelated donor, HLA disparities, stem cell source, and prophylaxis regimen, including the use of ATG or alemtuzumab, were not relevant to our cohort of patients. On univariate analysis of other clinical variables previously shown to confer an increased risk of grade II-IV aGVHD in sibling stem cell transplantations, including recipient and donor age, use of total body irradiation, sex-mismatched transplantations, and diagnosis of CML,22-26 only the latter was found to affect the occurrence of aGVHD (Table 2). Specifically, the cumulative incidence of grade II-IV aGVHD for patients with a diagnosis of CML was 61% compared with 41% for other malignancies (P = .038).
After multivariate regression analysis, CD4− iNKT-cell dose and CML diagnosis were the only independent factors associated with the occurrence of grade II-IV aGVHD (Table 3). A low CD4− iNKT-cell dose was associated with a relative risk (RR) of grade II-IV aGVHD of 4.27 (P = .0023; 95% CI, 1.68-10.85), whereas diagnosis of CML conferred a RR of 2.54 (P = .036; 95% CI, 1.06-6.04). Unlike aGVHD, we found no association between the iNKT-cell dose infused and occurrence of moderate-to-severe chronic GVHD (data not shown).
Variables from a multivariate analysis describing the probability of developing severe aGVHD
| Variable . | No. . | RR . | 95% CI . | P . |
|---|---|---|---|---|
| Diagnosis | 57 | |||
| Non-CML | 1 | |||
| CML | 2.54 | 1.06-6.04 | .036 | |
| CD8+ T-cell dose | 57 | |||
| < 47.7 × 106 | 1 | |||
| > 47.7 × 106 | 1.7 | 0.75-3.86 | .2 | |
| Total iNKT-cell dose | 57 | |||
| > 0.057 × 106 | 1 | |||
| < 0.057 × 106 | 1.36 | 0.58-3.18 | .48 | |
| CD4+ iNKT-cell dose | 57 | |||
| > 0.026 × 106 | 1 | |||
| < 0.026 × 106 | 5.51 | 0.87-35.1 | .07 | |
| CD4− iNKT-cell dose | 57 | |||
| > 0.031 × 106 | 1 | |||
| < 0.031 × 106 | 4.27 | 1.68-10.85 | .0023 |
| Variable . | No. . | RR . | 95% CI . | P . |
|---|---|---|---|---|
| Diagnosis | 57 | |||
| Non-CML | 1 | |||
| CML | 2.54 | 1.06-6.04 | .036 | |
| CD8+ T-cell dose | 57 | |||
| < 47.7 × 106 | 1 | |||
| > 47.7 × 106 | 1.7 | 0.75-3.86 | .2 | |
| Total iNKT-cell dose | 57 | |||
| > 0.057 × 106 | 1 | |||
| < 0.057 × 106 | 1.36 | 0.58-3.18 | .48 | |
| CD4+ iNKT-cell dose | 57 | |||
| > 0.026 × 106 | 1 | |||
| < 0.026 × 106 | 5.51 | 0.87-35.1 | .07 | |
| CD4− iNKT-cell dose | 57 | |||
| > 0.031 × 106 | 1 | |||
| < 0.031 × 106 | 4.27 | 1.68-10.85 | .0023 |
“Best” model from a backward-stepping procedure that included the variables found significant at the P < .1 level in univariate analyses: CML diagnosis, CD8+ T-cell dose, total iNKT-cell dose, CD4+ iNKT-cell dose, and CD4− iNKT-cell dose.
Function of CD4− iNKT cells in the in vitro alloresponse
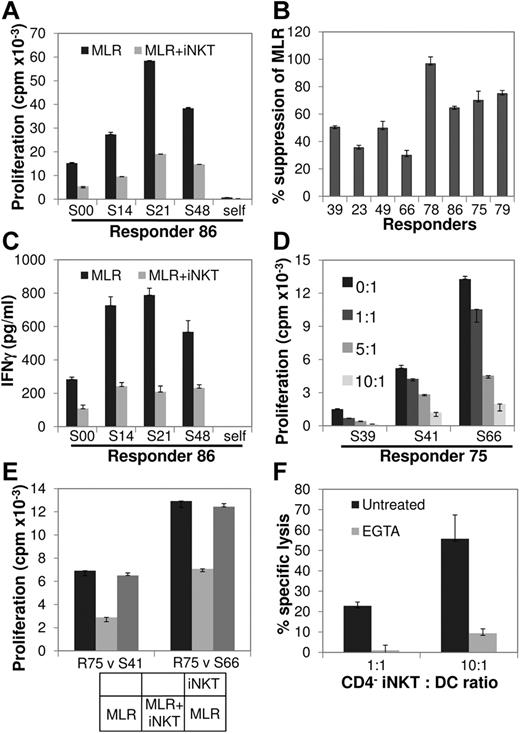
To directly show that human iNKT cells are able to inhibit the alloresponse, as shown in murine studies in which αGC-expanded donor iNKT cells ameliorate experimental aGVHD,15,16 we tested the ability of αGC-expanded human CD4+ and CD4− iNKT-cell subsets to regulate the MLR, an in vitro correlate of the alloresponse. For this purpose, highly purified (supplemental Figure 4) normal donor CD4+ and CD4− NKT cells, autologous to responder T cells, were added to the allogeneic, HLA-mismatched MLR at an iNKT-to-responder (R) ratio of 10:1. Although the effect of CD4+ iNKT cells was variable (supplemental Figure 5), the addition of CD4− iNKT cells had a consistently inhibitory effect (P = .0048; one sample z test), and titration of the CD4− iNKT-cell numbers resulted in inhibition of the MLR in a dose-dependent manner (Figure 2A-C). On subsequent studies that focused on the function of CD4− iNKT cells, their addition reduced IFN-γ secretion during MLR (Figure 2D; supplemental Figure 6), and, in a transwell coculture set up, their suppressive effect was found to be contact dependent (Figure 2E; supplemental Figure 6). In line with this and their known selective expression of perforin,2 CD4− iNKT cells efficiently lysed allogeneic mature myeloid DCs, an effect abolished by the Ca2+ chelator EGTA and consistent with cytotoxicity because of perforin (Figure 2F; supplemental Figure 6).
Effect of CD4− iNKT cells on MLR. (A) αGC-expanded, highly purified CD4− iNKT cells from a normal donor (R86) were placed in MLR comprising autologous CD3+ T cells (responders; R) against allogeneic irradiated PBMC (stimulators; S) at iNKT/R/S ratio of 10:1:1 and compared with R/S (baseline) and autologous (self) MLR. Cell proliferation was measured by 3H-thymidine incorporation 96 hours later. Bars represent means and error bars represent SEMs of triplicate assays. (B) In a series of MLRs involving 8 different responders, each placed against a panel of 3-4 allogeneic stimulators, the addition of autologous CD4− iNKT cells resulted in a 59% ± 8% (P = .0048; 1 sample z test) decrease in proliferation compared with baseline MLR. (C) IFN-γ secretion was significantly decreased on the addition of CD4− iNKT cells to the baseline MLR of R86 against the same panel of stimulators as in panel A. Data are shown as mean ± SEM of triplicate assays. (D) Dose-dependent inhibition of the MLR by CD4− iNKT cells. Responder 75 (R75) CD4− NKT cells were added in MLR at different iNKT/R ratios as shown. Data are shown as mean ± SEM of triplicate assays. (E) Contact-dependent inhibition of the MLR by CD4− iNKT cells. MLRs of R75 versus 2 stimulators were set up in a transwell coculture system as shown at the bottom and described in “Methods.” Contact of CD4− NKT cells with the responder and stimulator cells suppressed the MLR; when CD4− iNKT cells were separated from the MLR by the transwell membrane, cell proliferation was almost completely restored to the levels of the baseline MLR. Data are shown as mean ± SEM of triplicate assays. (F) CD4− iNKT cells are cytotoxic against allogeneic myeloid DCs. CD4− iNKT cells were cytotoxic against allogeneic DCs in a Cr51 release assay at the iNKT/DC ratios of 1:1 and 10:1, an effect that was abrogated by the Ca2+ chelator EGTA. Data are shown as mean ± SEM of triplicate assays. See also supplemental Figure 6.
Effect of CD4− iNKT cells on MLR. (A) αGC-expanded, highly purified CD4− iNKT cells from a normal donor (R86) were placed in MLR comprising autologous CD3+ T cells (responders; R) against allogeneic irradiated PBMC (stimulators; S) at iNKT/R/S ratio of 10:1:1 and compared with R/S (baseline) and autologous (self) MLR. Cell proliferation was measured by 3H-thymidine incorporation 96 hours later. Bars represent means and error bars represent SEMs of triplicate assays. (B) In a series of MLRs involving 8 different responders, each placed against a panel of 3-4 allogeneic stimulators, the addition of autologous CD4− iNKT cells resulted in a 59% ± 8% (P = .0048; 1 sample z test) decrease in proliferation compared with baseline MLR. (C) IFN-γ secretion was significantly decreased on the addition of CD4− iNKT cells to the baseline MLR of R86 against the same panel of stimulators as in panel A. Data are shown as mean ± SEM of triplicate assays. (D) Dose-dependent inhibition of the MLR by CD4− iNKT cells. Responder 75 (R75) CD4− NKT cells were added in MLR at different iNKT/R ratios as shown. Data are shown as mean ± SEM of triplicate assays. (E) Contact-dependent inhibition of the MLR by CD4− iNKT cells. MLRs of R75 versus 2 stimulators were set up in a transwell coculture system as shown at the bottom and described in “Methods.” Contact of CD4− NKT cells with the responder and stimulator cells suppressed the MLR; when CD4− iNKT cells were separated from the MLR by the transwell membrane, cell proliferation was almost completely restored to the levels of the baseline MLR. Data are shown as mean ± SEM of triplicate assays. (F) CD4− iNKT cells are cytotoxic against allogeneic myeloid DCs. CD4− iNKT cells were cytotoxic against allogeneic DCs in a Cr51 release assay at the iNKT/DC ratios of 1:1 and 10:1, an effect that was abrogated by the Ca2+ chelator EGTA. Data are shown as mean ± SEM of triplicate assays. See also supplemental Figure 6.
Discussion
This study addresses an unresolved question in the biology of iNKT cells, namely whether they have any functional significance in humans.4 Defining the role of iNKT cells in disease has been hampered by the difficulty of tracking them in time and in space during the course of human pathology such as autoimmunity and malignancy. Study of iNKT cells in donor PBSC grafts provides a unique opportunity to accurately identify and enumerate iNKT cells as well as other effector and regulatory immune cells in humans and thus define their role in aGVHD, a major source of morbidity and mortality in allogeneic HSCT. We addressed this important question by studying the effect of iNKT cells contained in G-CSF–mobilized PBSC grafts on the basis of evidence from the murine model showing that (1) G-CSF–mobilized iNKT cells protect from aGVHD and enhance the GVL effect14 and (2) adoptively transferred, unmanipulated,17 or in vitro αGC-expanded15,16 iNKT cells can ameliorate aGVHD.
By studying a larger cohort than in previous reports27 we confirmed that graft iNKT cells are as rare and as variable in their frequency as peripheral blood iNKT cells and found that, in terms of frequency, they are the most variable among all other graft cellular components. Consequently, for patients who would otherwise receive comparable doses of other cell types, there is a higher probability that they would receive considerably different doses of iNKT cells. Given the efficacy of donor iNKT cells in controlling murine aGVHD, we anticipated that higher doses of iNKT cells might also be protective in the clinical setting. Indeed, univariate analysis found the unique effect of total iNKT cells and subsets in predicting aGVHD. Multivariate analysis showed that a low median dose of CD4− iNKT cells was the only variable associated with grade II-IV aGVHD with a relative risk of 4.27. This level of risk prediction is one of the highest reported either for clinical parameters or graft cellular components and suggests a powerful influence of iNKT-cell dose on the incidence and severity of aGVHD.
In contrast to previous reports,22 clinical variables such as recipient age and conditioning regimen were not significant determinants of aGVHD in our study. This is probably because of the small effect that each of these parameters exerts on the risk of aGVHD (generally RR < 2), which our study was underpowered to detect. In line with previous reports,22 CML diagnosis was associated with higher risk of aGVHD (Table 2) than other hematologic malignancies.
To determine whether graft cellular subsets other than T cells might predict for the occurrence of aGVHD, the frequencies and contents of other effector and regulatory lymphocyte subsets have been intensively studied. In general, the reported effects of NK-8 and B-cell28 doses are either relatively small or not confirmed in all studies, and this was also the case in our study. Our results are also in agreement with several other reports showing that the dose of T cells, the undisputed and most powerful effectors of aGVHD, does not predict risk of aGVHD.29 This is consistent with the “all-or-none” notion that alloreactive T cells induce aGVHD only when their dose exceeds a threshold of 5-10 × 104 cells/kg.30
Whether and how graft FoxP3+ Tregs might protect from aGVHD has also been investigated extensively. Although a protective effect of high graft Treg content was suggested in some10,11 but not all31 reports, we found no evidence that the Treg dose affects the risk of aGVHD. Nevertheless, 2 phase 1 clinical trials designed to test the safety of Treg infusion for prevention of aGVHD have already been reported.32,33
CD4− iNKT cells formed the subset primarily associated with the protective effect against aGVHD. Human CD4− iNKT cells differ functionally from their CD4+ counterparts in terms of cytokine and cytotoxicity profiles: they secret higher amounts of IFN-γ than IL-4, having thus a Th1 bias and express preferentially perforin.2 Although both subsets inhibited MLR proliferation in our vitro alloreactivity studies, in line with their stronger association with protection from aGVHD in vivo, this effect was more consistently observed with CD4− iNKT cells, which in a contact-dependent manner displayed direct cytotoxicity against CD1d-expressing mature myeloid DCs. Furthermore, we previously showed that iNKT cells, despite the nonpolymorphic nature of CD1d, can display alloreactivity, an effect that requires CD1d and invariant TCR interaction but is instructed by activating KIR interacting with yet to be identified APC ligands.34 In this regard, iNKT cells resemble NK cells which in the context of donor-recipient KIR mismatch are activated and exert a selective GVL effect.35
We therefore propose that one possible mechanism for the protective effect of iNKT cells might involve donor CD4− iNKT cells interacting with host APCs, the cells that orchestrate aGVHD in secondary lymphoid organs.36 This interaction would lead to activating KIR-dependent iNKT-cell activation, followed by perforin-mediated cytolysis of APCs.
In the clinical setting, enhancing the iNKT-cell dose by adding back either ex vivo–selected or in vitro–expanded iNKT cells to iNKT cell–poor grafts might constitute a novel strategy for prevention of moderate-to severe aGVHD. To this end, clinical grade protocols for iNKT-cell selection and expansion are under development.37
In conclusion, this study shows that the dose of iNKT cells, and in particular of CD4− iNKT cells, delivered with the PBSC graft in the context of HLA-identical T cell–replete allogeneic HSCT is a significant determinant of the incidence and severity of aGVHD and provides a basis for developing new strategies for its prevention.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Leukaemia & Lymphoma Research, Leuka, and the National Institute for Health Research Biomedical Research Center.
Authorship
Contribution: A.C. performed research, analyzed data, and wrote the paper; S.P. performed research and analyzed data; R.S. analyzed data and wrote the paper; M.S.C. retrieved and analyzed data; F.D., E.K., D. McDonald, D. Marin, D. Milojkovic, J.P., A.R., K.R., and J.A. analyzed data and contributed to writing of the paper; J.D. contributed reagents; J.G. contributed to writing of the paper; I.R. analyzed data, contributed to the writing of paper, and supervised research; and A.K. designed and supervised research, analyzed data, and wrote the paper
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anastasios Karadimitris, Centre for Haematology, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 0NN, United Kingdom; e-mail: a.karadimitris@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal