Abstract
Acute graft-versus-host disease (aGVHD) remains a major complication of allogeneic hematopoietic stem cell transplant (alloHSCT), underscoring the need to further elucidate its mechanisms and develop novel treatments. Based on recent observations that microRNA-155 (miR-155) is up-regulated during T-cell activation, we hypothesized that miR-155 is involved in the modulation of aGVHD. Here we show that miR-155 expression was up-regulated in T cells from mice developing aGVHD after alloHSCT. Mice receiving miR-155–deficient donor lymphocytes had markedly reduced lethal aGVHD, whereas lethal aGVHD developed rapidly in mice recipients of miR-155 overexpressing T cells. Blocking miR-155 expression using a synthetic anti–miR-155 after alloHSCT decreased aGVHD severity and prolonged survival in mice. Finally, miR-155 up-regulation was shown in specimens from patients with pathologic evidence of intestinal aGVHD. Altogether, our data indicate a role for miR-155 in the regulation of GVHD and point to miR-155 as a novel target for therapeutic intervention in this disease.
Introduction
Acute GVHD (aGVHD) develops in 30% to 75% of recipients of allogeneic hematopoietic stem cell transplant (alloHSCT) and is associated with significant morbidity and mortality, representing a major barrier toward the wider and safer application of this potentially curative approach to hematologic malignancies.1 aGVHD develops when allogeneic donor T cells destroy HLA-mismatched host tissues by secreting inflammatory cytokines (IL-1, TNF-α, and IFN-γ) and/or inducing direct cytotoxic cellular response.1,2 Recent studies indicate that microRNAs (miRNAs) play critical roles in the development and function of the immune system.3-7 In particular, miR-155 is required for normal function of B and T lymphocytes.5,6 Mice deficient for miR-155 exhibit impaired B-cell responses (reduced immunoglobulin M [IgM], switched antigen-specific antibodies, and germinal center B-cell numbers) and decreased TNF-α production,5,6 a cytokine intricately involved in the pathogenesis of aGVHD.1,2 Moreover, CD4+ T cells lacking miR-155 exhibit bias toward Th2 differentiation, as evidenced by the high levels of IL-4 and IL-10 and low levels of TNF-α.6 In contrast, lymphocytes from miR-155 overexpressing transgenic mice produce higher TNF-α levels than their respective wild-type controls.8 Complementary to these findings, miR-155 is induced upon CD4+ activation and promotes Th1 differentiation.4,6 Based on these observations, we hypothesize that miR-155 is up-regulated in donor T cells during aGVHD and is required for the development of this process. Here we provide data that support a role of miR-155 in the regulation of aGVHD after HSCT.
Methods
All the animal and human samples studies were performed under institutional review board and Institutional Animal Care and Use Committee–approved protocols (OSUCCC 2005C0014 and IACUC2010A0000170).
Mice
C57/BL/6(H2b), (DBA/Ca) × C57BL/6) F1, B10.BR-H2k and B6.Cg-miR-155tm1.1Rsky/j mice were purchased from Jackson ImmunoResearch Laboratories. Bic-deficient (bicm2/m2) mice were obtained from Dr Tridandapani (The Ohio State University [OSU]).5 The miR155 tm1.RskyJ was developed using a modified bacterial artificial chromosome targeting vector to replace a 0.97-kb portion of exon 2 of the bic/mir-155 gene with an in-frame b-galactosidase (lacZ) reporter gene,6 whereas in the miR-155–deficientm2/m2, the miR-155 hairpin precursor within exon 2 of Bic was replaced by a PGK-neomycin-resistance cassette using the bacterial recombineering system.5 For the development of the LCK–miR-155 transgenic mouse model, a 318-bp DNA fragment containing the precursor sequence of mouse miR-155 was amplified from 129SvJ mouse DNA. The fragment was then cloned into the BamHI unique cloning site of a modified pUC-19 plasmid next to the LCK promoter. The transgene was linearized by digestion with NotI and injected into the male pronuclei of mouse zygotes. Pups where then screened by PCR for random insertion of the transgene. Fifteen founding lines were identified with 3 lines having high expression of the transgene. All lines were bred and expanded. All mice used in this proposal were bred and maintained in an OSU animal care facility. A group of B6 and all B10.BR mice were bred and maintained at the University of Minnesota animal care facility. For all experiments, mice were used between 8 and 12 weeks of age.
aGVHD murine models
Mice were transplanted under standard protocols approved by the University committee on Use and Care of Laboratory Animals at OSU. Only age- and sex-matched mice were used for transplant experiments. Briefly, 8- to 12-week recipient mice (F1) were irradiated with 1.1 Gy administered in 2 fractions to minimize toxicity. T cell–depleted BM cells (5 × 106) plus 20 × 106 total splenocytes from B6 WT (miR-155+/+) or B6 miR-155–deficient (Cg-miR-155tm1.1Rsky/j, from now on named miR-155−/−) donors were administered via tail vein injection after the radiation. One group of mice did not receive any splenocytes (BM only group) and a second group did not receive any cells (radiation only group). For other experiments, this protocol was modified; and instead of splenocytes, untouched T cells (2 × 106) from B6 miR-155 WT or deficient mice were used. This parent to F1 model of aGVHD was chosen to eliminate host versus graft rejection responses in attempt to eliminate differential engraftment kinetics. In addition, this is a full or major MHC-mismatched model, where aGVHD develops in response to class I, II HLA molecules, and is dependent on mainly CD4+ cells, although CD8+ cells could elicit additional pathology.9 There were no significant differences in the T-cell dose among the splenocyte preparations from miR-155−/− and the WT B6 mice. A second MHC-mismatched aGVHD murine model was performed at the University of Minnesota using university approved animal protocols. In this model, B10.BR recipients were lethally irradiated with 10 Gy and were divided in 3 groups: group 1 received only BM cells (10 × 106), group 2 received B6 WT BM (10 × 106), plus unfractionated splenocytes (25 × 106) from B6 WT mice, and group 3 received WT B6 BM (10 × 106) plus unfractionated splenocytes (25 × 106) from B6 miR-155−/− mice.
Clinical and histologic assessment of aGVHD
Recipient mice were weighed 4 times a week and monitored daily for clinical signs of aGVHD and survival. GVHD scores were performed according to Cooke et al.10 Briefly, this scoring system incorporates 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity. Individual mice were ear tagged and graded (in a scale from 0 to 10) 3 times a week by 2 researchers (one of them blinded). Mice who reached an aGVHD score of more than or equal to 7 were very sick and were killed and their tissues harvested. GVHD was also assessed by detailed histopathology analysis of liver, spleen, and gut tissues using a previously reported scoring system with a range of 0 (absence of signs of GVHD) to 4 (maximal GVHD damage).11 Two experienced pathologists read all the samples in a blinded fashion.
miR expression analysis
miR-155 expression levels were detected using the single tube TaqMan murine miRNA assays as previously described (Applied Biosystems).12 Normalization was performed using sno135 expression levels. Comparative real-time quantitative PCR was performed in triplicate, including no-template controls. Relative expression was calculated using the comparative Ct method.13
GVL experiments
Briefly, 1 × 104 firefly luciferase transduced P81514-17 (H-2d) cells (a mastocytoma derived from a DBA/2 mouse) were injected intravenously into F1 recipients on day 0 along with BM and B6 miR155+/+ or miR155−/− donor splenocytes. Control groups included BM and P815 cells (leukemia alone). Separate cohorts were used for imaging and survival. P815-induced leukemic death was defined by the occurrence of either macroscopic tumor nodules in liver or spleen or hind leg paralysis.14-17 GVHD death was defined by the absence of leukemia and the presence of clinical and pathologic signs of GVHD. Luciferase-transduced P815 mastocytoma cell line was a generous gift from Dr Marcel R. M. van den Brink and Vladimir Ponomarev (Memorial Sloan-Kettering Cancer Center, New York, NY).
In vivo imaging
Xenogen IVIS imaging system (Caliper Life Sciences) was used for live animal imaging. Mice were anesthetized using isofluorane (Webster Veterinary). Firefly luciferin substrate (150 mg/kg body weight; 5 mg/mL in PBS; Caliper Life Sciences) was injected intraperitoneally, and the IVIS imaging was performed 5 minutes after substrate injection. Whole body bioluminescent signal intensity was determined weekly, and pseudocolor images overlaid on conventional photographs are shown. Data were analyzed and presented as photon counts per area.
Patient samples
Histopathologic samples of human gut aGVHD were obtained from 5 patients treated with allogeneic stem cell transplants at OSU. These samples were retrieved from the OSU pathology archives, and the studies were approved by the institutional review board. In addition, 3 normal colon biopsies performed during screening for other diseases were used as controls.
In vivo treatment with LNA–anti–miR-155 oligonucleotides
The 8-mer seed-targeting locked nucleic acid (LNA)–anti–miR-155 (5′-TAGCATTA-3′) and 8-mer LNA scramble control (5′-TCATACTA-3′) oligonucleotides were synthesized as unconjugated, fully LNA-substituted oligonucleotides with a complete PS backbone as described.18 Lethally irradiated recipient F1 mice transplanted with BM (5 × 106) plus B6 WT spleen cells (20 × 106) were treated with LNA–anti–miR-155 (n = 10) or control LNA oligos (n = 10) starting at day 7 with a loading dose 25 mg/kg followed by 5 mg/kg intravenously twice weekly up to day 30 after infusion of donor B6 splenocytes. Clinical GVHD scores and survival were followed after transplantation.
Statistical analysis
Survival data were analyzed using Kaplan-Meier and long-rank test methods (SPSS and Graphma Prisma). Differences between continuous variables (eg, cytokine expression and miRNA expression) were analyzed using t tests. All P values are 2 sided.
Results
miR-155 expression is up-regulated in activated T cells from murine recipients with aGVHD
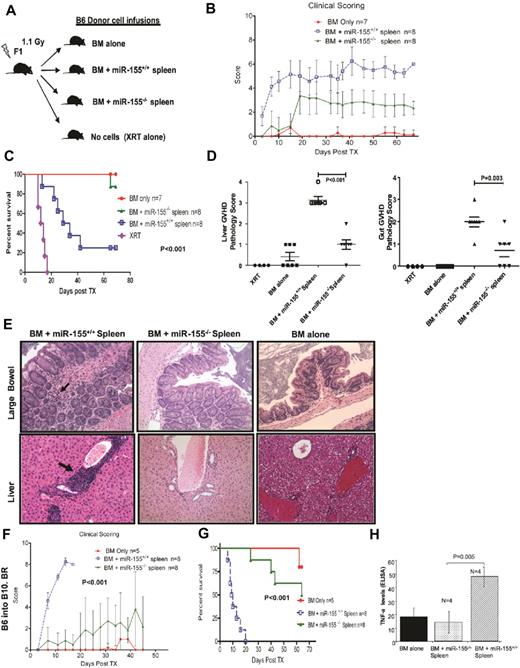
To investigate whether miR-155 expression is up-regulated in T-cell subsets during aGVHD, a MHC-mismatched HSCT model was used in which spleen cells (20 × 106) and T cell–depleted BM (5 × 106) from C57BL/6 (B6) donors were transferred intravenously into lethally irradiated B6D2F1 (F1) recipient mice (Figure 1A). Two additional groups were included as controls, with one group receiving no cell infusion (irradiation only) and a second group receiving only BM. We chose this model of haplotype-mismatched MHC (class I and II) because the aGVHD that develops is primarily dependent on CD4+ T cells and most of the T-cell alterations observed in miR-155–deficient mice have been described in CD4+ cells.9 However, CD8+ T cells also contribute to the development of aGVHD in this model because of class I and minor HLA disparities; thus, we may be able to investigate the expression of miR-155 in functionally important CD8+ subsets as well.9 Mice receiving donor BM plus spleen cells (n = 3) developed severe aGVHD that was confirmed by liver histology (Figure 1B). Mice were killed when they achieved a clinical GVHD score of more than or equal to 710 (median time 21 days after bone marrow transplantation [BMT]; range, 19-23 days). Control mice treated with BM only were killed at the same time point. CD4+CD62L− (memory effectors) and CD8+CD44+ (effectors active) cell subpopulations were isolated from the spleen of the killed mice using a combination of column magnetic bead and cell sorting as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Total RNA was extracted from these highly purified cell populations (Figure 1C; supplemental Figure 1). We found that both CD4+ and CD8+ cell populations isolated from mice with aGVHD exhibited increased miR-155 expression (6.5- and 5-fold, respectively) with respect to the same cell populations obtained from the controls (Figure 1C; supplemental Figure 1). These results were validated in a second independent experiment using CD4+CD62L− cells but using a different method of miRNA profiling (Nanostring). miR-155 was the top up-regulated miRNA in CD4+CD62L− cells from mice with aGVHD versus controls (BM alone; 4-fold increase, P < .001, supplemental Table 1; Figure 1D). Finally, we investigated the expression of miR-155 in the infiltrating lymphocytes of murine liver biopsies with aGVHD mice (n = 3) or controls (BM alone n = 3) using LNA-based in situ hybridization as described in supplemental Methods. Consistent with the previous results, a strong expression of miR-155 was observed within the lymphocyte infiltrates in the liver of all mice with aGVHD (Figure 1E).
miR-155 expression is up-regulated in effectors T cells of mice recipients with aGVHD. (A) Schematic presentation showing aGVHD murine model used. (B) Histopathologic evaluation of a representative liver sample collected from a mouse with clinical score of GVHD of more than or equal to 7. This section was stained with hematoxylin and eosin (original magnification ×40 and ×400). (C) Average miR-155 expression in CD4+ CD62L− T cells isolated from the spleen of 3 recipient mice with aGVHD (BM + spleen) or controls (BM alone). The results shown represent the average of 3 independent mouse samples performed each in triplicate. Values are expressed as relative miRNA expression after 2ΔCt calculations and normalization with sno135. Bars represent SD. (D) Hierarchical clustering analysis of miRNA expression obtained using Nanostring from CD4+ CD62L− T cells isolated from the spleen of recipient mice with aGVHD (BM + spleen) or controls (BM alone). Color areas indicate relative expression of each miRNA among the 2 type of samples (red high, blue low expression). (E) Histopathologic and molecular evaluation of a representative liver sample collected from a mouse with clinical score of GVHD of more than or equal to 7 or a control mouse (BM alone). At least 3 mice in each group were evaluated for staining with LNA anti–miR-155 and scramble control. These sections were stained with LNA anti–miR-155 probes or scrambled controls. Dark staining indicates positivity (original magnification ×400). Note the strong staining of the mononuclear cells in the periportal lymphoid aggregate in the BM + spleen mouse that is lost in the same cells in the serial section with the scrambled probe.
miR-155 expression is up-regulated in effectors T cells of mice recipients with aGVHD. (A) Schematic presentation showing aGVHD murine model used. (B) Histopathologic evaluation of a representative liver sample collected from a mouse with clinical score of GVHD of more than or equal to 7. This section was stained with hematoxylin and eosin (original magnification ×40 and ×400). (C) Average miR-155 expression in CD4+ CD62L− T cells isolated from the spleen of 3 recipient mice with aGVHD (BM + spleen) or controls (BM alone). The results shown represent the average of 3 independent mouse samples performed each in triplicate. Values are expressed as relative miRNA expression after 2ΔCt calculations and normalization with sno135. Bars represent SD. (D) Hierarchical clustering analysis of miRNA expression obtained using Nanostring from CD4+ CD62L− T cells isolated from the spleen of recipient mice with aGVHD (BM + spleen) or controls (BM alone). Color areas indicate relative expression of each miRNA among the 2 type of samples (red high, blue low expression). (E) Histopathologic and molecular evaluation of a representative liver sample collected from a mouse with clinical score of GVHD of more than or equal to 7 or a control mouse (BM alone). At least 3 mice in each group were evaluated for staining with LNA anti–miR-155 and scramble control. These sections were stained with LNA anti–miR-155 probes or scrambled controls. Dark staining indicates positivity (original magnification ×400). Note the strong staining of the mononuclear cells in the periportal lymphoid aggregate in the BM + spleen mouse that is lost in the same cells in the serial section with the scrambled probe.
Recipients of miR-155–deficient splenocytes do not develop severe aGVHD and show increased survival
To confirm a causal relationship between miR-155 and aGVHD severity, we performed the previously described MHC-mismatched murine experiment using B6 mice deficient for BIC/miR-155 expression (miR-155−/−) or wild-type (WT) miR-155+/+ as donors (Figure 2A).6 The miR-155−/− mice exhibit no miR-155 expression in CD4+ cells either at baseline or after cross-linking T-cell activation (supplemental Figure 2). aGVHD incidence and severity were significantly reduced in recipients of miR-155−/− spleen cells as evidenced by clinical GVHD scores (t test, P = .0007, Figure 2B). Overall survival was also improved in these mice with respect to WT (log-rank test, P = .001, Figure 2C). There was no difference in CD4+ and CD8+ T cell numbers between miR-155−/− or WT miR-155+/+ splenocytes using FACS analysis. Pathologic analysis performed in a second cohort of mice confirmed the clinical findings (Figure 2D). GVHD scores of the liver and colon obtained on day 15 after BMT were significantly lower in recipient mice transplanted with miR-155–deficient mice donors compared with mice receiving transplants from WT donors (Figure 2D-E). To further confirm these results, we performed a MHC-mismatched GVHD murine experiment as described in “aGVHD murine models” but using a second miR-155 knockout model in a B6 background (miR-155–deficientm2/m2) established by Rodriguez et al.5 These mice also do not express miR-155 in T cells and differs from the BIC/miR-155 mice in the strategy used to generate the knockout (see “Mice”).5 Lower aGVHD clinical scores and increased survival were observed for F1 recipients of B6-miR-155–deficientm2/m2 cells (supplemental Figure 3A-B). The lack of severe aGVHD in recipients of miR-155–deficient splenocytes was also independently validated in a different MHC-mismatched aGVHD murine model (B6 donors into lethally irradiated B10.BR [H-2k])19 performed in a different colony environment at the University of Minnesota. Recipients of miR-155–deficient spleen cells had significantly lower clinical GVHD scores and exhibited increased overall survival compared with recipients of WT spleen cells (Figure 2F-G). Last, to further identify the miR-155–deficient cell population that protects from aGVHD, we performed the B6 into F1 parental model described in “aGVHD murine models” but using donor untouched T cells (2 × 106) instead of unfractionated splenocytes. aGVHD incidence and severity were significantly reduced in recipients of miR-155−/− T cells as evidenced by clinical GVHD scores (t test P < .001, supplemental Figure 3C). Overall survival was also improved in these mice with respect to WT (log-rank test P < .001, supplemental Figure 3D).
Recipients from miR-155–deficient splenocytes do not develop severe aGVHD and have increased survival. (A) Schematic presentation showing the different donor cells used in lethally irradiated F1 mice. (B) Clinical scores from the different recipient mice groups after transplantation. (C) Survival rate of lethally irradiated mice receiving BM alone, BM + miR-155−/− spleen, BM + miR-155+/+ spleen, or XRT alone. Data represent 2 independent experiments. (D) Histopathologic GVHD scores of recipient tissues. A second cohort of mice was used for histopathologic analysis. Fifteen days after transplantation (except for XRT group), mice from the indicated groups were killed and sections of the large bowel and liver were stained with H&E. Data are pooled from 2 independent experiments, representing approximately 6 or 7 animals per group, the XRT group that had 4 mice. There was no significant difference in the liver GVHD scores between the BM alone and BM + miR-155−/− spleen recipients (P = .07). The GVHD scores in the gut were higher in the BM + miR-155−/− spleen recipients versus the BM alone (P = .04). (E) Histopathologic evaluation of representative liver and large-bowel samples collected from the different recipient mice groups at day 15 after transplantation. The arrows in the large-bowel slide from the B6 BM + miR-155+/+ spleen F1 recipients showed lymphoplasmacytic infiltration in the lamina propia with frequent apoptosis The arrows in the liver slide from the same F1 recipients showed abundant lymphocytes infiltrating around the portal vein. No significant pathology is shown in BM alone and miR-155−/− groups. (F) Clinical GVHD scores after transplantation from lethally irradiated B10.BR recipients transplanted with BM alone, BM + miR-155−/−, or BM + miR-155+/+ spleen. (G) Survival rate of the different recipient B10.BR mice groups. (H) TNF-α levels measured in the serum of lethally irradiated F1 recipients of BM alone, BM + miR-155−/− spleen, and BM + miR-155+/+ spleen, 15 days after transplantation. Samples were assayed in triplicate and results are shown as the means from 4 biologic samples within a group.
Recipients from miR-155–deficient splenocytes do not develop severe aGVHD and have increased survival. (A) Schematic presentation showing the different donor cells used in lethally irradiated F1 mice. (B) Clinical scores from the different recipient mice groups after transplantation. (C) Survival rate of lethally irradiated mice receiving BM alone, BM + miR-155−/− spleen, BM + miR-155+/+ spleen, or XRT alone. Data represent 2 independent experiments. (D) Histopathologic GVHD scores of recipient tissues. A second cohort of mice was used for histopathologic analysis. Fifteen days after transplantation (except for XRT group), mice from the indicated groups were killed and sections of the large bowel and liver were stained with H&E. Data are pooled from 2 independent experiments, representing approximately 6 or 7 animals per group, the XRT group that had 4 mice. There was no significant difference in the liver GVHD scores between the BM alone and BM + miR-155−/− spleen recipients (P = .07). The GVHD scores in the gut were higher in the BM + miR-155−/− spleen recipients versus the BM alone (P = .04). (E) Histopathologic evaluation of representative liver and large-bowel samples collected from the different recipient mice groups at day 15 after transplantation. The arrows in the large-bowel slide from the B6 BM + miR-155+/+ spleen F1 recipients showed lymphoplasmacytic infiltration in the lamina propia with frequent apoptosis The arrows in the liver slide from the same F1 recipients showed abundant lymphocytes infiltrating around the portal vein. No significant pathology is shown in BM alone and miR-155−/− groups. (F) Clinical GVHD scores after transplantation from lethally irradiated B10.BR recipients transplanted with BM alone, BM + miR-155−/−, or BM + miR-155+/+ spleen. (G) Survival rate of the different recipient B10.BR mice groups. (H) TNF-α levels measured in the serum of lethally irradiated F1 recipients of BM alone, BM + miR-155−/− spleen, and BM + miR-155+/+ spleen, 15 days after transplantation. Samples were assayed in triplicate and results are shown as the means from 4 biologic samples within a group.
Serum TNF-α expression levels are lower in recipients of miR-155–deficient splenocytes
High levels of soluble TNF-α are characteristic for aGVHD, promote T-cell activation and proliferation, and contribute to tissue damage.1,2,20 Moreover, murine miR-155–deficient CD4+ cells exhibit a Th2 cytokine profile opposite to the Th1 cytokine profile observed during aGVHD.5,6 Thus, we measured the expression of TNF-α in the serum of lethally irradiated F1 recipients of BM alone, BM + miR-155−/− splenocytes, and BM + miR-155+/+ splenocytes (WT), 15 days after transplantation using ELISA. Mice receiving miR-155–deficient spleen cells had significantly lower serum TNF-α level than WT controls (Figure 2H). Thus, aGVHD reduction in recipients of miR-155−/− versus miR-155+/+ splenocytes is associated with lower sera TNF-α levels.
Recipient mice of donor splenocytes overexpressing miR-155 in T cells exhibit rapidly evolving aGVHD and short survival
To further establish the regulatory role of miR-155 in aGVHD, we generated a transgenic mouse that overexpresses miR-155 in T cells under the LCK promoter (Figure 3A-B; supplemental Figure 4A) and performed the MHC-mismatched HSCT experiments as described previously, but using the LCK–miR-155 transgenic splenocytes as donors (Figure 3C). aGVHD incidence and severity were significantly increased in recipients of miR-155 transgenic cells with respect to miR-155 WT as evidenced by clinical GVHD scores (t test P = .002, Figure 3D). Recipients of miR-155 transgenic cells died earlier than miR-155 WT recipients after transplant (log-rank test, P = .007, Figure 3E). GVHD scores of the liver and colon obtained on day 14 after BMT were significantly higher in recipient mice transplanted with miR-155 overexpressing donor T cells compared with mice receiving transplants from WT donors (t test, P = .008 and P = .0003, respectively, Figure 3F-G). Lastly, cytokine measurements revealed higher levels of TNF-α, IL-2, and IFN-γ in recipients of LCK-miR-155 mice with respect to WT recipients (TNF-α, 45 ± 14 vs 96 ± 13, P = .004; IL-2, 36 ± 11 vs 140 ± 50, P = .003; IFN-γ, 133 ± 40 vs 440 ± 113, P = .007, supplemental Figure 4B).
Recipient mice from donor splenocytes overexpressing miR-155 in T cells exhibit rapidly evolving aGVHD and short survival. (A) Schematic presentation showing the construct used to generate the LCK-miR-155 mice. (B) miR-155 expression (fold change) in the thymus and spleen from age- and sex-matched B6 LCK–miR-155 transgenic mice and B6 miR-155−/− mice. Bars represent SD. (C) Schematic presentation depicting the different recipients F1 mice groups. (D) Clinical GVHD scores after transplantation from lethally irradiated F1 recipients transplanted with BM alone (n = 8), BM + LCK–miR-155 (n = 8), or BM + miR-155+/+ (n = 8) splenocytes. P values were obtained by using t test. (E) Survival rate of the different recipient F1 mice groups. Comparisons between BM + miR-155−/− spleen and BM + miR-155+/+ spleen survival curves were performed using log-rank test. (F) Histopathologic GVHD scores of recipient tissues. A second cohort of mice was used for histopathologic analysis. Fourteen days after transplantation, mice from the indicated groups were killed and sections of the large bowel and liver were stained with H&E. P values were obtained by using t test. (G) Histopathologic evaluation of representative liver samples obtained from recipients of BM alone, BM + miR-155+/+ spleen, and BM + LCK-miR-155 donor cells. Slides were stained with H&E. The arrows indicate lymphocytic infiltration in the recipient livers characteristic of GVHD.
Recipient mice from donor splenocytes overexpressing miR-155 in T cells exhibit rapidly evolving aGVHD and short survival. (A) Schematic presentation showing the construct used to generate the LCK-miR-155 mice. (B) miR-155 expression (fold change) in the thymus and spleen from age- and sex-matched B6 LCK–miR-155 transgenic mice and B6 miR-155−/− mice. Bars represent SD. (C) Schematic presentation depicting the different recipients F1 mice groups. (D) Clinical GVHD scores after transplantation from lethally irradiated F1 recipients transplanted with BM alone (n = 8), BM + LCK–miR-155 (n = 8), or BM + miR-155+/+ (n = 8) splenocytes. P values were obtained by using t test. (E) Survival rate of the different recipient F1 mice groups. Comparisons between BM + miR-155−/− spleen and BM + miR-155+/+ spleen survival curves were performed using log-rank test. (F) Histopathologic GVHD scores of recipient tissues. A second cohort of mice was used for histopathologic analysis. Fourteen days after transplantation, mice from the indicated groups were killed and sections of the large bowel and liver were stained with H&E. P values were obtained by using t test. (G) Histopathologic evaluation of representative liver samples obtained from recipients of BM alone, BM + miR-155+/+ spleen, and BM + LCK-miR-155 donor cells. Slides were stained with H&E. The arrows indicate lymphocytic infiltration in the recipient livers characteristic of GVHD.
miR-155 does not affect the alloreactive or homeostatic proliferative potential of T cells
Although we have shown that miR-155 modulates aGVHD in vivo, the mechanisms by which miR-155 exerts these effects are unknown. Because miR-155 is up-regulated during T-cell activation and promotes Th1 differentiation and robust TNF-α secretion,4-7 we hypothesized that miR-155 up-regulation during aGVHD may play a pivotal role in maintaining a robust Th1 response and effector T-cell proliferation and activation during this process. In contrast, miR-155–deficient T cells may have dampened responses to alloantigen stimulation and proliferate less than miR-155 WT counterparts, resulting in reduced aGVHD. To test this hypothesis, we performed in vitro experiments where CFSE labeled CD4+ T cells from miR-155−/− and WT B6 mice were incubated with BM-derived F1 dendritic cells and donor T-cell activation (CD25 and CD69 expression), lymphocyte proliferation, and cytokine secretion (IL-2 and TNF-α) were measured over time. We did not observe any differences in T-cell activation markers, proliferation, and cytokine secretion between miR-155−/− and WT B6 splenocytes after alloantigen stimulation in vitro up to 96 hours (supplemental Figure 5A-C). These results were confirmed in vivo. We performed the previously described B6 into F1 MHC-mismatched murine experiment, using T cell–depleted BM and CFSE-labeled B6 miR-155−/− or WT splenocytes as donors. Spleens were harvested on day 3 after transplantation, and the precursor frequency21 of donor T cells was determined. There was no difference between the alloreactive proliferative response of the miR-155−/− and miR-155+/+ donor T cells in vivo (supplemental Figure 5D-E). To extend these results, we investigated the homeostatic proliferative potential of miR-155−/− T cells. Congenic lethally irradiated B6 CD45.1 mice received BM cells as well as CFSE-labeled CD45.2 miR-155+/+ or miR-155−/−splenocytes. Three days later, spleens were harvested and the precursor frequency was determined on the donor CD45.2 T cells. There was no difference in the proliferative response between the WT B6 and miR-155–deficient T cells (supplemental Figure 5F). Altogether, our results indicate that miR-155 does not affect the alloreactive or homeostatic proliferative potential of T cells.
miR-155 modulates chemokine receptor expression of allogeneic donor T cells
One of the critical steps in the pathophysiology of aGVHD involves the migration of activated donor T cells to and from secondary lymphoid organs (SLO) to the aGVHD target organs.22,23 Two major families of G protein coupled receptors, including the chemokine receptors (CCR7, CXCR4, CCR5) and the sphingosine-1-phosphate (S1P1) receptors, play an important role in orchestrating the migration of leukocytes to and from the SLOs.24-27 Thus, alteration in donor T-cell trafficking patterns could be one of the major mechanisms via which miR-155 modulates aGVHD. To investigate this possibility, we determined the expression levels of the major homing receptors CCR5, CXCR4, CCR7, and S1P1 on donor T cells isolated from recipient splenocytes on early (day 4) and late (day 21) time points after transplant using flow cytometry. We found no significant differences in the expression pattern of CCR7 between T cells of the miR-155–deficient and B6 WT cells at the time points investigated. Although there were no differences in expression at the early time point, CXCR4 was significantly down-regulated on miR-155–deficient CD4+ (251.66 ± 14.01 vs 711 ± 188.83, P = .006) and CD8+ (310.33 ± 38.73 vs 940.66 ± 243.79, P = .005) T cells compared with WT B6 T cells at day 21 (Figure 4A). We also identified a significant decrease in the surface expression of S1P1 on splenic CD4+ miR-155–deficient T cells compared with WT B6 (253.33 ± 17.03 vs 476 ± 101.79; P = .01) at day 21, whereas no differences were observed at the earlier time point (Figure 4B). With respect to CCR5, its expression was significantly lower on CD4+ and CD8+ miR-155–deficient donor T cells compared with WT B6 (CD4+; 381.66 ± 38.03 vs 787.33 ± 268.06, P = .01; and CD8+: 495 ± 40.95 vs 969.66 ± 369.15, P = .007) at day 21 (Figure 4C). Complementary to the chemokine receptor findings, we also found reduced donor lymphocyte infiltration in aGVHD target organs of mice that received miR-155–deficient splenocytes compared with those that received WT B6 splenocytes (Figure 2D-E).
miR-155 modulates chemokine receptor expression and migration of allogeneic donor T cells. On days 4 and 21 after BMT, splenocytes were harvested from F1 recipients and H2Kd- CD4+/CD8+ donor cells were analyzed by flow cytometry for chemokine receptor expression. Chemokine receptor expression was quantified by measuring mean fluorescence intensity (MFI). P values < .05 were considered significant. n = at least 5 mice per group at each time point. (A) Expression of CXCR4 on CD4+ and CD8+ WT and miR-155−/− donor T cells on day 21 after transplantation. (B) Expression of S1P1 on CD4+ WT and miR-155−/− donor T cells on day 21 after transplantation. (C) Expression of CCR5 on CD4+ and CD8+ WT and miR-155−/− donor T cells on day 21 after transplantation. (D) Average IL-12Rb1 mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR. The results shown represent the average of 9 independent mouse samples performed each in triplicate. Values are expressed as relative expression after 2ΔCt calculations and normalization with 18s RNA. Bars represent SD. (E) Average STAT-4 mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR. (F) Average IFN-γ mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR. (G) Average CCR5 mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR.
miR-155 modulates chemokine receptor expression and migration of allogeneic donor T cells. On days 4 and 21 after BMT, splenocytes were harvested from F1 recipients and H2Kd- CD4+/CD8+ donor cells were analyzed by flow cytometry for chemokine receptor expression. Chemokine receptor expression was quantified by measuring mean fluorescence intensity (MFI). P values < .05 were considered significant. n = at least 5 mice per group at each time point. (A) Expression of CXCR4 on CD4+ and CD8+ WT and miR-155−/− donor T cells on day 21 after transplantation. (B) Expression of S1P1 on CD4+ WT and miR-155−/− donor T cells on day 21 after transplantation. (C) Expression of CCR5 on CD4+ and CD8+ WT and miR-155−/− donor T cells on day 21 after transplantation. (D) Average IL-12Rb1 mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR. The results shown represent the average of 9 independent mouse samples performed each in triplicate. Values are expressed as relative expression after 2ΔCt calculations and normalization with 18s RNA. Bars represent SD. (E) Average STAT-4 mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR. (F) Average IFN-γ mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR. (G) Average CCR5 mRNA expression in untouched T cells isolated from spleens of recipient mice on day 21 after transplantation by RT-PCR.
To gain an insight into how miR-155 regulates CCR5, the primary chemokine receptor that orchestrates migration of donor T cells to recipient peripheral target organs such as liver, we isolated T cells from recipient mice on day 21 and measured mRNA expression levels of candidate genes known to regulate CCR5 by RT-PCR. In particular, we focused on the IL-12 signaling pathway that is known to regulate CCR5 expression on activated T cells28 ; and because it has been shown that IL-12R expression on T cells is negatively regulated by SOCS-1,29 a validated direct target of miR-155 in T cells.30 As shown in Figure 4D, IL-12Rβ1 expression levels were down-regulated in miR-155−/− donor T cells with respect to controls. In addition, STAT-4, which is a major biologic mediator of the downstream signaling events initiated by IL-12R activation in lymphocytes31,32 and is known to be indispensible for the induction of CCR5 on T cells,33 was found down-regulated in miR-155−/− T cells (Figure 4E). Likewise, IFN-γ, which is another important IL-12 and STAT-4 regulated gene, was down-regulated in miR-155–deficient cells (Figure 4F). As expected, CCR5 mRNA expression levels were down-regulated as well in miR-155–deficient T cells (Figure 4G). In conclusion, we found a significant decrease in the expression of homing receptors CCR5, CXCR4, and S1P1 receptors on miR-155–deficient donor T cells. The down-regulation of these homing receptors in donor T cells could impair the migration of these cells to the peripheral targets organs resulting in less aGVHD.
Blocking miR-155 expression using a seed-targeting LNA–anti–miR-155 oligonucleotides decreases the severity of aGVHD and prolonged survival after HSCT in recipient mice
Having shown that miR-155 modulates aGVHD, we next asked whether blocking miR-155 expression using LNA–anti–miR-155 could prevent or delay the development of aGVHD disease after HSCT. A recent study has described an approach that enables inhibition of miRNA function in cultured cells and in vivo using fully LNA-modified 8-mer anti–miR oligonucleotides complementary to the miRNA seed region.18 Thus, we treated lethally irradiated recipient F1 mice with LNA–anti–miR-155 (n = 10) or an 8-mer LNA control oligonucleotide (n = 10; loading dose 25 mg/kg and 5 mg/kg intravenously twice weekly),34 starting at day 7 up to day 30 after infusion of donor B6 splenocytes (Figure 5A). Treatment with anti–miR-155 decreased the severity of clinical aGVHD (Figure 5B) and prolonged the survival of recipient mice after transplantation (Figure 5C). Down-regulation of miR-155 was observed in the spleen of the mice treated with anti–miR-155 with respect to the controls (Figure 5D).
Treatment with LNA–anti–miR-155 decreases the severity of aGVHD and prolongs the survival of MHC-mismatched recipient mice. (A) Schematic presentation of the schedule of oligonucleotide injections after transplantation. Briefly, lethally irradiated recipient F1 mice transplanted with BM (5 × 106) plus B6 WT spleen cells (20 × 106) were treated with LNA–anti–miR-155 (n = 10) or control LNA oligos (n = 10) starting at day 7 with a loading dose 25 mg/kg followed by 5 mg/kg intravenously twice weekly up to day 30 after infusion of donor B6 splenocytes. (A) Clinical GVHD scores after transplantation from lethally irradiated F1 recipients transplanted with B6 donor BM and splenocytes and treated with LNA–anti–miR-155 or control oligonucleotide. P values were obtained by using t test. (B) Survival rate of the different recipient F1 mice groups. Comparisons between survival curves were performed using log-rank test. (D) miR-155 expression in spleen from LNA–anti–miR-155 or control treated mice as measured by RT-PCR. Data represent the average of 3 mice. Experiments performed in triplicate. Bars represent SE.
Treatment with LNA–anti–miR-155 decreases the severity of aGVHD and prolongs the survival of MHC-mismatched recipient mice. (A) Schematic presentation of the schedule of oligonucleotide injections after transplantation. Briefly, lethally irradiated recipient F1 mice transplanted with BM (5 × 106) plus B6 WT spleen cells (20 × 106) were treated with LNA–anti–miR-155 (n = 10) or control LNA oligos (n = 10) starting at day 7 with a loading dose 25 mg/kg followed by 5 mg/kg intravenously twice weekly up to day 30 after infusion of donor B6 splenocytes. (A) Clinical GVHD scores after transplantation from lethally irradiated F1 recipients transplanted with B6 donor BM and splenocytes and treated with LNA–anti–miR-155 or control oligonucleotide. P values were obtained by using t test. (B) Survival rate of the different recipient F1 mice groups. Comparisons between survival curves were performed using log-rank test. (D) miR-155 expression in spleen from LNA–anti–miR-155 or control treated mice as measured by RT-PCR. Data represent the average of 3 mice. Experiments performed in triplicate. Bars represent SE.
miR-155 deficiency in donor T cells does not abrogate GVL effects
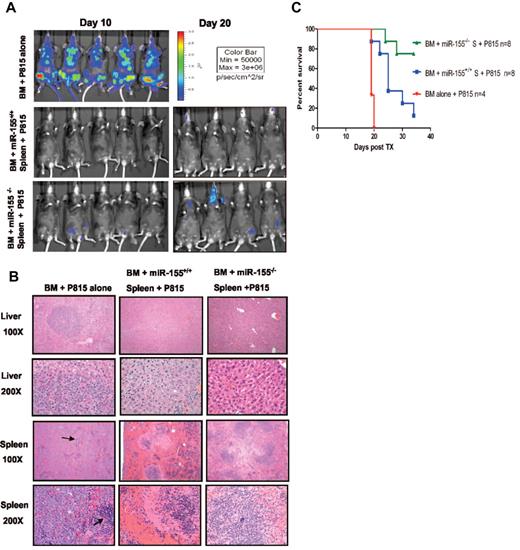
Because aGVHD and graft-versus-leukemia (GVL) effects are 2 highly linked immune reactions,1,2 we sought to determine whether miR-155 down-regulation may interfere with GVL effects.1,2 Therefore, to address the effects of miR-155 on GVL early after transplantation, we transplanted unfractionated splenocytes (20 × 106) from either miR-155−/− B6 (B6, H-2b) or WT B6 mice donors along with T cell–depleted BM (5 × 106) cells from WT B6 mice into lethally irradiated F1 mice. In addition, a luciferase-transduced murine leukemic cell line P81514-17 (1 × 104) was transplanted along with the splenocytes. Separate cohorts were used for imaging and survival. As shown in Figure 6A-B, there were no significant differences in the leukemic burden in recipient tissues as measured by bioluminescence assays and histopathology between miR-155−/− versus WT B6 splenocyte recipients, suggesting retention of GVL effect in miR-155−/− donor T cells. Recipients of miR-155−/− spleen cells plus P815 (n = 8) survived longer than WT spleen cell recipients plus P815 (n = 8; log-rank test P = .01; Figure 6C). Two mice from the miR-155−/− cohort died from leukemia as defined by the presence of hind limb paralysis, although, like the remaining 6 mice from that group, there was no significant evidence of leukemic infiltration in the spleen or liver by histopathology as shown in a representative case (Figure 6B). The cohort of mice who received miR-155+/+ WT spleen cells died earlier from aGVHD and in one case from leukemia defined only by hind limb paralysis. As expected, the BM only cohort died prematurely from disseminated leukemia (Figure 6B-C).
miR-155 deficiency in donor T cells does not abrogate GVL effects. (A) Whole body bioluminescent signal intensity of recipient mice. Mice were imaged on day 10 and day 20. (B) Histopathologic analysis of spleen and liver tissues of the different mice cohorts. Histopathologic examination at lower power (original magnification ×100) revealed extensive nodular leukemic infiltration in the liver in the BM alone recipients, whereas no infiltrates were seen in recipients who received splenocytes, either from WT or miR-155–deficient mice (top panels). Middle top panels: The same liver sections are shown at larger magnification (original magnification ×200). Note in the BM alone the many large anaplastic cells with pleomorphic, multilobular large nuclei and frequent mitosis figures. Last 2 bottom panels: Spleen sections at lower (top) and higher (bottom) magnification. Note the complete replacement of the spleen by the large anaplastic cells in the BM alone group. The arrows point to normal spleen. There were no leukemic infiltrations in the recipient groups who received either WT or miR-155–deficient splenocytes. (C) Survival of lethally irradiated mice transplanted with WT or miR-155–deficient splenocytes plus BM P815 leukemic cells. Survival comparisons were performed using the log-rank test.
miR-155 deficiency in donor T cells does not abrogate GVL effects. (A) Whole body bioluminescent signal intensity of recipient mice. Mice were imaged on day 10 and day 20. (B) Histopathologic analysis of spleen and liver tissues of the different mice cohorts. Histopathologic examination at lower power (original magnification ×100) revealed extensive nodular leukemic infiltration in the liver in the BM alone recipients, whereas no infiltrates were seen in recipients who received splenocytes, either from WT or miR-155–deficient mice (top panels). Middle top panels: The same liver sections are shown at larger magnification (original magnification ×200). Note in the BM alone the many large anaplastic cells with pleomorphic, multilobular large nuclei and frequent mitosis figures. Last 2 bottom panels: Spleen sections at lower (top) and higher (bottom) magnification. Note the complete replacement of the spleen by the large anaplastic cells in the BM alone group. The arrows point to normal spleen. There were no leukemic infiltrations in the recipient groups who received either WT or miR-155–deficient splenocytes. (C) Survival of lethally irradiated mice transplanted with WT or miR-155–deficient splenocytes plus BM P815 leukemic cells. Survival comparisons were performed using the log-rank test.
miR-155 expression is up-regulated in the intestinal tract from patients with aGVHD
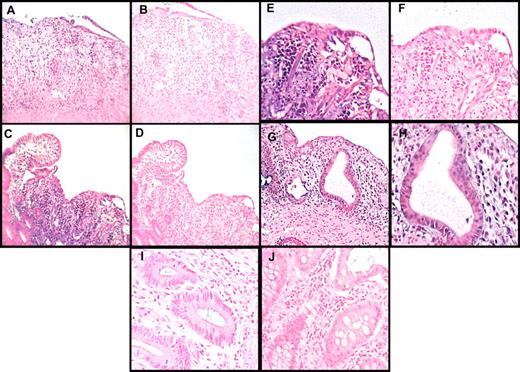
To establish the potential relevance of the mouse findings to the human system, we measured miR-155 expression using LNA-based in situ hybridization from clinically and histologically confirmed small- and large-bowel biopsies of aGVHD patients (n = 5), and healthy controls (n = 3; Figure 7). We found a strong up-regulation of miR-155 expression in the inflammatory cells from all patients with small- and large-bowel aGVHD, whereas miR-155 expression was absent in normal bowel (Figure 7). As expected, further immunohistochemistry studies confirmed that the inflammatory cells were T lymphocytes (supplemental Figure 8). Altogether, these results suggest that high levels of miR-155 are relevant in human aGVHD.
miR-155 expression is up-regulated in the intestinal tract from patients with aGVHD. Histopathologic assessment of human small- and large-bowel samples from patients with aGVHD or from control patients who had a bowel biopsy, but no pathology was observed (controls). In situ hybridization was performed using a digoxigenin-labeled LNA-modified probe complementary to miR-155 or a scrambled LNA control probe. (A) The colon tissue shows marked inflammation with a loss of glands and concomitant erosion. Note that the many inflammatory cells in the lamina propria were positive for miR-155 (dark staining; original magnification ×200). (B) The miR-155 in situ hybridization signal is lost in the same cells in the serial section on using the LNA scramble control probe. (C) A section of ileum shows a normal villous to the left side of the panel, and the loss of the villi in the rest of the section. Note the strong signal in the inflammatory cells in the area of the damaged villi with the miR-155 probe (original magnification ×200). (D) Negative control for panel C, showing loss of the signal with the scrambled probe in the same cells in the serial section. (E-F) The area to the right of the panel C is magnified (original magnification ×400) in panel E (miR-155) and panel F (scrambled probe) to underscore the localization of the signal to mononuclear cells. (G) An earlier stage of GVHD with degenerated/regenerating glands with mononuclear infiltrates, which are primarily located in the lamina propria adjacent to the damaged glands. The miR-155 signal is rare in the damaged epithelial cells but is strong in the adjacent inflammatory cells as shown in higher magnification (×1000) (H) where mononuclear infiltrate into the epithelia, typical of GVHD, is evident. (I-J) Normal colon mucosa negative for miR-155 staining (original magnification ×400).
miR-155 expression is up-regulated in the intestinal tract from patients with aGVHD. Histopathologic assessment of human small- and large-bowel samples from patients with aGVHD or from control patients who had a bowel biopsy, but no pathology was observed (controls). In situ hybridization was performed using a digoxigenin-labeled LNA-modified probe complementary to miR-155 or a scrambled LNA control probe. (A) The colon tissue shows marked inflammation with a loss of glands and concomitant erosion. Note that the many inflammatory cells in the lamina propria were positive for miR-155 (dark staining; original magnification ×200). (B) The miR-155 in situ hybridization signal is lost in the same cells in the serial section on using the LNA scramble control probe. (C) A section of ileum shows a normal villous to the left side of the panel, and the loss of the villi in the rest of the section. Note the strong signal in the inflammatory cells in the area of the damaged villi with the miR-155 probe (original magnification ×200). (D) Negative control for panel C, showing loss of the signal with the scrambled probe in the same cells in the serial section. (E-F) The area to the right of the panel C is magnified (original magnification ×400) in panel E (miR-155) and panel F (scrambled probe) to underscore the localization of the signal to mononuclear cells. (G) An earlier stage of GVHD with degenerated/regenerating glands with mononuclear infiltrates, which are primarily located in the lamina propria adjacent to the damaged glands. The miR-155 signal is rare in the damaged epithelial cells but is strong in the adjacent inflammatory cells as shown in higher magnification (×1000) (H) where mononuclear infiltrate into the epithelia, typical of GVHD, is evident. (I-J) Normal colon mucosa negative for miR-155 staining (original magnification ×400).
Discussion
Using a combination of gain and loss of function approaches, we show here that miR-155 regulates the severity of aGVHD after allogeneic HSCT. Our data are consistent with previous studies indicating a proinflammatory role of miR-155 in human disease.35-37 It has been reported that miR-155 expression is up-regulated in the inflamed colonic mucosa and in the synovial tissue of patients with active ulcerative colitis and rheumatoid arthritis, respectively.36,37 It was also shown that miR-155–deficient mice were highly resistant to experimental autoimmune encephalomyelitis and that miR-155 promotes the development of inflammatory T cells, including the T helper 17 (Th17) cell and Th1 cell subsets.35
Mechanistically, our results can be explained by a combination of miR-155–dependent effects on T-cell homing and TNF-α secretion. Notably, we observed a significant down-regulation of CCR5 mRNA and protein expression in miR-155–deficient donor T cells after engraftment (day 21), which is likely caused by reduced IL-12R levels and dampened downstream signaling pathways via STAT-4. CCR5 plays an important role in directing the migration of donor T cells toward the liver.23,38 We reasoned that the down-regulation of CCR5 on the donor miR155−/− T cells may contribute to the dramatic decrease of donor lymphocytic infiltration in the recipient liver as confirmed by histology.
In addition, we observed a marked decrease in the expression of SIP1 in donor miR-155-deficient T cells after HSCT. Previous studies showed that SIP1 down-regulation via administration of FTY720 leads to sequestration of T cells within secondary lymphoid organs, decreasing lymphocyte migration and tissue infiltration.39,40 Last, we found decreased levels of CXCR4 in donor miR-155–deficient T cells at day 21 after HSCT. CXCL12/SDF-1 and its receptor CXCR4 have a major role in directing the migration of progenitors during hematopoiesis25 and a minor role in T-cell activation, proliferation, and migration to peripheral lymph nodes.41 Thus, CXCR4 down-regulation in miR-155–deficient T cells might contribute to a reduction in the inflammatory function/phenotype and migration of these cells. Overall, the combined effects of CXCR4, CCR5, and S1P1 down-regulation in donor T cells suggests that alterations in cell trafficking and homing could be one of the mechanisms by which miR-155 modulates the severity of aGVHD in mice. Furthermore, our data indicate that miR-155 up-regulation during aGVHD may be important to maintain high levels of TNF-α and perpetuate inflammation during this process, in particular regarding gut aGVHD.20,42,43 High levels of TNF-α have been shown to be associated with aGVHD severity, and studies in animal models have established the relevance of TNF-α in the activation of APCs, T cells, and tissue damage.20,42-45
Altogether, our discoveries point to miR-155 as novel target for therapeutic intervention in aGVHD. Blocking miR-155 expression in donor T cells after HSCT resulted in less aGVHD and improved survival. Importantly, the loss of miR-155 expression in our model did not abrogate GVL effects. Because many hematologic malignancies overexpress miR-15546-48 and allogeneic HSCT is used frequently as consolidation therapy for these diseases, suppressing miR-155 expression in this setting may also be of potential beneficial not only for aGVHD prevention but also for minimal residual disease control.
In conclusion, we have shown that miR-155 expression is up-regulated in T cells from mice with aGVHD and modulation of miR-155 expression on these cells dramatically affects aGVHD incidence and severity in a murine model of aGVHD. More importantly, we show that blocking miR-155 expression in vivo by LNA–anti–miR-155 after allogeneic HSCT prevents lethal aGVHD in mice. Finally, up-regulation of miR-155 was found in clinical specimens from patients with intestinal aGVHD. Altogether, our data indicate a role for miR-155 in the modulation of aGVHD and thus point to miR-155 as a novel target for therapeutic intervention in aGVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Marcel R. M. van den Brink and Vladimir Ponomarev for the P815 cell line and Dr Susheela Tridandapani for providing the Bic-deficient (bicm2/m2) mice.
This work was supported by the National Institutes of Health (grants R0172669, P50CA140158, and other funding agency grants AI34495, HL56067) and Pelotonia Fellowship award (P.R).
National Institutes of Health
Authorship
Contribution: P.R., C.E.A.H., N.S., C.N., R.S., M.H., and P.A.T. performed all the transplant experiments; C.M. performed T-cell selection; P.R. performed T-cell assays and FACS studies; S.S. and G.J.N. performed in situ LNA hybridization; A.L. and L.C. performed experiments and analyzed data; S.C. developed the miR-155 transgenic mice; G.H. and A.S. performed pathologic studies; S.O., O.B., and S.K. synthesized LNA; B.R.B., J.C.B., G.A.H., G.M., D.P., S.M.D., C.M.C., and M.C. designed and supervised research performed in their laboratories; S.M.D. provided patient samples; and R.G. and P.R. wrote the manuscript.
Conflict-of-interest disclosure: S.O., O.B., and S.K. are employees of Santaris Pharma, a clinical stage biopharmaceutical company that develops RNA-targeted therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Ramiro Garzon, The Ohio State University, Comprehensive Cancer Center, Biomedical Research Tower Rm 1084, 460 West 12th Ave, Columbus, OH 43210; e-mail: ramiro.garzon@osumc.edu.
References
Author notes
P.R., C.E.A.H., and S.C. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal