Abstract
In rodent graft-versus-host disease (GVHD) models, anti–IL-21 neutralizing mAb treatment ameliorates lethality and is associated with decreases in Th1 cytokine production and gastrointestinal tract injury. GVHD prevention was dependent on the in vivo generation of donor-inducible regulatory T cells (Tregs). To determine whether the IL-21 pathway might be targeted for GVHD prevention, skin and colon samples obtained from patients with no GVHD or grade 2 to 4 GVHD were analyzed for IL-21 protein expression. By immunohistochemistry staining, IL-21 protein-producing cells were present in all gastrointestinal tract samples and 54% of skin samples obtained from GVHD patients but not GVHD-free controls. In a human xenogeneic GVHD model, human IL-21–secreting cells were present in the colon of GVHD recipients and were associated with elevated serum IL-21 levels. A neutralizing anti–human IL-21 mAb given prophylactically significantly reduced GVHD-associated weight loss and mortality, resulting in a concomitant increase in Tregs and a decrease in T cells secreting IFN-γ or granzyme B. Based on these findings, anti–IL-21 mAb could be considered for GVHD prevention in the clinic.
Introduction
Graft-versus-host disease (GVHD) is a severe complication and major cause of morbidity and mortality after HSCT.1 Although pro-inflammatory cytokine production has been associated with GVHD induction, the relative contribution of specific cytokines to GVHD has been difficult to establish because of seemingly paradoxical results. For example, whereas secretion of the Th1 inflammatory cytokine IFN-γ increases GVHD-related colon damage,2,3 CD4+ T cells from IFN-γ−/− mice cause even more severe GVHD.4 Similarly, although early experiments inferred a role for Th2 responses in GVHD,3 donor T cells from IL-4−/− mice cause severe disease in some,5 but not all,4 studies, and the cotransfer of Th2-skewed cells can mitigate GVHD pathology.6,7 Like Th1 and Th2 cells, the role of Th17 cells in GVHD is controversial. Whereas cotransfer of highly purified ex vivo polarized Th17 cells with naive T cells results in a more aggressive disease that is dependent on IL-17,8 CD4+ T cells from IL-17−/− or RORγt (Th17-deficient) donors attenuate, exacerbate, or had no effect on GVHD, depending on the model and knockout strain.9–12
IL-21 is produced by CD4+ T cells (especially Th17 cells13 ) and natural killer T cells14 and signals through the IL-2Rγc and IL-21R complex. The receptor for IL-21 is expressed on hematopoietic and epithelial cells and promotes the activation, differentiation, maturation, and expansion of NK cells, B cells, CD8+ and CD4+ T cells, dendritic cells, and macrophages.15,16 IL-21 contributes to autoimmunity in some,17–19 but not all,20,21 experimental models. Recent reports by us and others show that inhibiting IL-21 also decreases disease severity in murine models of GVHD.22–26 Specifically, disruption of IL-21 signaling, either genetically or via neutralizing mAbs, reduces transplant-related weight loss, tissue pathology, and mortality. Disease amelioration correlated with decreased numbers of CD4+ T cells secreting IFN-γ and a concomitant increase in the proportion of CD4+ T cells expressing Foxp3. Using regulatory T cell–depleted donor cells obtained from a FoxP3-GFP reporter mouse, we demonstrated that IL-21 blockade with anti–IL-21 mAb increased the frequency of FoxP3+ cells because of conversion of CD4 + 25− T cells into inducible regulatory T cells (iTregs) rather than preferential expansion of natural Tregs (nTregs) themselves.22
Although murine models indicated that IL-21 blockade was an attractive strategy to reduce GVHD-associated injury, this may not be the case for human GVHD because critical differences exist in how IL-21 functions in mouse and humans. For example, in humans, IL-21 can act as the second signal required for Th17 polarization27 whereas only IL-6, and not IL-21, has this function in mice.28,29
In the current study, we show that human IL-21 protein expression within GVHD target organs correlates with disease severity. Human IL-21–producing cells also can be found in the colon of mice undergoing a xenogeneic GVHD reaction. Using an anti–human IL-21–neutralizing mAb, we demonstrate that IL-21 blockade is also effective at suppressing GVHD-associated pathology and mortality induced by human cells. As observed in murine models, anti–IL-21 mAb-treated mice had a lower percentage of cells capable of secreting IFN-γ and granzyme B (GrB), and a higher percentage capable of secreting IL-4. Treated mice also exhibited significant increases in Tregs (both absolute number and percentage of CD4+Foxp3+ cells). Further, anti–IL-21 mAb treatment in the xenogeneic GVHD model decreased the percentage of cells secreting IL-17. These results provide proof of principle that human IL-21 is expressed in GVHD target organs and that anti–human IL-21 mAb may be an effective treatment for suppressing GVHD in the clinic.
Methods
Mice
NOD/SCID/γc−/− mice were purchased from The Jackson Laboratory and housed in a specific pathogen-free facility in micro-isolator cages. Mice were used at 8 to 12 weeks. Animal protocols were approved by Institutional Animal Care and Use Committee at the University of Minnesota.
Flow cytometry and antibodies
Human-specific antibodies used for flow cytometry CD4 (clone RPA-T4), CD8 (RPA-T8), CD14 (MSE2), CD19 (HIB19), CD25 (M-A251), CD45RA (HI100), and GrB (GB11) were purchased from BD Biosciences PharMingen or eBioscience. Antibodies to IFN-γ, IL-2, IL-4, and IL-17 were from eBioscience, whereas Foxp3 (clone 249D) is from BioLegend. For intracellular cytokine staining, spleens were excised and splenocytes isolated by ACK lysis and stimulated for 4 hours with or without phorbol myristate acetate (2 pg/mL) and ionomycin (1 μg/mL) in the presence of brefeldin A (100 ng/mL; all from Sigma-Aldrich). Cells were then stained for CD4, CD25, Foxp3, and cytokine (IL-2, IL-4, IL-17, and IFN-γ) or GrB using the standard Foxp3 intracellular staining kit. Acquisition was performed using a FACSCalibur or LSRII (BD Biosciences), and data were analyzed using FlowJo Version 8.8.6 software (TreeStar). Neutralizing human anti–hIL-21 mAb (clone 362.597.3.15; hIgG1) and isotype control mAb (trastuzumab; hIgG1) were provided by ZymoGenetics.
In the in vivo xenogeneic GVHD model, T-, B-, and NK-deficient and macrophage-defective NOD/ SCID/IL-2Rγc−/− mice (The Jackson Laboratory) were injected intravenously with human PBMCs at doses ranging from 30 to 60 × 106. Mice were assessed for survival daily and weighed thrice weekly. To document PBMC-associated peripheral T-cell expansion, animals were bled (10-40 μL), red blood cells lysed, and Treg and PBMC subsets were enumerated by flow cytometry by staining with mAbs to human CD4, Foxp3, CD8, and CD45 and acquired with a known number of counting beads (Sigma-Aldrich). Animal protocols were approved by Institutional Animal Care and Use Committee at the University of Minnesota.
IL-21 ELISA
To quantify human IL-21 levels in sera, mice were bled via facial vein on day 20 or at the time of death. In addition, serum samples (stored at −80°C and shipped on dry ice) were collected from 38 adult allo-HSCT patients at the University of Minnesota, 19 adult patients at the Universitätsspital Basel, and 38 adult patients at the Hospital Saint Louis, France (all transplanted for hematologic malignancies). All sample acquisition was approved by the individual institutional review boards. Patient characteristics and clinical status of patients at the time of serum collection are described in Results (Table 1).
Patient characteristics and clinical status at the time of serum collection for human IL-21 analysis
| Graft source . | Conditioning (full/reduced) . | Matching (related/unrelated) . | GVHD grade (0/1/2/3/4) . | Sample date post-treatment aGVHD history (active/none/inactive) . | Steroid refractory GVHD . |
|---|---|---|---|---|---|
| Adult | 37/14 | 26/25 | 20/14/10/5/2 | 23/35/24 | 5 |
| Cord | 44/0 | 0/44 | 16/1/17/4/6 | 13/18/13 | 0 |
| Graft source . | Conditioning (full/reduced) . | Matching (related/unrelated) . | GVHD grade (0/1/2/3/4) . | Sample date post-treatment aGVHD history (active/none/inactive) . | Steroid refractory GVHD . |
|---|---|---|---|---|---|
| Adult | 37/14 | 26/25 | 20/14/10/5/2 | 23/35/24 | 5 |
| Cord | 44/0 | 0/44 | 16/1/17/4/6 | 13/18/13 | 0 |
A total of 95 adult patients undergoing allo-HCT with cells from either adult or cord blood were used in this analysis.
To optimize the detection of IL-21 in human serum, several commercial IL-21 assays as well as bead-based assays developed at ZymoGenetics were tested. The most sensitive assay was the human IL-21 Platinum ELISA by eBioscience which had a lower limit of quantification of 156 pg/mL when conducted according to the manufacturer's instructions. The anti–hIL-21 ELISA was validated before use for GVHD patient samples by doing spike and recovery experiments with broad dilutions of recombinant IL-21 in human serum and using a recombinant soluble neutralizing IL-21 receptor (IL-21R/γc-Fc) as a specificity control. Quality control (QC) samples (recombinant human IL-21 [rhIL-21]; ZymoGenetics) spiked into serum at 2000 pg/mL, 500 pg/mL, and 200 pg/mL) were included in each assay. QC samples were made by diluting rhIL-21 into a custom pool of normal human donor serum previously confirmed by validated bioanalytical assays to contain undetectable endogenous levels of IL-21 (ZymoGenetics).
IHC staining and pathology data
To identify human IL-21–secreting cells in the xenogeneic model of GVHD, samples of intestine were harvested on days 10 and 20 from animals that received 30 × 106 PBMCs and were flash-frozen in Tissue-Tek OCT compound (VWR); 5-μm cryosections were cut and air dried for 1 hour then placed with desiccant at −80°C. For staining, slides were brought to room temperature and fixed in acetone/ethanol (3:1) and washed with PBS. Slides were then incubated with avidin/biotin blocking reagents (Vector Laboratories), 10% normal human serum, peroxidase, and alkaline phosphatase (Dako North America). IL-21–secreting cells were detected with biotinylated human anti–hIL-21 (clone 366.552.11, hIgG1 ZymoGenetics lot no. E10435), followed by HRP-conjugated streptavidin (Dako North America) and diaminobenzidine (Vector). A control biotinylated hIgG1 Fc protein was used as a negative control (ZymoGenetics). Finally, the tissue sections were counterstained with hematoxylin. Images were captured with a Nikon DS-Fi1 digital camera attached to a Nikon Eclipse 80i microscope using NIS-Elements BR Version 3.0 software. Slides were subjectively evaluated by an American College of Veterinary Pharmacists boarded veterinary pathologist blinded to treatment. Staining was scored as follows: +/− indicates weak; +, mild; ++, moderate; and +++, strong. In addition, the number of immunoreactive mononuclear cells (MNCs) in the colons were graded as follows (entire length of samples examined): 1, less than 50% of 20× fields contained at least 1 immunoreactive cell; 2, 50% to 75% of 20× fields contained at least 1 immunoreactive cell; or 3, all or nearly all 20× fields contained at least 1 immunoreactive cell.
IL-21–secreting cells were quantified in adult patients after allo-HSCT at the Hospital Saint-Louis. All patients had biopsies performed as diagnostic procedures for digestive or cutaneous GVHD symptoms.30 The Hospital Saint-Louis ethical review board approved the design of this study. Human tissue samples were fixed in 4% formaldehyde, embedded in paraffin, and 6-μm cryosections were cut, dewaxed, and subjected to citrate antigen retrieval before slides were washed. Human tissues were stained and recorded as with the xenogeneic GVHD tissues and were analyzed by 2 independent pathologists who were blinded of the pathologic results when quoting the intensity of the staining and number of MNCs stained. Patient descriptions and details of HSCT are provided in “IL-21 protein is expresed in the GI tract and skin, but not the serum, of allo-HSCT patients with GVHD.”
Statistical analysis
Data were analyzed by ANOVA or Student t test. P values ≤ .05 were considered statistically significant.
Results
Human IL-21 protein is expressed in the GI tract and sera of murine recipients with xenogeneic GVHD
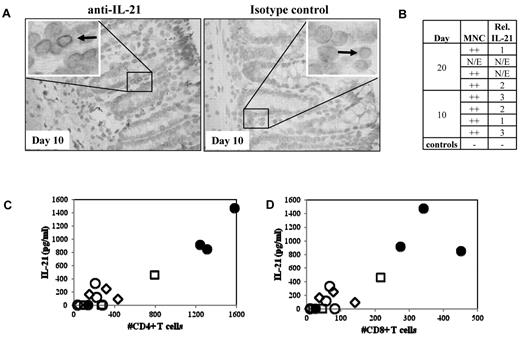
We previously demonstrated that IL-21 is produced by donor T cells and functions in an autocrine loop to drive murine GVHD.22 To test whether IL-21 might play a similar role in human GVHD, we used a xenogeneic model of GVHD resulting from adoptive transfer of human PBMCs (30 × 106) into NOD/SCID/γc−/− mice. Because blocking IL-21 signaling in mice preferentially ameliorated only gut pathology associated with murine GVHD, colon and ileum samples from mice receiving human PBMCs that were electively killed on day 10 or day 20 after transplantation were assayed for the presence of human IL-21–secreting cells (Figure 1A-B). MNCs with cytoplasmic IL-21 staining were readily visible in all evaluable samples (n = 6, Figure 1A). Greater numbers of IL-21 immunoreactive cells were observed in the mouse large intestine than in the small intestine (not shown).
IL-21 protein is expressed in the GI tract and found in serum in recipient mice with xenogeneic GVHD. To determine whether IL-21 is produced by human cells in a xenogeneic model of GVHD, colon, small intestine, and blood were removed from NOD/SCID/γc−/− mice receiving 30 or 60 × 106 human PBMCs and assayed for IL-21. (A) Left: Representative large intestine from mice receiving 30 × 106 PBMCs, probed with anti–IL-21. Arrow points to an immunoreactive MNC. Right: Same tissue probed with a biotinylated Fc control. (B) Quantitative analysis comparing the degree of MNC infiltrate and the relative number of IL-21–secreting cells that are present in colon sections. N/E indicates not evaluable. (C) Comparison of the IL-21 concentration in sera with the number of CD4+ (C) or CD8+ (D) T cells present in the blood on day 10. Serum samples taken from animals receiving 30 or 60 × 106 PBMCs (open and filled symbols, respectively) on day 10 (diamonds), day 20 (squares), or when animals became moribund (circles).
IL-21 protein is expressed in the GI tract and found in serum in recipient mice with xenogeneic GVHD. To determine whether IL-21 is produced by human cells in a xenogeneic model of GVHD, colon, small intestine, and blood were removed from NOD/SCID/γc−/− mice receiving 30 or 60 × 106 human PBMCs and assayed for IL-21. (A) Left: Representative large intestine from mice receiving 30 × 106 PBMCs, probed with anti–IL-21. Arrow points to an immunoreactive MNC. Right: Same tissue probed with a biotinylated Fc control. (B) Quantitative analysis comparing the degree of MNC infiltrate and the relative number of IL-21–secreting cells that are present in colon sections. N/E indicates not evaluable. (C) Comparison of the IL-21 concentration in sera with the number of CD4+ (C) or CD8+ (D) T cells present in the blood on day 10. Serum samples taken from animals receiving 30 or 60 × 106 PBMCs (open and filled symbols, respectively) on day 10 (diamonds), day 20 (squares), or when animals became moribund (circles).
Because human IL-21–producing cells were observed in gut sections from mice in this xenogeneic model of GVHD, we assessed whether human IL-21 could be detected in the serum by ELISA. Because disease severity in this model is proportional to the number of PBMCs transferred, we assayed serum for one group of animals receiving 3 × 107 PBMCs electively killed on day 20, and other groups of animals receiving 3 × 107 or 6 × 107 PBMCs after they became moribund (median survival times, 23 vs 12 days, respectively; range, 21-24 and 12-33 days, respectively; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). IL-21 was present in serum, and the concentration of IL-21 correlated with day 10 peripheral CD4+ and CD8+ T-cell expansion (Figure 1C; r = 0.95 and 0.87, respectively).
Anti–IL-21 mAb treatment significantly reduces lethality and weight loss in a xenogeneic model of GVHD
Because human IL-21 protein is expressed in mice with xenogeneic GVHD and disrupting IL-21/IL-21R signaling ameliorates disease in mouse models of GVHD,22 we tested whether an anti–IL-21 mAb could ameliorate disease in our xenogeneic model of GVHD. On day 0, 30 × 106 PBMCs were injected intravenously, and weight and health were monitored through day 45. Anti–IL-21 antibody or isotype control (200 μg) was injected intraperitoneally on day −1, then 3 times weekly until day 41 (Figure 2A). As shown in Figure 2B, anti–IL-21 mAb treatment significantly decreased mortality. Moreover, whereas no animals from the PBMCs only or control mAb-treated mice survived past day 31, 60% (3 of 5) of mice receiving anti–IL-21 mAb survived until the end of the experiment (Figure 2B). GVHD-associated weight loss was also significantly reduced after day 14 by anti–IL-21 mAb treatment (Figure 2C). Liver GVHD was reduced in mice (n = 4) receiving anti–IL-21 (2.5 ± 0.6) compared with PBMCs only (3.4 ± 0.1; P = .08) and IgG control treated animals (3.4 ± 0.3; P = .09). Similar to our fully murine GVHD studies,22 survival of the anti–IL-21 mAb-treated animals in the xenogeneic GVHD model diminished after discontinuation of mAb treatment, although the decreasing weight curve indicates these animals had active GVHD before day 41.
Anti–IL-21 mAb treatment reduces lethality and weight loss in a xenogeneic model of GVHD. NOD/SCID/γc−/− mice receiving human PBMCs (30 × 106 cells) were treated with anti–IL-21 mAb or isotype control to assess potency for preventing xenogeneic GVHD. (A) Schematic showing the antibody dosing schedule: 200 μg per injection; injections starting on day −1, and 3 times weekly for 6 weeks. (B) Kaplan-Meier survival curves for mice receiving PBMCs only (solid gray line) or PBMCs plus anti–IL-21 mAb (solid black line) or isotype control (dashed black line). (C) Average weight (percentage of initial) for mice surviving on a given day for different groups of mice. *P ≤ .05 for anti–IL-21 from days 14 to 24. n = 5 mice for each group. Two independent experiments were performed with similar results.
Anti–IL-21 mAb treatment reduces lethality and weight loss in a xenogeneic model of GVHD. NOD/SCID/γc−/− mice receiving human PBMCs (30 × 106 cells) were treated with anti–IL-21 mAb or isotype control to assess potency for preventing xenogeneic GVHD. (A) Schematic showing the antibody dosing schedule: 200 μg per injection; injections starting on day −1, and 3 times weekly for 6 weeks. (B) Kaplan-Meier survival curves for mice receiving PBMCs only (solid gray line) or PBMCs plus anti–IL-21 mAb (solid black line) or isotype control (dashed black line). (C) Average weight (percentage of initial) for mice surviving on a given day for different groups of mice. *P ≤ .05 for anti–IL-21 from days 14 to 24. n = 5 mice for each group. Two independent experiments were performed with similar results.
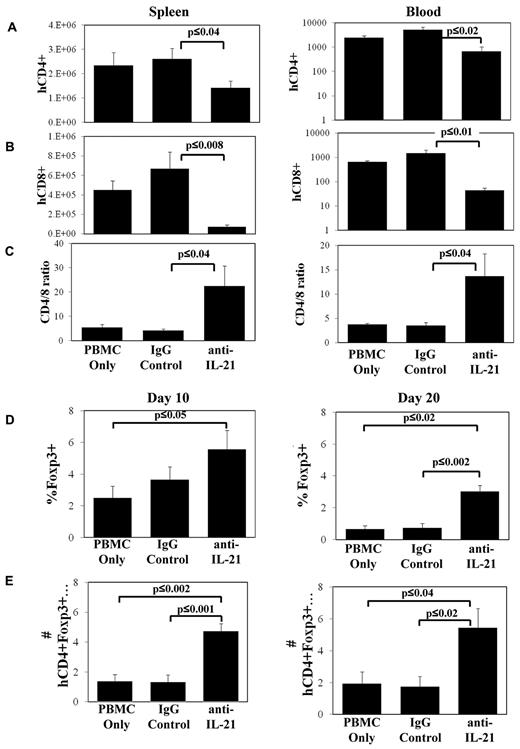
IL-21 blockade decreases CD8+ T-cell numbers in xenogeneic GVHD and increases the percentage of CD4+ cells expressing Foxp3
To determine the mechanisms associated with anti–IL-21 amelioration of GVHD, we assayed the expansion of human CD4+ and CD8+ T cells in the spleen and in circulation on days 10 and 20. Similar to murine models of GVHD, blocking IL-21 signaling had no effect on the number of CD4+ or CD8+ T cells present in spleen or blood 10 days after transplantation (data not shown). However, mice treated with anti–IL-21 mAb had decreased CD4+ and CD8+ T-cell numbers in spleen and blood on day 20 (Figure 3A-B). Anti–IL-21 mAb treatment was more effective at decreasing CD8+ compared with CD4+ T-cell expansion, resulting in an increased CD4/CD8 T-cell ratio (Figure 3C).
Anti–IL-21 mAb treatment decreases CD8+ T-cell numbers in xenogeneic GVHD and increases the percentage of CD4+ cells expressing Foxp3. NOD/SCID/γc−/− mice receiving human HLA-A2− PBMCs (30 × 106 cells) were treated with anti–IL-21 mAb, isotype control antibody, or in vitro expanded, HLA-A2+ nTregs and were electively killed on days 10 and 20. Average number (± SEM) of human CD4+HLA-A2− (A), CD8+HLA-A2− (B) T cells in spleen or microliter of blood, or ratio of CD4/CD8 (gated for HLA-A2−) on day 20 (C). Relative (D) or total (E) number (× 104; ± SEM) of human CD4+HLA-A2−Foxp3+ cells in spleen on day 10 or day 20. n = 3 or 4 mice for each time point (day 10 and day 20) and each treatment group, and data shown are representative of 2 independent experiments.
Anti–IL-21 mAb treatment decreases CD8+ T-cell numbers in xenogeneic GVHD and increases the percentage of CD4+ cells expressing Foxp3. NOD/SCID/γc−/− mice receiving human HLA-A2− PBMCs (30 × 106 cells) were treated with anti–IL-21 mAb, isotype control antibody, or in vitro expanded, HLA-A2+ nTregs and were electively killed on days 10 and 20. Average number (± SEM) of human CD4+HLA-A2− (A), CD8+HLA-A2− (B) T cells in spleen or microliter of blood, or ratio of CD4/CD8 (gated for HLA-A2−) on day 20 (C). Relative (D) or total (E) number (× 104; ± SEM) of human CD4+HLA-A2−Foxp3+ cells in spleen on day 10 or day 20. n = 3 or 4 mice for each time point (day 10 and day 20) and each treatment group, and data shown are representative of 2 independent experiments.
In our prior studies, we demonstrated that IL-21 blockade suppresses disease in murine GVHD models primarily by increasing iTreg generation.22 To determine whether treatment with anti–IL-21 mAb supports an increase in human Tregs in vivo in the xenogeneic model, we enumerated Foxp3+ cells in the spleen on day 10 and day 20 in animals treated with anti–IL-21 mAb. Anti–IL-21 mAb treatment increased the percentage and absolute number of splenic-localized CD4+ cells that also expressed Foxp3+ as early as day 10 compared with the PBMC control (Figure 3D-E). An approximate 3-fold increase in the percentage and absolute number of CD4+ T cells that coexpressed Foxp3 was noted in the spleen on day 20 compared with either PBMCs or irrelevant mAb-treated groups (Figure 3D-E).
Splenocytes from mice receiving anti–IL-21 mAb have decreased numbers of human cells secreting IFN-γ, IL-17, and GrB
In addition to preventing Treg induction, previous literature indicates that IL-21 contributes to GVHD-induced alloresponses by skewing effector cell secretion from IL-4 and IL-17 to IFN-γ, which can mediate GVHD-related colon damage. To determine the extent and type of effector cell differentiation with or without anti–IL-21 mAb treatment, splenocytes were isolated from animals 10 or 20 days after receiving 30 × 106 PBMCs, and the percentage of cells secreting IFN-γ, IL-4, or IL-17 determined by flow cytometry after restimulation with phorbol myristate acetate and ionomycin. Whereas anti–IL-21 mAb treatment had no effect on these cytokines at the early time point (Figure 4A,C), anti–IL-21 mAb suppressed the generation of IFN-γ–secreting cells and actually increased the percentage of cells secreting IL-4 as assessed on day 20 after transplantation (Figure 4B,D). As might be expected, albeit in contrast to our previous murine GVHD data,22 anti–IL-21 mAb treatment also significantly decreased the percentage of cells secreting IL-17 at both 10 and 20 days (Figure 4E-F).
Splenocytes from mice receiving anti–IL-21 mAb have decreased numbers of cells secreting IFN-[gamma], IL-17, and GrB. Spleens were isolated on day 10 and day 20 from NOD/SCID/γc−/− mice receiving human PBMCs (30 × 106 cells) that were treated with nothing, anti–IL-21 mAb, or isotype control antibody, and splenocytes were stimulated with phorbol myristate acetate and ionomycin and assayed using intracellular cytokine staining. Average percentage (± SEM) of cells secreting IFN-γ (A-B), IL-4 (C-D), IL-17 (E-F), and GrB (G-H) on day 10 (A,C,E,G) or day 20 (B,D,F,H). n = 4 mice for each time point (day 10 and day 20) and for each treatment group.
Splenocytes from mice receiving anti–IL-21 mAb have decreased numbers of cells secreting IFN-[gamma], IL-17, and GrB. Spleens were isolated on day 10 and day 20 from NOD/SCID/γc−/− mice receiving human PBMCs (30 × 106 cells) that were treated with nothing, anti–IL-21 mAb, or isotype control antibody, and splenocytes were stimulated with phorbol myristate acetate and ionomycin and assayed using intracellular cytokine staining. Average percentage (± SEM) of cells secreting IFN-γ (A-B), IL-4 (C-D), IL-17 (E-F), and GrB (G-H) on day 10 (A,C,E,G) or day 20 (B,D,F,H). n = 4 mice for each time point (day 10 and day 20) and for each treatment group.
GrB secretion by T cells also contributes to xenogeneic GVHD-induced colon injury.31–33 Therefore, we assessed whether anti–IL-21 mAb decreased the generation of human GrB-secreting cells in the xenogeneic GVHD model. Whereas IL-21/IL-21R blockade did not decrease the frequency of GrB-secreting cells in murine models, GrB secreting cells were reduced more than 1.5- and 10-fold in the xenogeneic GVHD model on day 10 and 20, respectively (Figure 4G-H).
IL-21 protein is expressed in the GI tract and skin, but not the serum, of allo-HSCT patients with GVHD
To determine whether IL-21 might play a role in the pathogenesis of human GVHD, we sought to determine whether IL-21 was present in the serum of patients undergoing allo-HSCT, although past efforts to detect IL-21 in human serum have revealed that this cytokine is only rarely present in the circulation (ZymoGenetics, S.R.D., unpublished data, August 2011). Serum IL-21 concentrations were assayed for 95 adult patients (median age, 51 ± 13 years) undergoing allogeneic transplant with matched related (n = 26), unrelated adult donor (n = 25), or unrelated umbilical cord blood (n = 44; Table 1). Samples for 28 patients who developed at least grade 1 GVHD were evaluated on multiple days, including the day treatment for GVHD started, and 3 to 15 days later (supplemental Table 1). Serum IL-21 levels were elevated (332 pg/mL, not shown) in one patient with steroid-resistant, stage IV acute GVHD of the gastrointestinal (GI) tract and skin. A second patient with prior stage IV acute GVHD, resolved at the time of sample collection, had a detectable level of 39 pg/mL, which was below the linear range of the curve (not shown). The remaining 93 patients had undetectable IL-21 levels, which might be attributed to the detection level in the assay or to the likelihood that IL-21 is produced and acts at sites of inflammation within the tissues.
In rodents, IL-21 had a more profound effect on T effector cells and Tregs within the GI tract compared with the peripheral lymphoid organs. Thus, IL-21 may have its major GVHD effect at the tissue level in GVHD recipients. If this were the case, then the absence of IL-21 in the sera would not exclude a biologic effect mediated by IL-21 at the level of the GVHD target organ. Therefore, GVHD tissue biopsies were examined for the presence of IL-21-secreting cells present in allo-HSCT recipients with or without active GVHD. Twenty-seven adult patients (median age, 39 years; range; 17-60 years) who underwent allogeneic HSCT at the Hospital Saint Louis were included in this study; all patients had biopsies performed as diagnostic procedures for digestive or cutaneous symptoms.34 There were 19 males and 8 females. Primary diagnoses included acute myeloid leukemia (n = 5), chronic myelogenous leukemia and myelofibrosis (n = 7), acute lymphoblastic leukemia (n = 5), severe aplastic anemia (n = 2), non-Hodgkin lymphoma/chronic lymphocytic leukemia (n = 6), and multiple myeloma (n = 2). Twenty patients were transplanted for advanced diseases and 7 for early (first complete remission) diseases. Conditioning regimens were myeloablative in 12 patients and of reduced intensity in 15. Sources of cells included bone marrow (n = 9), peripheral blood stem cells (n = 14), and cord blood (n = 4). GVHD prophylaxis included cyclosporine A and methotrexate for patients who underwent transplant after myeloablative conditioning, and cyclosporine A plus mycophenolate after reduced intensity conditioning, as previously reported.35
For the 27 patients, clinical GVHD was graded as 0 (n = 3), I (n = 6), II (n = 11), III (n = 3), and IV (n = 4) using the Glucksberg scoring system.36 Twelve patients had clinical GI GVHD (stage I, n = 6; stage II, n = 2; stage III, n = 3; stage IV, n = 1). Fourteen patients had GI (duodenal) biopsies that were scored as follows: grade 0 (n = 4), grade 2 (n = 4), and grade 3 (n = 6). Nineteen had clinical skin GVHD (stage I, n = 4; stage II, n = 6; stage III, n = 7; stage IV, n = 2). Skin biopsies from 16 patients were analyzed for IL-21 protein expression. Pathology grading for those biopsies was grade 0 (n = 5), grade 2 (n = 9), or grade 3 (n = 2). Staining of human GI biopsies for IL-21 revealed both cytoplasmic expression in MNCs with morphology compatible with lymphocytes and extracellular staining in vessels and the GI tissue (Figure 5A-B). Of note, endovascular positive staining was found in 9 patients, all with biopsy-proven GI GVHD (pathologic stage: stage I, 1 patient; stage II, 4 patients; stage III, 4 patients). All 9 patients had dense MNC infiltrate with IL-21+ cells. For patients with skin GVHD, IL-21 MNCs were detected in 6 of 11 (54%) GVHD patients, including 5 of 9 (55%) patients with grade 2 and 1 of 2 (50%) patients with grade 3 GVHD. In contrast, 0 of 5 patients without pathologic evidence of skin GVHD had IL-21–expressing MNCs. Overall, IL-21+ MNCs were more numerous in the GI tract than in skin (Figure 5A). Finally, it is notable that, in 8 cases with severe GI GVHD and in 1 case of skin GVHD, strong IL-21 staining was observed in vessels (not shown).
IL-21 protein is expressed in the GI tract and skin, but not the serum, of allo-HSCT patients with GVHD. Samples of colon and skin were biopsied from transplant patients and were stained with anti–IL-21 mAb, counterstained with hematoxylin, and then analyzed in a blinded fashion. (A) Representative examples of gut and skin staining with anti–IL-21 mAb or isotype control antibody. Counts of double immunostained cells were independently assessed by 2 pathologists (P.R. and A.J.) on an Olympus AX 70 microscope with wide-field eyepiece number 26.5 using CellSens software (Olympus). At 400× magnification, this wide-field eyepiece provided a field size of 0.344 mm2. (B) High magnification example of gut staining showing numerous IL-21–expressing MNCs. Graphs comparing the relative amount of anti–IL-21 staining in the gut (C) and skin (D) with the GVHD pathology grade. n = 14 and 16 for GI and skin biopsies, respectively.
IL-21 protein is expressed in the GI tract and skin, but not the serum, of allo-HSCT patients with GVHD. Samples of colon and skin were biopsied from transplant patients and were stained with anti–IL-21 mAb, counterstained with hematoxylin, and then analyzed in a blinded fashion. (A) Representative examples of gut and skin staining with anti–IL-21 mAb or isotype control antibody. Counts of double immunostained cells were independently assessed by 2 pathologists (P.R. and A.J.) on an Olympus AX 70 microscope with wide-field eyepiece number 26.5 using CellSens software (Olympus). At 400× magnification, this wide-field eyepiece provided a field size of 0.344 mm2. (B) High magnification example of gut staining showing numerous IL-21–expressing MNCs. Graphs comparing the relative amount of anti–IL-21 staining in the gut (C) and skin (D) with the GVHD pathology grade. n = 14 and 16 for GI and skin biopsies, respectively.
Discussion
Here we show that IL-21 plays an important role in GVHD mediated by human cells. IL-21–producing cells were found in skin and colon biopsies of allo-HSCT patients exhibiting grade 2 to 4 GVHD, but not in those without active disease. IL-21 was not present in serum regardless of GVHD status, reinforcing our hypothesis that the tissues are the main site of production and action of this cytokine. Human IL-21–producing cells were detected in the colon of mice in a xenogeneic model of GVHD. In contrast to allo-HSCT patients, human IL-21 was detected in the serum of the xenogeneic GVHD mice, and it correlated with disease severity. This discrepancy is probably the result of disease activity when the samples were collected, as serum was collected from moribund mice, whereas patient samples were mostly obtained at GVHD diagnosis or shortly thereafter. Although the IL-21–secreting cells were not co-stained for T-cell markers, the cytoplasmic staining is in agreement with previous results suggesting they are either Th17 or natural killer T cells.13,14 The physiologic relevance of IL-21 in the xenogeneic GVHD model was demonstrated by showing that treatment with anti–hIL-21–neutralizing mAb ameliorated GVHD-induced weight loss, pathology, and mortality. Moreover, IL-21 neutralization inhibited the generation of both IFN-γ– and IL-17–secreting cells, and also increased the number and percentage of CD4+ cells that are Foxp3+. However, mice continued to have evidence of active GVHD during anti–IL-21 mAb treatment. Thus, anti–IL-21 mAb-associated increase in iTregs was of sufficient magnitude to inhibit lethality but only incompletely suppress GVHD. Because increased iTregs were readily detectable on day 20 and not examined later after transplantation, whether the increase in iTregs was stable during anti–IL-21 mAb administration is unknown. Moreover, because anti–IL-21 mAb was given during the entire observation period, it also is unknown whether discontinuation and clearance of anti–IL-21 mAb would have led to an inhibition of iTregs that may then have exacerbated GVHD. These data are consistent with the previously established rodent allogeneic model that IL-21 probably enhances GVHD by suppressing peripheral iTreg generation or nTreg expansion, and by skewing T effector cell differentiation away from a Th2 (IL-4) response and toward an inflammatory Th1 (IFN-γ) response.22
Although anti–IL-21 mAb treatment had many similar effects in the murine and xenogeneic models, some differences existed that suggest IL-21 neutralization may be more effective in humans. For example, although Foxp3+ T cells were rarely seen in tissues other than the lamina propria in the murine model, they were readily detected in the blood and spleen in the xenogeneic GVHD model. Blocking IL-21 signaling in human cells also decreased the secretion of GrB which, in addition to possessing cytolytic activity, has recently been show to be involved in inflammatory diseases in a perforin-independent manner.37 Although the generation of Th17 cells is not affected in murine models, blocking IL-21 signaling in human T cells does inhibit the differentiation of these potentially pathogenic cells that are also some of the primary producers of IL-21. This is probably because, in contrast to murine Th17 cells, which require IL-6 as a cofactor for differentiation, human Th17 cells can use IL-6, IL-21, or IL-23 for differentiation.13 Although the extent that each cytokine is used in vivo during GVHD induction is not known, the data presented here suggest that IL-21 is an important in vivo factor for human Th17 differentiation. Blocking IL-21 significantly ameliorates pathology in the small intestine and colon in multiple murine models.22,23,26 However, although IL-21–secreting cells are present in the small intestine and colon of animals in the xenogeneic GVHD model, the pathology observed in animals electively killed on day 10 or 20 was not severe enough to assess the actions of anti–IL-21 (not shown). Blocking IL-21 also decreases liver pathology in some,23,26 but not all,22,23 murine GVHD models, and had some beneficial effects in the xenogeneic model.
Results in murine GVHD models have not always translated to human disease and, similar to our findings with IL-21, discrepancies between serum and in situ cytokine levels have previously been reported.34 In the xenogeneic GVHD model, human IL-21 levels correlated with disease severity, although mice were analyzed at a time when they were moribund, in contrast to the samples obtained from patients who, for the most part, were assayed at GVHD diagnosis or shortly thereafter (not shown). Moreover, despite the fact that the one patient with highly elevated levels of serum IL-21 had steroid-refractory GVHD, it is unlikely that steroids played a role in the lack of high levels of serum IL-21 as no increase was observed in 4 other patients with steroid-refractory GVHD, and a total of 19 patients were assayed while on steroids. In contrast, IL-21–secreting cells were found in GVHD-associated organs in both the xenogeneic model and in patients with active GVHD. Furthermore, the frequency of cells secreting IL-21 was a sensitive marker of GI GVHD severity, although the density of the infiltrate was often relatively low. In contrast, the frequency of IL-17 secreting cells only correlated with disease severity when analyzed within the context of Treg frequency (ie, Th17/Treg ratio).35,38
We previously showed that disrupting IL-21 signaling in mice undergoing an allogeneic GVHD reaction resulted in an increase in Foxp3+ Tregs.22 Using highly sorted CD4+FoxP3− T cells obtained from FoxP3-GFP knockin reporter mice, we demonstrated that anti–IL-21–neutralizing mAb markedly increased the frequency of CD4+FoxP3+ T cells, in contrast to irrelevant mAb-treated controls, consistent with iTreg generation in vivo. We further demonstrated that T cells from chimeras generated using FoxP3-deficient and congenic wild-type donors and given to secondary recipients caused lethal GVHD despite neutralizing anti–IL-21 mAb, indicating that Foxp3 expression is critical for the protective effect of IL-21 inhibition. Increased numbers of Foxp3+ cells were also observed in a recent study by the Ozawa group using IL-21R−/− mice, although they postulated that attenuated GVHD might be caused by an impairment of effector T-cell differentiation itself, rather than by an increase in Tregs.26 These 2 possibilities are not mutually exclusive, as T cells do not differentiate into effector cells if activated in the presence of Treg.39 However, our current data show that effector T-cell differentiation on day 10 is not significantly influenced by anti–IL-21 mAb treatment. Instead, the delayed effect on T effector differentiation suggests that anti–IL-21 mAb must induce Tregs before T effector cell generation can be inhibited and GVHD symptoms ameliorated. In other studies using IL-21R−/− donor T cells,23 the authors concluded that the lack of IL-21R signaling permitted nTreg expansion and not iTreg generation. Although each of the 3 published reports had a different conclusion regarding the mechanism responsible for higher numbers of CD4+FoxP3+ cells in settings in which IL-21/R signaling was blocked, our studies in the xenogeneic GVHD model provide additional support for an inverse relationship between IL-21 signaling and absolute Treg numbers.
The mechanisms of anti–IL-21 mAb inhibition of GVHD lethality at later time points (ie, day 20) are largely similar to nTreg treatment (decreased secretion of IFN-γ, IL-17, and GrB; and Treg induction from PBMCs). Although GVHD can be ameliorated by adoptive transfer of nTregs or iTreg,40–42 a therapy based on IL-21 neutralization would have several advantages over cellular transfer approaches. First, murine and xenogeneic studies showed that high doses of Treg (∼ 1:1 with donor T cells) were required to efficiently and reproducibly suppress disease43,44 ; and although Treg cell number can be greatly increased by ex vivo expansion, this requires a costly and time-consuming Treg purification and culture period. In our recently completed phase 1 clinical trial studying the safety of expanded nTregs,45 the targeted Treg dose was not uniformly achieved because of inadequate ex vivo expansion. Moreover, it is important to note that high numbers of Tregs are not always beneficial. Several studies have now demonstrated, either by adoptive transfer or with Foxp3-transgenic animals, that an overabundance of nTreg can cause generalized immunosuppression and impair tumor or pathogen clearance.46–48 In our previous report, we noted that, despite inhibiting GVHD, blockade of IL-21/IL-21R signaling did not impair the elimination of a concurrently administered cell line, P815 mastocytoma cells,22 which was confirmed and extended to the A20 lymphoma model by Hanash et al, who found this was the result of tissue-specific effects whereby GI GVHD is reduced while peripheral T-cell function is maintained.23 Should such studies be translatable into the human setting, the transient neutralization of IL-21 during the period of greatest acute GVHD risk (ie, 4-8 weeks after transplantation) might be sufficient to reduce acute GVHD while potentially permitting anti–tumor T effector cells to develop, especially given our findings that IFN-γ– and GrB-expressing cells are present at substantial frequencies at least for early time periods (ie, day 10) after transplantation in xenogeneic GVHD recipients. Anti–IL-21 mAb treatment may be preferable to nTreg infusion because CD4+ T-cell numbers were not as drastically reduced in studies in which nTreg infusion was concurrently studied (data not shown), which may decrease the risk of lymphopenia-associated infections after transplantation. The retention of higher levels of human donor CD4+ T cells may allow patients to be less immunocompromised after transplantation. However, the effects of longer-term anti–IL-21 mAb treatment and potential immunosuppression in the context of allogeneic BMT are not known. Although GVHD prevention, treatment, and the GVHD process itself are highly immunosuppressive,49 low IL-21 RNA expression in children also correlates with an inability to clear chronic infections, such as hepatitis B.50 How these findings will extrapolate to the clearance of other relevant viral pathogens in allogeneic transplantation recipients is unknown.
In conclusion, IL-21 appears to play a significant role in human GVHD. Full suppression of xenogeneic GVHD with interventional therapies is a difficult challenge. Taken in this context, these data with neutralizing anti–IL-21 mAbs are striking. Therefore, anti–IL-21 mAb therapy could be considered for application as a GVHD preventive agent in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Todd Defor for University of Minnesota clinical data, Sarah Merkel for laboratory management, and Christine Nelson for excellent assistance with animal husbandry.
This work was supported in part by the Children's Cancer Research Fund (research grants, K.L.H.) and Leukemia and Lymphoma Translational Research (grant R6029-07, B.R.B.; and P01 CA067493, B.R.B. and J.S.M.). This work was also supported in part by the National Institutes of Health (P30 CA77598) using the shared resource Flow Cytometry Core from the Masonic Cancer Center, University of Minnesota.
National Institutes of Health
Authorship
Contribution: D.K.S., A.M.B., T.B., K.A.M., K.S.W., R.P.d.L., A.J., and J.M.C. performed the experiments, interpreted the data, and assisted with the paper; and K.L.H., C.B., S.R.D., J.S.M., G.S., and B.R.B. designed the research, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: S.R.D., T.B., K.A.M., and K.S.W. are paid employees of ZymoGenetics Inc (a Bristol-Myers Squibb Company). The remaining authors declare no competing financial interests.
Correspondence: Keli L. Hippen University of Minnesota, 460 MCRB, 425 East River Rd, Minneapolis, MN 55455; e-mail: hippe002@umn.edu; and Bruce R. Blazar, University of Minnesota, 460 MCRB, 425 East River Rd, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
Author notes
G.S. and B.R.B. contributed equally to this study.


![Figure 4. Splenocytes from mice receiving anti–IL-21 mAb have decreased numbers of cells secreting IFN-[gamma], IL-17, and GrB. Spleens were isolated on day 10 and day 20 from NOD/SCID/γc−/− mice receiving human PBMCs (30 × 106 cells) that were treated with nothing, anti–IL-21 mAb, or isotype control antibody, and splenocytes were stimulated with phorbol myristate acetate and ionomycin and assayed using intracellular cytokine staining. Average percentage (± SEM) of cells secreting IFN-γ (A-B), IL-4 (C-D), IL-17 (E-F), and GrB (G-H) on day 10 (A,C,E,G) or day 20 (B,D,F,H). n = 4 mice for each time point (day 10 and day 20) and for each treatment group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/2/10.1182_blood-2011-07-368027/5/m_zh89991184110004.jpeg?Expires=1765892407&Signature=uizIxSYCNcfguw3PEAVepTb8jih5-R0nm~d9SRrqDkjBS8ICIo-740ZjO3Y2XrZE7AdYZJ9Wo4H-ims9MCeMNBk5TNMI2sJv99hp6JCC4xlZHDsqvdU2tT4OUErw8LYPUWI30yDw8L0-UHUxw74LmX~9HUzjIdwJz8-U~k59nUxMeZ5xBB2RKE71Ex-Uk6boKJdcsZe2hZ-LBGKqib7kNv8onfVum~qcXehNOlYmBolXoHtb-lCXGjZuCVjwSEjCq4qrMhVyHtClLjy2zOZLtdgJ8ZPeOzPWW2ZvqaoThR3xc8~deAnR2hMce9bMZuJkSPA0GTQ8JK4hVcgMHfXBgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
