The complex of the serine protease factor IX (FIX) and its cofactor, factor VIII (FVIII), is crucial for propagation of the intrinsic coagulation cascade. Absence of either factor leads to hemophilia, a disabling disorder marked by excessive hemorrhage after minor trauma. FVIII is the more commonly affected protein, either by X-chromosomal gene mutations or in autoimmune-mediated acquired hemophilia. Whereas substitution of FVIII is the mainstay of hemophilia A therapy, treatment of patients with inhibitory Abs remains challenging. In the present study, we report the development of FIX variants that can propagate the intrinsic coagulation cascade in the absence of FVIII. FIX variants were expressed in FVIII-knockout (FVIII-KO) mice using a nonviral gene-transfer system. Expression of the variants shortened clotting times, reduced blood loss after tail-clip assay, and reinstalled clot formation, as tested by in vivo imaging of laser-induced vessel injury. In addition, we confirmed the therapeutic efficacy of FIX variants in mice with inhibitory Abs against FVIII. Further, mice tolerant to wild-type human FIX did not develop immune responses against the protein variants. Our results therefore indicate the feasibility of using variants of FIX to bypass FVIII as a novel treatment approach in hemophilia with and without neutralizing FVIII Abs.
Introduction
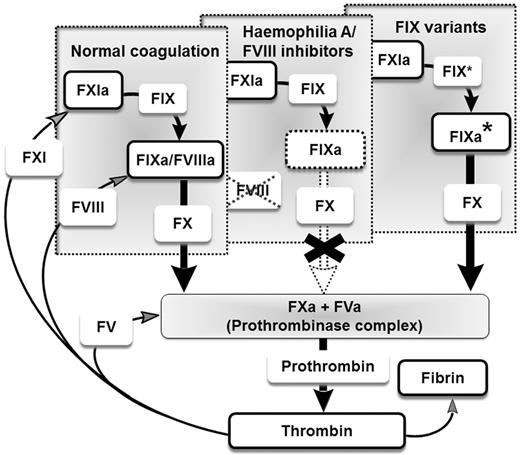
The intrinsic coagulation cascade is a tightly regulated protease- and cofactor-dependent amplification system that ensures the formation of stable clots after injury.1 Within this system, deficiencies of the coagulation cofactor, factor VIII (FVIII), or the corresponding coagulation protease, factor IX (FIX), lead to the X-chromosomal inherited bleeding disorders hemophilia A and hemophilia B, respectively. Hemophilia A occurs approximately in 1 of 5000 newborn boys, whereas hemophilia B is less common.2 Untreated, hemophilia presents with spontaneous bleeding preferentially into large joints and skeletal muscle, and internal and intracranial bleedings can also occur. Substitution of deficient coagulation factors by intravenous infusion of plasma-derived or recombinant coagulation factor concentrates is the therapy of choice. In the last decades, treatment has evolved from so-called on-demand treatment for acute injury/hemorrhage to a secondary preventative approach with regular prophylactic infusions. Prophylactic treatment, securing plasma levels above 1% of normal, already prevents the major long-term consequences of the disease: joint damage and muscular atrophy.3,4 A major obstacle for protein substitution therapy is the occurrence of neutralizing Abs directed against the FVIII protein. This can either be a result of an immune response after exogenous protein exposure5 or may appear in adult patients as a spontaneous auto-immune event.6 In these cases, FVIII infusion is often ineffective and so-called bypassing agents are used. These agents consist of constitutively activated proteases such as activated factor VII (FVIIa), and promote clot formation directly without restoring the intrinsic amplification loop. Although these therapeutics are efficient at stopping acute bleeding, limitations include the relatively short half-lives of activated proteases in the circulation and potential vascular risks in long-term treatment.7,8 In the present study, we report on the generation of FIX variants with FVIII-independent clotting activity that we propose to use as novel therapeutics to restore the intrinsic coagulation pathway for patients with congenital (with or without neutralizing Abs) or acquired forms of FVIII deficiency (Figure 1).
Restoring the intrinsic amplification loop. Illustration of the intrinsic amplification loop in the presence of FVIII for hemophilia A, in the case of inhibitors to FVIII, and in the absence of FVIII when the coagulation cascade is restored by FIX variants (FIX* or IXa* for activated FIX variants).
Restoring the intrinsic amplification loop. Illustration of the intrinsic amplification loop in the presence of FVIII for hemophilia A, in the case of inhibitors to FVIII, and in the absence of FVIII when the coagulation cascade is restored by FIX variants (FIX* or IXa* for activated FIX variants).
We based our approach on recently described models for the transition of activated FIX (FIXa) from a zymogen-like form to the active protease.9,10 One specific feature of FIXa is its minimal activity toward its natural substrate, factor X (FX), and toward small peptide substrates. Only after assembly in the tenase complex, which consists of FIXa, activated FVIII (FVIIIa), and FX, is relevant protease activity achieved, exceeding the activity of FIXa alone by 100 000- to 1 000 000-fold.10,–12 The exchange of amino acid residues specific for FIX with those of FX and the modification of the 99-loop, which is unique to FIX and believed to shield the FIX active site before assembly in the tenase complex, led to FIXa variants with several hundred- to several thousand-fold higher amidolytic activities.10 We hypothesized that these modifications could mimic the effect of FVIIIa binding to FIXa. Therefore, we expressed the recombinant full-length variants of FIX and observed measurable clotting activity in FVIII-deficient plasma. Consecutively, we tested each of the introduced variations for their effect on FVIII-independent clotting activity and combined them with additional candidate mutations. Finally, we obtained novel variants with a FVIII-independent activity of up to 16%-22% compared with wild-type FIX (FIX-WT) in the presence of FVIII (100%). Further, we confirmed the efficacy of FIX variants, even in the presence of neutralizing Abs to FVIII, using models for hemorrhage and clot formation in vivo. Therefore, a novel new treatment strategy using FIX variants to bypass FVIII seems feasible.
Methods
Nonviral vectors and DNA constructs
The CMV.FIX expression cassette containing the human FIX (hFIX) mini-gene, including a 1.4-kb fragment of intron A, was derived from plasmid pAAV.CMV.hFIX13 (kindly provided by Dr Katherine High, Children's Hospital of Philadelphia). The construct was used for in vitro testing of hFIX variants and as a vaccination vector to induce immune responses against hFIX variants after intramuscular injection. For this, we introduced CMV.hFIX into a pcDNA3.1 backbone. For in vivo expression of hFIX, we constructed a strong liver-specific expression plasmid consisting of the hepatic locus control region 1 (HCR), the human α-1-antitrypsin promoter (hAAT), the hFIX mini-gene including a 1.4-kb fragment of intron A, and the bovine growth hormone polyadenylation signal with pSL1180 backbone.14 The hFIX expression cassette was then excised from the intermediary plasmid and inserted into a minicircle producer plasmid15 (kindly provided by Mark Kay, Stanford University). This system allows the elimination of the bacterial plasmid backbone sequence for circular DNA vectors, resulting in the formation of the minicircle vector MC.HCR/hAAT-hFIX, consisting of the pure episomal hFIX liver-specific expression cassette after intramolecular recombination at recognition sequences in Escherichia coli cells and 1-step affinity purification, as described previously.15 B domain–deleted FVIII (FVIIIΔB) cDNA was generated as described previously16 and inserted into plasmid pSL1180 containing the HCR/hAAT promoter, resulting in the vector pSL1180.HCR/hAAT-FVIIIΔB (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). pSL1180.HCR/hAAT-FVIIIΔB was used for induction of inhibitory anti–human FVIII (anti-hFVIII) Ab formation in FVIII-KO mice after liver-specific expression.
Generation of FIX variants
Amino acid substitutions were introduced in the hFIX cDNA using the QuickChange II site-directed mutagenesis kit (Agilent Technologies) as specified by the manufacturer. Introduced substitutions were confirmed by sequencing and restriction digest.
Recombinant protein production
For transient expression of hFIX variants, human embryonic kidney 293T cells were transfected with CMV-hFIX plasmids using the calcium phosphate–mediated precipitation method. Protein expression was performed in serum-free medium (Opti-MEM; Invitrogen) containing vitamin K at a concentration of 10 μg/mL for 24-36 hours. hFIX protein (or an hFIX variant protein) was concentrated using Vivaspin 20 centrifugal concentrators (MWCO 30-kDa; Sartorius Group) and then used for protein and activity testing. Recombinant hFIX (BeneFIX; Pfizer) and FVIII (KogenateFS; Bayer Schering Pharma) preparations were used as controls.
FIX and coagulation assays
Protein activity was determined by a modified 1-stage clotting assay; 25 μL of test sample (when required, samples were diluted in imidazole buffer) and 25 μL of either FIX-deficient or FVIII-deficient plasma were mixed with 25 μL of activated partial thromboplastin time (aPTT) reagent (Dade Behring) and incubated for 3 minutes at 37°C. Next, 25 μL of CaCl2 (25mM) was added and the time to clot formation was measured using a fibrometer (MC1-Vet; Merlin). Nonactivated partial thromboplastin time (NAPTT) was determined by modified 1-stage clotting assay using NAPTT reagent (Haematex) instead of aPTT regent in FIX-deficient plasma. We tested for FIXa in concentrated supernatants after transient expression of hFIX variants using the chromogenic Biophen Factor IXa kit according to the manufacturer's specifications. hFIX concentrations were determined using an ELISA assay in which an mAb to hFIX, clone HIX-1 (Sigma-Aldrich) was used as a capture Ab. Peroxidase-conjugated polyclonal goat anti-hFIX (Affinity Biologicals) was used as the detecting Ab.13 For visualization of the activated or nonactivated FIX heavy chain, 300 ng of protein from either hFIX-WT or hFIX variants (hFIX-T or hFIX-ITV) was applied for Western blot analysis after transient expression in human embryonic kidney 293T cells and determination of the hFIX concentration. After SDS-PAGE (4%-20%) and transfer onto nitrocellulose membrane, we detected hFIX using a mouse anti-hFIX mAb (ThermoFisher Scientific). Visualization of hFIX bands was performed after exposure in the Bio-Rad ChemiDoc XRS imaging system after staining with horseradish peroxidase–conjugated rabbit anti–mouse Ab (ThermoFisher Scientific). Thrombin-antithrombin (TAT) complexes were measured in citrated plasma with ELISA (Enzygnost TAT; Siemens Healthcare Diagnostics). This immunoassay, which was developed to detect human TAT, is highly cross-reactive to murine TAT complexes, as described previously.13 Abs to hFIX were measured by a specific ELISA to murine Ig subclasses (IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA), as described previously14 with minor modifications, by coating plates with 1 μg/mL of purified recombinant hFIX (BeneFIX; Pfizer) or the hFIX variants T or ITV. We tested for functionally inhibitory Abs against hFIX-WT and hFIX variants with a modified Bethesda assay in which we mixed plasma samples from BALB/c mice with equal volumes of hFIX-WT or variants at different dilutions. After incubation at 37°C for 2 hours, remaining FIX activity was determined using a 1-stage clotting assay in FIX-deficient plasma. Where indicated, recombinant hFIX (BeneFIX; Pfizer) or recombinant FVIII (KogenateFS; Bayer Schering Pharma) were used as controls.
Mouse models
All animal procedures were approved by the local animal care, protection, and use authorities (University and Regierungspräsidium Darmstadt). FVIII-deficient mice containing a disruption of the murine FVIII gene in exon 16 were ordered from Charles River Laboratories. C57Bl/6 and BALB/c mice were purchased from Harlan Laboratories. The nonviral gene-transfer vectors MC.HCR/hAAT-hFIX or pSL1180.HCR/hAAT-FVIIIΔB were administered by hydrodynamic injection. In short, 2 mL of saline solution containing vector doses of 2.5-50 μg/mouse were rapidly (5-8 seconds) injected into a lateral tail vein. FVIII-KO mice received 50 μg of pSL1180-HCR/hAAT-FVIIIΔB by the same procedure for induction inhibitory Abs against FVIII. Vaccination of naive BALB/c mice or mice tolerant to hFIX-WT was performed by intramuscular injection of 500 μg of pCDNA3.1.CMV-hFIX into the tibialis anterior muscle of each hind limb in a final volume of 50 μL at 3 different time points. Blood was collected either by retroorbital bleeding or by bleeding after tail cut.
In vivo bleeding assays
FVIII-KO mice were anesthetized and the distal part of the tail (at a 1.5- or 3-mm diameter) was cut and immediately immersed into a 15-mL Falcon tube containing prewarmed saline solution at 37°C. Blood was collected for 10 minutes. Blood loss was determined by measuring the absorbance of hemoglobin (A492 nm) after lysis of the collected RBCs from the saline solution in which the tail was placed, as described previously.13
Intravital microscopy of laser-induced injury thrombus formation
FVIII-KO mice were anesthetized and the cremaster muscle was exposed, stretched, and pinned across the intravital microscopy tray as described previously.17,18 Labeled Abs for visualization of platelets (anti-CD41 Alexa Fluor 647) and fibrinogen (anti-fibrin Alexa Fluor 555) were administered via the catheterized jugular vein as described previously.19,20 After infusion of the labeled Abs, controlled vessel wall damage to arterioles and venules of the fixed cremaster muscle was inflicted using a pulse-nitrogen dye laser applied through the micropoint laser system (Phototonic Instruments). Real-time imaging of thrombus formation was recorded using an Olympus BX6IWI fixed-stage motorized upright fluorescence microscope with a long-distance condenser and a 40× water-immersion objective. Progression of laser-induced thrombus formation was followed over a course of 3 minutes at 3.7 frames per second. A maximum of 4 arteriolar and 4 venular injuries per mouse were induced.
Statistical analysis
Student t test or ANOVA with the Dunnett test for multiple comparisons to the corresponding control group was used for parametric data, and the Fisher exact test was used for categorical data, including Bonferroni adjustment for multiple comparisons using Instad Version 3.06 software (GraphPad).
Results
FIX variants shorten clotting time in the absence of FVIII and in the presence of FVIII-neutralizing Abs
To test our hypothesis that the modifications of FIXa described in previous studies10 resemble FVIIIa influences on FIXa, we introduced the mutations Y259F, K265T, and Y345T into our full-length hFIX cDNA expression construct and expressed the mutation in cell culture. We then tested its activity using modified one-stage assays in FIX-, FX-, and FVIII-deficient plasma compared with hFIX-WT. The variant had almost the same activity compared with WT in FIX-deficient plasma. Neither protein shortened the clotting time in FX-deficient plasma, but we could detect a small but distinct shortening of the clotting time in FVIII-deficient plasma using the variant compared with hFIX-WT. We also examined each of the 3 mutations Y259F, K265T, and Y345T separately. Results are summarized in Table 1. Briefly, of the 3 mutations, the K265T mutation alone was responsible for the FVIII-independent clotting activity. In contrast, the other 2 substitutions diminished FVIII-independent FIX function.
FIX specific activity and FVIII bypassing activity of hFIX variants derived from hFIX Y259F/K265T/Y345T
| FIX variant . | FIX act. (%) ± SEM . | FVIII byp. act. (%) ± SEM . |
|---|---|---|
| hFIX Y259F/K265T/Y345T | 73.2 ± 6.3 | 2.0 ± 0.2 |
| hFIX Y259F | 96.9 ± 11.1 | 0 |
| hFIX K265T | 191.1 ± 14.0 | 6.6 ± 0.7 |
| hFIX Y345T | 50.9 ± 8.1 | 0 |
| hFIX Y259F/K265T | 141.0 ± 20.5 | 5.8 ± 0.9 |
| FIX variant . | FIX act. (%) ± SEM . | FVIII byp. act. (%) ± SEM . |
|---|---|---|
| hFIX Y259F/K265T/Y345T | 73.2 ± 6.3 | 2.0 ± 0.2 |
| hFIX Y259F | 96.9 ± 11.1 | 0 |
| hFIX K265T | 191.1 ± 14.0 | 6.6 ± 0.7 |
| hFIX Y345T | 50.9 ± 8.1 | 0 |
| hFIX Y259F/K265T | 141.0 ± 20.5 | 5.8 ± 0.9 |
Positions Y259, K265, and Y345 correspond to Y94, K98, and Y177 in chymo-trypsinogen numbering. Values represent activity measured by 1-stage assay in FIX-deficient plasma for FIX specific activity and in FVIII-deficient plasma for FVIII bypass activity divided by FIX antigen levels. One hundred percent representing the specific activity of 200 IU/mg measured in presence of FVIII, that is, normal plasma–derived FIX has 100% FIX specific activity and close to 0 % FVIII bypassing activity. Mean values were obtained from at least 6 independent measurements ± SEM.
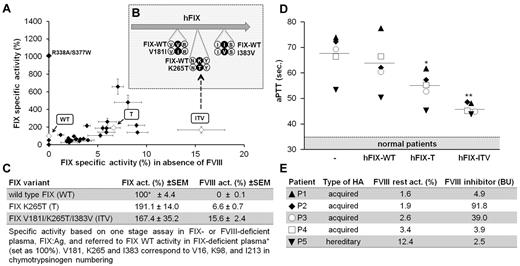
We then added additional mutations and screened the hFIX variants for their specific clotting activity in both the presence and absence of FVIII (Figure 2A). Substitutions were chosen based on homology comparisons with other serine proteases, preferentially picking substitutions with known effects on the function of a closely related protease. In this manner, 2 additional mutations, V181I and I383V, were identified, which improved FVIII-independent activity but had the desirable minimal effect on FIX activity in the presence of FVIII. The triple variant containing all 3 substitutions, hFIX V181I/K265T/I383V (ITV), exhibited 15.6% FVIII-bypassing activity compared with 100% activity of hFIX-WT in normal plasma (Figure 2B-C). As hypothesized, the hFIX variants T and ITV similarly shortened clotting times when added to the plasma of patients with inhibitory Abs to FVIII (Figure 2D-E). The effect in patient plasma was proportional to FVIII-bypassing potency, as shown by studies with FVIII-deficient plasma (Figure 2C).
Generation of FIX variants to bypass FVIII. (A) Screening of hFIX variants for FVIII-independent clotting. Each mark represents an hFIX variant tested. hFIX variants of interest used for in vivo testing are highlighted. Values represent means of specific FIX activity calculated as FIX activity divided by FIX antigen given as a percentage. 100% is defined as full FIX activity of the WT protein in the presence of FVIII (ie, a specific activity of 200 U/mg). Plotted on the y-axis are specific activity values calculated from measurements in FIX-deficient plasma; on the x-axis are values calculated from measurements in FVIII-deficient plasma. Each dot represents the mean value from at least 6 independent tests for each variant with error bars showing SEM. (B) Illustration of amino acid substitutions for variant ITV. (C) Specific FIX- and FVIII-bypassing activity of hFIX variants (hFIX-T and hFIX-ITV) compared with hFIX-WT. (D-E) Effect of hFIX variants on clotting times by one-stage FVIII assay in the presence of inhibitory anti-FVIII Abs. Plasma samples of patients with inhibitory FVIII Abs were mixed 1:1 with FIX variant solution or buffer with a final hFIX variant concentration of 2.5 μg/mL (50% of normal FIX antigen levels) and incubated for 2 hours at 37°C to allow Ab binding. A range for normal coagulation was established using identical incubation with control plasma from healthy volunteers without inhibitory Ab–containing samples (n = 5) using the same procedure. *P < .05 and **P < .01 (ANOVA with Dunnett test for multiple comparisons with the FIX-WT group). (E) Remaining coagulation activity, inhibitor levels in Bethesda units (BU), and underlying disease (acquired hemophilia with autoimmune response against FVIII and hereditary hemophilia A with inhibitory Abs) are shown for each patient.
Generation of FIX variants to bypass FVIII. (A) Screening of hFIX variants for FVIII-independent clotting. Each mark represents an hFIX variant tested. hFIX variants of interest used for in vivo testing are highlighted. Values represent means of specific FIX activity calculated as FIX activity divided by FIX antigen given as a percentage. 100% is defined as full FIX activity of the WT protein in the presence of FVIII (ie, a specific activity of 200 U/mg). Plotted on the y-axis are specific activity values calculated from measurements in FIX-deficient plasma; on the x-axis are values calculated from measurements in FVIII-deficient plasma. Each dot represents the mean value from at least 6 independent tests for each variant with error bars showing SEM. (B) Illustration of amino acid substitutions for variant ITV. (C) Specific FIX- and FVIII-bypassing activity of hFIX variants (hFIX-T and hFIX-ITV) compared with hFIX-WT. (D-E) Effect of hFIX variants on clotting times by one-stage FVIII assay in the presence of inhibitory anti-FVIII Abs. Plasma samples of patients with inhibitory FVIII Abs were mixed 1:1 with FIX variant solution or buffer with a final hFIX variant concentration of 2.5 μg/mL (50% of normal FIX antigen levels) and incubated for 2 hours at 37°C to allow Ab binding. A range for normal coagulation was established using identical incubation with control plasma from healthy volunteers without inhibitory Ab–containing samples (n = 5) using the same procedure. *P < .05 and **P < .01 (ANOVA with Dunnett test for multiple comparisons with the FIX-WT group). (E) Remaining coagulation activity, inhibitor levels in Bethesda units (BU), and underlying disease (acquired hemophilia with autoimmune response against FVIII and hereditary hemophilia A with inhibitory Abs) are shown for each patient.
FIX variants are the source of FVIII-bypassing activity
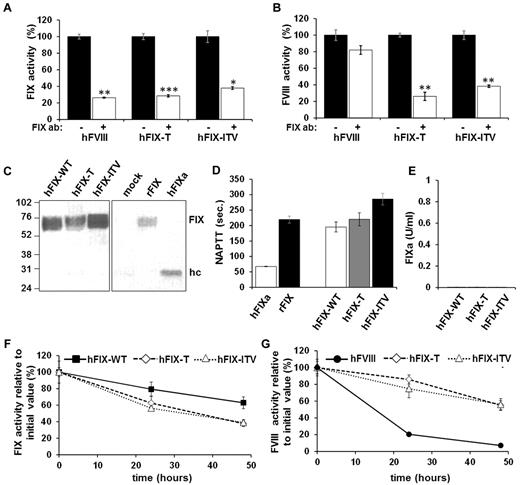
To confirm that the shortening of the clotting time is specifically caused by the FVIII-bypassing activity of FIX variants and not by any a possible contamination of our protein preparation, we incubated hFIX variants with an hFIX-neutralizing Ab and tested activity in FIX- or FVIII-deficient plasma in a one-stage assay (Figure 3A-B). Ab incubation partially neutralized the procoagulant effect of hFIX-T, hFIX-ITV, and control hFIX-WT in FIX-deficient plasma (Figure 3A). Similarly, the Ab inhibited FVIII-bypassing activity of FIX-T and FIX-ITV, but not of the control sample spiked with FVIII.
FIX variants are the source of FVIII-bypassing activity. (A-B) Neutralization of hFIX variants by mouse anti-hFIX Ab (clone HIX-1). Aliquots of hFIX-WT, hFIX-T, and hFIX-ITV or hFVIII were incubated with (white bars) or without (black bars) anti-hFIX Ab for 2 hours at 37°C. Subsequently, activities in FIX-deficient (A) or FVIII-deficient (B) plasma were determined by one-stage assay. Each bar represents the mean value of 3 independent measurements ± SEM. *P < .05, **P < .01, and ***P < .001 (Student t test) for comparison between measurements with or without Ab incubation for each protein. (C) Western blotting: lane 1 is hFIX-WT; lane 2, hFIX-T; lane 3, hFIX-ITV; lane 4, supernatant of mock-transfected 293T cells; lane 5, recombinant hFIX control (BeneFIX); and lane 6, hFIXa control. Zymogen or FIXa was detected using an anti-hFIX mAb directed toward the heavy chain of hFIX. After denaturation of the protein on SDS-PAGE, the uncleaved zymogen FIX can be identified at 70 kDa or the cleaved heavy chain at 27 kDa. Recombinant FIX (rFIX; BeneFIX) was used as a control for the FIX zymogen and FIX activated by FXIa as a control for FIXa. (D) Spurious contamination of FIX preparations by FIXa were excluded using 5 μg/mL of FIX-WT, FIX-T, FIX-ITV, or BeneFIX in an NAPTT assay sensitive to activated proteases. FIXa at a concentration of 0.2 μg/mL was used as positive control. Each bar represents the mean value of 6 independent tests with error bars ± SEM. (E) Residual FIXa activity was quantified via chromogenic assay specific for FIXa. Proteins were applied at a concentration of 2.5 μg/mL. Measured FIXa activity was negligible (no bars). (F-G) Stability of hFIX variants, hFIX-WT, or hFVIII controls in human FIX- or FVIII-deficient plasma. After incubation, samples were assayed for FIX (F) or FVIII (G) activity. Each dot represents the mean value of 3 independent tests for each sample with error bars indicating the SD.
FIX variants are the source of FVIII-bypassing activity. (A-B) Neutralization of hFIX variants by mouse anti-hFIX Ab (clone HIX-1). Aliquots of hFIX-WT, hFIX-T, and hFIX-ITV or hFVIII were incubated with (white bars) or without (black bars) anti-hFIX Ab for 2 hours at 37°C. Subsequently, activities in FIX-deficient (A) or FVIII-deficient (B) plasma were determined by one-stage assay. Each bar represents the mean value of 3 independent measurements ± SEM. *P < .05, **P < .01, and ***P < .001 (Student t test) for comparison between measurements with or without Ab incubation for each protein. (C) Western blotting: lane 1 is hFIX-WT; lane 2, hFIX-T; lane 3, hFIX-ITV; lane 4, supernatant of mock-transfected 293T cells; lane 5, recombinant hFIX control (BeneFIX); and lane 6, hFIXa control. Zymogen or FIXa was detected using an anti-hFIX mAb directed toward the heavy chain of hFIX. After denaturation of the protein on SDS-PAGE, the uncleaved zymogen FIX can be identified at 70 kDa or the cleaved heavy chain at 27 kDa. Recombinant FIX (rFIX; BeneFIX) was used as a control for the FIX zymogen and FIX activated by FXIa as a control for FIXa. (D) Spurious contamination of FIX preparations by FIXa were excluded using 5 μg/mL of FIX-WT, FIX-T, FIX-ITV, or BeneFIX in an NAPTT assay sensitive to activated proteases. FIXa at a concentration of 0.2 μg/mL was used as positive control. Each bar represents the mean value of 6 independent tests with error bars ± SEM. (E) Residual FIXa activity was quantified via chromogenic assay specific for FIXa. Proteins were applied at a concentration of 2.5 μg/mL. Measured FIXa activity was negligible (no bars). (F-G) Stability of hFIX variants, hFIX-WT, or hFVIII controls in human FIX- or FVIII-deficient plasma. After incubation, samples were assayed for FIX (F) or FVIII (G) activity. Each dot represents the mean value of 3 independent tests for each sample with error bars indicating the SD.
Because preactivation due to protein instability had been a concern in previous studies of modified FIX21 and could lead to erroneous results in clotting assays, we tested FIX preparations with Western blot analysis (Figure 3C); NAPTT (Figure 3D), which detects trace amounts of activated coagulation proteases; and by a specific FIXa assay (Figure 3E). None of the tests showed signs of FIX preactivation in our hFIX variant preparations. To determine the stability of hFIX variants, we spiked hFIX-WT, hFIX-T, or hFIX-ITV into FIX-deficient plasma and hFIX-T, hFIX-ITV, or hFIX-FVIII into FVIII-deficient plasma. Test preparations were incubated at 37°C and sampled at various time points. In all samples, clotting activity measured by one-stage assays decreased over time. However, irrespective of whether FIX activity (Figure 3F) or FVIII-bypassing activity (Figure 3G) was measured, the decline of activity in all 3 hFIX preparations (hFIX-WT, hFIX-T, and hFIX-ITV) was similar. In contrast, the decrease in FVIII activity in the sample spiked with the heat-sensitive FVIII protein was much more pronounced (Figure 3G).
Expression of FIX variants improves clotting activity and corrects the bleeding phenotype in FVIII-KO mice
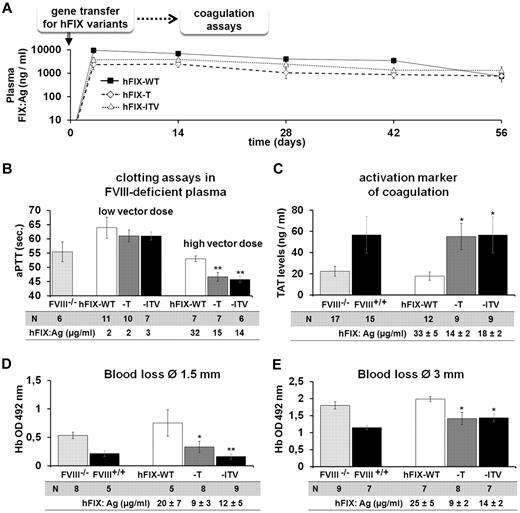
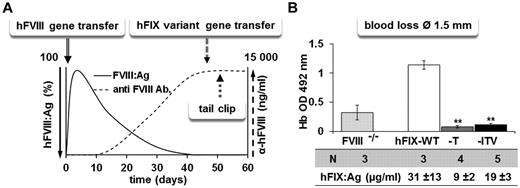
To explore the expression of the of hFIX variants in vivo, we introduced the mutations into a nonviral minicircle vector system with a strong liver-specific expression cassette for hFIX. We injected the minicircle vectors encoding hFIX-WT, hFIX-T, or hFIX-ITV into C57Bl/6 mice hydrodynamically (Figure 4A). This gene-transfer system offers the possibility of exploring the hemostatic effect of hFIX variants at stable circulating levels in the blood. Expression levels were slightly lower for the variants compared with the hFIX-WT, but were equally maintained over time. We next treated FVIII-KO mice with “hFIX variant” expression vectors at 2 different vector concentrations (7.5 and 25 μg/mouse). At the lower vector dose, which resulted in hFIX expression levels between 2 and 3 μg/mL, aPTT-based 1-stage assay was only marginally affected. The higher vector dose resulted in FIX variant levels between 14 and 15 μg/mL, and significantly shortened aPTT in hFIX variant–treated mice (Figure 4B; for comparison, 5 μg/mL is the physiologic concentration of FIX in human plasma). We then investigated the effect of hFIX variants on TAT complex levels as a general activation markers of the coagulation system. High expression levels of hFIX-T and hFIX-ITV raised TAT levels in FVIII-KO mice to levels observed in hemostatically normal control mice (Figure 4C). TAT levels of hFIX-WT–expressing mice remained similar to untreated FVIII-KO mice. Furthermore, expression of hFIX variants resulted in correction of the bleeding phenotype after dissection of the distal part of the tail at a 1.5-mm diameter (Figure 4D). Similarly, in a more severe hemorrhage model in which the tail is dissected at a 3-mm diameter, the variants reduced blood loss almost to values observed in hemostatically normal controls (Figure 4E).
In vivo efficacy of hFIX variants. (A) hFIX variant expression over time in groups of C57Bl/6 mice after hydrodynamic minicircle gene transfer to the liver at a dose of 7.5 μg/mouse for FIX-T (n = 5), FIX-ITV (n = 7), and FIX-WT (n = 5). (B) aPTT-based 1-stage assay in mouse plasma from groups of FVIII-KO mice treated with hFIX-WT or variant vectors at doses of 7.5 or 25 μg/mouse. (C) TAT complex levels in FVIII-KO mice after hFIX variant minicircle gene transfer at 25 μg/mouse. (D-E) Blood loss assay after tail dissection at 1.5-mm (D) or 3-mm (E) diameter in FVIII-KO mice receiving minicircle doses of 25 μg/mouse. Blood loss was measured by optic density measurement at 492 nm of hemoglobin lost over a period of 10 minutes. For all experiments, hFIX expression levels were determined by FIX ELISA. *P < .05 and **P < .01 according to ANOVA with Dunnett test for multiple comparisons with the corresponding FIX-WT group.
In vivo efficacy of hFIX variants. (A) hFIX variant expression over time in groups of C57Bl/6 mice after hydrodynamic minicircle gene transfer to the liver at a dose of 7.5 μg/mouse for FIX-T (n = 5), FIX-ITV (n = 7), and FIX-WT (n = 5). (B) aPTT-based 1-stage assay in mouse plasma from groups of FVIII-KO mice treated with hFIX-WT or variant vectors at doses of 7.5 or 25 μg/mouse. (C) TAT complex levels in FVIII-KO mice after hFIX variant minicircle gene transfer at 25 μg/mouse. (D-E) Blood loss assay after tail dissection at 1.5-mm (D) or 3-mm (E) diameter in FVIII-KO mice receiving minicircle doses of 25 μg/mouse. Blood loss was measured by optic density measurement at 492 nm of hemoglobin lost over a period of 10 minutes. For all experiments, hFIX expression levels were determined by FIX ELISA. *P < .05 and **P < .01 according to ANOVA with Dunnett test for multiple comparisons with the corresponding FIX-WT group.
FIX variants reconstitute clot formation in the microvasculature
Because bleeding episodes in hemophilia typically originate in small vessels, we also examined the efficacy of the hFIX variants for microvascular hemorrhage. We used intravital microscopy of clot formation after laser-induced injury of small cremaster arterioles and venules as an established model for this purpose.17,,–20 Platelet and fibrin accumulation were monitored by real-time imaging of the forming clot in hFIX variant or WT control-treated FVIII-KO mice over a 3-minute period. Few clots with slow clot progression were observed in untreated FVIII-KO mice or mice expressing hFIX-WT (Figure 5A-B). In contrast, hFIX-T– and hFIX-ITV–treated FVIII-KO mice displayed solid clot formation and strong clot progression (Figure 5A-B). Even injection of a low minicircle vector dose for hFIX-ITV (2.5 μg/mouse, resulting in hFIX expression levels of approximately 1 μg/mL) was sufficient to reconstitute clot formation after every single induced injury.
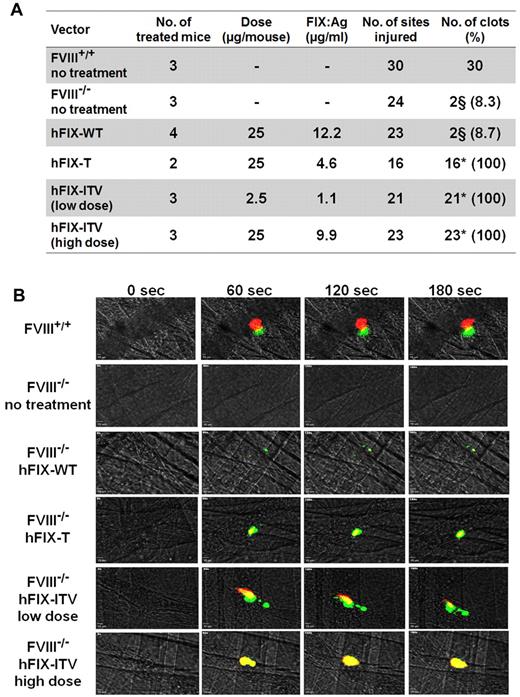
Effect of hFIX variants on thrombus formation after laser-induced injury of small cremaster vessels in FVIII-KO mice. (A) Results overview. (B) Representative immune fluorescence images showing the progression of thrombus formation by intravital microcopy after laser-induced vessel wall damage over the course of 180 seconds for each treatment group. Platelet deposition and fibrin generation in arterial and venous thrombi were visualized using an anti-CD41 Alexa Fluor 647– (red) and an anti-fibrin Alexa Fluor 555–labeled Ab (green). §Small, slow progressing clots. *P < .001 according to Fisher exact test including Bonferroni adjustment for multiple comparisons.
Effect of hFIX variants on thrombus formation after laser-induced injury of small cremaster vessels in FVIII-KO mice. (A) Results overview. (B) Representative immune fluorescence images showing the progression of thrombus formation by intravital microcopy after laser-induced vessel wall damage over the course of 180 seconds for each treatment group. Platelet deposition and fibrin generation in arterial and venous thrombi were visualized using an anti-CD41 Alexa Fluor 647– (red) and an anti-fibrin Alexa Fluor 555–labeled Ab (green). §Small, slow progressing clots. *P < .001 according to Fisher exact test including Bonferroni adjustment for multiple comparisons.
FIX variants prevent bleeding in the presence of FVIII inhibitors in vivo
FVIII-KO mice were immunized against hFVIII using plasmid gene transfer of a hFVIII expression vector. In this inhibitor model,22 nonviral gene transfer leads to hepatic hFVIII expression, which induces a strong hFVIII-neutralizing Ab response. Abs and hFVIII levels were followed over time until FVIII activity or antigen was undetectable and titers of anti-hFVIII Abs had increased to above 10 000 ng/mL, which corresponds to a Bethesda titer above 5 units (Figure 6A). Because hepatic expression of hFVIII in the mice continues, the situation in the mice resembles the situation in patients with acquired hemophilia A. In a second step, mice with inhibitory Abs were treated with minicircles for expression of hFIX-WT, hFIX-T, or hFIX-ITV. Figure 6A illustrates the schedule of the experiment. Similar to results in FVIII-KO mice without Abs, treatment with hFIX-T and hFIX-ITV corrected blood loss after hemostatical challenge by tail dissection at the 1.5-mm diameter (Figure 6B).
Testing of hFIX variants in the presence of FVIII-inhibitory Abs. (A) Schematic illustration of the experiment. Anti-hFVIII Abs were induced by nonviral gene transfer of 50 μg/mouse of pSL1180-HCR/hAAT-FVIIIΔB vector by hydrodynamic liver-directed delivery into FVIII-KO mice. After confirming the absence of FVIII antigen and activity and the presence of high titers of anti-hFVIII Abs, mice received hFIX-T, hFIX-ITV, or hFIX-WT minicircle gene transfer (25 μg/mouse). (B) Blood loss assay after tail dissection at 1.5-mm diameter in FVIII-KO mice with inhibitory Abs. Blood loss was measured by optic density measurement at 492 nm of hemoglobin lost over a period of 10 minutes. hFIX expression levels were determined by FIX ELISA. **P < .01 according to ANOVA with Dunnett test for multiple comparisons with the corresponding FIX-WT group.
Testing of hFIX variants in the presence of FVIII-inhibitory Abs. (A) Schematic illustration of the experiment. Anti-hFVIII Abs were induced by nonviral gene transfer of 50 μg/mouse of pSL1180-HCR/hAAT-FVIIIΔB vector by hydrodynamic liver-directed delivery into FVIII-KO mice. After confirming the absence of FVIII antigen and activity and the presence of high titers of anti-hFVIII Abs, mice received hFIX-T, hFIX-ITV, or hFIX-WT minicircle gene transfer (25 μg/mouse). (B) Blood loss assay after tail dissection at 1.5-mm diameter in FVIII-KO mice with inhibitory Abs. Blood loss was measured by optic density measurement at 492 nm of hemoglobin lost over a period of 10 minutes. hFIX expression levels were determined by FIX ELISA. **P < .01 according to ANOVA with Dunnett test for multiple comparisons with the corresponding FIX-WT group.
FIX variants do not cause immune responses in FIX-WT–tolerant BALB/c mice
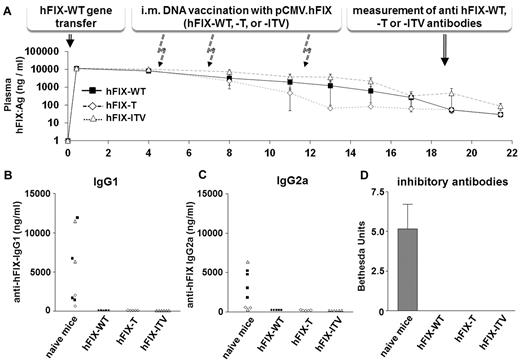
Challenging mice with the human FIX protein causes an immune response and leads to the induction of anti-FIX–specific Ab formation, as seen in Figure 7B through D. Conversely, nonviral FIX gene transfer to the liver is known to induce immune tolerance toward human FIX in mice.14,23 To examine whether the introduced amino acid substitutions lead to novel immunogenic epitopes in FIX, BALB/c mice were either not previously treated or were made tolerant to human FIX-WT by nonviral gene transfer to the liver (Figure 7A). After this, mice were challenged 3 times by intramuscular DNA vaccination with FIX-WT or the FIX-T or FIX-ITV variants. We used anti–hFIX-T, anti–hFIX-ITV, and anti–hFIX-WT–specific Ab ELISAs to detect epitope-specific Abs or Abs directed against the WT protein. Data for FIX-variant–specific IgG1 and IgG2a Abs are shown in Figure 7B. In additional ELISA tests, anti–hFIX-WT Abs were measured in variant-treated mice. As expected, we did not detect Abs in mice tolerant to the FIX-WT. No or only very weak Ab formation against FIX was found in other Ig classes and subclasses (data not shown). Neutralizing Abs were detected using a modified Bethesda assay in which an hFIX variant that included all tested amino acid substitutions (hFIX-ITV) was used as the Ab target (Figure 7D). All mice without tolerogenic pretreatment developed an immune response against hFIX. Ab specificity did not discriminate between hFIX-WT and variants. In contrast, none of the mice tolerant to the human hFIX-WT developed an immune response against hFIX variants. This suggests that the tested variants do not introduce readily recognized neoantigens, at least for the MHC H2d background tested.
Immunogenicity of hFIX variants. Mice were either made tolerant to hFIX-WT by nonviral gene transfer into the liver or were left naive as controls. (A) Graph showing hFIX-WT expression in groups of BALB/c mice over time. The different arrows indicate the vaccination events with variants T (n = 5), ITV (n = 6), and WT (n = 5) and the time point for Ab measurement. For vaccination leading to the induction of Abs, we injected 500 μg/mouse of pCDNA3.1.CMV-hFIX-WT, hFIX-T, or hFIX-ITV into both tibialis anterior muscles. The procedure was repeated twice at the indicated time points to boost Ab development. Four weeks after the last immunization boost, Ab titers were measured by anti-hFIX Ig-specific ELISAs for IgG1 (B) and IgG2a (C). Depending on the treatment group, hFIX-T, hFIX-ITV, or hFIX-WT was used for coating to allow detection of epitope-specific Abs. (D) For the Bethesda assay, we used an hFIX variant (ITV) that included all tested amino acid exchanges for the detection of functional epitope-specific Abs.
Immunogenicity of hFIX variants. Mice were either made tolerant to hFIX-WT by nonviral gene transfer into the liver or were left naive as controls. (A) Graph showing hFIX-WT expression in groups of BALB/c mice over time. The different arrows indicate the vaccination events with variants T (n = 5), ITV (n = 6), and WT (n = 5) and the time point for Ab measurement. For vaccination leading to the induction of Abs, we injected 500 μg/mouse of pCDNA3.1.CMV-hFIX-WT, hFIX-T, or hFIX-ITV into both tibialis anterior muscles. The procedure was repeated twice at the indicated time points to boost Ab development. Four weeks after the last immunization boost, Ab titers were measured by anti-hFIX Ig-specific ELISAs for IgG1 (B) and IgG2a (C). Depending on the treatment group, hFIX-T, hFIX-ITV, or hFIX-WT was used for coating to allow detection of epitope-specific Abs. (D) For the Bethesda assay, we used an hFIX variant (ITV) that included all tested amino acid exchanges for the detection of functional epitope-specific Abs.
Discussion
In the present study, we report for the first time the in vivo therapeutic efficacy of an approach to bypassing FVIII with FIX variants engineered to directly activate FX. Previous studies21,24 showing FVIII-independent activity of FIX variants had already suggested the feasibility of this approach. However, the identification of individual functional mutations and the additive to synergistic effect of the combination of 3 of them (V181I/K265T/I383V) was required to achieve the reported dramatic increase in FVIII-independent clotting activity. Because no clotting activity of FIX-WT was detected in the absence of FVIII, the -fold increase in activity can only be grossly estimated. Based on reports indicating that the presence of FVIII leads to a 500 000-fold boost in FIX activity,10,–12 the ITV variant with 16% FVIII-independent activity increases FIX activity in the absence of FVIII by approximately 80 000-fold. This activity is sufficient for the prevention and/or treatment of hemorrhage at FIX variant circulating plasma levels, which can reasonably be achieved. In FVIII-KO mice, hFIX variant expression levels of approximately 200% of normal human plasma levels sufficed to induce a significant shortening of the clotting time (aPTT). In a model of large-vessel injury (tail clip), therapeutic effects were achieved with similar plasma levels. In contrast, the small-vessel injury model (laser injury of small cremaster vessels), a model for the spontaneous hemorrhage associated with hemophilia A and thus resembling clinical bleeding prophylaxis, 10-fold lower expression levels (approximately 1000 ng/mL, 20% FIX in normal plasma) sufficed for reconstitution of clot formation. Based on these values, a prediction of the levels necessary for the treatment of patients is difficult. Using normal murine plasma as a standard to determine the bypassing activity of the hFIX variants hFIX-T and hFIX-ITV by 1-stage assay, we calculated a bypassing activity of 4.7% ± 2.1% for the hFIX-ITV and 2.9% ± 1.3% for hFIX-T variant at 5000 ng/mL pf protein in murine FVIII-deficient plasma. The lower activity compared with results in human plasma may just reflect the known higher specific activity of murine FVIII in this setting,25 or it could be caused by a hindered interaction of hFIX variants in the absence of FVIII with other murine coagulation factors. In any case, the variants should have a 4-fold greater efficacy in the human system. As mentioned, 1% FVIII activity is the target range for prophylactic treatment of hemophilia A in humans.3,4 Therefore, given the approximately 16% bypassing (or FVIII-like) activity of hFIX-ITV, circulating hFIX-ITV levels of 6% of normal FIX levels would be required to reach functionally equivalent levels. This calculates as 300 ng/mL, which seems feasible.
A major issue for any novel treatment strategy is safety, even more so if alternative regimens are available. Using FIX variants to bypass FVIII, the main safety concerns are the risk of spontaneous activation of coagulation, predisposing for arterial or venous thrombosis, and a possible immune response induced by the variant proteins. In mice tolerant to hFIX-WT, we could not induce an immune response against the specific hFIX variant nor against the WT protein. Although the occurrence of an immune response in humans to specific FIX epitopes cannot be definitively predicted because of MHC diversity, these results suggest that the proposed mutations do not affect highly immunogenic epitopes in the MHC H2d background tested, and may not affect “universal” epitopes for FIX inhibitor formation.
Furthermore, patients with hemophilia A have normal FIX levels, and the risk for Ab formation to FIX-WT is almost zero in hemophilia B patients with point mutations in FIX.26 As a case in point, commercially available recombinant FIX carries a threonine at position 148, whereas approximately 70% of the patient population has an alanine at this position, and an increased risk for inhibitor induction has not been described.27,28 Of the 3 amino acids at positions 181, 265, and 383, which are mutated in our best FIX variant, point mutations are only listed at position 181 in the hemophilia B mutation database.29 Inhibitory Abs for patients carrying these mutations have not been reported. Concerning the thrombotic risk, we measured TAT levels as a surrogate marker for general activation of the coagulation system in FVIII-KO mice. There was a significant increase in hFIX-variant–treated mice over untreated hemophilic mice, but even at hFIX ITV levels at a concentration of 400% of normal hFIX plasma levels, values did not increase above levels observed in hemostatically normal control mice. Similarly, we previously measured an increase in TAT up to levels of control mice after FIX substitution in FIX-KO mice.13 We did not observe any signs of thrombosis or an increased mortality in hFIX-variant–treated mice. Although a protein with high FIX-specific activity has been reported in the context of familiar thrombophilia,30 the case of the presented variants is different. FIX-specific activity of the proteins is grossly normal and the FVIII-independent coagulation activity only accounts for a fraction of the physiologic activity in the presence of FVIII. In addition, clot formation observed by in vivo imaging was similar to clot formation in presence of FVIII. Therefore, our data suggest that FIX variant treatment is safe, both with respect to antigenicity and to activation of coagulation.
We found that the mutation K265T was the single most important change leading to FVIII-independent coagulation activity and the essential modification that enabled us to compose the triple variant. Previously, cooperating alterations of the 99-loop, K265T and residue Y345 belonging to the 170 helix, had been proposed to mimic cofactor binding.10,21,31 In contrast, we observed that mutation of Y345 decreased clotting activity in FVIII-deficient plasma (Table 1). The use of the entire protein and the full natural substrate FX, rather than a truncated version of the protein and small peptide substrates,10,31,32 might lead to these differences in results. We therefore suggest a modified mechanism by which FIXa progresses from an inactive to an active protease. Whereas FVIIIa binding leads to a rearrangement of the 99-loop, FX binding could be responsible for the changes involving position Y345, which together open the active site. We also did not observe FIXa contamination during recombinant protein expression of FIX variants, unlike what was previously reported.21 Eventually, not including modifications of the 170 helix (Y345) might help to preserve FIX protein stability.
The second substitution, I383V, is located in the S1 pocket of FIX in proximity to the active site.9 Although valine at position 383 only modestly modified the amidolytic properties of FIXa, combination of I383V and other protein modifications increased amidolytic activity by 130-fold.9 This reported effect might reflect the synergistic effect of I383V together with K265T.
The third amino acid substitution included in the ITV variant is the first amino acid of the COOH-terminal heavy chain and is located immediately adjacent to the activation peptide cleavage site (R180-V181). Substitution of V181I does not affect activation or activity of FIX.33,34 However, several other mutations at this position lead to severe to moderate hemophilia B.33,35,36 Hamaguchi et al also reported normal antithrombin III binding for variant V181I but not for other substitutions at this position.34 After cleavage of the activation peptide, amino acid 181 flips into the activation pocket, where it forms a salt bridge with D364, which is located right next to the central serine at position 365. Bode described the importance of isoleucine at position 181, I16 in chymotrypsin numbering, in the trypsinogen to trypsin transition. Peptides with N-terminal isoleucine exhibited the strongest binding affinity to trypsinogen and enabled its transition into a trypsin-like state.37 V181I substitution in FIX might therefore increase protein efficiency.
Several other FVIII-bypassing strategies have been reported over the last years. These include FVIIa with a prolonged half-life38,,,–42 and increased activity,43,–45 activated FX mutations,46 and FX variants that can be activated directly by thrombin.47 We believe that FIX variants could have several advantages compared with these approaches. Both the use of a zymogen (inactive) form and the high functional specificity (only bypassing FVIII) are properties that suggest low prothrombotic risk, especially when used prophylactically. Moreover, tests to measure FIX and FVIII are established in all coagulation laboratories, thus allowing for easy monitoring of FIX variant therapy. In the future, the proposed FIX-ITV could be easily combined with existing technologies to improve FIX therapy. These include modern production procedures, molecules with prolonged half-lives, and gene-therapy approaches.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Valder R. Arruda, Rodney M. Camire, Denise E. Sabatino, and Katherine A. High at the Children's Hospital of Philadelphia for helping with mice, critical reagents, and in vivo coagulation studies, and Halvard Bönig at the German Red Cross Blood Donor Service for critical comments and review of the manuscript.
This research was supported by the “Stiftung Hämotherapie-Forschung” (Hemotherapy Research Foundation), by the German Red Cross Blood Donor Service Baden-Württemberg–Hessen, by a grant from the German Society for Thrombosis and Hemostasis Research, and by a Bayer Hemophilia Award from Bayer HealthCare to J.S. P.L. and D.A. are students within the graduate study program GK-1172 funded by the Deutsche Forschungsgemeinschaft, and E.S. received funding through Excellence Cluster Cardio-Pulmonary System of the Deutsche Forschungsgemeinschaft. E.S., M.G., and J.S. received support from the LOEWE Center for Cell and Gene Therapy (Frankfurt, Germany) funded by the Hessisches Ministerium für Wissenschaft und Kunst (2010, funding reference no. III L 4-518/17.004).
Authorship
Contribution: P.M. and J.S. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript; M.G., T.T., and E.S. designed the experiments and contributed to the preparation of the manuscript; W.M. and S.A. provided the patient samples; D.A. and P.Q.-L. performed the in vitro experiments; and L.I. performed the in vivo experiments using the laser-injury model.
Conflict-of-interest disclosure: J.S. receives research funding from Bayer Healthcare. E.S. and J.S. applied for intellectual property protection for FIX variants, patent pending. The remaining authors declare no competing financial interests.
Correspondence: Dr med Jörg Schüttrumpf, Institute for Transfusion Medicine and Immune Hematology, Clinics of the Johann Wolfgang Goethe University, German Red Cross Blood Donor Service Baden-Wuerttemberg–Hessen, Sandhofstr 1, D-60528 Frankfurt, Germany; e-mail: j.schuettrumpf@blutspende.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal