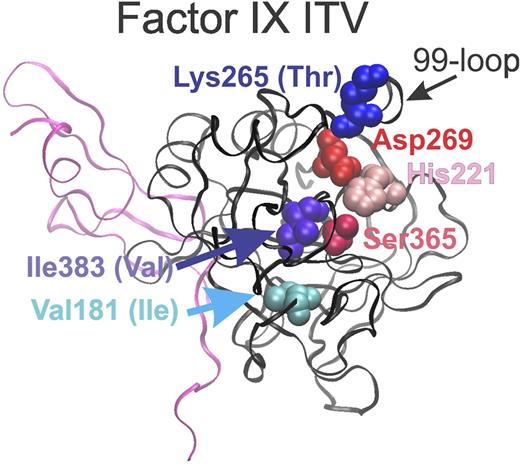
The stucture of FIX is based on that of Wang et al.6 The ribbon structure of the heavy chain of activated FIX appears in black and part of the light chain in pink. The amino acids of the catalytic triad (His221, Asp269, and Ser365) are identified in reds and pinks. The amino acids of normal FIX that were mutated to generate FIX-ITV are identified in blues and purples, with the amino acids present in FIX-ITV shown in parenthesis. PDB code is 3LC3. Jay W. Ponder assisted in preparing the figure.
The stucture of FIX is based on that of Wang et al.6 The ribbon structure of the heavy chain of activated FIX appears in black and part of the light chain in pink. The amino acids of the catalytic triad (His221, Asp269, and Ser365) are identified in reds and pinks. The amino acids of normal FIX that were mutated to generate FIX-ITV are identified in blues and purples, with the amino acids present in FIX-ITV shown in parenthesis. PDB code is 3LC3. Jay W. Ponder assisted in preparing the figure.
Inhibitors of FVIII are antibodies that block its coagulation function. For congenital hemophilia A, inhibitors develop in ∼ 20% of all patients, for an overall incidence of 1:50 000 of the general population, or ∼ 6000 people in the United States. Acquired FVIII inhibitors can develop spontaneously in patients with a normal FVIII gene, and occur in ∼ 1 per 1 million people.2 Bleeding episodes in such patients are often treated with so-called bypass agents, which allow coagulation to occur in the absence of FVIII activity.3 One bypass agent is recombinant FVIIa, which activates FX of the common pathway of coagulation, and FIX of the extrinsic pathway. A second bypass agent is Factor 8 Inhibitor Bypassing Activity (FEIBA), a prothrombin complex concentrate that contains activated vitamin K–dependent coagulation factors. FVIIa and FEIBA appear to be roughly equivalent in their efficacy for minor bleeding episodes.4 FVIIa is given up to every 2 hours at a cost of ∼ $8000 per dose for an adult, while FEIBA is given up to every 8 hours at a cost of ∼ $8500 per dose.5 One of the major drawbacks for both of these treatments is the short half-life of the active protein(s), and the cost of treatment for a single major bleed or surgery can easily exceed $250 000. The development of a hemostatic agent that is active in the presence of an inhibitor but has a longer half-life could reduce the cost of treating bleeding episodes.
Here, Milanov et al report the development and in vivo testing of a FIX variant with activity in the absence of FVIIIa.1 A FIX protein designated FIX-ITV that contained 3 mutations (V181I, K265T, and I383V; see figure based on the wild-type FIX structure6 ). FIX-ITV was demonstrated to promote coagulation in vitro in an aPTT assay when present at wild-type FIX levels in the absence of FVIII at ∼ 16% of the activity found when normal levels of wild-type FIX were present with normal levels of wild-type FVIII. The secreted form of wild-type FIX has 415 amino acids, and is cleaved between Arg145 and Ala146, and between Arg180 and Val181 to generate the activated 2-chain FIXa protein for which the heavy chain (181-415) contains the proteolytic domain and the light chain (1-145) contains γ-carboxyglutamate (GLA) and epidermal growth factor (EGF) domains.3 The amino acids for the catalytic triad are residues His221, Asp269, and Ser365 (see figure). It is believed that FVIIIa functions by altering the conformation of the so-called 99-loop, which are amino acids 94 to 99 using the chymotrypsin numbering system, which represent amino acids 259 to 266 of FIX.7 The 99-loop is proposed to block the active site in the absence of FVIIIa. Although it was not definitely proven in this or prior studies with related proteins, the alterations in FIX-ITV were proposed to move the 99-loop and open up the FIX active site in the absence of FVIIIa, which would allow FIX to have access to its substrate, FX, and cleave FX more efficiently.8,9
Expression of human FIX-ITV using gene transfer allowed hemostasis to be achieved in hemophilia A mice as assessed by a laser-induced injury thrombus formation assay. Furthermore, hemophilia A mice with inhibitory antibodies still achieved hemostasis after tail-clip assay. The half-life of this protein at 37°C in plasma was roughly equal to that of wild-type FIX. If the half-life of FIX-ITV in vivo in humans indeed resembles that of wild-type FIX of ∼ 18 to 24 hours,2 FIX-ITV could result in a prolonged hemostatic activity in patients, which could translate into less-frequent dosing and perhaps a cheaper overall cost of treatment in patients. In addition, because AAV8-mediated gene therapy with a wild-type FIX gene has recently been shown to be effective for patients with hemophilia B,10 it is feasible that gene therapy with a sequence that encodes FIX-ITV could result in a long-term therapeutic effect in hemophilia A patients with inhibitors.
The major concern with a FIXa protein that does not require FVIIIa for activity is that this could induce thrombosis. Although there was no evidence of spontaneous clot formation in mice that expressed FIX-ITV in this study, the duration of expression was relatively short, and the risk of thrombosis will need to be evaluated carefully in the future. Despite this concern, there is a real need for new bypass reagents that have a more-prolonged duration of action than FVIIa or FEIBA, and the benefits of this protein may well outweigh its risk.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■