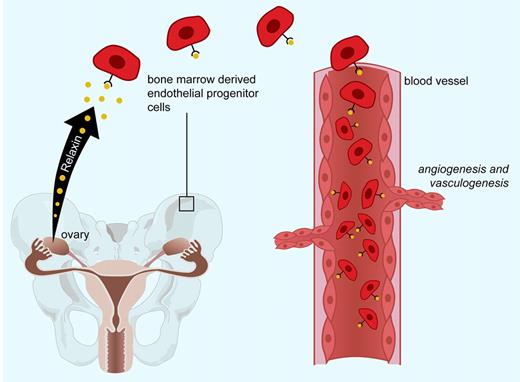
Relaxin mobilizes bone marrow–derived endothelial progenitor cells to sites of vascularization.
Relaxin mobilizes bone marrow–derived endothelial progenitor cells to sites of vascularization.
Originally isolated by Hisaw and colleagues in 1926, relaxin was named for its capacity to soften the pubic syphysis.2 Produced primarily by the corpus luteum in both pregnant and nonpregnant women, relaxin peaks during the luteal phase of the menstrual cycle, declines in the absence of pregnancy, and rises again early in gestation under the stimulus of human chorionic gonaotrophin. During pregnancy, additional relaxin is produced by the chorion and the decidua. Gestational functions of relaxin include softening the cervix, relaxing the uterine musculature, and enhancing endometrial angiogenesis.3 As a modulator of the cardiovascular changes of pregnancy, relaxin is both a potent systemic and renal vasodilator. Increased vascular gelatinase activity and activation of the endothelin B receptor–nitric oxide pathway help mediate the vasodilatory effects of relaxin.4,5
Pregnancy is characterized by dense vasculogenesis and angiogenesis in the placenta.4 Segal and colleagues have shown that relaxin may contribute to vascularization by mobilizing, activating, and enhancing homing of CD34+ bone marrow–derived endothelial cells (BMDECs) to sites of neoangiogenesis, thus demonstrating a novel function for relaxin during pregnancy and in vascular homeostasis (see figure). Relaxin also increases intracellular nitric oxide in human endothelial progenitor cells, which has previously been shown to be critical for BMDEC function. Moreover, Segal et al demonstrate that the NO inhibitor L-NAME and inhibitors blocking the downstream PI3K/AKT pathway can prevent relaxin from stimulating BMDEC chemotaxis. Segal et al have also shown that effects of relaxin on BMDEC function are mediated through the major relaxin receptor RXFP1.6
The findings by Segal et al have important implications for vascular homeostasis during health and in disease. Typically, factors that mobilize endothelial progenitor cells, such as G-CSF, are associated with an entire orchestra of like-minded pro-inflammatory players; however, relaxin is unique in its ability to mobilize and activate BMDECs as a putative anti-inflammatory molecule.7 While this provides a significant advantage for relaxin as an intriguing therapeutic agent in vascular injury states, we do not fully understand the nature of this phenomenon. Although Segal et al have demonstrated that relaxin-induced neovessels are lined by BMDECs, the role of relaxin as the primary conductor of vasculogenesis or as an accompanist remains to be determined. It would be important to evaluate whether relaxin may in addition promote the production of paracrine cues important for angiogenesis and endothelial repair. Nitric oxide has been shown to be important in mediating relaxin's biologic effects; however, there are other, equally important molecular pathways that were not investigated, such as vascular endothelial growth factor signaling that is downstream of relaxin.8 Finally, the dosing of recombinant relaxin used by Segal et al was supra-physiologic. Future studies evaluating either relaxin or its receptor-deficient mice during states of physiologic angiogenesis and in diseases (such as in tumor angiogenesis) are needed to fully assess the role of relaxin in vascular health.
Because of relaxin's potent effects on the cardiovascular system, it has been studied as a therapy for congestive heart failure. A recent randomized phase 2 clinical trial reporting favorable results demonstrated a decrease in cardiovascular mortality and improvement in dyspnea relief.9 Not only because relaxin is a systemic and renal vasodilator, but also because it is a naturally occurring peptide in pregnancy, a phase 1 safety study of relaxin in women with severe preeclampsia is ongoing, with the goal of increasing placental blood flow and improving maternal hypertension and renal dysfunction.10 Although circulating levels of relaxin are not altered in pre-eclampsia,11 relaxin therapy may be particularly beneficial in ameliorating the increased risk of pre-eclampsia and fetal growth restriction noted in women with egg donor pregnancies (who get pregnant using in vitro fertilization and have no corpus luteum). Circulating numbers of endothelial progenitor cells in pre-eclamptic pregnancies are decreased,12 which now provides another reason why relaxin therapy may prove useful in pre-eclampsia. Relaxin may also be useful in treating cardiovascular disease in postmenopausal women who have lost normal ovarian function.
In summary, relaxin now joins a distinguished ensemble of molecules that have the capacity to regulate endothelial progenitor cell number and function, with a prospect of being identified as a principal player in gestational vasculogenesis, angiogenesis, and vascular remodeling. Insights from this study by Segal et al as well as prior work suggest that recombinant relaxin may be useful in treating diverse vascular diseases.
Conflict-of-interest disclosure: A.W. declares no competing financial interests. S.A.K. is a co-inventor on multiple patents held by Beth Israel Deaconess Medical Center related to the use of angiogenic proteins for the diagnosis and therapy of pre-eclampsia, is a consultant to Beckman Coulter and Roche, and has a financial interest in Aggamin LLC. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal