Abstract
Cancer stem cells represent the most important target cells for antitumor therapy. TRAIL (TNF-related apoptosis-inducing ligand) is a potential anticancer agent that induces apoptosis in a wide variety of tumor cells, but its ability to target cancer stem cells is currently unknown. Here we investigated whether TRAIL targets leukemia-initiating cells. Limiting dilution transplantation assays were performed on xenografts from pediatric patients with precursor B-cell acute lymphoblastic leukemia (pre-B ALL) in NSG mice. In vitro treatment of xenograft cells with TRAIL significantly reduced and delayed their engraftment and procrastinated animal death from leukemia. Systemic TRAIL treatment of mice injected with patient-derived pre-B ALL xenograft cells abrogated leukemia in 3 of 5 mice in 1 sample. In conclusion, our data suggest that TRAIL targets leukemia-initiating cells derived from pre-B ALL xenografts in vitro and in vivo, and hence constitutes an attractive candidate drug for treatment of ALL.
Introduction
The cancer stem cell (CSC) model suggests that a subset of cells is uniquely able to sustain tumor growth and relapse. Therefore, CSCs represent the most important targets for anticancer therapy.1,2 The cancer stem cell hypothesis was first demonstrated in studies on acute myeloid leukemia, which serves as suitable model disease.3,4 CSCs seem to display high resistance to conventional cytotoxic drugs5-7 ; therefore, new therapies are needed.
TRAIL (TNF-related apoptosis-inducing ligand) triggers apoptosis preferentially in solid tumor8 and leukemia cells9,10 while sparing normal cells, including hematopoietic progenitors11 and, therefore, is considered a promising anticancer agent. TRAIL showed antitumor activity in several preclinical models12 and in phase 1 and 2 clinical trials,13,14 but so far no studies have assessed its ability to target CSCs.
Acute lymphoblastic leukemia (ALL) is a frequent disease in children. Despite its favorable prognosis in children, the demand for new therapies persists, especially for relapsed and adult ALL patients.15 Here we asked whether TRAIL targets leukemia-initiating cells (LICs) in precursor B-cell acute lymphoblastic leukemia (pre-B ALL) cells of patients using limiting dilution transplantation assays and a preclinical mouse model. LICs are functionally defined as cells that regenerate the patients' leukemia in mice. In this model, we show that treatment with TRAIL in vitro or in vivo inhibits LICs and their capacity to recapitulate disease in NSG mice.
Methods
Pre-B ALL samples were obtained from children at the Dr von Haunersches Kinderspital, Ludwig-Maximilians-University Munich. Cells were injected intravenously into NSG (NOD-scid IL2Rgammanull) mice,16 and engraftment was monitored by FACS staining and/or immunohistochemistry. Recombinant human TRAIL was produced in Escherichia coli. For determining LIC frequencies, limiting dilution transplantation assays were performed in mice after in vitro treatment with TRAIL or vehicle for 48 hours. In vivo, engrafted mice were treated with TRAIL or vehicle intravenously. For a detailed description, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The study was performed in accordance with the ethical standards of the responsible committee on human experimentation (written approval by Ethikkommission des Klinikums der Universität of Ludwig Maximilians Universität München, April 15, 2008, #068-08) and with the Declaration of Helsinki (1975), as revised in 2000. Written informed consent was obtained from all patients and from parents/caregivers in the cases where the patients were minors.
Results and discussion
CSCs constitute the most important target for anticancer therapy.1,2,17 Here we aimed at determining whether the candidate anticancer drug TRAIL12 targets LICs in pre-B ALL. Studying the sensitivity of CSCs to drugs is technically challenging. In vitro studies are hampered by the fact that cell lines do not represent adequate models. In addition, all attempts to phenotypically characterize LICs in patient samples have failed to identify discrete populations of LICs in ALL.18-20 Here we studied the effect of TRAIL on LICs within unsorted leukemia xenograft cells. We transplanted leukemia xenografts into NSG mice in limiting dilutions and determined engraftment. In ALL, studies on LICs require transplantation assays, as phenotypic characterization of LICs is currently unfeasible in this disease.18-20 Only LICs support serial transplantation of leukemia xenografts. However, the functional approach to study LICs does not allow mechanistic or signaling studies.
In previous studies, TRAIL induced apoptosis in 25% of primary pediatric ALL samples.21 Here, primary ALL samples were engrafted and passaged as xenografts in NSG mice.22 We chose to study 3 pre-B ALL xenografts ALL-54, ALL-169, and ALL-177 (clinical data in supplemental Table 1), in which TRAIL induced 97% (ALL-54), 60% (ALL-169), and 98% (ALL-177) specific apoptosis after 48 hours in vitro.
ALL is known for its erratic clonal nature.23,24 Nevertheless and according to published data,25 sensitivity of leukemia bulk cells toward TRAIL-induced apoptosis remained unchanged on xenotransplantation and passaging through mice (supplemental Figure 1A), and cells of different passages remained indistinguishable from primary tumor cells regarding expression of surface markers (supplemental Figure 1B). In all samples, bulk leukemia cells expressed at least 1 agonistic TRAIL receptor (supplemental Figure 2).
To study the effect of TRAIL on LICs, fresh splenic isolates from passage 3 to 5 were incubated with TRAIL or vehicle (1000 ng/mL) for 48 hours in vitro. In leukemia bulk cells, TRAIL treatment slightly increased the percentage of CD34+ cells suggesting increased sensitivity in CD34− cells (supplemental Figure 3). Serial dilutions were performed and the cells injected into groups of mice (supplemental Table 2). Twelve to 16 weeks after injection, mice were analyzed for engraftment and LIC frequencies were calculated.
Mice injected with vehicle-treated cells showed typical signs of leukemia in a cell number-dependent manner, including spleen enlargement and infiltration of other organs. In contrast, in vitro TRAIL treatment before transplantation significantly reduced leukemic burden and delayed or even abrogated engraftment as a function of the number of transplanted cells (Figure 1A-B; supplemental Figure 4; supplemental Table 2). TRAIL exposure before injection resulted in a 75% to 99.9% reduction of LIC frequencies in the samples tested (Figure 1C; supplemental Table 3) and clearly documented that TRAIL affected LICs at least as efficiently as it affected the bulk of leukemia cells. The effect of TRAIL against LICs was independent from the number of passages in mice arguing toward low clonal evolution in the model (Figure 1C). These data suggest that TRAIL might affect LICs, which are known for their resistance against conventional drugs.5-7,26
In vitro treatment with TRAIL delays engraftment of pre-B ALL xenograft cells. Xenograft ALL-177 cells freshly isolated from the spleen of a donor mouse were treated in vitro with vehicle or TRAIL (1000 ng/mL) for 48 hours. Cells were serially diluted (range, 1 × 106 to 3 × 103) and injected into a total of 39 mice. (A-B) Half of the 39 mice (13 mice in the vehicle group, 10 mice in the TRAIL group, raw data are shown in supplemental Table 2) were killed 15 weeks after transplantation to evaluate dose-response. (A) Representative spleen sizes (left); immunohistochemistry of spleens stained for cells expressing human CD10 (middle; original magnification ×200); and bone marrow infiltration by human leukemia cells using flow cytometric analysis of human CD38 (right); numbers on top of the panels indicate amount of human leukemia cells injected per mouse. (B) Leukemic infiltration in bone marrow (BM) and spleen for all mice, as estimated by flow cytometric analysis of human CD38; each mouse is represented by a single dot. (C) From analysis of bone marrow and spleen, engraftment was considered positive if ≥ 1% human cells were detected in at least 1 organ (raw data listed in supplemental Table 2). LIC frequencies were calculated using Poisson statistics. Similar experiments were performed for ALL-54 and ALL-169 (supplemental Figure 3; raw data listed in supplemental Table 2). Decrease in LIC frequency by TRAIL treatment was calculated and indicated above bars. **P < .01 (Student t test). ***P < .001 (Student t test). Filled bars represent late mouse passage (passage 3 in ALL-54 and passage 4 in ALL-177); and bars with dots, early passage (cells that have been collected from a mouse injected with the primary sample). (D-E) To evaluate the kinetics of leukemic growth, the second half of the 39 animals transplanted with ALL-177 cells were observed over time (n = 8 mice per group; supplemental Table 4). Appearance of leukemic cells in peripheral blood (PB) was the readout, which was regularly determined by flow cytometry detecting human CD38. (D) Kinetics in 6 representative mice injected with 3 different cell numbers, for TRAIL and vehicle. (E) The relation between time to engraftment/time to death and numbers of leukemia cells injected per mouse; each mouse is represented by a single dot. Leukemic engraftment was defined as ≥ 1% leukemia cells in PB; overt leukemia/time to death was defined as ≥ 35% leukemia cells in PB. p.i. indicates postinjection. Regression curves and equations were calculated thereof and are indicated.
In vitro treatment with TRAIL delays engraftment of pre-B ALL xenograft cells. Xenograft ALL-177 cells freshly isolated from the spleen of a donor mouse were treated in vitro with vehicle or TRAIL (1000 ng/mL) for 48 hours. Cells were serially diluted (range, 1 × 106 to 3 × 103) and injected into a total of 39 mice. (A-B) Half of the 39 mice (13 mice in the vehicle group, 10 mice in the TRAIL group, raw data are shown in supplemental Table 2) were killed 15 weeks after transplantation to evaluate dose-response. (A) Representative spleen sizes (left); immunohistochemistry of spleens stained for cells expressing human CD10 (middle; original magnification ×200); and bone marrow infiltration by human leukemia cells using flow cytometric analysis of human CD38 (right); numbers on top of the panels indicate amount of human leukemia cells injected per mouse. (B) Leukemic infiltration in bone marrow (BM) and spleen for all mice, as estimated by flow cytometric analysis of human CD38; each mouse is represented by a single dot. (C) From analysis of bone marrow and spleen, engraftment was considered positive if ≥ 1% human cells were detected in at least 1 organ (raw data listed in supplemental Table 2). LIC frequencies were calculated using Poisson statistics. Similar experiments were performed for ALL-54 and ALL-169 (supplemental Figure 3; raw data listed in supplemental Table 2). Decrease in LIC frequency by TRAIL treatment was calculated and indicated above bars. **P < .01 (Student t test). ***P < .001 (Student t test). Filled bars represent late mouse passage (passage 3 in ALL-54 and passage 4 in ALL-177); and bars with dots, early passage (cells that have been collected from a mouse injected with the primary sample). (D-E) To evaluate the kinetics of leukemic growth, the second half of the 39 animals transplanted with ALL-177 cells were observed over time (n = 8 mice per group; supplemental Table 4). Appearance of leukemic cells in peripheral blood (PB) was the readout, which was regularly determined by flow cytometry detecting human CD38. (D) Kinetics in 6 representative mice injected with 3 different cell numbers, for TRAIL and vehicle. (E) The relation between time to engraftment/time to death and numbers of leukemia cells injected per mouse; each mouse is represented by a single dot. Leukemic engraftment was defined as ≥ 1% leukemia cells in PB; overt leukemia/time to death was defined as ≥ 35% leukemia cells in PB. p.i. indicates postinjection. Regression curves and equations were calculated thereof and are indicated.
In addition to the dose-response analysis, kinetics of engraftment were investigated using ALL-177 in limiting dilution transplantation assays (supplemental Table 4). Here the animals were monitored until death by overt leukemia. The known relationship between cell number and 2 variables (time to engraftment and time to death) was clearly visible (Figure 1D-E). On in vitro treatment with TRAIL, onset and death from leukemia were significantly delayed. For example, TRAIL treatment of 106 injected cells delayed onset of and leukemic death by 8 and 10 weeks, respectively (Figure 1E). Eventually, all mice died from leukemia, except the animal injected with low numbers of TRAIL-treated cells (4 × 104). Surprisingly, TRAIL not only delayed engraftment (P = .004) and death (P = .003) but also slowed down leukemic growth kinetics in engrafted mice. In Figure 1D and E, slopes of regression differed between both groups; the mean time between engraftment and death differed significantly between animals injected with TRAIL-treated (6.3 weeks) and vehicle-treated cells (4.6 weeks; P = .03, Student t test).
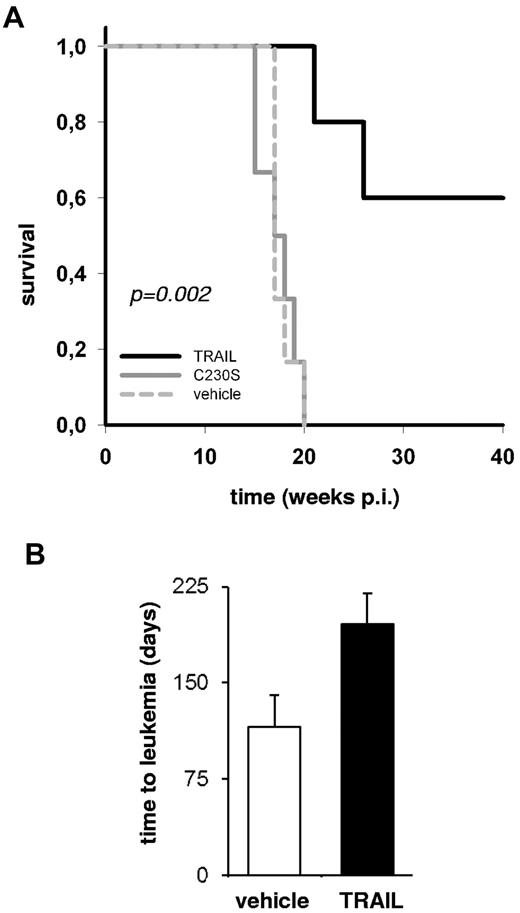
In a second approach, mice transplanted with ALL xenografts were treated with TRAIL systemically to determine its effect in vivo in a proof-of-concept approach. In the preclinical xenograft mouse model, 5 × 104 pre-B ALL xenograft cells ALL-54 or ALL-177 were injected per mouse. Five or 6 mice per group were treated with TRAIL, nonfunctional TRAIL mutant C230S-TRAIL, or vehicle starting on day 5 after injection, after leukemia cell homing.26 In sample ALL-177, TRAIL significantly extended the survival of the animals (5 weeks, P = .007, supplemental Figure 5). In sample ALL-54, mice treated with the nonfunctional TRAIL showed similar kinetic of engraftment and death as the vehicle-treated animals, but TRAIL completely prevented development of leukemia in 3 of 5 mice (Figure 2A; supplemental Figure 6). Surviving mice did not show any signs of leukemia until the end of their life spans and in postmortem analyses. As TRAIL abrogated development of leukemia in these mice, TRAIL must have affected LICs in vivo. From a TRAIL-treated mouse that nevertheless developed leukemia, secondary transplant recipients showed retarded leukemic growth, again arguing that systemic TRAIL treatment had affected LICs in the primary recipients (Figure 2B).
In vivo treatment with TRAIL prolongs life in animals engrafted with pre-B ALL xenograft cells. NSG mice were injected with 5 × 104 ALL-54 cells and treated on days 5 to 9 and days 12 to 16 (10 doses) with 7.5 mg/kg TRAIL, vehicle, or mutant C230S-TRAIL by intravenous bolus injection. Five mice were treated with TRAIL; 6 mice each were treated with vehicle or the TRAIL mutant C230S. (A) Survival curve. The P value is for the log-rank test. p.i. indicates postinjection. (B) For secondary transplantations, 106 leukemia splenic cells of 1 mouse treated with vehicle and 1 diseased mouse treated with TRAIL were injected into 2 mice each. Secondary transplant recipients were monitored for engraftment as described in Figure 1D and E. The mean time to overt leukemia is shown together with the SD from the 2 mice.
In vivo treatment with TRAIL prolongs life in animals engrafted with pre-B ALL xenograft cells. NSG mice were injected with 5 × 104 ALL-54 cells and treated on days 5 to 9 and days 12 to 16 (10 doses) with 7.5 mg/kg TRAIL, vehicle, or mutant C230S-TRAIL by intravenous bolus injection. Five mice were treated with TRAIL; 6 mice each were treated with vehicle or the TRAIL mutant C230S. (A) Survival curve. The P value is for the log-rank test. p.i. indicates postinjection. (B) For secondary transplantations, 106 leukemia splenic cells of 1 mouse treated with vehicle and 1 diseased mouse treated with TRAIL were injected into 2 mice each. Secondary transplant recipients were monitored for engraftment as described in Figure 1D and E. The mean time to overt leukemia is shown together with the SD from the 2 mice.
Both approaches did not reveal the mechanisms by which TRAIL targeted LICs (eg, by reducing engraftment, affecting graft survival, inducing cell death) but, based on current knowledge about TRAIL, it most probably induced apoptosis in LICs.
Taken together, our data show, for the first time, that TRAIL significantly reduces the leukemia-initiating capacity of pre-B ALL xenografts derived from patients, pointing toward a beneficial use of TRAIL for treatment of ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Volker Groiß for all mouse work, Wolfgang Hammerschmidt for helpful discussions and for reading the manuscript, Katja Schneider for establishing the ALL xenograft model, the animal facility for caring for the mice, Ulrike Borgmeier for technical help, Harald Ehrhardt for TRAIL-receptor staining, and Claudia Kloss for immunohistochemical analysis.
This work was supported by Deutsche Forschungsgemeinschaft (SFB684, TP22), Deutsche José Carreras Leukämie Stiftung (R10/26), and Bettina Bräu Stiftung (all I.J.).
Authorship
Contribution: C.C.A. designed and carried out experiments and wrote the manuscript; N.T. and S.G collected data; M.G. prepared recombinant TRAIL; U.G. provided samples; L.Q.-M. performed and interpreted the immunohistochemical analysis; I.J. designed the experiments and wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irmela Jeremias, Department of Gene Vectors, Helmholtz Zentrum München, German Research Center for Environmental Health, Marchioninistraße 25, 81377 Munich, Germany; e-mail: irmela.jeremias@helmholtz-muenchen.de.