Abstract
Standardized criteria for diagnosis and response evaluation in chronic lymphocytic leukemia (CLL) are essential to achieve comparability of results and improvement of clinical care. With the increasing range of therapeutic options, the treatment context is important when defining refractory CLL. Refractory CLL has been defined as no response or response lasting ≤ 6 months from last therapy. This subgroup has a very poor outcome, and many trials use this group as an entry point for early drug development. With the intensification of first-line regimens, the proportion of patients with refractory CLL using these criteria decreases. This has immediate consequences for recruitment of patients into trials as well as salvage strategies. Conversely, patients who are not refractory according to the traditional definition but who have suboptimal or short response to intense therapy also have a very poor outcome. In this Perspective, we discuss recent results that may lead to a reassessment of risk categories in CLL focusing on fit patients who are eligible for all treatment options. We cover aspects of the history and biologic basis for refractory CLL and will focus on how emerging data on treatment failure from large trials using chemoimmunotherapy may help to define risk groups in CLL.
Introduction
The definition of treatment response and estimation of outcome are of central importance in chronic lymphocytic leukemia (CLL) and cancer in general. The current CLL guidelines for diagnosis and treatment have recently been updated in this journal.1 Because of the clinical consequences, the definition of “refractory” CLL is of particular importance.2-4 The terms “refractory” CLL and “fludarabine-refractory” CLL are often used interchangeably, but with the variety of treatment options available, the consideration of the particular treatment context is of crucial importance.5-8 Clearly, the selective pressure of fludarabine monotherapy is different from that of fludarabine, cyclophosphamide, and rituximab combination (FCR) and, correspondingly, the consequences of refractoriness or suboptimal response to these regimens are different.5,6,9-14
The clinical importance of refractory CLL is based on the fact that, unlike most CLL patients, this subgroup was shown to have very poor prognosis (median, 1-2 years overall survival [OS] in most studies) despite various salvage therapy strategies.3,15-17 In addition, many trials of investigational agents use this definition as an entry point for early drug development. The Food and Drug Administration and European Medicines Agency have licensed drugs for this particular subgroup of patients based on phase 2 trials (nonrandomized) because of the unmet medical need.16 Furthermore, recent trials use similar definitions for refractoriness to other drugs (eg, alemtuzumab) and have resulted in the approval of novel agents based on interim analyses of phase 2 trial data.18
Early work on standardizing diagnostic and response criteria in CLL were motivated by the realization that heterogeneous response or diagnostic criteria would impede the comparability of treatment results and hold up improvement in clinical care.19,20 Historically, these categorizations stem from times when complete remissions were almost unattainable.21,22
The most recent guidelines1 define refractory CLL as treatment failure (no partial remission [PR]/complete remission [CR]), or disease progression within 6 months to the last antileukemic therapy and correspond in part to the definition coined in times when CLL treatment was based on chlorambucil and fludarabine monotherapy.16 In current standard practice, young and fit patients failing therapy will have been exposed to purine analogs, but being refractory to chlorambucil or fludarabine (or other purine analogs, or bendamustine), monotherapy is different from being refractory to combined chemoimmunotherapy, such as FCR. With the evolution of first-line treatment, the overall response rate has increased from 31%-72% with chlorambucil (CR rate, 0%-7%), to 59%-83% with fludarabine (CR rate, 7%-15%), to ca. 85% with FC (CR rate, 24%-38%), to 90%-95% with FCR (CR rate, 44%-50%).5,6,9,10,23 This also means that the proportion of patients not responding in the first-line setting has decreased to approximately 10%, leading to slower accrual into trials for refractory patients.
There is accumulating evidence that patients who are formally not refractory based on the current definition, but relapse early after chemoimmunotherapy (ie, within 24-36 months),14,17 have a very poor outcome as the relapse is postponed without changing the biology underlying the poor response.
This Perspective is explicitly not intended as an update of current guidelines but as a discussion of historical aspects and the biologic basis for refractory CLL with the perspective of the need to develop new stratification schemes. We will focus on potential risk categories in CLL derived from data of trials using chemoimmunotherapy as the current standard of care in fit patients with CLL.5-7,17
Historical perspective and current definition of refractory CLL
In initial guidelines for “protocol studies” written in 1978, response was categorized into CR, PR, clinical improvement, no response, and progressive disease.22 “Failure of chemotherapy” was suggested to be considered if no benefit was obtained after 3 months of treatment, if localized disease appeared requiring radiation, if relapse occurred without favorable response to drug increase, if drug toxicity precluded its further use, and if disease progressed. The authors acknowledged that “revisions will continue to be made as new knowledge accumulates.”22 It was also conceded that “it is difficult to be categorical about the design of protocols for the treatment of chronic lymphocytic leukemia (CLL) and for evaluating response to therapy.”22
The recommendations of the National Cancer Institute–sponsored Working Group from 1988 defined CR, PR, and progressive disease as well as compiling the diverse response criteria in use at the time. Refractory disease or failure was not separately defined.24 The International Workshop on CLL formulated general practice recommendations, which put emphasis on change in clinical stage.25 The 1996 National Cancer Institute-sponsored Working Group revised guidelines defined treatment failure as responses other than CR, nodular PR, or PR but did not comment on length or intensity of treatment. Refractory disease was defined as failure to achieve at least a PR or progression while on therapy.26
In the key studies on “fludarabine refractory” CLL, the definition was adopted as failure to achieve a CR/PR or progression within 6 months of last dose of therapy.16 In a recent review on the treatment of refractory CLL, the definition followed this term but also suggested that “patients progressing or relapsing shortly (eg, within 12 months) after stem cell transplantation should be considered as having refractory disease.”3
The most recently updated CLL guidelines defined refractory disease as treatment failure (stable disease, nonresponse, progressive disease, or death from any cause) or disease progression within 6 months from the last antileukemic therapy (Table 1).1 Although this definition is also derivable from the 1996 guideline, it is now summed up more explicitly; and indeed, it is accepted and used in most clinical trial protocols as well as clinical practice. In addition, the authors also took up the category of “high-risk CLL” justifying the consideration of allogeneic stem cell transplantation (SCT).1,27
Overview of recent definitions of “refractory,” “high-risk,” and “ultra-high risk” CLL
| Source . | Disease state defined . | Definition . |
|---|---|---|
| IWCLL (2008)1 | “Refractory” CLL | Failure to achieve CR/PR, relapse within 6 mo of last treatment |
| EBMT guideline (2007)27 | “High-risk” (consider alloSCT) | Nonresponse or early relapse (within 12 mo) after purine analogs |
| Relapse within 24 mo after having achieved a response with purine analog-based combination therapy or autologous transplantation | ||
| Patients with p53 abnormalities requiring treatment | ||
| Stilgenbauer and Zenz (2010)17 | “Ultra-high risk” | Purine analog (or similar; ie, bendamustine)–refractory CLL |
| Early relapse (within 24 mo) after FCR (or FCR-like) with treatment indication | ||
| TP53 deletion/mutation and indication for treatment |
| Source . | Disease state defined . | Definition . |
|---|---|---|
| IWCLL (2008)1 | “Refractory” CLL | Failure to achieve CR/PR, relapse within 6 mo of last treatment |
| EBMT guideline (2007)27 | “High-risk” (consider alloSCT) | Nonresponse or early relapse (within 12 mo) after purine analogs |
| Relapse within 24 mo after having achieved a response with purine analog-based combination therapy or autologous transplantation | ||
| Patients with p53 abnormalities requiring treatment | ||
| Stilgenbauer and Zenz (2010)17 | “Ultra-high risk” | Purine analog (or similar; ie, bendamustine)–refractory CLL |
| Early relapse (within 24 mo) after FCR (or FCR-like) with treatment indication | ||
| TP53 deletion/mutation and indication for treatment |
Working model for risk categories in CLL
Defining risk groups and guiding treatment accordingly are central aims of clinical research in cancer. It is important to define the biologic basis of risk groups because this serves as the basis for targeted treatment approaches. Similarly important is the definition of standard clinical approaches to different risk groups. Only the availability of treatment alternatives will make risk stratification useful. For the definition of “high-risk” CLL, it is important to stress that this term is currently used without clear definition and it is foreseeable that there will be more than 2 risk groups in CLL.
For the current Perspective, we suggest 3 risk categories in CLL based on current treatment scenarios. Importantly, this concept will undergo reconsideration with new prognostic markers as well as improved treatments. Currently, a practical distinction of risk categories could be made based on the predicted effectiveness of FCR-like treatment. It is important to appreciate that treatment suggestions based on these categories have not demonstrated superiority in prospective trials but are based on data from the retrospective analysis of subgroups and the experience of the authors as well as many other experts in the field. Ideally, the acceptance of this risk hierarchy will pave the way for trials exploring optimal treatment strategies.
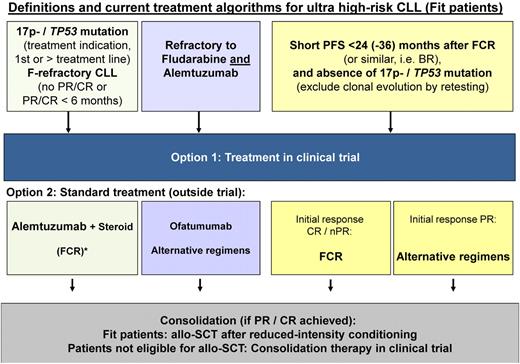
In the “highest-risk” category (TP53 loss/mutation, “purine analog-refractory,” very short [< 24 months] response to prior FCR and no CR on prior exposure), treatment with FCR is unlikely to yield acceptable response, relevant CR rates, or prolonged survival (Table 2). These patients are prime candidates for drugs with proven activity in TP53 deleted/mutant cells, investigational agents in clinical trials, and allogeneic SCT (Figure 1). In CLL, an allogeneic SCT as a treatment should be considered in patients who fulfill the definition of a “highest-risk” group. Although the use and the timing of allogeneic SCT has not been defined based on comparative treatment trials, there is overwhelming evidence that allogeneic SCT is beneficial in subgroups of patients.2,3,27 The Chronic Leukemia Working Party of the European Bone Marrow Transplant have published the recommendations of the Consensus Group with regard to their recommended indications where allogeneic SCT can be considered as an option with evidence-based efficacy (Table 1).27 This proposal has been confirmed by recent data from clinical trials.12,27
Potential risk model, definitions, and suggested treatment algorithms in CLL
| Risk group . | Definition . | Treatment . |
|---|---|---|
| Highest-risk | F-refractory CLL Early relapse (≤ 24 mo) after FCR (or FCR-like) treatment TP53 deletion/mutation and indication for treatment | Alternative induction with novel agent in clinical trial/alemtuzumab in general practice Consolidation with allogeneic SCT Maintenance (in clinical trial) |
| High-risk | Unmutated IGHV, 11q deletion, high β2-MG, no highest-risk traits | FCR + investigational agent (in induction/maintenance) |
| Low-risk | None of the above, no prior treatment | FCR, consider de-escalation, potentially MRD-based |
| Risk group . | Definition . | Treatment . |
|---|---|---|
| Highest-risk | F-refractory CLL Early relapse (≤ 24 mo) after FCR (or FCR-like) treatment TP53 deletion/mutation and indication for treatment | Alternative induction with novel agent in clinical trial/alemtuzumab in general practice Consolidation with allogeneic SCT Maintenance (in clinical trial) |
| High-risk | Unmutated IGHV, 11q deletion, high β2-MG, no highest-risk traits | FCR + investigational agent (in induction/maintenance) |
| Low-risk | None of the above, no prior treatment | FCR, consider de-escalation, potentially MRD-based |
The risk model takes into account 3 groups of patients. The “highest-risk” group may be defined based on predicted failure to respond to FCR (TP53 loss/mutation; short or no response to FCR). There are a number of exciting substances in clinical development (eg, CAL-101; PCI 32765), which make inclusion into trials an attractive option. The high-risk category is expected to benefit from FCR (or similar), but relapse risk is high and there remains room for improvement in efficacy by investigational approaches. At relapse, these patients are still expected to respond to FCR or similar regimens. The low-risk group includes all other patients. In these, FCR (or similar) is of high efficacy, and the aim of future trials may be to achieve similar outcomes with less treatment toxicity.
Treatment algorithm in different “highest-risk” scenarios of CLL. Patients with 17p deletion or TP53 mutation, refractory CLL (refractory to fludarabine, fludarabine combination, and similar regimens) as well as patients with short PFS after FCR (or similar regimens, PCR, BR) have a very high risk of death within 2 years from treatment indication.14,15,17 *The recommendations are not based on comparative trial data.
Treatment algorithm in different “highest-risk” scenarios of CLL. Patients with 17p deletion or TP53 mutation, refractory CLL (refractory to fludarabine, fludarabine combination, and similar regimens) as well as patients with short PFS after FCR (or similar regimens, PCR, BR) have a very high risk of death within 2 years from treatment indication.14,15,17 *The recommendations are not based on comparative trial data.
A second scenario of “high-risk” CLL may relate to the fact that, with increasing remission depth and duration, it will also be important to identify subgroups that will relapse relatively early with standard treatment, even if not in the “highest-risk” group. Separation of this high-risk group will have the advantage of potentially more biologic homogeneity and statistical power (because of the higher likelihood of early events). Obvious candidates within this group could be patients with high β-2-microglobulin (β2-M; or thymidine kinase), unmutated immunoglobulin heavy chain variable region (IGHV), or 11q deletion (Table 2).5,28,29 In these patients, the addition of rituximab to FC leads to improved response, progression-free survival (PFS), and OS (CLL8 trial: hazard ratio for OS FCR group: 0.62, unmutated IGHV, P = .023; or 0.42, 11q−, P = .036).5,13 Nonetheless, these patients have a short PFS. This subgroup of patients may be particularly suited for investigational agents combined with FCR or maintenance strategies (Table 2).
Although IGHV status could be replaced by alternate markers (eg, ZAP70), the inclusion of IGHV status in the current model is based on the availability of most data for this factor from FCR trials.
It is important to separate this group from “low-risk” patients (no 11q deletion, no TP53 deletion/mutation, mutated IGHV, low β2-M, no prior therapy). This group of patients has a very favorable outcome despite treatment indication (Table 2).
Improvement of response and outcome with current chemoimmunotherapy approaches
A simple summary of the overall response rate of current treatment regimens shows the improvement of outcome over the last 20 years (Table 3).2 It is important to note that less intense and less toxic regimens maintain their place in current treatment approaches for elderly/unfit patients.2,23
| Trial . | A . | Clb . | B . | F . | FC . | FCR . |
|---|---|---|---|---|---|---|
| UKCLL49 (n = 777) | 28 | 20 | 6 | |||
| Clb vs Benda63 (n = 314) | 69 | 32 | ||||
| CAM30762 (n = 297) | 17 | 45 | ||||
| GCLLSG CLL410 (n = 375) | 17 | 5 | ||||
| ECOG39 (n = 278) | 41 | 26 | ||||
| GCLLSG CLL85 , n = 817 (Binet C) | 20 (23) | 10 (16) | ||||
| MDACC6 (n = 300; nonrandomized) | 5 |
| Trial . | A . | Clb . | B . | F . | FC . | FCR . |
|---|---|---|---|---|---|---|
| UKCLL49 (n = 777) | 28 | 20 | 6 | |||
| Clb vs Benda63 (n = 314) | 69 | 32 | ||||
| CAM30762 (n = 297) | 17 | 45 | ||||
| GCLLSG CLL410 (n = 375) | 17 | 5 | ||||
| ECOG39 (n = 278) | 41 | 26 | ||||
| GCLLSG CLL85 , n = 817 (Binet C) | 20 (23) | 10 (16) | ||||
| MDACC6 (n = 300; nonrandomized) | 5 |
A indicates alemtuzumab; Clb, chlorambucil; B, bendamustine; F, fludarabine; C, cyclophosphamide; and R, rituximab.
The results of chemoimmunotherapy (eg, FCR) have been unique in the demonstration of improved OS in historical and randomized comparison.5,7,30 The increased intensity of treatment will influence selective pressure on the tumor clones. Indeed, the proportion of nonresponders has decreased to 10% in the FCR arm compared with 20% in the FC arm of the CLL8 trial.5 In patients with Binet C disease, the proportion of patients without response is higher (27% FC; 16% FCR arm).5
The outcome of patients failing F, FC, and FCR has not been formally compared. Based on the small but consistent differences in outcome with respect to OS, the differences may not be expected to be striking, but even in the absence of comparative data, early failure after FCR appears to be a challenging disease.3,4,8,14,15
In high-grade lymphoma, the outcome of patients relapsing after rituximab-containing treatment has been shown to be much poorer than for patients initially treated without rituximab.31 In an early analysis from the CLL8 trial, the outcome of patients in the FC arm failing therapy and receiving second-line treatment appears better than patients failing in the FCR arm (T.Z., M.H., S.S., unpublished data, November 2011).
In a recent analysis from the MD Anderson Cancer Center (MDACC) assessing the outcome of FCR therapy in previously treated patients, patients with 17p deletion (OS = 10.5 months), more than 3 prior lines of treatment (OS = 25 months), and F-refractory CLL (OS = 38 months) were unlikely to respond to FCR (CR rate, 0%-7%).8 Data on patient cohorts failing FCR early (ie, within 24-36 months) are currently emerging. Results from MDACC7 and Keating et al (M.K., written communication, November 2010) as well as from the CLL8 trial (FC vs FCR) suggest that OS is very poor for patients who need early retreatment after first-line therapy in this setting.48
In the MDACC trial, which established FCR as the treatment with the highest efficacy in CLL,6,7 patients needing re-treatment within 36 months had a short OS (< 24 months from time point of subsequent treatment) compared with patients re-treated later than 36 months (P < .01). Outcome and response were compared after diverse salvage treatments. In an analysis of 2010, 114 of 300 patients (treated with FCR in first line) had been re-treated and 55% responded. An important predictor of response to second-line treatment was response after first-line FCR: Whereas patients with PR or no response to first-line FCR only responded in 30% to 33%, patients with initial CR/nodular PR responded in 68% to 78%.
In an analysis of all patients in the CLL8 trial (both FC and FCR arms), patients with refractory CLL, patients with a remission duration of 6 to 12 months (n = 42), and patients with a remission duration of 12 to 24 months (n = 63) showed short OS from the time point of second-line treatment, which was almost identical to that of patients with refractory CLL (n = 50; OS from second-line treatment: 24.7, 24.9, and 21.9 months, respectively).14,17
Although based on exploratory analysis, these data suggest that, taking a conservative approach, patients with remission duration less than 24 months may be considered “highest-risk” at the time of recurring treatment indication.17 The data also suggest that, although surrogate endpoints will remain important in first-line treatment trials, it will also be of key importance to assess response and outcome with subsequent treatment lines and the overall impact on OS from the time of treatment and from the time of diagnosis. It is important to stress that treatment indication in all disease phases should be based on the presence of “active disease” as defined by current guidelines.1 In clinical practice as well as in the interpretation of data from clinical trial cohorts at relapse and beyond first-line treatment, rigorous adherence to the response criteria and criteria used to reinitiate therapy is crucial.
In an update of the long-term results of FCR, the analysis of response to treatment suggested that, within the group of patients with PR, different subgroups could be distinguished based on the reasons for inclusion in this response group (nodular PR, patients with PR because of residual disease, and those who met all criteria for CR except for incomplete recovery of blood counts).7 Patients in PR because of persistent cytopenia (referred to as PR-i in the publication) experienced longer time to disease progression than patients in PR because of persistent disease. The outcome of this latter group of patients was poor (6-year OS 42% after first-line FCR). Therefore, although separation of patients in PR into several remission categories by further disease status is not a validated endpoint under the current guidelines, it may improve current risk models.
In the light of these data, current treatment decisions may be based on the length and depth of remission. In the future, this is likely to include minimal residual disease (MRD) assessment.32,33 In an analysis from the CLL8 trial, although more profound reductions of MRD were observed in the FCR arm, patients who attained low level MRD by FC chemotherapy had very similar PFS as patients who achieved the same low CLL cell levels using FCR.34,35 The superiority of the more active FCR regimen over FC was reflected by a greater chance to achieve this low-level (< 10−4) disease. At the same time, these data and prior studies suggest that early risk stratification will be possible based on MRD in the near future.32,35
It is important to stress that similar outcome might be expected in patients treated with comparable regimens using alternative chemotherapy components, such as pentostatin and cyclophosphamide, mitoxantrone, and bendamustine (eg, pentostatin, cyclophosphamide, rituximab [PCR], fludarabine, cyclophosphamide, mitoxantrone, rituximab [FCM-R], fludarabine, rituximab [FR], and bendamustine combined with rituximab [BR]), even if the data are currently not available.36-38 In the absence of respective analysis from different treatment regimens, one can only assume that overall response rate of a particular regimen will correlate with its selective pressure on the tumor clone, the ability to be salvaged by subsequent treatment, and therefore outcome after next therapy.
In the design of future trials, it will be prudent to distinguish individual response and remission duration subgroups (refractory, early relapse, late relapse) after intense therapy and separate these away from failure to respond and early relapses after nonintense treatment.
Molecular mechanisms underlying refractory CLL
It is desirable to have precise molecular markers and comprehensive biologic understanding of refractory CLL as well as in the additional risk categories in CLL. Well-defined lesions, ideally defining clear categories, could make any discussion about how to best define CLL risk groups superfluous. Unfortunately, these molecular markers have not been fully defined.
Although it is intuitive to consider nonresponders or patients progressing on treatment separately from those with early (eg, within 6 months of last treatment) relapse, this is not generally done in the studies assessing the outcome or biology of patients with refractory CLL. In a recent analysis of the CLL8 trial, most of the patients who fell in the refractory cohort had failed treatment because of the lack of CR/PR. In addition to the fact that refractory CLL is quite rare in first-line treatment situations, the potential heterogeneity within the group may hold up advances in understanding their biology. In general, the incidence of TP53 mutation/loss is strongly associated with refractory CLL and is highest in this subgroup.4,10,39-45 Whereas the incidence of TP53 mutation or 17p deletion ranges from 8% to 12% in first-line treatment situation,4,46 it increases to an incidence of approximately 50% in patients with refractory disease (after intense therapy, such as FCR).4,14 Poor outcome of patients with 17p deletion has been known for decades (single-center experience) and has been confirmed in all prospective trials.5,9,39,42,47-50 Patients with these aberrations are beginning to be channeled into separate (genotype specific) treatment trials in CLL.
The observation that 17p deletion and TP53 mutation are found only in approximately 50% of FCR-refractory patients raises questions regarding the biologic basis of refractoriness in the remaining cases. So far, there is some evidence that additional cases are associated with p53 pathway defects, but it is also clear that not all refractory cases have a defect in the DNA damage response.44,51 Lesions in the ATM gene are not as clearly associated with refractory CLL. The recently discovered of mutations in SF3B1, NOTCH1, and BIRC3 appear to be associated with refractory CLL.52-56
A subgroup of patients with 17p deletion, who present with early-stage disease without classic indication for treatment and mutated IGHV, may exhibit stable disease for a prolonged period of time.48,57 These patients should not be treated “prematurely” (before classic criteria of active disease are met). However, once treatment is required, the overwhelming majority will have a dismal outcome, irrespective of the IGHV status.
Nonetheless, even in the clearest scenario of genetic lesions causing refractory CLL, overlap remains and models will be far from perfect. Cases with refractory CLL may not exhibit genetic changes; and conversely, cases with TP53 mutation/deletion may have a somewhat variable response in a small subgroup. Part of this phenomenon may be explained by the normal heterogeneity in the phenotype of genetic lesions.
Along these lines of difficulty to categorically assign patients to risk groups because of biologic variables, it is also important to reconsider that the standardization and categorization of response was designed based on clinical trial necessity and not on genetic or biologic principles. In the future, one would like to have quantitative variables for response assessment used in addition to the clinically indispensible response criteria. In many ways, MRD assessment and novel imaging techniques may be particularly suited for such approaches of closely monitoring tumor load and response. We would predict that the biologic basis for inferior response may correlate much more closely to these quantifiable measures of disease control.
Future strategy
Bringing new drugs into the clinical arena is a formidable task in the light of very effective combination therapies for the majority of patients. Examples of large randomized trials failing despite encouraging mechanism of action and concepts are relatively common.58 The practical challenges are inherent to the very high response rate and long remission duration in the majority of patients in the first-line setting.5,6 To advance the field, it will be crucial to build stronger models of CLL subgroups. In these models, it will be important to consider genetic risk (TP53 mutation and 17p deletion) alongside clearer clinical subgroups based on suboptimal response. With our increasing understanding of genetic lesions in CLL (eg, NOTCH1, ATM, TP53, SF3B1, BIRC3 mutation), it will be crucial to exploit these mutations as therapeutic targets and novel biologic risk markers.56,59 This will lead to the discovery of predictive factors and genotype-specific approaches.
CLL may be an ideal disease where pretreatment (eg, genomic aberrations, TP53 mutation, IGHV status, stage, comorbidity) and after treatment factors (MRD level, response depth and duration) could be integrated into novel models. These models can be foreseen to be used in clinical decision making as well as adaptive trial design. This would be one way to improve speed of translation of new drugs into clinical trials and routine patient care. To develop such models, it will be crucial to closely monitor patients for many years. This monitoring will need to include new lines of treatment as well as competing risks for death.
For current clinical use, key questions remain as the indication of allogeneic SCT along with optimal salvage treatment. For the time being and in the absence of randomized trials assessing the use of allogeneic SCT in CLL, the current guidelines can be fully endorsed based on recent results of allogeneic SCT in CLL.12,27,60,61 The question of optimal salvage regimens remains complicated, but early analysis as done by the MDACC group will help to define new trial questions in this area.8 Moreover, a wealth of novel treatment options targeting specific biologic disease characteristics of CLL are currently in early clinical development and may entirely change our approach to therapy in the near future.
In conclusion, treatment of patients with CLL today faces several challenges. On one end of the spectrum, there is a small but challenging group of patients with poor response to FCR, which can be defined as a highest-risk group based on genetic and clinical criteria. This group will be of particular importance in reconsideration of the current definitions of refractory CLL. The fact that the majority of patients respond very favorably poses problems at the design of trials aiming to improve these standards. To achieve this, a separate risk category (high-risk) distinct from the highest risk group, may prove important to allow distinction of effects of novel agents used with standard therapy (ie, FCR). Based on the current genetic models, this group may include patients with unmutated IGHV, 11q deletion or high β2-M, with none of the lesions defining highest risk. In addition, future models should integrate quantifiable response measures, such as MRD levels, with more advanced profiling of genetic lesions in CLL. We hope that this Perspective serves as an initiative toward refined risk models.
Acknowledgments
This work was supported by the CLL Global Research Foundation, the Helmholtz Society, the Harald Huppert Stiftung, (DFG STI296 4-1), the Deutsche Jose Carreras Leukämiestiftung (R08/26f), Virtual Helmholtz Institute (VH-VI-404), and the Deutsche Krebshilfe (109674).
Authorship
Contribution: T.Z. designed the study, analyzed data, and wrote the paper; J.G.G. edited and wrote the paper; M.H. contributed data and edited the paper; M.J.K. contributed data, analyzed data, and edited the paper; H.D. analyzed data and edited the paper; and S.S. analyzed data and edited and wrote the paper.
Conflict-of-interest disclosure: T.Z. has honoraria from GSK, Boehringer-Ingelheim, and Roche. J.G.G. has honoraria from Roche/Genentech, Celgene, Mundipharma and GSK. S.S. has research support and honoraria from Amgen, Bayer, Boehringer-Ingelheim, Celgene, Genmab, Genzyme, GSK, Mundipharma, Roche, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Thorsten Zenz, Department of Translational Oncology, National Center for Tumor Diseases, and German Cancer Research Center, Im Neuenheimer Feld 460, 69120 Heidelberg, Germany; e-mail: thorsten.zenz@nct-heidelberg.de; or Stephan Stilgenbauer, Department of Internal Medicine III, University Hospital of Ulm, Albert Einstein Allee 23, 89081 Ulm, Germany; e-mail: stephan.stilgenbauer@uniklinik-ulm.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal