Abstract
Because single nucleotide polymorphisms (SNPs) in platelet endothelial aggregation receptor 1 (PEAR1) are associated with differential functional platelet responses in healthy subjects, we studied the function of PEAR1 in human platelets. During platelet aggregation by various agonists, the membrane expression of PEAR1 and its tyrosine phosphorylation increased. The recombinant PEAR1 EMI domain (GST-EMI) competitively reduced platelet adhesion to surface-coated PEAR1, diminished platelet aggregation, and eliminated PEAR1 phosphorylation. Polyclonal antibodies against the extracellular PEAR1 domain triggered PEAR1 phosphorylation in a src family kinase (SFK)–dependent manner. Such resulted in downstream signaling, culminating in extensive platelet degranulation and irreversible aggregation reactions interrupted by excess monovalent anti–GST-EMI F(ab) fragments. In resting platelets, the cytoplasmic tail of PEAR1 was found complexed to c-Src and Fyn, but on its phosphorylation, phospho-PEAR1 recruited p85 PI3K, resulting in persistent activation of PI3K and Akt. Thus, αIIbβ3 activation was amplified, hence stabilizing platelet aggregates, a signaling cascade fully interrupted by the SFK inhibitor PP1 and the PI3K inhibitor LY294002. This study is the first demonstration of a functional role for PEAR1 in platelet activation, underpinning the observed association between PEAR1 and platelet function in genome-wide association studies.
Introduction
PEAR1 (platelet endothelial aggregation receptor-1; also known as MEGF12 or JEDI) is a transmembrane protein of the multiple epidermal growth factor (EGF)–like domain protein family, first described in 2005.1 PEAR1 is mainly expressed in platelets and endothelial cells, as well as satellite glial cell precursors, where it is necessary for apoptotic neuron clearance in the embryonic dorsal root ganglia via an engulfment-dependent activity.2 PEAR1 is composed of an extracellular EMI domain (protein-protein interaction domain), 15 extracellular EGF-like repeats, and multiple cytoplasmic tyrosines and prolines.1 The intracellular domain structure contains 5 proline-rich domains and an NPXY922 motif, which may serve as a phosphotyrosine binding site. During platelet aggregation, PEAR1 is phosphorylated at Tyr-925 and Ser-953/1029, on its oligomerization, in an αIIbβ3-dependent manner; correspondingly, the inhibition of platelet aggregation abrogates PEAR1 phosphorylation.1 PEAR1 phosphorylation can also be triggered during physical platelet approximation via centrifugation, independently of αIIbβ3. Thus, PEAR1 is believed to be a platelet-platelet contact receptor.1
Platelet responses to different agonists are highly variable within healthy donors.3,4 The wide range of responses to standard platelet agonists correlate with quantitative trait loci (QTL) associated with functional control of platelet function and are identified as single nucleotide polymorphisms (SNPs) in genes that encode platelet proteins.3,4 Numerous SNPs point toward proteins with previously unknown function in platelets.5 A few studies have linked SNPs in PEAR1 with increased or decreased platelet responses to various agonists.3,5-8 A PEAR1 promoter-region variant (rs2768759) was associated with increased platelet aggregation, most strongly in response to epinephrine.7 The enhanced expression of PEAR1 would be an important cause of platelet hyperreactivity7 and genetic variation within PEAR1, particularly rs41299597 may lead to increased membrane expression of PEAR1 in activated platelets and elevated responsiveness to GPVI ligands.3 In addition, a variant in intron 1 of the PEAR1 gene (rs12041331) is associated with increased PEAR1 protein expression and platelet aggregation triggered by multiple agonists and potentially represents a functional variant.8 In contrast, genome-wide meta-analyses linked the minor allele of the PEAR1 SNP rs12566888 to a drop in the aggregation response to ADP (adenosine 5′-diphosphate) and epinephrine in a European and African ancestry sample.5
Although these genetic studies point toward an important regulatory role for PEAR1 in human platelet activation, its exact function and intracellular signaling pathway still remain completely unknown. In this study, we investigated the fate of PEAR1 during platelet activation. Via a functional recombinant PEAR1 EMI domain, the importance of PEAR1 in platelet aggregation by various agonists has been elucidated. Furthermore, the use of anti-PEAR1 and anti-PEAR1 EMI domain antibodies as pseudo-ligands has largely clarified the underlying signal transduction coupled to PEAR1 phosphorylation. We report how PEAR1 plays a role in platelet activation and how its engagement sustains the activation of αIIbβ3 in aggregating platelets.
Methods
Reagents
Apyrase, dimethylsulfoxide (DMSO), thrombin receptor agonist peptide-6 (TRAP-6), and protease-activated receptor-4 activating peptide (PAR4-AP) were from Sigma-Aldrich; prostaglandin E1 came from Pfizer; aspirin was from Sanofi-Aventis, and eptifibatide from GSK (Integrilin). LY294002 was purchased from Calbiochem and PP1 from Enzo Life Sciences. Bovine thrombin was from Siemens and BSA albuMAX from Gibco. The monoclonal antibody IV.3 producing hybridoma HB-217 was from the ATCC and the monoclonal FITC–PAC-1, PE-CD62-P antibodies from BD Bioscience. 4G10 platinum and 4G10-HRP were from Millipore. A rabbit monoclonal antibody against the phosphorylated form of Akt (pSer473) and antibodies against total Akt, β3 integrin, p85 phosphatidylinositol 3-kinase (PI3K), Fyn and Syk were from Cell Signaling Technology. Polyclonal antibodies against Lyn were from Santa Cruz and antibodies against c-Src, phosphorylated form of c-Src (pTyr529, inhibition loop), Src family kinase (SFK) activation loop (pTyr418), rhodamine phalloidin, and Alexa Fluor 488/647 donkey anti–goat/anti–rabbit immunoglobulin (Ig) antibodies were from Invitrogen. Polyclonal antibodies against the phosphorylated form of Fyn (pTyr530, inhibition loop) were from Abcam. Enhanced chemiluminescence (ECL) and Rap1 detection kits came from Pierce. Horseradish peroxidase-conjugated secondary antibodies, rabbit FITC-polyclonal anti–goat antibodies and rabbit anti–human von Willebrand factor (VWF) antibodies were from Dako. A polyclonal antibody against the extracellular domain of PEAR1 (referred to as PEAR1-EC Ab) and a recombinant PEAR1 extracellular domain protein (PEAR1-EC) were purchased from R&D Systems. Polyclonal anti-PEAR1 antibodies against the intracellular domain were raised in our laboratory in rabbits, injected with a recombinant fusion protein between glutathion-S-transferase and a fragment encoding residue V769 to R1037 of PEAR1, made on inserting a polymerase chain reaction (PCR) fragment in the pGEX 4T-2 vector (GE Healthcare) and expression in the Escherichia coli strain BL21. This antibody is referred to as PEAR1-IC Ab.
PEAR1 EMI domain production
A Glutathion-S-transferase fusion protein was generated, GST-EMI, by subcloning the PEAR1 EMI domain (residues N22-P103) into the pGEX vector. The resulting construct was transformed into the E coli strain BL21. Recombinant fusion proteins were purified using glutathione sepharose beads as previously described.9 Polyclonal anti-PEAR1 EMI domain antibodies were raised in our laboratory in rabbits, immunized with GST-EMI, referred to as EMI Ab. Treatment of these antibodies with papain yielded Fab fragments, isolated via protein A chromatography and referred to as EMI F(ab).10
Platelet aggregation
Venous blood was collected from healthy donors. Permission was given by the ethical committee of the Leuven University Hospital to use blood from healthy individuals for further analysis. Platelet-rich plasma (PRP) was prepared by centrifugation for 15 minutes at 150 × g. Platelets were pelleted at 800g for 15 minutes in the presence of 1 U/mL apyrase and 1μM prostaglandin E (PGE)1 and washed twice in platelet-washing buffer (36mM citric acid, 5mM glucose, 5mM KCl, 1mM MgCl2, 103mM NaCl, pH 6.5). Washed platelets were resuspended in Tyrode buffer (137mM NaCl, 12mM NaHCO3, 2mM KCl, 0.34mM Na2HPO4, 1mM MgCl2, 2mM CaCl2, 5.5mM glucose, and 5mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid], pH 7.4).
Platelet aggregation was monitored by measuring light transmission through the stirred suspension of washed platelets at 37°C with a Chronolog dual-beam aggregometer. In some experiments, platelets (4 × 105/μL) were preincubated with antibody IV.3 (10 μg/mL), in the absence or presence of GST-EMI (0-6 μg/mL), the PI3K inhibitor LY294002 (5 minutes, 50μM), the SFK inhibitor PP1 (10 minutes, 10μM) or dimethylsulfoxide (DMSO), the ADP-degrading enzyme apyrase (0.4 U/mL), aspirin (550μM), or the αIIbβ3 antagonist eptifibatide (10-20 μg/mL), at 37°C. The DMSO concentration never exceeded 0.2% (vol/vol). Platelet aggregation was triggered by various agonists or by PEAR1-EC Ab (azide free, reconstituted with sterile PBS) and measured and expressed as the percentage of change in light transmission, with the value for the blank sample (buffer without platelet) set at 100%. All experiments using PEAR1-EC Ab as an agonist were done in the presence of IV.3 (10 μg/mL).
Static platelet deposition
Platelet adhesion to PEAR1 was investigated in a microtiter plate adhesion assay as described.11 A washed platelet suspension (105/μL) was stimulated or not with TRAP-6 (50μM) and immediately plated (50 μL) at 37°C for 15, 30, and 45 minutes in wells precoated with PEAR1-EC (50 μg/mL) or with fibrinogen (200 μg/mL). Adhesion was studied in the presence of GST-EMI (0-7 μg/mL) and/or eptifibatide (0-10 μg/mL). Nonspecific adhesion was determined in wells precoated with 0.5% BSA in PBS. Platelet deposition was quantified by an acid phosphatase assay.11
Platelet analysis via flow cytometry
Washed platelets (105/μL), stimulated with a range of agonists, were incubated with appropriate fluorophore-conjugated antibodies for 30 minutes at room temperature. To measure PEAR1 expression, nonactivated or activated platelets (30 minutes) were fixed with 1% paraformaldehyde for 15 minutes. Platelets were then washed with platelet washing buffer and incubated with 2.5 μg/mL PEAR1-EC Ab for 20 minutes in Tyrode buffer, and washed and incubated with secondary FITC-rabbit anti–goat antibodies for 20 minutes. The reaction was stopped by adding 2% paraformaldehyde, and samples were analyzed with a FACSCantoII flow cytometer (BD Bioscience).
Western blotting
Platelets were lysed with ice-cold Triton lysis buffer (10mM Tris-HCl pH 8, 125mM NaCl, 2 mM EDTA, 1% Triton X-100, 10mM NaF, 2mM Na3VO4, protease inhibitor cocktail). For some experiments, platelets were lysed in SDS denaturing buffer (10 mM Tris-HCl pH 8, 125mM NaCl, 2mM EDTA, 1% sodium dodecyl sulfate (SDS), 10mM NaF, 2mM Na3VO4, protease inhibitor cocktail). Proteins were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose. Membranes were incubated with various primary antibodies, that is, PEAR1-EC Ab (1/1000), PEAR1-IC Ab (2 μg/mL), anti–Akt-P (1/500), anti-Akt (1/1000), anti-β3 (1/1000), anti-SFK activation loop (1/1000), anti-Fyn (1/1000), anti–Fyn-P inhibition loop (1/1000), anti-Lyn (1/1000), anti-Syk (1/1000), anti–c-Src (1/1000), anti–c-Src-P inhibition loop (1/1000), antiphosphoprotein (P-Tyr) 4G10 platinum (1/1000), and 4G10-HRP (1/500). After adding horseradish peroxidase–conjugated secondary antibodies, immunoreactive bands were visualized by ECL (Amersham Biosciences).
PEAR1 phosphorylation on Tyr residues, referred to as PEAR1-P, was evaluated after immunoprecipitation with PEAR1-EC Ab and detection of P-Tyr by 4G10 platinum; membranes were stripped and reprobed for total PEAR1.
Rap1b activation
Washed platelets (4 × 105/μL) were stimulated with TRAP-6 (10μM) in the presence or the absence of GST-EMI, lysed with 4× buffer (100mM Tris-HCl pH 7.2, 600mM NaCl, 20mM MgCl2, 4% NP-40, 20% glycerol with protease inhibitor cocktail); 30 μL of the samples of the cell lysate were saved as a Rap1b control. Then, samples were incubated for 1 hour with glutathione S-transferase (GST)–RalGDS-RBD (20 μg/mL) and 50 μL glutathione-sepharose at 4°C while rotating. The beads were then washed 3 times with lysis buffer 1x. Guanosine 5′-triphosphate (GTP)–bound Rap1 was eluted with SDS sample buffer, separated by 12% SDS-PAGE, and immunoblotted with anti-Rap1 antibody (1/1000).
Confocal immunofluorescence microscopy
Coverslips were coated with fibrinogen (100 μg/mL) at room temperature for 2 hours. After washing twice with PBS, coverslips were blocked with BSA (5 mg/mL) for 1 hour, and washed twice with PBS. Washed platelets were deposited for 30 minutes at 37°C, and then fixed with 4% paraformaldehyde in cytoskeleton buffer pH 6.9 (0.1M PIPES, 2M glycerol, 1mM EGTA [ethylene glyco-bis(b-aminoethyl ester)-N,N,N′,N′-tetraacetic acid], 1mM MgCl2) for 15 minutes. Platelets were permeabilized in cytoskeleton buffer, containing 0.2% Triton X-100 for 5 minutes and incubated in PBS containing either 10% serum or BSA 0.5%, for 1 hour. Filamentous actin was visualized by incubation with rhodamin-labeled phalloidin. Platelets were incubated overnight at 4°C with primary antibodies, and with the appropriate Alexa 488– or 647–labeled secondary antibody for 45 minutes at 37°C. Coverslips were mounted and sealed on glass slides and analyzed with a Zeiss CLSM510 confocal microscope and Imager LSM510 Version 3.2 SP2 software.
Results
PEAR1 is expressed in human platelets on the membrane and in α-granules
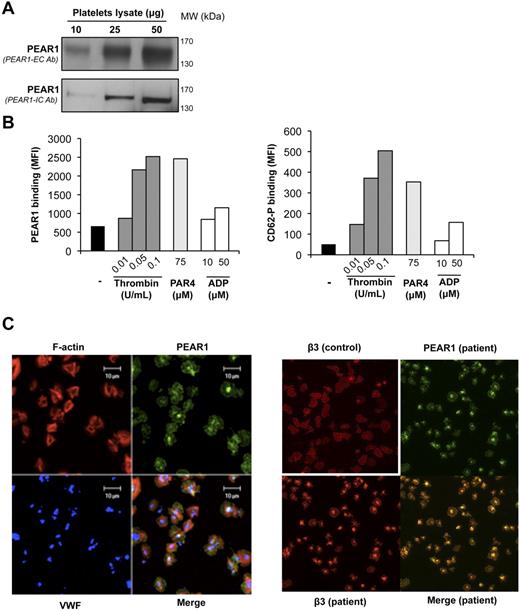
A homogeneous protein band around Mr 150 000 is found in platelet lysates, during Western blotting with antibodies against the extracellular (PEAR1-EC) and intracellular (PEAR1-IC) domain, respectively (Figure 1A). When analyzed in flow cytometry, PEAR1-EC Ab detected PEAR1 on resting platelets, but its expression was raised 4- to 5-fold on platelet stimulation with thrombin or PAR4-AP, paralleling agonist-induced CD62-P expression (Figure 1B). In contrast, ADP, triggering platelet secretion weakly,12 induced faint PEAR1 and CD62-P elevation in activated platelets (Figure 1B). Confocal immunofluorescence microscopy of fibrinogen-spread platelets further revealed PEAR1 expression on the platelet membrane and in the platelet center, compatible with its location in granules (Figure 1C). Costaining of PEAR1 and VWF, a marker of α-granules, demonstrated partial coincidence of PEAR1 and VWF in α-granules (Figure 1C). Costaining and merging for PEAR1 and β3 integrin (another marker of α-granules and the outer membrane) in normal control and in uncharacterized-secretion defective patient platelets (Figure 1C) confirmed that both proteins coincide in granules and in the platelet membrane. Incubation of PEAR1-EC Ab with an excess of recombinant PEAR1, before staining abolished PEAR1 detection in cytometry and in immunofluorescence microscopy (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, PEAR1, already present in the resting platelet membrane, can readily be up-regulated in the membrane on platelet degranulation.
PEAR1 in the platelet membrane and in α-granules. (A) Western blot of lysate from resting platelets, revealing PEAR1 by antibodies against the extracellular (PEAR1-EC Ab, upper lanes) and intracellular (PEAR1-IC Ab, lower lanes) domains, for the indicated amounts of loaded protein. (B) Flow cytometric measurement as mean fluorescence intensity (MFI) of PEAR1 and P-selectin (CD62-P) on the membrane of resting washed platelets and platelets stimulated with thrombin, PAR4-activating peptide (PAR4) and ADP, at the indicated concentrations. Data shown represent one experiment, representative of at least 3 analyses. (C) Confocal immunofluorescence microscopy of human platelets spread over a fibrinogen matrix; left panel: stained with rhodamin-phalloidin (F-actin, red), and labeled for PEAR1 (green), and VWF (blue) and for the colocalization of PEAR1/VWF (cyan), PEAR1/actin (yellow), and PEAR1/VWF/actin (white). Bar, 10 μm. Original magnification ×630; right panel: human platelets (control and uncharacterized-secretion defective patient platelets) spread over a fibrinogen matrix, labeled for β3 integrin (red) and for PEAR1 (green). Original magnification ×400.
PEAR1 in the platelet membrane and in α-granules. (A) Western blot of lysate from resting platelets, revealing PEAR1 by antibodies against the extracellular (PEAR1-EC Ab, upper lanes) and intracellular (PEAR1-IC Ab, lower lanes) domains, for the indicated amounts of loaded protein. (B) Flow cytometric measurement as mean fluorescence intensity (MFI) of PEAR1 and P-selectin (CD62-P) on the membrane of resting washed platelets and platelets stimulated with thrombin, PAR4-activating peptide (PAR4) and ADP, at the indicated concentrations. Data shown represent one experiment, representative of at least 3 analyses. (C) Confocal immunofluorescence microscopy of human platelets spread over a fibrinogen matrix; left panel: stained with rhodamin-phalloidin (F-actin, red), and labeled for PEAR1 (green), and VWF (blue) and for the colocalization of PEAR1/VWF (cyan), PEAR1/actin (yellow), and PEAR1/VWF/actin (white). Bar, 10 μm. Original magnification ×630; right panel: human platelets (control and uncharacterized-secretion defective patient platelets) spread over a fibrinogen matrix, labeled for β3 integrin (red) and for PEAR1 (green). Original magnification ×400.
PEAR1 binds to activated platelets through its EMI domain independently of αIIbβ3
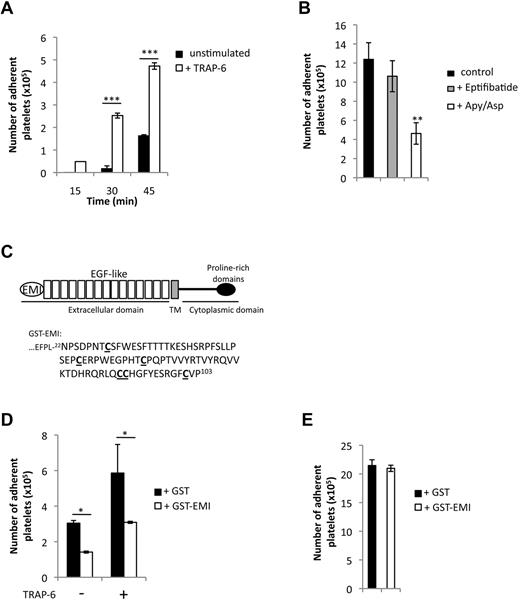
Because PEAR1 is a “platelet contact” receptor,1 platelet adhesion to the coated PEAR1-EC protein was investigated, at platelet numbers where their aggregation did not occur (108/mL).13 Activation by TRAP-6 induced enhanced platelet adhesion at all time-points investigated, that is from 1.6 × 105 to 4.7 × 105 platelets at 45 minutes (Figure 2A). We then investigated the role of secondary platelet amplification, including αIIbβ3 mediated outside-in signaling, by activating platelets in the presence of apyrase (0.4 U/mL) plus aspirin (550μM) or eptifibatide (10 μg/mL). Platelet adhesion depended on amplification pathways (ADP/ATP, TXA2) necessary to platelet secretion but did not depend on αIIbβ3, excluding outside-in signaling in the adhesive process (Figure 2B). Figure 2C shows the structural organization of PEAR1 and shows in detail how the EMI domain was fused to GST. GST-EMI partially inhibited the adhesion of platelets to PEAR1-EC (Figure 2D), suggestive of competition between PEAR1-EC and GST-EMI for a platelet surface ligand, itself enriched in activated platelets. In contrast, platelet adhesion on coated fibrinogen was not affected by GST-EMI, in the same conditions (Figure 2E). These findings explicit that interactions between PEAR1 and platelets depend on platelet activation and that the PEAR1 EMI domain is the main functional domain responsible for this binding, further excluding that PEAR1 would interact with (activated) αIIbβ3 directly.
Platelet adhesion to PEAR1 extracellular domain. (A,B,D) Washed human platelets were plated by incubation for the indicated times at 37°C in wells precoated with PEAR1 extracellular domain (50 μg/mL) or (E) fibrinogen (200 μg/mL). (A) Kinetics of the adhesion of platelets stimulated or not with TRAP-6 (50μM). B) Role of αIIbβ3 and amplification pathways (ADP/ATP, TXA2) during platelet adhesion to PEAR1 for platelets activated with TRAP-6 (50μM). Platelets were either treated with apyrase (0.4 U/mL) and aspirin (550μM) before TRAP-6 stimulation or with eptifibatide (10 μg/mL) after TRAP-6 stimulation. Platelet adhesion was measured at 60 minutes. (C) Schematic representation of the modular architecture of PEAR1. Human PEAR1 is composed of an extracellular domain (comprising 1 EMI domain and 15 EGF-like domains), a transmembrane domain (TM, gray box) and a cytoplasmic domain (comprising 5 proline-rich domains). The sequence of the EMI domain N22-P103 is fused to GST (GST-EMI). (D) Platelet adhesion at 60 minutes in the presence or the absence of either GST or GST-EMI (6 μg/mL), with or without platelet activation by TRAP-6 (50μM). (E) Effect of GST-EMI (6 μg/mL) on platelet adhesion to fibrinogen after TRAP-6 stimulation (t test: *P < .05; **P < .01; ***P < .001).
Platelet adhesion to PEAR1 extracellular domain. (A,B,D) Washed human platelets were plated by incubation for the indicated times at 37°C in wells precoated with PEAR1 extracellular domain (50 μg/mL) or (E) fibrinogen (200 μg/mL). (A) Kinetics of the adhesion of platelets stimulated or not with TRAP-6 (50μM). B) Role of αIIbβ3 and amplification pathways (ADP/ATP, TXA2) during platelet adhesion to PEAR1 for platelets activated with TRAP-6 (50μM). Platelets were either treated with apyrase (0.4 U/mL) and aspirin (550μM) before TRAP-6 stimulation or with eptifibatide (10 μg/mL) after TRAP-6 stimulation. Platelet adhesion was measured at 60 minutes. (C) Schematic representation of the modular architecture of PEAR1. Human PEAR1 is composed of an extracellular domain (comprising 1 EMI domain and 15 EGF-like domains), a transmembrane domain (TM, gray box) and a cytoplasmic domain (comprising 5 proline-rich domains). The sequence of the EMI domain N22-P103 is fused to GST (GST-EMI). (D) Platelet adhesion at 60 minutes in the presence or the absence of either GST or GST-EMI (6 μg/mL), with or without platelet activation by TRAP-6 (50μM). (E) Effect of GST-EMI (6 μg/mL) on platelet adhesion to fibrinogen after TRAP-6 stimulation (t test: *P < .05; **P < .01; ***P < .001).
PEAR1 and its EMI domain in platelet aggregation
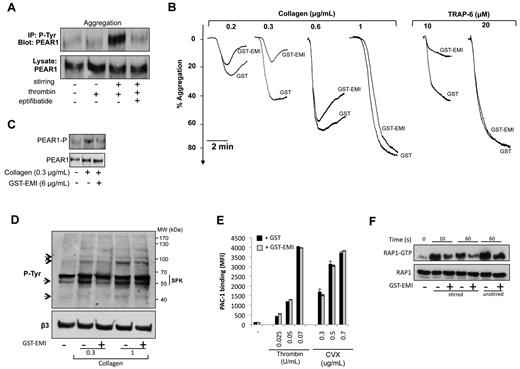
PEAR1 phosphorylation was studied in unstirred and stirred platelets. Despite platelet activation (Figure 1B), thrombin (0.1 U/mL) did not trigger PEAR1 phosphorylation in unstirred platelets (Figure 3A). During platelet aggregation it induced strong tyrosine phosphorylation, as soon as 30 seconds after aggregation induction. Eptifibatide abrogated PEAR1 phosphorylation, in agreement with previous findings1 (Figure 3A). These observations can be explained by the lack of interaction between PEAR1 and its putative ligand present on activated platelets, when these are unstirred (Figure 2) or αIIbβ3-antagonized. At low concentrations of collagen and TRAP-6, platelet aggregation (Figure 3B) and PEAR1 Tyr phosphorylation (Figure 3C) were impaired by GST-EMI, compared with GST. At increasing collagen and TRAP-6 concentrations, the inhibitory effect of GST-EMI was gradually lost, that is contributions of PEAR1 to platelet aggregation were gradually overcome at higher agonist concentrations. The corresponding total platelet tyrosine phosphorylation profiles showed that 4 phosphobands at ≈ 100, 90, 60, and 45 kDa were affected by GST-EMI, during collagen-induced aggregations (Figure 3D). In the absence of stirring, that is in the absence of a role for PEAR1 in signaling, GST-EMI has no impact on αIIbβ3 activation, measured in flow cytometry by PAC-1 binding, for thrombin and convulxin-activated platelets, in further support of the specificity of GST-EMI (Figure 3E).
The EMI domain of PEAR1 in platelet aggregation and signaling. (A) PEAR1 phosphorylation during platelet activation by thrombin (0.1 U/mL, 30s) in the absence or presence of eptifibatide (20 μg/mL), after immunoprecipitation of PEAR1-P with the 4G10 platinum antiphosphotyrosine antibody and Western blotting for PEAR1. (B) Washed platelet aggregation by collagen and TRAP-6 in the presence of GST or GST-EMI (6 μg/mL). Aggregation traces are representative of at least 3 independent experiments. (C) PEAR1 phosphorylation in platelets aggregated with collagen after 1 minute, in the presence of GST or GST-EMI. (D) Tyr-phosphorylation of washed human platelets, activated with collagen in the presence of GST (−) or GST-EMI (+), 5 minutes after start of the aggregation. (E) Flow cytometric measurement as MFI of activated αIIbβ3 (PAC-1) on activation of washed unstirred platelets with thrombin and convulxin (CVX) in the presence of GST or GST-EMI. (F) Rap1-GTP after TRAP-6 stimulation of platelets (10 or 60 seconds), with or without stirring, as indicated, in the presence of GST (−) or GST-EMI (+).
The EMI domain of PEAR1 in platelet aggregation and signaling. (A) PEAR1 phosphorylation during platelet activation by thrombin (0.1 U/mL, 30s) in the absence or presence of eptifibatide (20 μg/mL), after immunoprecipitation of PEAR1-P with the 4G10 platinum antiphosphotyrosine antibody and Western blotting for PEAR1. (B) Washed platelet aggregation by collagen and TRAP-6 in the presence of GST or GST-EMI (6 μg/mL). Aggregation traces are representative of at least 3 independent experiments. (C) PEAR1 phosphorylation in platelets aggregated with collagen after 1 minute, in the presence of GST or GST-EMI. (D) Tyr-phosphorylation of washed human platelets, activated with collagen in the presence of GST (−) or GST-EMI (+), 5 minutes after start of the aggregation. (E) Flow cytometric measurement as MFI of activated αIIbβ3 (PAC-1) on activation of washed unstirred platelets with thrombin and convulxin (CVX) in the presence of GST or GST-EMI. (F) Rap1-GTP after TRAP-6 stimulation of platelets (10 or 60 seconds), with or without stirring, as indicated, in the presence of GST (−) or GST-EMI (+).
In aggregating platelets, the Ras-related protein Rap1 is involved in αIIbβ3 activation.14 Platelet agonists increase GTP-bound Rap1b either by turning on (or recruiting) a guanine nucleotide exchange factor (GEF) or by inhibiting a Rap1 GTPase-activating protein (GAP). We selected this system to measure how GST-EMI would affect PEAR1-dependent signaling during platelet aggregation and to demonstrate that PEAR1 plays a functional role in platelet aggregation via controlling αIIbβ3 activation. Figure 3F shows that during TRAP-6–mediated platelet aggregation, GST-EMI potently inhibited the rapid TRAP-6–triggered Rap1b activation by 60%, as early as 10 seconds. In the absence of stirring (and aggregation), Rap1 activation was weakly inhibited by GST-EMI (approximately 20%). These findings demonstrated that PEAR1 contributes to platelet aggregation via intracellular signaling, leading to αIIbβ3 activation.
Molecular partners in complex with PEAR1
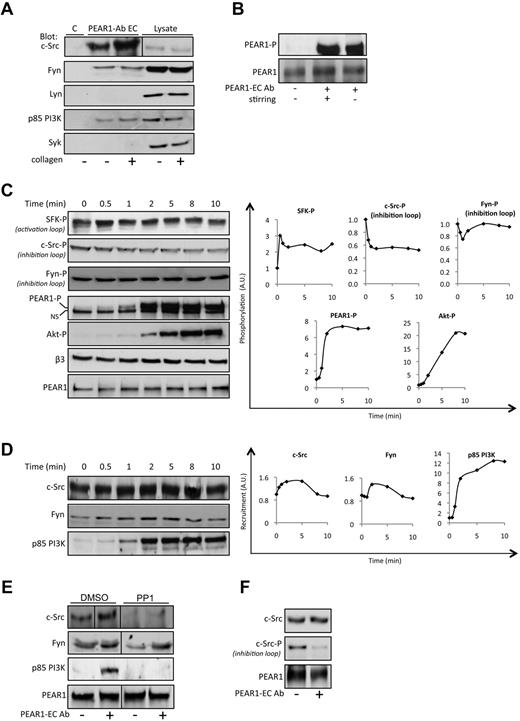
The PEAR1 cytoplasmic tail comprises 5 proline-rich regions, various tyrosines, and a putative hemi-ITAM site (supplemental Table 1), hinting toward several potential molecular interactions. Therefore, we investigated whether src family kinase proteins (SFK: c-Src, Fyn, and Lyn), p85 PI3K, and Syk are in complex with PEAR1, in resting platelets or with PEAR1-P, 2 minutes into a collagen-induced platelet aggregation, conditions leading to PEAR1 tyrosine phosphorylation (Figure 3C). Immunoprecipitation of PEAR1 revealed coprecipitation primarily of c-Src in resting, but more so in activated platelets. Furthermore, Fyn and the p85 PI3K subunit, but neither Lyn nor Syk, were found complexed to PEAR1 (Figure 4A). During platelet aggregation, structural cytoskeleton proteins and enzymes associate with membrane receptors into large insoluble complexes.15 We found that PEAR1 shifted from the Triton-soluble to the Triton-insoluble fraction, over a time interval of 5 minutes after initiation of platelet activation (supplemental Figure 2). To avoid this translocation, complicating the study of PEAR1-related signaling, we investigated whether PEAR1 phosphorylation could be brought about by PEAR1-EC Ab. As previously described,1 we found that PEAR1-EC Ab triggers PEAR1 phosphorylation equipotently in unstirred and stirred platelets (Figure 4B), independently of platelet aggregation. More importantly, when left unstirred, PEAR1 did not translocate to the cytoskeleton (supplemental Figure 2).
Molecular partners in human platelets in complex with PEAR1. (A) Western blots of c-Src, Fyn, Lyn, p85 PI3K, and Syk after coimmunoprecipitation with PEAR1. Stirred platelets were stimulated or not with collagen and lysed at 2 minutes (30% aggregation); the immunoprecipitate by PEAR1-EC Ab is shown, together with total platelet lysate, in comparison to an IgG goat control antibody (C). (B) PEAR1 phosphorylation in platelets activated with PEAR1-EC Ab (1 μg/mL) after 3 minutes, with or without stirring. (C) Western blot analysis of the kinetics of the phosphorylation status of SFK activation loop (SFK-P), c-Src, and Fyn phosphorylation in the inhibitory loop, PEAR1-P, PEAR1, Akt-P, and β3 for washed unstirred human platelets treated with apyrase (0.4 U/mL), aspirin (550μM) and eptifibatide (10 μg/mL) on platelet activation with PEAR1-EC Ab (7 μg/mL) for different times (0-10 minutes), as indicated. Right panel: quantitative imaging for the evolution of the phosphorylation status of SFK, c-Src, Fyn, PEAR1 and Akt after PEAR1-EC Ab stimulation with time (NS, nonspecific band). (D) Western blot analysis of the kinetics of recruitment of c-Src, Fyn, and p85 PI3K in the PEAR1 complex after coimmunoprecipitation with PEAR1-EC Ab. Unstirred platelets were stimulated with PEAR1-EC Ab (7 μg/mL) for different times (0-10 minutes), as indicated. Right panel: quantitative imaging for the evolution of the recruitment of c-Src, Fyn, and p85 PI3K after PEAR1-EC Ab stimulation. (E) Western blot analysis of the recruitment of c-Src, Fyn, and p85 PI3K in the PEAR1 complex after coimmunoprecipitation with PEAR1-EC Ab. Unstirred platelets were stimulated with PEAR1-EC Ab (7 μg/mL) for 5 minutes in the presence or the absence of PP1 (10μM). (F) Western blot analysis of the recruitment of c-Src and c-Src phosphorylated in the inhibitory loop in the PEAR1 complex after coimmunoprecipitation with PEAR1-EC Ab. Unstirred platelets were stimulated with PEAR1-EC Ab (7 μg/mL) for 5 minutes. Vertical lines indicate a repositioned gel lane.
Molecular partners in human platelets in complex with PEAR1. (A) Western blots of c-Src, Fyn, Lyn, p85 PI3K, and Syk after coimmunoprecipitation with PEAR1. Stirred platelets were stimulated or not with collagen and lysed at 2 minutes (30% aggregation); the immunoprecipitate by PEAR1-EC Ab is shown, together with total platelet lysate, in comparison to an IgG goat control antibody (C). (B) PEAR1 phosphorylation in platelets activated with PEAR1-EC Ab (1 μg/mL) after 3 minutes, with or without stirring. (C) Western blot analysis of the kinetics of the phosphorylation status of SFK activation loop (SFK-P), c-Src, and Fyn phosphorylation in the inhibitory loop, PEAR1-P, PEAR1, Akt-P, and β3 for washed unstirred human platelets treated with apyrase (0.4 U/mL), aspirin (550μM) and eptifibatide (10 μg/mL) on platelet activation with PEAR1-EC Ab (7 μg/mL) for different times (0-10 minutes), as indicated. Right panel: quantitative imaging for the evolution of the phosphorylation status of SFK, c-Src, Fyn, PEAR1 and Akt after PEAR1-EC Ab stimulation with time (NS, nonspecific band). (D) Western blot analysis of the kinetics of recruitment of c-Src, Fyn, and p85 PI3K in the PEAR1 complex after coimmunoprecipitation with PEAR1-EC Ab. Unstirred platelets were stimulated with PEAR1-EC Ab (7 μg/mL) for different times (0-10 minutes), as indicated. Right panel: quantitative imaging for the evolution of the recruitment of c-Src, Fyn, and p85 PI3K after PEAR1-EC Ab stimulation. (E) Western blot analysis of the recruitment of c-Src, Fyn, and p85 PI3K in the PEAR1 complex after coimmunoprecipitation with PEAR1-EC Ab. Unstirred platelets were stimulated with PEAR1-EC Ab (7 μg/mL) for 5 minutes in the presence or the absence of PP1 (10μM). (F) Western blot analysis of the recruitment of c-Src and c-Src phosphorylated in the inhibitory loop in the PEAR1 complex after coimmunoprecipitation with PEAR1-EC Ab. Unstirred platelets were stimulated with PEAR1-EC Ab (7 μg/mL) for 5 minutes. Vertical lines indicate a repositioned gel lane.
Therefore, signaling studies were performed on platelets activated by PEAR1-EC Ab in static conditions. Furthermore, to avoid secondary platelet amplification, including αIIbβ3-mediated outside-in signaling, activation was carried out in the presence of apyrase (0.4 U/mL), aspirin (550μM) and eptifibatide (10 μg/mL). Time-wise analysis of whole platelet lysates via western blots revealed SFK activation loop Tyr-phosphorylation and c-Src dephosphorylation of the inhibitory loop (Tyr529) as rapidly as 30 seconds (Figure 4C) followed by Tyr-phosphorylation of PEAR1 at 1 minute and Akt Ser473 phosphorylation at 2 minutes. Fyn dephosphorylation of the inhibitory loop was barely seen. In addition, we found in PEAR1 immunoprecipitates that PEAR1-EC Ab transiently and rapidly raised the association of c-Src and Fyn with PEAR1, but also that the p85 subunit of PI3K quantitatively associated with PEAR1, reaching a plateau at 5 minutes after platelet activation by PEAR1-EC Ab (Figure 4D). This recruitment is dependent on SFK activity for c-Src and p85 PI3K but not for Fyn, because PP1 treatment inhibits c-Src and p85 PI3K recruitment completely, but not that of Fyn (Figure 4E). In the PEAR1 complex, c-Src is activated by PEAR1-EC Ab, because the inhibitory loop is dephosphorylated (Figure 4F). These results demonstrate that PEAR1 forms a molecular complex comprising c-Src and Fyn, and that p85 PI3K recruitment and PI3K activation are downstream of PEAR1 phosphorylation.
PEAR1-EC Ab as a pseudo-ligand for PEAR1
In addition to triggering PEAR1 phosphorylation and signal transduction in unstirred platelets, PEAR1-EC Ab also induced potent and dose-dependent aggregation of washed platelets, when stirred in the presence of the anti-FcγRIIA receptor antibody IV.3 (Figure 5A). Aggregation was potentiated by EMI Ab, but largely inhibited by EMI F(ab) (supplemental Figure 3B-D), compatible with a mechanism in which the PEAR1-EC Ab activation depends on divalency of the antibody (supplemental Figure 3A), a mode of action disabled by excess monovalent EMI F(ab). Aggregation was specific, because abrogated by prior incubation of PEAR1-EC Ab with an excess of PEAR1-EC (5 μg/mL; supplemental Figure 1C-D) and was αIIbβ3-mediated, that is fully inhibited by eptifibatide, requiring δ-granule secretion and TXA2 synthesis, largely eliminated by apyrase and aspirin, at 1 μg/mL PEAR1-EC Ab (Figure 5B). Flow cytometry confirmed that PEAR1-EC Ab properly and dose-dependently also activated unstirred platelets, detected via enhanced membrane expression of activated αIIbβ3 (PAC-1 binding) and P-selectin (CD62-P binding; Figure 5C). Because c-Src (and Fyn) associate with PEAR1 in platelets (Figure 4) and because phosphorylation of the SFK activation loop preceded the Tyr phosphorylation of PEAR1, we investigated the role of SFK in PEAR1 phosphorylation. Indeed, the SFK inhibitor PP1 fully blocked PEAR1-EC Ab induced platelet aggregation (Figure 5D), as well as the tyrosine phosphorylation of several proteins (≈ 200 kDa, 150 kDa, 120 kDa, 72 kDa, 60 kDa, 50 kDa, and 45 kDa), including PEAR1 (Figure 5E). Combined, these results demonstrate that PEAR1-EC Ab triggers SFK activation (and further recruitment), leading to PEAR1 phosphorylation and subsequent p85 PI3K association with PEAR1-P, in turn leading to downstream PI3K-dependent Akt activation.
PEAR1-EC Ab-induced platelet aggregation. Washed platelet aggregation by PEAR1-EC Ab (A) at 0.25 to 2 μg/mL, (B) at 0.5 μg/mL, in the presence of eptifibatide (10 μg/mL) or apyrase (0.4 U/mL) and aspirin (550μM). Aggregations with PEAR1-EC Ab in the presence of IV.3 (10 μg/mL), traces representative of at least 5 independent experiments. (C) Flow cytometric measurement as MFI of activated αIIbβ3 (PAC1) and P-selectin (CD62-P) on activation of washed platelets with PEAR1-EC Ab (5-10 μg/mL). Activated integrin and CD62-P exposure are expressed as percentage of positive platelets in the fluorescence histogram for one experiment, representative of 3 independent analyses. (D) Inhibition of aggregation by PP1 (10μM) versus DMSO, as indicated. (E) Phosphorylation of total tyrosine (P-Tyr, 1 and 5 minutes) and PEAR1 (PEAR1-P, 5 minutes). Phosphorylation was analyzed in platelets incubated with PP1 (10μM) or DMSO for 10 minutes before activation with PEAR1-EC Ab (7 μg/mL) in unstirred conditions, in the presence of aspirin (550μM), apyrase (0.4 U/mL) and eptifibatide (10 μg/mL). Total PEAR1 was determined after stripping and reprobing. Data are representative of 3 experiments.
PEAR1-EC Ab-induced platelet aggregation. Washed platelet aggregation by PEAR1-EC Ab (A) at 0.25 to 2 μg/mL, (B) at 0.5 μg/mL, in the presence of eptifibatide (10 μg/mL) or apyrase (0.4 U/mL) and aspirin (550μM). Aggregations with PEAR1-EC Ab in the presence of IV.3 (10 μg/mL), traces representative of at least 5 independent experiments. (C) Flow cytometric measurement as MFI of activated αIIbβ3 (PAC1) and P-selectin (CD62-P) on activation of washed platelets with PEAR1-EC Ab (5-10 μg/mL). Activated integrin and CD62-P exposure are expressed as percentage of positive platelets in the fluorescence histogram for one experiment, representative of 3 independent analyses. (D) Inhibition of aggregation by PP1 (10μM) versus DMSO, as indicated. (E) Phosphorylation of total tyrosine (P-Tyr, 1 and 5 minutes) and PEAR1 (PEAR1-P, 5 minutes). Phosphorylation was analyzed in platelets incubated with PP1 (10μM) or DMSO for 10 minutes before activation with PEAR1-EC Ab (7 μg/mL) in unstirred conditions, in the presence of aspirin (550μM), apyrase (0.4 U/mL) and eptifibatide (10 μg/mL). Total PEAR1 was determined after stripping and reprobing. Data are representative of 3 experiments.
PEAR1-dependent PI3K activation stabilizes platelet aggregates
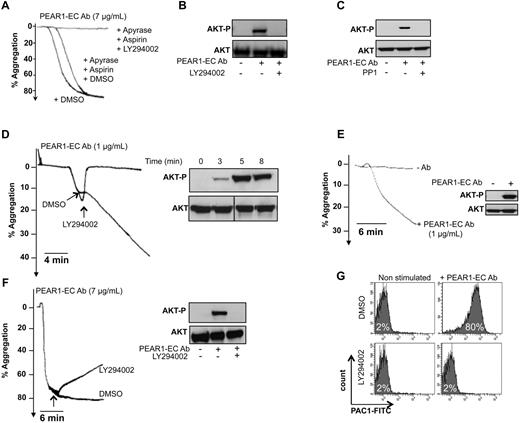
Because p85 PI3K association with PEAR1-P leads to Akt phosphorylation, we investigated how PEAR1 signaling activates PI3K in platelet aggregation. In the presence of apyrase and aspirin, at 7 μg/mL, PEAR1-EC Ab triggered amplification pathway-independent platelet aggregation, fully inhibited by the PI3K inhibitor LY294002 (Figure 6A). As expected, in these conditions, PEAR1-EC Ab triggered potent Akt phosphorylation as soon as 3 minutes after its addition, fully prevented by LY294002 (Figure 6B). SFKs are necessary for the phosphorylation of PEAR1 (Figure 5F), but PP1 also completely inhibited the phosphorylation of Akt induced by PEAR1-EC Ab (Figure 6C), confirming the link in PEAR1 signaling between SFK activation, PEAR1 phosphorylation, and Akt activation.
PEAR1 activation induces sustained PI3K signaling. (A) Aggregation of washed platelets by PEAR1-EC Ab (7 μg/mL), in the presence of apyrase (0.4 U/mL) and aspirin (550μM), with added DMSO or LY294002 (50μM). (B) Corresponding blots for Akt phosphorylation (Akt-P vs total Akt) for platelets activated with PEAR1-EC Ab in the absence or presence of LY294002. (C) Akt phosphorylation (Akt-P vs total Akt) was analyzed in platelets incubated with PP1 (10μM) or DMSO for 10 minutes before activation with PEAR1-EC Ab (2 μg/mL) in unstirred conditions, in the presence of aspirin (550μM), apyrase (0.4 U/mL), and eptifibatide (10 μg/mL) for 5 minutes. (D) Washed platelet aggregation with a “low dose” of PEAR1-EC Ab (1 μg/mL) with LY294002 (50μM), added during early aggregation, as indicated. The inset shows the corresponding Akt phosphorylation during the aggregation at corresponding time points. (E) Washed platelet aggregation by PEAR1-EC Ab (1 μg/mL) supplemented with aspirin (550μM) and apyrase (0.4 U/mL), for 12 minutes. The inset shows the corresponding Akt phosphorylation at the end of the aggregation (12 minutes). (F) Washed platelet aggregation with a high dose of PEAR1-EC Ab (7 μg/mL) with LY294002 (50μM), added during late aggregation, as indicated. The insert shows the corresponding Akt phosphorylation at the end of the aggregation (12 minutes). (G) Flow cytometric measurement as MFI of activated αIIbβ3 (PAC1) on activation of washed platelets with PEAR1-EC Ab (7 μg/mL), in the presence of apyrase (0.4 U/mL) and aspirin (550μM), with or without LY294002 (50μM). Vertical lines indicate a repositioned gel lane.
PEAR1 activation induces sustained PI3K signaling. (A) Aggregation of washed platelets by PEAR1-EC Ab (7 μg/mL), in the presence of apyrase (0.4 U/mL) and aspirin (550μM), with added DMSO or LY294002 (50μM). (B) Corresponding blots for Akt phosphorylation (Akt-P vs total Akt) for platelets activated with PEAR1-EC Ab in the absence or presence of LY294002. (C) Akt phosphorylation (Akt-P vs total Akt) was analyzed in platelets incubated with PP1 (10μM) or DMSO for 10 minutes before activation with PEAR1-EC Ab (2 μg/mL) in unstirred conditions, in the presence of aspirin (550μM), apyrase (0.4 U/mL), and eptifibatide (10 μg/mL) for 5 minutes. (D) Washed platelet aggregation with a “low dose” of PEAR1-EC Ab (1 μg/mL) with LY294002 (50μM), added during early aggregation, as indicated. The inset shows the corresponding Akt phosphorylation during the aggregation at corresponding time points. (E) Washed platelet aggregation by PEAR1-EC Ab (1 μg/mL) supplemented with aspirin (550μM) and apyrase (0.4 U/mL), for 12 minutes. The inset shows the corresponding Akt phosphorylation at the end of the aggregation (12 minutes). (F) Washed platelet aggregation with a high dose of PEAR1-EC Ab (7 μg/mL) with LY294002 (50μM), added during late aggregation, as indicated. The insert shows the corresponding Akt phosphorylation at the end of the aggregation (12 minutes). (G) Flow cytometric measurement as MFI of activated αIIbβ3 (PAC1) on activation of washed platelets with PEAR1-EC Ab (7 μg/mL), in the presence of apyrase (0.4 U/mL) and aspirin (550μM), with or without LY294002 (50μM). Vertical lines indicate a repositioned gel lane.
PI3K is important for the stabilization of platelet aggregates.16 Correspondingly, LY294002 (50μM) caused a rapid and complete disassembly of platelet aggregates during the early PEAR1-EC Ab (1 μg/mL) induced platelet aggregation (Figure 6D). In contrast to ADP, which is a weak platelet agonist causing only reversible washed platelet aggregation17 and weak Akt phosphorylation (supplemental Figure 4A-B), PEAR1-EC Ab induced rapid and sustained Akt phosphorylation at 12 minutes, even when at 1 μg/mL PEAR1-EC Ab, aggregation only reached 25% (Figure 6E). Yet, the PEAR1-EC Ab aggregation further increased up to 30 minutes, with maintained Akt phosphorylation (data not shown). When added to PRP, 1 μg/mL PEAR1-EC Ab did not cause platelet aggregation by itself, but enhanced platelet aggregation by low ADP concentrations, in a fully PI3K-dependent manner (supplemental Figure 4C-D). Moreover complete aggregation of washed aspirin/apyrase-treated platelets by 7 μg/mL PEAR1-EC Ab could be reversed only in part, although Akt was fully dephosphorylated 12 minutes after addition of LY294002 at 5 minutes (Figure 6F). Correspondingly, the PEAR1-EC Ab-induced activation of αIIbβ3 was abrogated by LY294002 (Figure 6G), compatible with the critical role of PI3K in αIIbβ3 activation. All of these results demonstrate that phosphorylation of PEAR1 ultimately promotes late platelet aggregate stabilization via sustained activation of PI3K.
Discussion
This study demonstrates that PEAR1, already present in the membrane of resting platelets, is also released from α-granules during platelet activation, further raising its membrane expression. Platelet contacts between the PEAR1 EMI domain and an unidentified surface ligand (itself equally exposed during platelet activation) triggers rapid PEAR1 phosphorylation, thus initiating a signaling cascade that culminates in PI3K activation, reinforcing αIIbβ3 activation and, consequently, platelet aggregation (Figure 7). As reported for the activation of human CLEC-2,18 SFK-dependent tyrosine phosphorylation of PEAR1 appears to be sufficient to initiate downstream signaling events, requiring 2 PEAR1 receptors, a mechanism recapitulated by divalent PEAR1-EC Ab.
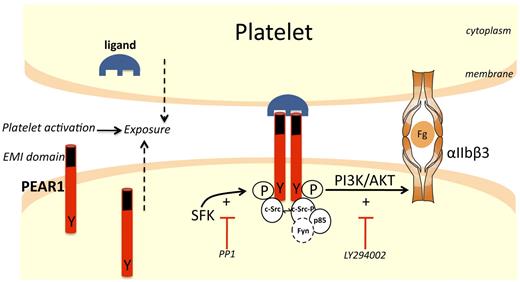
Scheme for PEAR1 signaling pathway. In resting platelets, low amounts of PEAR1 are found on the platelet surface. During platelet activation with various platelet agonists, the expression of both PEAR1 and its ligand increase at the surface. Interactions between PEAR1 and its ligand on adjacent platelets induces formation of a complex comprising at least 2 PEAR1 receptors. Dimeric (or oligomeric) PEAR1 complexes are rapidly tyrosine-phosphorylated in a SFK-dependent manner (inhibited by the SFK inhibitor PP1), transiently recruiting additional c-Src and Fyn. PEAR1-P avidly binds p85 PI3K, leading to strong and sustained activation of Akt at Ser473 (inhibited by the PI3K inhibitor LY294002 and by PP1). PI3K activation amplifies αIIbβ3 activation, sustaining platelet aggregation. Because c-Src is already strongly bound to PEAR1 in resting platelets, c-Src may be the SFK rapidly activated and responsible for PEAR1 phosphorylation, on ligand binding, but this was not formally shown. (Fg, fibrinogen; Y, tyrosine; P, phosphorylation). The EMI domain in PEAR1 is depicted in black.
Scheme for PEAR1 signaling pathway. In resting platelets, low amounts of PEAR1 are found on the platelet surface. During platelet activation with various platelet agonists, the expression of both PEAR1 and its ligand increase at the surface. Interactions between PEAR1 and its ligand on adjacent platelets induces formation of a complex comprising at least 2 PEAR1 receptors. Dimeric (or oligomeric) PEAR1 complexes are rapidly tyrosine-phosphorylated in a SFK-dependent manner (inhibited by the SFK inhibitor PP1), transiently recruiting additional c-Src and Fyn. PEAR1-P avidly binds p85 PI3K, leading to strong and sustained activation of Akt at Ser473 (inhibited by the PI3K inhibitor LY294002 and by PP1). PI3K activation amplifies αIIbβ3 activation, sustaining platelet aggregation. Because c-Src is already strongly bound to PEAR1 in resting platelets, c-Src may be the SFK rapidly activated and responsible for PEAR1 phosphorylation, on ligand binding, but this was not formally shown. (Fg, fibrinogen; Y, tyrosine; P, phosphorylation). The EMI domain in PEAR1 is depicted in black.
PEAR1 has been classified as a receptor tyrosine kinase (RTK).19 Most RTKs such as the insulin receptor are single subunit receptors, which on ligand binding to their extracellular domain form receptor dimers.20 The extracellular N-terminal region of such receptors exhibits conserved elements, including Ig-like or EGF-like domains, fibronectin type III repeats, or cysteine-rich regions. The intracellular C-terminal region displays the highest level of conservation and comprises catalytic domains endowed with kinase activity, catalyzing receptor autophosphorylation and tyrosine phosphorylation of substrates. Our analyses using Blast and Pfam (http://pfam.sanger.ac.uk/) did not reveal any tyrosine kinase domain in the PEAR1 sequence. Moreover, the molecular PEAR1 phosphorylation mechanism, coupled to SFK activation, eliminates the candidacy of PEAR1 as a tyrosine kinase receptor, in spite of receptor dimerization.
PEAR1 has an N-terminal EMI domain. EMI domains, first named after their presence in EMILIN family members, are small cysteine-rich modules of around 75 amino acids. The EMI domain possesses 6 highly conserved cysteine residues, usually forming disulphide bonds. It is most often found in extracellular and multimeric proteins, such as multimerin, a platelet protein,21,22 in association with other domains, such as C1q, EGF-like, collagen-like, or fibronectin type 3 structures.23 The EMI domain can act as a protein binding module, as illustrated by the interaction between the Emilin-1 EMI domain and the C1q domain of Emilin-2.21 Via the use of GST-EMI, EMI Ab, and EMI F(ab), we demonstrated for the first time that the EMI domain of PEAR1 is implicated in platelet aggregation, confirming that PEAR1 is a functional platelet contact receptor, requiring cross-linking to become active. Such findings may help understand the role of EMI domains in contact-dependent signaling pathways in platelets and beyond.
The cytoplasmic tail of PEAR1 contains various proline-rich domains such as PXXP, PXPPPP, PPPXP, PPXXXPP, and various tyrosine residues (supplemental Table 1). The Proteome-wide prediction software of cell signaling interactions using short sequence motifs Scansite 2.024 predicted an interaction between PEAR1 and Src via its Src homology-3 (SH3) group (with a pro-rich region in PEAR1), and p85 PI3K via its SH2 and SH3 groups (with a Tyr of PEAR1 and a pro-rich region, respectively). Binding of c-Src and p85 PI3K with PEAR1 were experimentally confirmed. Furthermore Fyn as well, but not Lyn or Syk, was found in association with PEAR1 (Figure 7). Interestingly, the PXXP motif is also present in platelet GPVI, where it confers binding and activation of Lyn and Fyn in transfected cells by placing the receptor in a “ready to go” state.25 However, unlike the role played by Lyn, Fyn and Syk in the phosphorylation of LAT, responsible for the activation of phospholipase Cγ2 (PLCγ2) during collagen and/or CLEC-2–mediated signaling,18,26 we did not find phospho-LAT during platelet activation by PEAR1-EC Ab, even though Fyn seems capable of LAT phosphorylation,26 nor did we find noticeable phosphorylation of PLCγ2. Thus, even when PEAR1 signaling and GPVI, respectively CLEC-2 activation mechanisms share some analogy in the SFK-mediated signal transduction, Syk and Lyn probably are not involved in early PEAR1 signaling, and at best, Fyn plays a minor role in early platelet activation.
Thrombus formation not only requires platelet activation via inside-out signaling pathways, but several positive feedback loops exist, which reinforce and sustain platelet activation.16,27 Thus, secreted ADP, Gas6, synthesized TXA2 and ephrin receptors amplify initial stimuli actively stabilizing platelet aggregates, allowing them to withstand shear forces in flowing blood.27,28 Several signaling pathways involving protein kinase C (PKC),29 PI3K,30 MAPK (ERK2, JNK1),31 Rap1b,32 talin,33,34 and kindlin-2/335,36 are mobilized to maintain the activated state of αIIbβ3. Phosphorylation of PEAR1 by PEAR1-EC Ab led to progressive and long-lasting Src-dependent PI3K/Akt signaling, coupled to its binding to p85 PI3K and gradual displacement to the platelet cytoskeleton. The docking of PI3K in the vicinity of the plasma membrane through p85 recognition to receptors with phosphorylated YxxM domains activates the PI3K pathway. Indeed, the PI3K p85 subunit contains 2 Src homology-2 (SH2) domains, an N and a C-terminal SH2 domain, that bind to 2 closely spaced YxxM motifs,37 where tyrosine phosphorylation creates binding sites for the SH2 domains of the p85 subunit. Because PEAR1 contains only one YxxM motif, the recruitment of the p85 PI3K subunit on PEAR1 may be linked to PEAR1 engagement, during aggregation, by associating one p85 PI3K to at least 2 PEAR1 molecules, a step also recapitulated during divalent PEAR1-EC Ab triggered platelet activation. The engagement of SH2 domains appears to induce a conformational change that converts PI3K from a closed low-activity state into an open high-activity state.38 As a result, PI3K will generate the lipid second messenger phosphoinositol-3,4,5-trisphosphate (PIP3).
PIP3 formation recruits pleckstrin homology domain-containing proteins, most notably the protein kinase Akt (reviewed in Engelman et al39 ) to the plasma membrane and initiates downstream signaling, modulating different cellular processes. PEAR1 promoted potent Akt phosphorylation, also in the absence of ADP/ATP, which contrasts to the case of Gas6.27 Whereas secreted ADP suffices to prevent embolization via continuously maintained P2Y12-elicited signaling,16 the role of autocrine ADP via P2Y12 receptor stimulation is time-restricted, because of the limited amount of ADP in platelets and its rapid degradation by ectonucleotidases. ADP-P2Y12 and Gas6-receptor activation pathways have to synergize to achieve persistent αIIbβ3 activation and platelet aggregation. It is tempting to speculate that the long-lasting PEAR1-induced PI3K activation, presently reported, is relevant for persistent activation of αIIbβ3. This is substantiated by the observation that the weak aggregation by low ADP concentrations is potently stimulated by PEAR1-EC Ab, at concentrations too low to trigger platelet aggregation by themselves (supplemental Figure 4). In this setting of absent platelet secretion, a poor up-regulation of membrane PEAR1 and probably of its ligand, and only weak PEAR1 phosphorylation were observed (data not shown). By reconstituting PEAR1 phosphorylation and PI3K activation, low PEAR1-EC Ab concentrations strongly potentiate the weak platelet aggregation by low ADP concentrations.
In conclusion, the use of GST-EMI has allowed us to define a role for PEAR1 in physiologic conditions of platelet activation. Hence, the existence of PEAR1 polymorphisms, associated with the modulation of platelet function correspond to a reality. Thus, a variant in intron 1 of the PEAR1 gene (rs12041331) is associated with increased PEAR1 protein expression and up-regulated platelet aggregation responses toward multiple agonists.6 This finding can now be understood on the basis of intensified PI3K-dependent sustained αIIbβ3 activation. This study is the first demonstration of a functional role for PEAR1 in platelet activation, underpinning the observed association between PEAR1 and platelet function in genome-wide association studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors appreciate the skillful assistance of Soetkin Van kerckhoven, Christine Vranckx, Katrien Cludts, and Chantal Thys.
This work was supported by research grant “Krediet aan Navorsers 1.5.229.11N” from the FWO Vlaanderen and by FWO Vlaanderen grant G.0601.12N. The CMVB is supported by the “Programmafinanciering KULeuven (PF/10/014), and by the “Geconcerteerde Onderzoeksacties” (GOA 2009/13) from the University of Leuven.
Authorship
Contribution: A.K., M.F.H., and P.V. designed and performed research, analyzed data, and wrote the paper; M.D.M. performed protein identification; S.L. performed research; and K.F. advised on platelet research methodology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc F. Hoylaerts, Center for Molecular and Vascular Biology, University of Leuven, Herestraat 49, B3000 Leuven, Belgium; e-mail: marc.hoylaerts@med.kuleuven.be.