Abstract
The pan-deacetylase inhibitor panobinostat (LBH589) recently has been shown to have significant clinical activity in patients with relapsed Hodgkin lymphoma, but its mechanism of action in Hodgkin lymphoma remains unknown. In this study, we demonstrate that panobinostat has potent antiproliferative activity against Hodgkin lymphoma–derived cell lines. At the molecular level, panobinostat activated the caspase pathway, inhibited STAT5 and STAT6 phosphorylation, and down-regulated hypoxia-inducible factor 1 α and its downstream targets, glucose transporter 1 (GLUT1) and vascular endothelial growth factor. Paradoxically, panobinostat inhibited LKB1 and AMP-activated protein kinase, leading to activation of mammalian target of rapamycin (mTOR) that promotes survival. Combining panobinostat with the mTOR inhibitor everolimus (RAD001) inhibited panobinostat-induced mTOR activation and enhanced panobinostat antiproliferative effects. Collectively, our data demonstrate that panobinostat is a potent deacetylase inhibitor against Hodgkin lymphoma–derived cell lines, and provide a mechanistic rationale for combining panobinostat with mTOR inhibitors for treating Hodgkin lymphoma patients. Furthermore, the effect of panobinostat on GLUT1 expression suggests that panobinostat may modulate the results of clinical diagnostic imaging tests that depend of functional GLUT1, such as fluorodeoxyglucose positron emission tomography.
Introduction
Deacetylases (DACs) are a group of enzymes that regulate a variety of cell functions, including survival, cell-cycle progression, angiogenesis, and immunity; therefore, DACs are considered promising targets for cancer therapy.1,2 DACs are classified into 4 major classes: class I includes DACs 1, 2, 3, 8, and 11; class II includes DACs 4, 5, 6, 7, 9, and 10; class III includes homologs of yeast SIRT 1 to 7; and class IV currently includes only DAC 11.3,4 Current DAC pharmacologic inhibitors either preferentially affect class I enzymes (eg, mocetinostat and entinostat) or more broadly affect several enzymes that belong to class I and class II DACs (also known as pan-DAC inhibitors; eg, vorinostat, panobinostat, and romidepsin). The lack of selectivity of currently available DAC inhibitors results in the modulation of a wide range of protein targets that may enhance the therapeutic response but may also cause undesired molecular and clinical effects. Better understanding of the molecular mechanisms of different DAC inhibitors may improve their use in the clinical setting by providing insights into improving their therapeutic efficacy and may provide a rationale for future combination strategies.
Clinical trials have demonstrated that DAC inhibitors have promising clinical activity in patients with relapsed Hodgkin lymphoma (HL).5-7 A recently completed phase 2 study of panobinostat reported a 27% response rate in heavily pretreated patients with relapsed HL.6 Despite this clinical activity, little is known about panobinostat's mechanism of action in this disease.8 In this study, we examined the in vitro antiproliferative activity of panobinostat in HL-derived cell lines and examined its potential molecular mechanisms of action. We found that panobinostat activated opposing mechanisms that regulate cell death and survival. On the one hand, panobinostat induced cell death by modulating several molecular mechanisms, including caspase pathway activation and inhibition of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. On the other hand, panobinostat inhibited LKB1 and adenine monophosphate-activated protein kinase (AMPK), leading to activation of mammalian target of rapamycin (mTOR), which promotes cell survival. Inhibiting of the mTOR function by everolimus potentiated the antiproliferative activity of panobinostat, providing a mechanistic rationale for evaluating this novel combination in the clinical setting. Moreover, panobinostat down-regulated hypoxia-inducible factor 1 α (HIF-1α) and its downstream targets glucose transporter 1 (GLUT1) and vascular endothelial growth factor (VEGF), suggesting that panobinostat therapy may influence the results of diagnostic imaging tests that depend on functional GLUT1, such as fluorodeoxyglucose-positron emission tomography (FDG-PET).
Methods
Cell lines and culture conditions
The human HL-derived cell lines HDLM-2, L-428, and KM-H2 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures. All cell lines were cultured in Gibco RPMI medium 1640 (Invitrogen) supplemented with 10% heat-inactivated FBS,1% l-glutamine, and penicillin/streptomycin in a humid environment of 5% CO2 at 37°C.
Reagents and antibodies
Antibodies specific for AKT, p70 S6 kinase, 4EBP1, HIF-1α, mTOR, AMPKα, LKB1, TSC2, STAT3, STAT5, and STAT6 and their respective phosphorylated forms, caspase 3, caspase 9, and poly ADP ribosome polymerase (PARP) were obtained from Cell Signaling Technology. Antibody to β-actin was from Sigma-Aldrich, and antibody to GLUT1 was purchased from Santa Cruz Biotechnology. The DAC inhibitor panobinostat (LBH589) and everolimus (RAD001) were provided by Novartis Pharmaceuticals.
In vitro proliferation assay
Cells were cultured in 24-well plates at a concentration of 0.5 × 106 cells/mL. Cell viability was assessed with the nonradioactive cell proliferation MTS assay using CellTiter96 Aqueous One solution reagent (Promega), as described previously.9 Briefly, 80-μL cell suspension and 20 μL were incubated in 96-well plates for 1 hour at 37°C in 5% CO2, and formazan absorbance was measured at 490 nm on a μQuant plate reader equipped with Gene5 Version 1.06 software (BioTek Instruments). Each measurement was made in triplicate and, the mean value was calculated.
Detection of apoptosis and cell-cycle fractions by flow cytometry
Apoptosis was determined by annexin V–FITC and propidium iodide double staining according to the manufacturer's instructions (BD Biosciences).
Cell-cycle fractions were determined by propidium iodide nuclear staining. Briefly, cells were harvested, washed in PBS, fixed in 70% ethanol for 30 minutes on ice, and incubated in propidium iodide solution (20 μg/mL propidium iodide, 0.2 mg/mL RNAse A in PBS) for 30 minutes at 37°C. Data were collected on a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo Version 7.5.5 software (TreeStar). Results represent the mean value of 3 independent experiments.
Western blots analysis
Total proteins were extracted from cells on iced lysis buffer (Cell Signaling Technology). Lysates were centrifuged at 13 000g for 20 minutes at 4°C, and the protein concentration in the supernatants was determined using a colorimetric assay (Bio-Rad DC Protein Assay; Bio-Rad). A total of 30 μg of protein was denatured in Laemmli buffer at 95°C and separated by SDS-PAGE. Proteins were then electrotransferred onto nitrocellulose membranes and submitted to immunodetection with the relevant antibody. Membrane-bound secondary antibodies (HRP-conjugated goat anti–rabbit or anti–mouse; Bio-Rad) were detected using SuperSignal West Dura Extended Duration Substrate (Pierce Chemical).
Measurement of cytokines and chemokines
HL cell lines were incubated with DMSO (0.1%), RAD001 (0.1μM), or LBH589 (0.02 and 0.1μM) for 72 hours. Supernatants were then collected and examined for production of MCP-1, IL-8, IL-13, IL-15, and TNF-α using the human cytokine 30-plex panel (Invitrogen) according to the manufacturer's instructions. Each experiment was performed in triplicate, and each result is the mean of 3 independent experiments plus or minus SEM.
Statistical analysis
The effectiveness of various drug combinations was analyzed by the Calcusin Version 2.1 software (Biosoft). The combination index was calculated according to the Chou-Talalay method.10 A combination index of 1 indicates an additive effect of the 2 drugs. Combination index values less than 1 indicate synergy, and combination index values more than 1 indicate antagonism. Procedures to determine the effects of certain conditions on cell proliferation, apoptosis, and cytokine production were performed in 3 independent experiments. The 2-tailed Student t test was used to estimate the statistical significance of the differences between results from the 3 experiments. Significance was set at P < .05.
Results
Panobinostat inhibits proliferation of HL cell lines
HL-derived cell lines were treated with increasing doses of panobinostat (0.01-1μM) for 72 hours and assessed for effects on proliferation and survival. Panobinostat demonstrated a significant antiproliferative activity in all 3 cell lines in a dose- and time-dependent manner, with an IC50 ranging between 20 and 40nM at 72 hours (Figure 1A-B). The antiproliferative effect was primarily the result of induction of apoptosis (Figure 1C-D). After incubation with 0.05μM of panobinostat for 48 hours, apoptosis was detected in 32%, 30%, and 35% of HDLM-2, L-428, and KM-H2 cells, respectively. At the molecular level, apoptosis induction was associated with down-regulation of the X-linked inhibitor of apoptosis protein (XIAP) and Bcl-2, activation of caspase 9 and cleavage of PARP (Figure 1E). Collectively, these data demonstrate that panobinostat has a potent antiproliferative activity in HL-derived cell lines associated with induction of apoptosis.
Panobinostat (LBH589)–induced death by apoptosis and autophagy in HL cell lines. (A) Three HL cell lines (HDLM-2, L-428, and KM-H2) were incubated with increasing doses of LBH589 (0.01-1μM). After 24, 48, and 72 hours, cell viability was determined by an MTS assay. Each value represents the mean ± SEM of the results of 3 independent experiments performed in triplicate. (B) IC50 for LBH589 is reduced in all HL cell lines after 72 hours. Data represent the mean of 3 independent experiments performed in triplicate. (C) HL cell lines were treated or not with 0.05μM of LBH589 for 48 hours. Apoptosis was determined by annexin V–FITC/propidium iodide double staining. Percentages of cells showing apoptosis (combined right quadrants) are shown in the boxes. (D) Summary of the results of annexin V–FITC/propidium iodide double staining from 3 independent experiments. Each value represents the mean of 3 independent experiments performed in triplicate. **P < .005. ns indicates not significant. (E) HL cell lines were incubated for 48 hours with diluent control or LBH589 (0.02-0.1μM), and intracellular protein levels were examined by Western blot. LBH589 induced cleavages of caspase 9 and PARP and decreased XIAP and Bcl2 levels in all cell lines.
Panobinostat (LBH589)–induced death by apoptosis and autophagy in HL cell lines. (A) Three HL cell lines (HDLM-2, L-428, and KM-H2) were incubated with increasing doses of LBH589 (0.01-1μM). After 24, 48, and 72 hours, cell viability was determined by an MTS assay. Each value represents the mean ± SEM of the results of 3 independent experiments performed in triplicate. (B) IC50 for LBH589 is reduced in all HL cell lines after 72 hours. Data represent the mean of 3 independent experiments performed in triplicate. (C) HL cell lines were treated or not with 0.05μM of LBH589 for 48 hours. Apoptosis was determined by annexin V–FITC/propidium iodide double staining. Percentages of cells showing apoptosis (combined right quadrants) are shown in the boxes. (D) Summary of the results of annexin V–FITC/propidium iodide double staining from 3 independent experiments. Each value represents the mean of 3 independent experiments performed in triplicate. **P < .005. ns indicates not significant. (E) HL cell lines were incubated for 48 hours with diluent control or LBH589 (0.02-0.1μM), and intracellular protein levels were examined by Western blot. LBH589 induced cleavages of caspase 9 and PARP and decreased XIAP and Bcl2 levels in all cell lines.
Opposing effects of panobinostat and everolimus on mTOR activity
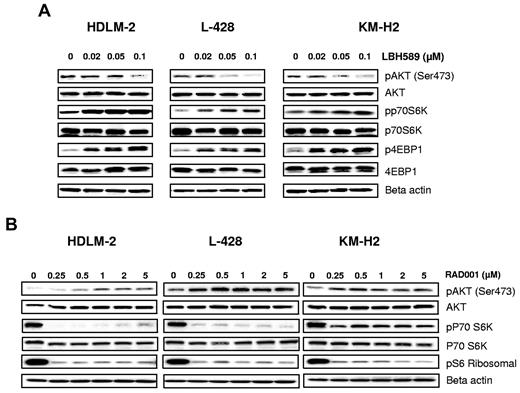
A variety of DAC inhibitors can induce and/or inhibit a variety of signal transduction pathways, including JAK/STAT, NF-κB, and PI3/AKT/mTOR pathway. Because regulation of these signaling pathways can be cell type dependent and may vary depending on the DAC inhibitor used, we first examined the effect of panobinostat on the PI3K/AKT/mTOR pathway, which is known to be aberrantly activated in HL-derived cell lines.9 Resting HL cells express the phosphorylated form of AKT. When cells were incubated with increasing concentrations of panobinostat, AKT phosphorylation was inhibited in all cell lines (Figure 2A). Surprisingly, AKT inhibition did not result in inhibition of mTOR complex 1 (mTORC1), a downstream target. Panobinostat activated mTORC1, as evident by phosphorylation of its downstream targets p70S6 kinase (S6K) and 4E binding protein 1 (4E-BP1; Figure 2A). Conversely, everolimus (rapalog inhibitor of mTORC1) decreased p70S6K phosphorylation in a dose-dependent manner (Figure 2B). As previously reported,11 everolimus inhibition of mTORC1 induced a negative feedback loop by phosphorylating AKT on serine 473 (Figure 2B). Collectively, these data indicate that panobinostat and everolimus have opposing effects on AKT and mTOR function, suggesting that combining both drugs may enhance their therapeutic value.
Effects of panobinostat and everolimus (RAD001) on mTOR activity. (A) Cells were incubated with diluent control or LBH589 (0.02-0.1μM); and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with (A) anti–phospho-AKT (Ser473), anti-AKT, anti–phospho-p70S6K, anti-p70S6K, anti–phospho-4EBP1, anti-4EBP1, and anti–β-actin antibodies. Representative blots from 3 independents experiments are shown. (B) Cells were incubated with diluent control or RAD (0-5μM); and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti–phospho-AKT (Ser473) anti-AKT, anti–phospho-p70S6K, anti-p70S6K, anti-pS6 ribosomal, and anti–β-actin antibodies. Representative blots from 3 independents experiments are shown.
Effects of panobinostat and everolimus (RAD001) on mTOR activity. (A) Cells were incubated with diluent control or LBH589 (0.02-0.1μM); and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with (A) anti–phospho-AKT (Ser473), anti-AKT, anti–phospho-p70S6K, anti-p70S6K, anti–phospho-4EBP1, anti-4EBP1, and anti–β-actin antibodies. Representative blots from 3 independents experiments are shown. (B) Cells were incubated with diluent control or RAD (0-5μM); and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti–phospho-AKT (Ser473) anti-AKT, anti–phospho-p70S6K, anti-p70S6K, anti-pS6 ribosomal, and anti–β-actin antibodies. Representative blots from 3 independents experiments are shown.
Panobinostat-induced mTOR activation is mediated by LKB1/AMPK
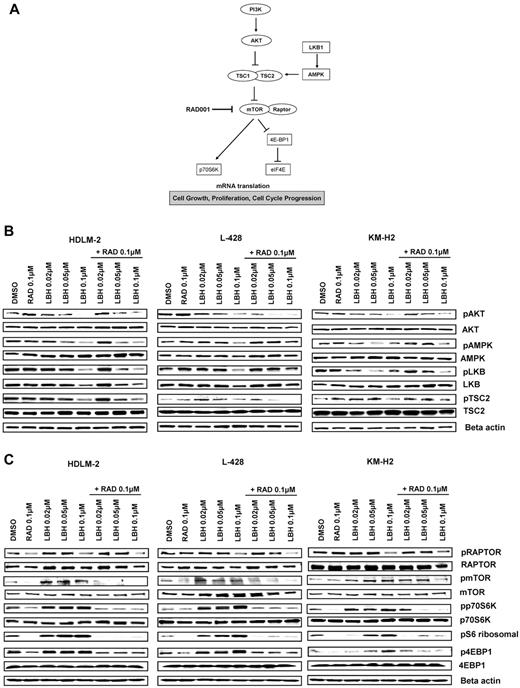
Accumulating evidence indicates that 5′ AMPK not only regulates fatty acids and glycogen metabolism but also modulates protein synthesis and cell growth through the tuberous sclerosis protein 2 (TSC2)/mTOR pathway (Figure 3A).12 The serine/threonine LKB1 kinase directly phosphorylates AMPK and activates its kinase activity. Treatment with panobinostat induced a dose-dependent decrease in the phosphorylation levels of LKB1, AMPKα, and TSC2, whereas everolimus did not affect the phosphorylation status of these proteins (Figure 3B). Consequently, panobinostat treatment resulted in activation of mTORC1, as evident by an increase in the phosphorylation status of p70S6K and 4EBP1. Importantly, everolimus treatment inhibited panobinostat-induced mTOR activation, as evident by inhibition of p70S6K and 4EBP1 phosphorylation (Figure 3C). Collectively, these data demonstrate that panobinostat and everolimus reciprocally inhibit negative feedback loops induced by these agents; everolimus-induced AKT phosphorylation is inhibited by panobinostat, and panobinostat-induced mTOR activation is inhibited by everolimus.
Effects of panobinostat (LBH589) and everolimus (RAD001) combination on the PI3K/AKT/mTOR pathway in HL cell lines. (A) Model showing PI3K/AKT/mTOR signaling. Everolimus inhibits mTOR/RAPTOR and leads to a down-regulation of p70S6K and eiF4E. (B-C) Cells were incubated with diluent control, LBH589 (0.02-0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with (B) anti–phospho-AKT (Ser473), anti-AKT, anti–phospho-LKB1, anti-LKB1, anti–phospho-AMPKα, anti-AMPKα, anti–phospho-TSC2, anti-TSC2 antibodies, and (C) anti–phospho-RAPTOR, anti-RAPTOR, anti–phospho-mTOR, anti-mTOR, anti–phospho-p70S6K, anti-p70S6K, anti–phospho-4EBP1, anti-4EBP1, and anti–β-actin antibodies. Representative blots from 3 independents experiments are shown.
Effects of panobinostat (LBH589) and everolimus (RAD001) combination on the PI3K/AKT/mTOR pathway in HL cell lines. (A) Model showing PI3K/AKT/mTOR signaling. Everolimus inhibits mTOR/RAPTOR and leads to a down-regulation of p70S6K and eiF4E. (B-C) Cells were incubated with diluent control, LBH589 (0.02-0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with (B) anti–phospho-AKT (Ser473), anti-AKT, anti–phospho-LKB1, anti-LKB1, anti–phospho-AMPKα, anti-AMPKα, anti–phospho-TSC2, anti-TSC2 antibodies, and (C) anti–phospho-RAPTOR, anti-RAPTOR, anti–phospho-mTOR, anti-mTOR, anti–phospho-p70S6K, anti-p70S6K, anti–phospho-4EBP1, anti-4EBP1, and anti–β-actin antibodies. Representative blots from 3 independents experiments are shown.
Everolimus enhances panobinostat's antiproliferative activity
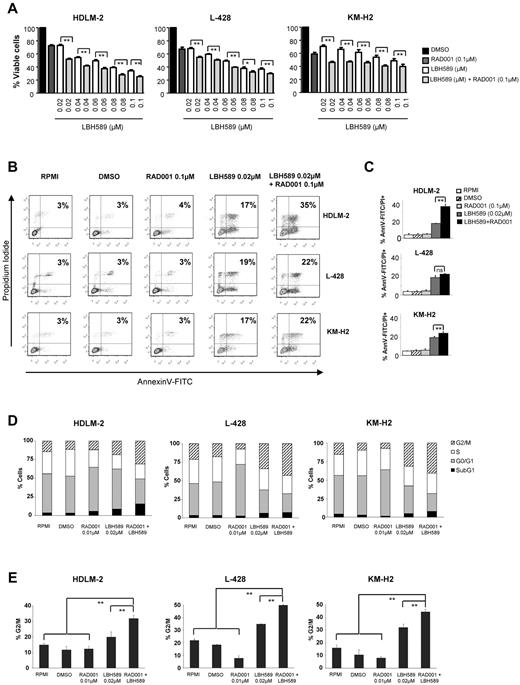
On the basis of our observations that panobinostat and everolimus inhibit each other via feedback loops, we hypothesized that this molecular “synergy” would translate into an enhanced antiproliferative effect in HL. To test this hypothesis, HL cells were incubated with increasing concentrations of panobinostat and everolimus (0.02-0.1μM) and the percentages of viable cells were determined by an MTS assay after 48 hours (Figure 4A). As described previously in Figure 1A, panobinostat induced cell death in a dose-dependent way. As expected, everolimus, which is known to be a cytostatic drug, produced an average inhibition of cell survival of 30% in all cell lines. Combined treatment of HL cell lines with everolimus and panobinostat significantly reduced the percentage of surviving cells (Figure 4A). Calculation of the combination index using the Chou-Talalay equation indicated that everolimus and panobinostat were synergistic, as indicated by combination index values of less than 1.0 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Effects of panobinostat (LBH589) and everolimus (RAD001) combination on the proliferation and the viability of HL cell lines. (A) Cells were treated with 0.02 to 0.1μM of LBH589 or 0.1μM of RAD001 or in combination for 48 hours, and cell viability was determined by an MTS assay. Each value represents the mean ± SEM of the results of 3 independent experiments performed in triplicate. **P < .005. (B) HL cell lines were treated or not with 0.02μM of LBH589 and/or RAD001 for 48 hours. Apoptosis was determined by annexin V–FITC/propidium iodide double staining. Percentages of cells showing apoptosis (combined right quadrants) are shown in the boxes. (C) Summary of the results of annexin V–FITC/propidium iodide double staining from 3 independent experiments. Each value represents the mean of 3 independent experiments performed in triplicate. **P < .005. (D) Cells were incubated with LBH589 (0.02μM) and/or RAD001 (0.1μM) for 48 hours, and the cell cycle was analyzed by flow cytometry using propidium iodide staining. The combination of panobinostat with everolimus significantly increased the percentage of cells in the G2/M phase. Each value is the mean of 3 independent experiments. (E) Summary of the percentage of cells in G2/M phase. **P < .005.
Effects of panobinostat (LBH589) and everolimus (RAD001) combination on the proliferation and the viability of HL cell lines. (A) Cells were treated with 0.02 to 0.1μM of LBH589 or 0.1μM of RAD001 or in combination for 48 hours, and cell viability was determined by an MTS assay. Each value represents the mean ± SEM of the results of 3 independent experiments performed in triplicate. **P < .005. (B) HL cell lines were treated or not with 0.02μM of LBH589 and/or RAD001 for 48 hours. Apoptosis was determined by annexin V–FITC/propidium iodide double staining. Percentages of cells showing apoptosis (combined right quadrants) are shown in the boxes. (C) Summary of the results of annexin V–FITC/propidium iodide double staining from 3 independent experiments. Each value represents the mean of 3 independent experiments performed in triplicate. **P < .005. (D) Cells were incubated with LBH589 (0.02μM) and/or RAD001 (0.1μM) for 48 hours, and the cell cycle was analyzed by flow cytometry using propidium iodide staining. The combination of panobinostat with everolimus significantly increased the percentage of cells in the G2/M phase. Each value is the mean of 3 independent experiments. (E) Summary of the percentage of cells in G2/M phase. **P < .005.
To further investigate the underlying molecular mechanisms involved in this enhanced inhibition of proliferation, we examined whether the combination of panobinostat and everolimus was more effective than either drug alone in inducing apoptosis. As shown in Figure 4C, there were more apoptotic HDLM-2 cells when panobinostat and everolimus were combined (35% vs 17% with panobinostat alone). This increase could be associated with a more important level of cleaved PARP, activated caspase 9 (data not shown). There was a light increase of the apoptosis in L-428 and KM-H2 cells. Panobinostat induced the activation of caspase 9 and PARP cleavage and down-regulated XIAP and Bcl-2. Adding everolimus did not enhance these molecular changes. Cell-cycle analysis performed by flow cytometry using propidium iodide staining revealed a marked increase in the G2/M fraction in all 3 cell lines after 48 hours of incubation with 0.02μM of panobinostat (Figure 4D-E). The percentage of cells blocked in G2/M was significantly increased when panobinostat was combined with everolimus.
Collectively, these data suggest that the enhanced antiproliferative effect observed with the combination of panobinostat and everolimus is predominantly the result of a cytostatic rather than a cytotoxic effect.
Panobinostat inhibits JAK/STAT pathway
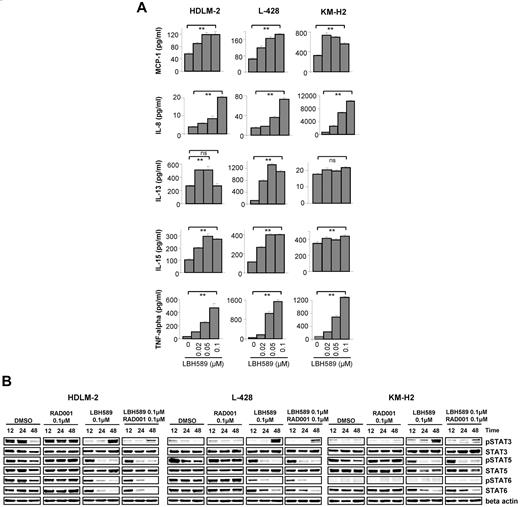
Both DAC inhibitors, vorinostat and MGCD0103, can inhibit STAT6 and alter cytokine and chemokine expression and production in HL cell lines.13 Therefore, we investigated whether panobinostat also may have similar properties, and examined whether the enhanced antiproliferative effect observed with the combination of panobinostat and everolimus was mediated by a coordinated effect on the JAK/STAT pathway. First, we examined the effect of panobinostat on the levels of 30 cytokines and chemokines in HL cell line supernatants and compared the results with those obtained with everolimus alone or with the combination (supplemental Figure 3). Panobinostat increased the production of MCP-1, IL-8, IL-15, IL-13, and TNF-α in a dose-dependent way (Figure 5A), whereas everolimus had no significant effect on the levels of these cytokines (data not shown). Because the JAK/STAT pathway is the principal signaling mechanism for a wide array of cytokines, we then measured the effect of the panobinostat/everolimus treatment on the activation of STATs. As shown in Figure 5B, panobinostat decreased the phosphorylation of STAT5 in the 3 cell lines after 24 hours. STAT6 phosphorylation was also decreased in HDLM-2 and L-428, but there was no phosphorylation of STAT6 in KM-H2 whatever the tested conditions. The panobinostat effect on STAT3 activation was more contrasted. After 12 and 24 hours, STAT3 phosphorylation was decreased, whereas it was increased after 48 hours. However, this late activation of STAT3 might be a consequence of the increase of TNF-α level observed in Figure 5A as previously reported.14 Indeed, experiments performed in 2 other HL cell lines, L-540 and HD-Myz, showed that STAT3 activation was not up-regulated in these cells after treatment with panobinostat for 48 hours, and there was no increase in the TNF-α level (supplemental Figure 5). Everolimus trended to increase STAT5 and STAT6 phosphorylation, and this effect was attenuated when everolimus was used in combination with panobinostat. Collectively, these data indicate that panobinostat is an effective inhibitor of STATs activity in HL cells and these changes may have contributed to the antiproliferative activity of panobinostat.
Effects of panobinostat (LBH589) on cytokine secretion in HL cell lines. (A) Cells were incubated with diluent control or LBH589 (0.02-0.1μM); and after 48 hours, supernatants were examined for cytokine levels using a multiplex assay. Each value represents a mean of 3 independent experiments ± SEM performed in triplicate. **P < .005. (B) Cells were incubated with diluent control or LBH589 (0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 12, 24, and 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti–phospho-STAT3 (Tyr 705), anti-STAT3, anti–phospho-STAT5 (Tyr 694), anti-STAT5, anti–phospho-STAT6 (Tyr 641), anti-STAT6, and anti–β-actin antibodies. Data are representative of 3 independent experiments showing similar results.
Effects of panobinostat (LBH589) on cytokine secretion in HL cell lines. (A) Cells were incubated with diluent control or LBH589 (0.02-0.1μM); and after 48 hours, supernatants were examined for cytokine levels using a multiplex assay. Each value represents a mean of 3 independent experiments ± SEM performed in triplicate. **P < .005. (B) Cells were incubated with diluent control or LBH589 (0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 12, 24, and 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti–phospho-STAT3 (Tyr 705), anti-STAT3, anti–phospho-STAT5 (Tyr 694), anti-STAT5, anti–phospho-STAT6 (Tyr 641), anti-STAT6, and anti–β-actin antibodies. Data are representative of 3 independent experiments showing similar results.
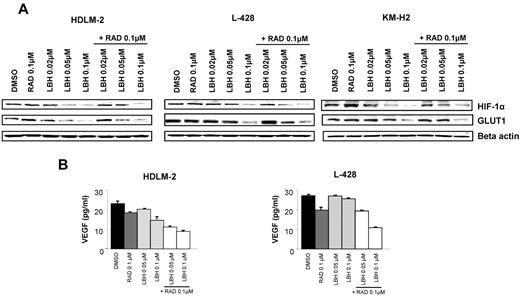
Panobinostat regulates angiogenesis and metabolism
Both mTOR and HDACs have been reported to regulate angiogenesis and tumor metabolism.15-18 Therefore, we examined the effects of everolimus and panobinostat on HIF-1α and its downstream targets GLUT1 and VEGF, and determined whether these 2 agents act synergistically on these proteins. As shown in Figure 6A, treatment with panobinostat inhibited the expression of HIF-1α, whereas 0.1μM everolimus did not. Panobinostat continued to inhibit HIF-1α expression in the presence of everolimus. HIF-1α has been implicated in regulating many of the genes involved in glucose metabolism, in particular GLUT1.18 Accordingly, panobinostat inhibition of HIF-1α expression was associated with down-regulation of GLUT1 expression in HL cell lines (Figure 6A). Furthermore, panobinostat significantly decreased VEGF production in the supernatants of HL cell lines (Figure 6B). Everolimus had also a significant effect on VEGF levels and enhanced the panobinostat's effect on VEGF production.
Effects of panobinostat (LBH589) and everolimus (RAD001) combination on angiogenesis and glucose metabolism. (A) Cells were incubated with diluent control, LBH589 (0.02-0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti–HIF-1α and anti-GLUT1 antibodies. (B) Cells were incubated with diluent control or LBH589 (0.02-0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 48 hours, supernatants were examined for VEGF levels using a multiplex assay. Each value represents a mean of 3 independent experiments ± SEM performed in triplicate.
Effects of panobinostat (LBH589) and everolimus (RAD001) combination on angiogenesis and glucose metabolism. (A) Cells were incubated with diluent control, LBH589 (0.02-0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 48 hours, cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti–HIF-1α and anti-GLUT1 antibodies. (B) Cells were incubated with diluent control or LBH589 (0.02-0.1μM), RAD001 (0.1μM), or the combination of LBH589 and RAD001; and after 48 hours, supernatants were examined for VEGF levels using a multiplex assay. Each value represents a mean of 3 independent experiments ± SEM performed in triplicate.
Discussion
Panobinostat has been evaluated in phase 1 and 2 trials of patients with relapsed HL. In a phase 1 study, 5 of 13 patients (38%) achieved partial response.19 On the basis of this promising clinical activity, a multicenter, international phase 2 study of panobinostat in relapsed HL was initiated, and preliminary results have demonstrated a significant clinical activity.6 Despite these clinical results, to date, there is no information on the mechanisms of action of panobinostat in HL.
In this study, we describe, for the first time, the complex biologic and molecular activity of panobinostat in HL cell lines. Our data provide insights on the mechanisms of the recently reported clinical activity of panobinostat in patients with relapsed HL. We demonstrated that panobinostat is among the most active HDAC inhibitors against HL cell lines in vitro, with IC50 in the nanomolar ranges.20 The antiproliferative activity involved regulation of several oncogenic pathways, including caspase activation and induction of apoptosis, inhibition of the activation of STAT5 and STAT6, and inhibition of tumor angiogenesis and regulation of glucose uptake. Our data also provide a mechanistic rationale for combining panobinostat with everolimus for the treatment of patients with HL.
A recent phase 2 trial of everolimus has demonstrated overall response rates of approximately 47% for relapsed HL.21 This single-agent activity in relapsed/refractory HL provides rationale that target the mTOR pathway is clinically relevant. Nevertheless, it has been previously reported that everolimus can also activate the mTORC2 target AKT in diffuse large B-cell lymphoma cells and the subsequent increase in AKT phosphorylation can probably contribute to attenuating the antitumor activity of everolimus and induce a potential mechanism of everolimus resistance.11 Our results are consistent with this everolimus potential resistance. Indeed, although everolimus can suppress phosphorylation of p70S6K and the activation of 4EBP1, this results in only a modest antiproliferative effect. In addition, we also found an increase of the AKT phosphorylation with everolimus treatment in HL cell lines. Gupta et al reported that there is a synergistic activity between an mTOR inhibitor and panobinostat because inhibition of HDAC overcomes rapamycin-mediated resistance by inhibiting AKT signaling through mTORC2.11 In our study, panobinostat was able to abrogate everolimus-induced AKT activation as well as constitutive activation of AKT. Surprisingly, panobinostat had unanticipated effects on mTORC1. Panobinostat induced an activation of mTORC1 and led to an activation of p70S6K and 4EBP1. This finding is in contrast to the study by Gupta et al11 in diffuse and large B-cell lymphoma in which panobinostat effectively inhibited mTORC1 and underlines the divergences that can occur between different cell lines. As previously reported in other cancer types,22,23 panobinostat induced a cell-cycle arrest in G2/M in HL cell lines. Moreover, the combination of panobinostat with everolimus was also more effective to induce this cell-cycle arrest in G2/M than the use of panobinostat alone. The antiproliferative synergistic activity observed with these drugs can thus be related to the effectiveness of the combination of panobinostat with everolimus to induce the cell-cycle arrest and may partly be explained by the complementary action of these drugs on the PI3K/AKT/mTOR pathway (Figure 7). Indeed, a previous study from Georgakis et al reported that an inhibition of this pathway in HL cell lines promotes cell-cycle arrest.9
Model of the effect of panobinostat (LBH589) and everolimus (RAD001) on the PI3K/AKT/mTOR pathway. This model shows the complementary inhibition of panobinostat and everolimus on the PI3K/AKT/mTOR pathway. LBH589 inhibits the AKT activation induced by RAD001, whereas RAD001 inhibits the mTOR activation induced by LBH589.
Model of the effect of panobinostat (LBH589) and everolimus (RAD001) on the PI3K/AKT/mTOR pathway. This model shows the complementary inhibition of panobinostat and everolimus on the PI3K/AKT/mTOR pathway. LBH589 inhibits the AKT activation induced by RAD001, whereas RAD001 inhibits the mTOR activation induced by LBH589.
In our study, HIF-1α expression was only affected with the use of panobinostat. Everolimus has been previously shown to exert an antiangiogenic activity via down-regulation of HIF-1α.24 Nevertheless, this finding was performed in different cell lines, and results may differ depending on the cell sensitivity to everolimus.
Cancer cells metabolize glucose differently from normal cells and preferentially switch to aerobic glycolysis rather than oxidative phosphorylation. For this reason, targeting the glucose metabolism may have significant value in treating cancer.25,26 Treatment with panobinostat alone also affected the expression of GLUT1, another HIF-1α target gene. This finding is of clinical interest as it suggests that panobinostat therapy may cause false-negative FDG-PET results. To further investigate this possibility, we are currently performing core needle biopsies from HL lesions that become FDG-PET negative while receiving HDAC inhibitors.
The PI3K/AKT/mTOR pathway is one of the most commonly activated oncogenic pathways in cancer cells, including HL. In this study, we demonstrated that panobinostat and everolimus act in a coordinated and complimentary manner on different key components of this pathway, resulting in inhibition of reciprocal feedback loops, leading to an enhanced antiproliferative activity. This finding provides a mechanistic rationale for combining these 2 active agents, as we have implemented in an ongoing phase 1 study in patients with relapsed HL. Early results from this study are demonstrating a significant clinical activity in heavily pretreated patients with HL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kristi M. Speights for editorial assistance.
This work was supported in part by the National Institutes of Health (SPORE grant 1P50CA136411-01A1), the MD Anderson Cancer Center (support grant CA016672), the Clay Chiles Lymphoma Fund, and the Jack L. Stotsky Memorial Fund (all A.Y.).
National Institutes of Health
Authorship
Contribution: M.L. conceived the study, performed experiments, analyzed data, prepared the figures, and wrote the paper; E.D. and D.B. performed experiments and analyzed data; L.J.M. and R.E.D. evaluated results and assisted in writing the manuscript; J.Z. assisted in the statistical analysis; Y.J. assisted in the statistical analysis; and A.Y. conceived the study, helped design experiments, evaluated the results, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anas Younes, Department of Lymphoma and Myeloma, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ayounes@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal