Abstract
The Wiskott-Aldrich syndrome protein (WASP) is a key cytoskeletal regulator of hematopoietic cells. Although WASP-knockout (WKO) mice have aberrant B-cell cytoskeletal responses, B-cell development is relatively normal. We hypothesized that N-WASP, a ubiquitously expressed homolog of WASP, may serve some redundant functions with WASP in B cells. In the present study, we generated mice lacking WASP and N-WASP in B cells (conditional double knockout [cDKO] B cells) and show that cDKO mice had decreased numbers of follicular and marginal zone B cells in the spleen. Receptor-induced activation of cDKO B cells led to normal proliferation but a marked reduction of spreading compared with wild-type and WKO B cells. Whereas WKO B cells showed decreased migration in vitro and homing in vivo compared with wild-type cells, cDKO B cells showed an even more pronounced decrease in the migratory response in vivo. After injection of 2,4,6-trinitrophenol (TNP)–Ficoll, cDKO B cells had reduced antigen uptake in the splenic marginal zone. Despite high basal serum IgM, cDKO mice mounted a reduced immune response to the T cell–independent antigen TNP-Ficoll and to the T cell–dependent antigen TNP–keyhole limpet hemocyanin. Our results reveal that the combined activity of WASP and N-WASP is required for peripheral B-cell development and function.
Introduction
B cells are generated via sequential differentiation steps in the BM and enter the circulation as immature, surface IgM-expressing cells.1 Immature B cells migrate into the spleen, where they differentiate into mature, naive B cells through highly regulated developmental steps. Naive, mature B cells recirculate through the bloodstream and enter into peripheral lymph nodes, peritoneal or pleural cavities, gut-associated lymphatic tissue, and the spleen, where they differentiate into effector cells in response to specific antigenic challenge. In the spleen, B cells can undergo an important cell-fate decision to become either a follicular (FO) or a marginal zone (MZ) B cell.1 FO B cells reside inside B-cell follicles, where they can undergo affinity maturation and class-switch recombination in response to antigenic challenge.2 MZ B cells reside in the splenic MZ, a location that provides a first line of defense against blood-borne pathogens. Peripheral B-cell development, activation, and function require both migration and adhesive properties. FO B cells depend on signaling by the chemokine receptor CXCR5 to localize to the follicles, whereas MZ B cells are sensitive to sphingosine-1-phosphate (S1P), which is highly concentrated in blood.1 Adhesion by MZ B cells to ICAM-1 and α4β1 integrin is critical for MZ B-cell retention in the MZ, an area that is exposed to the sheer stress of blood flow.1
The Wiskott-Aldrich syndrome protein (WASP) coordinates cell-surface signaling to changes in the actin cytoskeleton and is a key organizer of migration and adhesion in hematopoietic cells.3,4 In recent years, it has become clear that WASP deficiency affects specific aspects of B-cell biology. Although WASP seems dispensable for B-cell development in the BM, WASP serves a critical role in peripheral B-cell homeostasis and lack of WASP leads to a specific reduction of MZ precursor cells and MZ B cells.5-8 WASP-deficient MZ B cells fail to respond to S1P and show aberrant integrin clustering downstream of BCR engagement during formation of the B-cell immunologic synapse.5,8 Two recent papers show that cell-intrinsic loss of WASP in B cells cause breakdown of B-cell tolerance in the setting of normal T-cell function.9,10
WASP belongs to the family of proteins that includes N-WASP and several WAVE molecules.11 WASP is expressed exclusively in leukocytes. N-WASP is the closest homolog and shares 50% homology with WASP; it is ubiquitously expressed and is critical for development because N-WASP deficiency is embryonically lethal.12 Conditional deletion of N-WASP in keratinocytes has revealed that N-WASP deficiency leads to epidermal hyperproliferation and progressive loss of hair follicle cycling.13,14 Although WASP plays a key role in the function of most leukocytes, the functional contribution of N-WASP in these cell types is less clear. Compared with WASP deficiency alone, combined deletion of WASP and N-WASP in T cells leads to a profound block in thymopoiesis, resulting in marked reduction of CD4+ and CD8+ T cells in the periphery and a more pronounced defect in T-cell migration.15 N-WASP deletion alone had no apparent effect on T-cell function. The role of N-WASP in the development and function of other hematopoietic cells, including B cells, remains unknown.
In the present study, we sought to explore the unique and redundant activity of WASP and N-WASP in B cells, and found that the combined activity of WASP and N-WASP is required for peripheral B-cell development and for the capacity of B cells to take up and respond to antigens.
Methods
Animals
Mice were housed at Boston's Children's Hospital and at Massachusetts General Hospital under specific pathogen-free conditions. Animal experiments were carried out after approval and in accordance with guidelines from the Subcommittee on Research Animal Care of Children's Hospital and Massachusetts General Hospital. Wild-type (WT), WASP-knockout (WKO), conditionally targeted N-WASP–knockout (cNWKO), and WASP and N-WASP conditional double-knockout (cDKO) mice were littermates from breedings of WT 129Sv mice, WKO mice on a129Sv background, conditional N-WASP KO mice on a 129Sv background, and CD19-Cre mice on a C57Bl/6 background.
Proliferation, spreading, chemotaxis, and in vivo homing
The proliferative response was assessed in vitro as described previously using [3H]thymidine incorporation.15,16 B cells were purified with the CellSep B-cell enrichment kit (StemCell Technologies) and cultured with Abs for IgM and CD40 (eBiosciences), lipopolysaccharide (Sigma-Aldrich), and IL-4 (PeproTech). For in vivo proliferation/expansion, mice were fed with bromodeoxyuridine (BrdU; Sigma-Aldrich) for 6 days, and BrdU+ cells were identified using a BrdU-labeling kit (BD Biosciences). For B-cell spreading, B cells were cultured for 48 hours in lipopolysaccharide and IL-4 and incubated on anti-CD44 Ab–coated slides that had been precoated with poly-L-lysine for 8 hours. Cells were fixed and stained with Alexa Fluor 488–phalloidin. Spread B cells were defined as having at least one protrusion longer than one cell diameter in length compared with nonspread cells. In vitro migration of B cells to CXCL12 was assessed as described previously.7,15 In vivo homing of B cells was performed as described previously.17 Single-cell suspensions of spleen B cells were prepared from WT, WKO, and cDKO mice. Cells were labeled with either CFSE or tetramethylrhodamine isothiocyanate (both from Invitrogen) and injected IV into WT recipient mice at a 1:1 ratio. After 12-15 hours, spleen, peripheral, and mesenteric lymph nodes, Peyer patches, BM, and blood lymph nodes were analyzed by flow cytometry. The relative frequency of the 2 donor-cell populations was determined for each individual organ, and a homing index was calculated as described previously.17 Similar results were obtained when WT, WKO, and cDKO cells were stained with the alternative labeling agent.
Flow cytometry and immunohistochemistry
For flow cytometry, single-cell suspensions were labeled with fluorescently conjugated anti–mouse Abs including B220, CD5, CD11b, CD19, CD21, CD23, CD43, CD93, IgD, and IgM (all eBiosciences) and Fas, GL7, LFA-1, and 2,4,6-trinitrophenol (TNP, all BD Biosciences). Data were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo Version 8.2 software for Mac (TreeStar). For immunohistochemistry, sections were fixed in ice-cold acetone and labeled with fluorescently conjugated anti–mouse Abs including MOMA, MARCO, SIGN-R1 (Serotec) CD1d, B220, and TNP (all BD Biosciences) and peanut agglutinin (Vector Laboratories). All slides were viewed at room temperature with an Olympus Provis AX70 research system microscope using an UplanFl lens at 100× and Mowiol medium (Calbiochem). Images were acquired using a U-PHOTO Universal Photo System camera (Olympus) model U-CMAD-2 and were processed with MagnaFire 2.1c (Optronics) and Adobe Photoshop CS Version 8.0. Germinal center (GC) areas were measured on images of random sections using ImageJ 1.45 software for Mac and were calculated as a percentage of total spleen area in a particular image. A mean value of measurements from 3 images of each spleen was then determined.
Immunizations
For TI-2 antigen responses, mice were injected intravenously with TNP-Ficoll (Biosearch Technologies) and uptake of TNP-Ficoll in the MZ and by MZ B cells was examined 30 minutes and 3 hours after injection. For T cell–dependent antigen response, mice were immunized by IP injection of TNP–keyhole limpet hemocyanin (TNP-KLH) in alum. Three weeks later, mice were boosted with a second injection of TNP-KLH. GC cells and GC areas were quantified by flow cytometry and immunohistochemistry. To determine anti-TNP Ab titers in response to TNP-Ficoll and TNP-KLH immunization, anti-TNP IgM, IgG3, and IgG1 were measured by ELISA. The samples were run in triplicate and corrected for background binding.
Statistics
Data are expressed as means ± SD where indicated. Statistical significance between groups was assessed by 2-tailed Student t test and ANOVA. Differences were considered significant when P < .05.
Results
Specific deletion of WASP and N-WASP in B cells results in decreased number of peripheral B cells
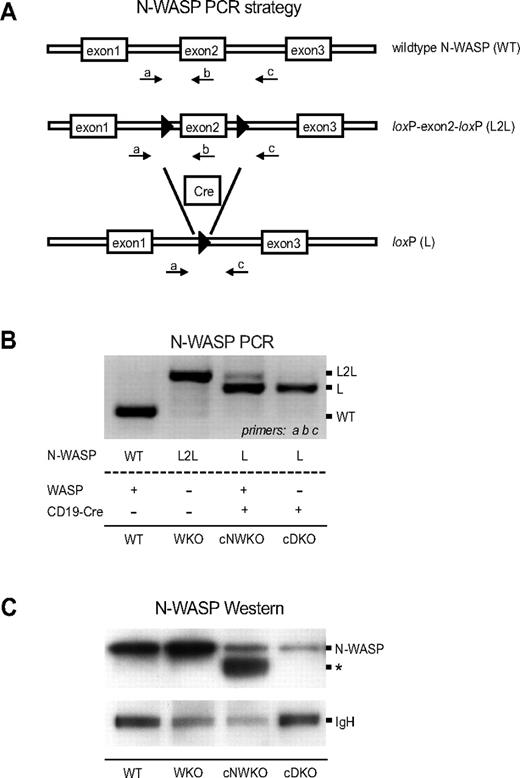
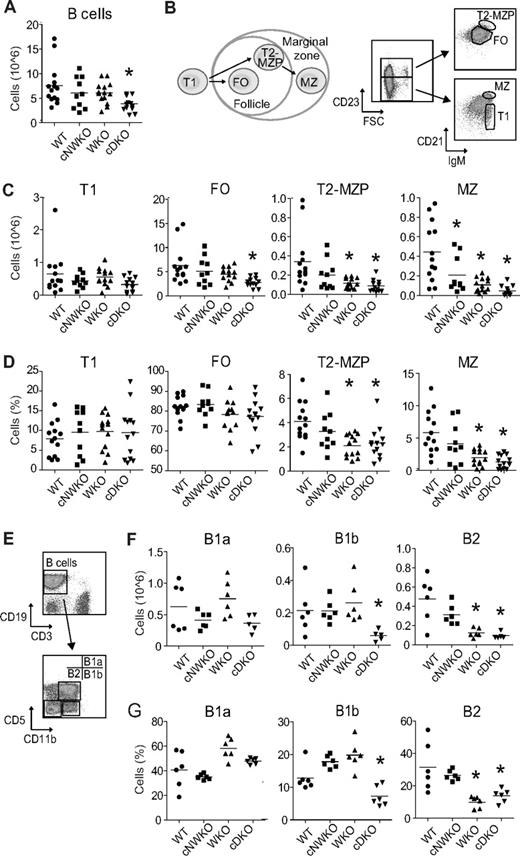
WKO mice have normal B-cell development except for a marked decrease of MZ B cells.5,7,8,16 We have demonstrated previously that both WASP and N-WASP are critical for T-cell development.15 We hypothesized that N-WASP may also have overlapping function with WASP in B-cell development and in the present study sought to address the unique and redundant function of N-WASP during B-cell lymphopoiesis. To circumvent the embryonic lethality associated with N-WASP deficiency in the mouse germline,12,18 we inactivated N-WASP specifically in B cells using the Cre-loxP system. We bred cNWKO mice generated previously15 with WKO mice and transgenic mice expressing Cre recombinase under the B cell–specific CD19 promoter (CD19-Cre) to generate cDKO mice. The use of the CD19-Cre–transgenic mouse results in deletion of loxP-flanked genes in BM-derived pre-B cells.19 PCR and Western blot analyses showed nearly complete excision of N-WASP exon 2 and reduced N-WASP protein expression, respectively, in splenic B cells expressing the Cre protein (Figure 1A-C). B-cell development in the BM was similar in WT, WKO, and cDKO mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Peripheral B cells were present at normal numbers in WT, cNWKO, and WKO mice, whereas the absolute B-cell number in cDKO mice was reduced (Figure 2A). We next analyzed the absolute and relative number of cellular subsets in peripheral B-cell development (Figure 2B-D). WT, cNWKO, and WKO mice had normal numbers of transitional type 1 (T1) cells and FO B cells (Figure 2C-D). As shown previously, transitional type 2 (T2)–MZP and MZ B cells were decreased in WKO mice (Figure 2C-D).5,8 cDKO mice had a normal number of T1 B cells, a decreased number of FO and T2-MZP B cells, and a markedly decreased number of MZ B cells (Figure 2C-D). Whereas there was no difference in the number of T2-MZP B cells in cNWKO mice, these mice did have a decreased number of MZ B cells (Figure 2C). To address the reason that cNWKO, WKO, and cDKO mice had reduced numbers of MZ B cells, we examined the expression of LFA-1, an adhesion molecule critical for retention of MZ B cells in the MZ. As expected, we detected increased expression of LFA-1 as WT B cells progressed from T1 to MZ B cells (supplemental Figure 2A). The expression of LFA-1 was modestly decreased in T2-MZP and MZ B cells from cNWKO, WKO, and cDKO mice compared with WT mice (supplemental Figure 2B). To delineate if the decreased number of precursor cells may explain the reduced number of FO B cells in cDKO mice, we examined FO precursor cells: T2 and transitional type 3 (T3) cells. Whereas WKO mice had decreased numbers of T3 cells, cDKO mice had decreased numbers of both T2 and T3 subsets, indicating a decreased number of FO precursor cells (supplemental Figure 3). We next examined the B-cell compartment in the peritoneum. Whereas WT, cNWKO, WKO, and cDKO mice had similar numbers and frequencies of B1a cells, cDKO mice had a decreased number of B1b cells (Figure 2F-G). Both WKO and cDKO mice had decreased numbers of peritoneal B2 cells, which are an intermediate between splenic B2 and peritoneal B1 cells (Figure 2F-G).
Generation of mice devoid of WASP and N-WASP in B cells. (A) Schematic of the PCR strategy used to monitor conditional targeting of N-WASP. Displayed is a portion of the WT N-WASP allele, the conditionally targeted allele with exon 2 flanked by loxP sites (L2L), and the conditionally targeted allele after Cre-mediated excision in B cells (L). LoxP sites are denoted by black arrowheads. Labeled arrows denote primers used in the PCR strategy to simultaneously detect L2L and L using primers a, b, and c. (B) PCR analysis of N-WASP deletion in splenic B cells from WT, WKO, cNWKO, and cDKO mice. Note that the WKO mouse in this experiment has the N-WASP L2L allele but lacks expression of CD19-Cre for deletion of N-WASP L2L, and therefore expresses N-WASP. (C) N-WASP protein detection by Western blotting in splenic B cells from WT, WKO, cNWKO, and cDKO mice using an Ab for N-WASP (top panel). IgH was used as loading control (bottom panel). The weak expression of N-WASP seen in cells from cNWKO and cDKO mice may reflect the incomplete deletion of N-WASP by CD19-Cre or the contribution of other hematopoietic cells left after B-cell purification (90%-95% B-cell purity). The asterisk denotes a band present in cNWKO B cells that may represent WASP, because the anti–N-WASP Ab shows some cross-reactivity with WASP.
Generation of mice devoid of WASP and N-WASP in B cells. (A) Schematic of the PCR strategy used to monitor conditional targeting of N-WASP. Displayed is a portion of the WT N-WASP allele, the conditionally targeted allele with exon 2 flanked by loxP sites (L2L), and the conditionally targeted allele after Cre-mediated excision in B cells (L). LoxP sites are denoted by black arrowheads. Labeled arrows denote primers used in the PCR strategy to simultaneously detect L2L and L using primers a, b, and c. (B) PCR analysis of N-WASP deletion in splenic B cells from WT, WKO, cNWKO, and cDKO mice. Note that the WKO mouse in this experiment has the N-WASP L2L allele but lacks expression of CD19-Cre for deletion of N-WASP L2L, and therefore expresses N-WASP. (C) N-WASP protein detection by Western blotting in splenic B cells from WT, WKO, cNWKO, and cDKO mice using an Ab for N-WASP (top panel). IgH was used as loading control (bottom panel). The weak expression of N-WASP seen in cells from cNWKO and cDKO mice may reflect the incomplete deletion of N-WASP by CD19-Cre or the contribution of other hematopoietic cells left after B-cell purification (90%-95% B-cell purity). The asterisk denotes a band present in cNWKO B cells that may represent WASP, because the anti–N-WASP Ab shows some cross-reactivity with WASP.
Specific deletion of WASP and N-WASP in B cells results in a decreased number of peripheral B cells. (A) Total absolute number of spleen B cells in WT, WKO, cNWKO, and cDKO mice. (B) Schematic diagram depicting B-cell development in the spleen (left) and flow cytometric analysis to define T1, FO, T2-MZP, and MZ B cells (right) in absolute number (C) and relative number (D) of cells. (E) Flow cytometry of peritoneum cells to define B1a, B1b, and B2 cells in absolute (F) and relative (G) numbers. Each group represents averages ± SD from 14 (WT, WKO, and cDKO) and 10 (cNWKO) mice for panels A through D and 6 mice per group in panels E through G. *P < .05 compared with WT.
Specific deletion of WASP and N-WASP in B cells results in a decreased number of peripheral B cells. (A) Total absolute number of spleen B cells in WT, WKO, cNWKO, and cDKO mice. (B) Schematic diagram depicting B-cell development in the spleen (left) and flow cytometric analysis to define T1, FO, T2-MZP, and MZ B cells (right) in absolute number (C) and relative number (D) of cells. (E) Flow cytometry of peritoneum cells to define B1a, B1b, and B2 cells in absolute (F) and relative (G) numbers. Each group represents averages ± SD from 14 (WT, WKO, and cDKO) and 10 (cNWKO) mice for panels A through D and 6 mice per group in panels E through G. *P < .05 compared with WT.
WASP- and N-WASP–deficient B cells alter the MZ architecture
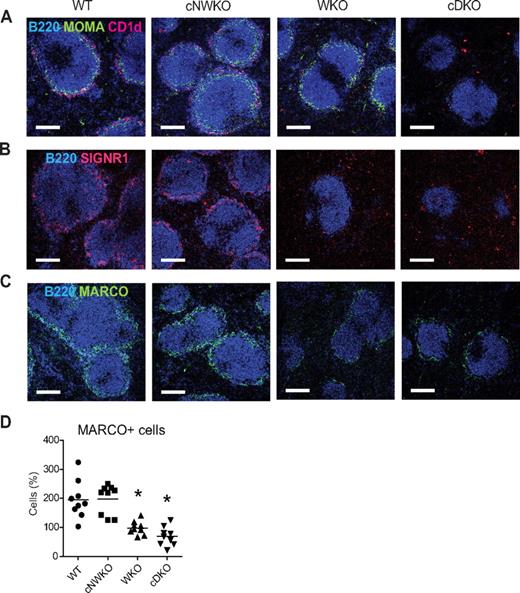
The splenic MZ is the site where blood flows into the spleen. MZ-resident cells include MZ B cells and MZ macrophages that express scavenger receptors for rapid uptake of blood-borne substances and apoptotic cells. Another subset of macrophages, metallophilic macrophages, delineates the border between the outer MZ and the inner B-cell follicle. WKO mice have a decreased number of both MZ B cells and MZ macrophages and fairly normal numbers of metallophilic macrophages.6,8,20 To address whether the combined activity of WASP and N-WASP in B cells is important for MZ architecture, we examined spleen sections of WT, cNWKO, WKO, and cDKO mice to identify MZ B cells, MZ macrophages, and metallophilic macrophages. MZ B cells (CD1d+) were present in the MZ of WT and cNWKO mice and not detectable in WKO and cDKO mice (Figure 3A). Metallophilic macrophages (MOMA+) were present at normal numbers in WT, cNWKO, and WKO mice, whereas cDKO mice showed a marked reduction in metallophilic macrophages (Figure 3A). To identify MZ macrophages, we used staining for the scavenger receptors SIGN-R1 and MARCO. The rim of MZ macrophages surrounding the B-cell follicle was clearly identified in WT and cNWKO mice, whereas WKO and cDKO mice had a decreased number of MZ macrophages (Figure 3B-D).
WASP and N-WASP-deficient B cells alter the MZ architecture. Immunohistochemistry of spleen sections from WT, WKO, and cDKO mice. (A) FO B cells were visualized with B220, MZ B cells with CD1d, and metallophilic macrophages with MOMA-1 Ab staining. MZ macrophages were visualized with SIGNR1 (B) and MARCO (C) Ab staining. (D) Quantification of MARCO+ cells from spleen sections shown in panel C. Each group represents quantification from 3 mice and 3 sections from each mouse. Note the marked reduction of FO B cells, MZ B cells, metallophilic macrophages, and MZ macrophages in cDKO mice. Original magnification was 10×. This experiment is representative of analysis of at least 3 WT, WKO, and cDKO mice.
WASP and N-WASP-deficient B cells alter the MZ architecture. Immunohistochemistry of spleen sections from WT, WKO, and cDKO mice. (A) FO B cells were visualized with B220, MZ B cells with CD1d, and metallophilic macrophages with MOMA-1 Ab staining. MZ macrophages were visualized with SIGNR1 (B) and MARCO (C) Ab staining. (D) Quantification of MARCO+ cells from spleen sections shown in panel C. Each group represents quantification from 3 mice and 3 sections from each mouse. Note the marked reduction of FO B cells, MZ B cells, metallophilic macrophages, and MZ macrophages in cDKO mice. Original magnification was 10×. This experiment is representative of analysis of at least 3 WT, WKO, and cDKO mice.
WASP and N-WASP regulate B-cell spreading and migration
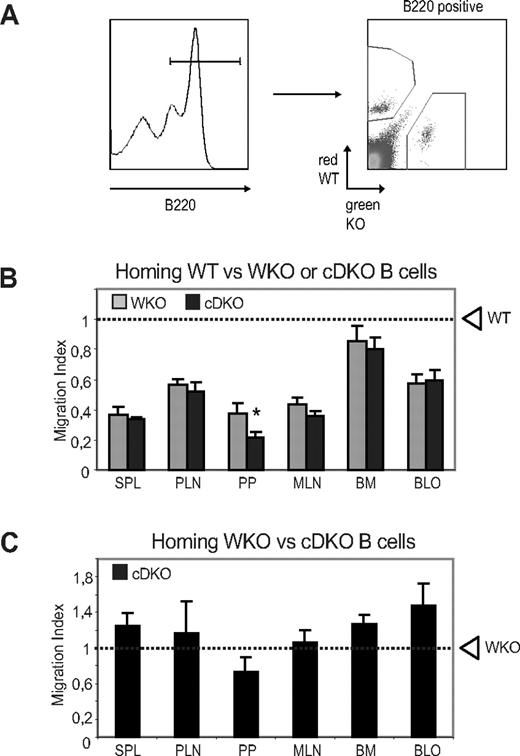
To evaluate whether the reduced number of peripheral B cells might result from decreased survival or proliferation, we first investigated whether WASP and N-WASP are required for receptor-mediated B-cell proliferation and found that WT, WKO, cNWKO, and cDKO B cells showed similar proliferative responses (Figure 4A). To address how B-cell turnover/proliferation in vivo may be affected by WASP and N-WASP double deficiency, we fed mice with BrdU for 6 days and analyzed BrdU+ cells in different B-cell compartments. We detected no differences in expansion of T1, FO, and T2-MZP B cells comparing WT, cNWKO, WKO, and cDKO mice (supplemental Figure 4). A higher proliferation of MZ B cells was detected in WKO and cDKO mice, suggesting increased homeostatic expansion of MZ B cells (supplemental Figure 4). We next examined cell death in cultured B cells and determined that the frequency of necrotic (7-amino-actinomycin D positive and annexin V positive) and apoptotic (7-amino-actinomycin D negative and annexin V positive) cells was similar in B cells from all mice (supplemental Figure 5). Because WASP family members play a critical role in cytoskeletal reorganization and in trafficking of immune cells, the receptor-mediated cytoskeletal responses of B cells was evaluated. To assess the spreading response, activated B cells were incubated on surfaces coated with Abs to CD44. WT and cNWKO B cells showed a high percentage of spread cells, defined as having at least 1 long protrusion, whereas WKO B cells had a reduced number of spread cells,21 and cDKO B cells showed an even more pronounced defect in the formation of long protrusions (Figure 4B-C). An in vitro chemotaxis assay was used to determine whether WASP and N-WASP double deficiency influenced the migration of B cells to the chemokine CXCL12, which is critical for the homing of mature B cells into lymphoid organs. Migration to CXCL12 was reduced in both WKO and cDKO B cells compared with WT and cNWKO B cells (Figure 4D). Although the analysis of cNWKO mice showed a reduction of MZ B cells (Figure 2C-D), in functional assays such as migration and spreading, cNWKO B cells responded similarly to WT B cells and we therefore did not analyze the cNWKO mice further. To evaluate how reduced spreading and migratory capacity of cDKO B cells would influence B-cell trafficking into lymphoid organs, we performed an in vivo homing assay. WT and KO (WKO or cDKO) B cells were labeled with tetramethylrhodamine isothiocyanate (red fluorescence) or CFSE (green fluorescence) and mixed at a ratio of 1:1 immediately before IV injection into a WT recipient mouse. To monitor the homing of labeled cells, recipient mice were killed 12 hours after the adoptive B-cell transfer and the percentage of labeled cells was analyzed using flow cytometry (Figure 5A). Compared with WT cells, both WKO and cDKO B cells showed reduced homing into secondary lymphoid organs including the spleen and the peripheral and mesenteric lymph nodes (Figure 5B). Homing defects were more pronounced in cDKO B cells compared with WKO B cells in Peyer patches (Figure 5B). To address competitive homing between WKO and cDKO B cells directly, a 1:1 ratio cell ratio was injected into WT recipient mice and homing into lymphoid organs was assessed. We found no difference in the homing capacity of WKO and cDKO B cells (Figure 5C). The pronounced defect of homing into Peyer patches by cDKO B cells may result from the increase in flow rates (ie, shear stress) unique to this lymphoid compartment.
WASP and N-WASP are dispensable for B-cell proliferation, but regulate B-cell spreading and migration. (A) Proliferation. Splenic B cells were stimulated for 48 hours with the indicated stimulus, followed by a 16-hour pulse with [3H]thymidine to determine the proliferative response. Bars represent mean values of cpm ([3H]thymidine) ± SD of triplicate wells from 1 of at least 3 independent experiments. (B) Spreading. Spreading of activated B cells was assessed on anti-CD44 Ab-coated surfaces. White arrowhead depicts the formation of long protrusions of spread B cells. (C) Graphs showing the average of relative numbers (± SD) of spread B cells in triplicate representative of 3 experiments. (D) Migration. Splenic B or T cells were allowed to migrate to CXCL12 for 3 hours using an in vitro chemotaxis chamber. Migrating cells were collected and enumerated by flow cytometry with reference beads. The percentage of migrating cells is shown as mean values ± SD of triplicate wells and data are representative of at least 3 experiments. *P < .05 compared with WT.
WASP and N-WASP are dispensable for B-cell proliferation, but regulate B-cell spreading and migration. (A) Proliferation. Splenic B cells were stimulated for 48 hours with the indicated stimulus, followed by a 16-hour pulse with [3H]thymidine to determine the proliferative response. Bars represent mean values of cpm ([3H]thymidine) ± SD of triplicate wells from 1 of at least 3 independent experiments. (B) Spreading. Spreading of activated B cells was assessed on anti-CD44 Ab-coated surfaces. White arrowhead depicts the formation of long protrusions of spread B cells. (C) Graphs showing the average of relative numbers (± SD) of spread B cells in triplicate representative of 3 experiments. (D) Migration. Splenic B or T cells were allowed to migrate to CXCL12 for 3 hours using an in vitro chemotaxis chamber. Migrating cells were collected and enumerated by flow cytometry with reference beads. The percentage of migrating cells is shown as mean values ± SD of triplicate wells and data are representative of at least 3 experiments. *P < .05 compared with WT.
WASP and N-WASP regulate in vivo homing of B cells. (A) Splenic B cells from WT, WKO, and cDKO mice were labeled with tetramethylrhodamine isothiocyanate (WT, red) or CFSE (WKO or cDKO, green) and mixed at a 1:1 ratio before IV injection to WT mice. (B) Competitive homing of WT and WKO or cDKO B cells. Spleen, peripheral and mesenteric lymph nodes, Peyer patches, BM, and blood of recipient mice were harvested after 12-15 hours, and the percentage of WKO and cDKO B-cell homing relative to WT cells was determined. (C) Competitive homing of WKO (tetramethylrhodamine isothiocyanate–labeled) and cDKO (CFSE-labeled) B cells. Shown are averages ± SD of combined data from 2 experiments including 4 mice per group. Dashed line represents the input percentage. *P < .05 compared with WKO.
WASP and N-WASP regulate in vivo homing of B cells. (A) Splenic B cells from WT, WKO, and cDKO mice were labeled with tetramethylrhodamine isothiocyanate (WT, red) or CFSE (WKO or cDKO, green) and mixed at a 1:1 ratio before IV injection to WT mice. (B) Competitive homing of WT and WKO or cDKO B cells. Spleen, peripheral and mesenteric lymph nodes, Peyer patches, BM, and blood of recipient mice were harvested after 12-15 hours, and the percentage of WKO and cDKO B-cell homing relative to WT cells was determined. (C) Competitive homing of WKO (tetramethylrhodamine isothiocyanate–labeled) and cDKO (CFSE-labeled) B cells. Shown are averages ± SD of combined data from 2 experiments including 4 mice per group. Dashed line represents the input percentage. *P < .05 compared with WKO.
cDKO mice show a reduced immune response to TNP-Ficoll
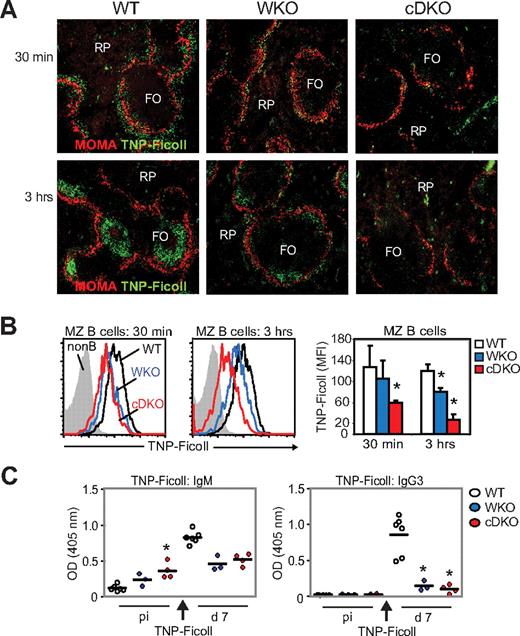
Positioning of MZ B cells in the MZ is critical for the uptake of blood-borne antigens and for antigen delivery to follicular dendritic cells.22 We reasoned that the pronounced decrease in MZ B cells and defects in B-cell adhesion and migration in cDKO mice would reduce the uptake and delivery of blood-borne antigen to the B-cell follicle. WT, WKO, and cDKO mice were immunized with TNP-Ficoll, a type 2 T-independent antigen, and antigen uptake was examined on splenic sections by immunohistology. In WT mice, as has been shown previously, TNP-Ficoll was detected in the MZ 30 minutes after injection and was localized exclusively to the B-cell follicles 3 hours after injection (Figure 6A).22 In WKO mice, less TNP-Ficoll was detected in the MZ at 30 minutes and in the follicle at 3 hours (Figure 6A).8 In cDKO mice, only scattered TNP-Ficoll was detected in the MZ at 30 minutes, and TNP-Ficoll was undetectable in the follicle at 3 hours (Figure 6A). To complement these studies, we next examined specific antigen uptake by MZ B cells using flow cytometry. Compared with WT MZ B cells, we found that WKO MZ B cells had reduced uptake of TNP-Ficoll, and this uptake defect was even more pronounced in cDKO MZ B cells (Figure 6B). To evaluate the specific Ab response to TNP-Ficoll, we measured TNP-specific serum titers by ELISA. Unchallenged WT mice had low TNP-reactive IgM Abs that increased at day 7 after immunization with TNP-Ficoll (Figure 6C left panel). As shown previously, unchallenged WKO mice had increased serum titers of TNP-reactive IgM Abs and a decreased specific immune response to TNP-Ficoll at day 7 compared with WT mice (Figure 6C left panel).8 Unchallenged cDKO mice had markedly elevated, TNP-reactive IgM Abs and, after immunization, there was no increased specific response to TNP-Ficoll (Figure 6C left panel). To determine whether cDKO B cells could undergo class-switch recombination after antigenic challenge, we examined TNP-specific IgG3 Abs after TNP-Ficoll immunization. WT mice responded to TNP-Ficoll with increased TNP-specific IgG3 Abs at day 7, whereas WKO and cDKO mice showed a significantly reduced response (Figure 6C right panel).
cDKO mice show a reduced immune response to TNP-Ficoll. WT, WKO, and cDKO mice were injected IV with 2.5 μg of TNP-Ficoll. (A) Uptake of TNP-Ficoll in the spleen 30 minutes and 3 hours after injection. Spleen sections were labeled to detect TNP-Ficoll and MOMA+ metallophilic macrophages to define the MZ. Note the marked reduction of TNP in the MZ at 30 minutes (top panel) and in the follicle at 3 hours (bottom panel) in cDKO mice compared with WT mice. Original magnification was 10×. (B) MZ B cells were labeled with anti-TNP Abs and analyzed by flow cytometry. (C) Anti-TNP IgM and IgG3 Ab titers were determined at day 7 after immunization by ELISA. Test samples were corrected for background binding. Each group represents 6 WT, 3 WKO, and 4 cDKO mice. Black bar represents the mean value for the group. Non-B indicates lymphocytes negative for CD21 and IgM; and RP, red pulp. *P < .05 compared with WT.
cDKO mice show a reduced immune response to TNP-Ficoll. WT, WKO, and cDKO mice were injected IV with 2.5 μg of TNP-Ficoll. (A) Uptake of TNP-Ficoll in the spleen 30 minutes and 3 hours after injection. Spleen sections were labeled to detect TNP-Ficoll and MOMA+ metallophilic macrophages to define the MZ. Note the marked reduction of TNP in the MZ at 30 minutes (top panel) and in the follicle at 3 hours (bottom panel) in cDKO mice compared with WT mice. Original magnification was 10×. (B) MZ B cells were labeled with anti-TNP Abs and analyzed by flow cytometry. (C) Anti-TNP IgM and IgG3 Ab titers were determined at day 7 after immunization by ELISA. Test samples were corrected for background binding. Each group represents 6 WT, 3 WKO, and 4 cDKO mice. Black bar represents the mean value for the group. Non-B indicates lymphocytes negative for CD21 and IgM; and RP, red pulp. *P < .05 compared with WT.
WASP and N-WASP are required for the B-cell immune response to TNP-KLH
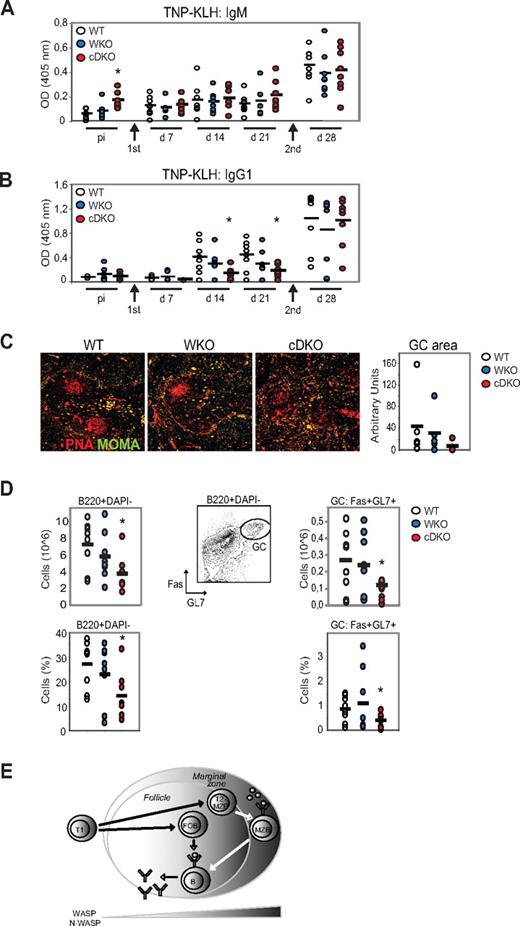
To assess the responsiveness of cDKO mice to a T-dependent antigen, WT and mutant mice were immunized with TNP-KLH in alum. TNP-specific serum Ab titers were measured by ELISA at days 7, 14, and 21 in the primary immune response and after a second administration of TNP-KLH to assess the secondary response at day 28. Unchallenged WT mice had low TNP-reactive IgM Abs and showed increased TNP-specific Abs at day 7 after immunization with TNP-KLH (Figure 7A). WKO mice had a delayed response with increased TNP-specific IgM Abs present first at day 14 (Figure 7A). In contrast, unchallenged cDKO mice had markedly elevated, TNP-reactive IgM Abs and lacked a specific immune response to TNP-KLH at day 7-21 (Figure 7A). WT, WKO, and cDKO mice had similar specific IgM Ab titers in the secondary response at day 28 (Figure 7A). To determine whether cDKO B cells could undergo class-switch recombination to a T-dependent antigen, we next examined TNP-specific IgG1 Abs after TNP-KLH immunization. WT and WKO mice responded to TNP-KLH with increased TNP-specific IgG1 at days 14 and 21, whereas cDKO mice showed a significantly reduced response (Figure 7B). At day 28, WT, WKO, and cDKO mice showed similar titers of TNP-specific IgG1 (Figure 7B). To determine whether the high TNP-specific IgG1 titers in cDKO mice at day 28 represented a delayed primary immune response or a normal secondary response, we investigated whether GCs had developed normally by immunohistochemistry and flow cytometry. Well-organized GCs were frequently detected in WT and WKO spleens, whereas cDKO mice showed reduced GC areas with loosely organized peanut agglutinin–positive GC cells (Figure 7C). To assess the frequency of GC B cells directly, we examined B cells in the spleen after TNP-KLH immunization at day 28. cDKO mice showed reduced frequency of B cells compared with WT and WKO mice (Figure 7D left panel). Moreover, the frequency and absolute number of GC B cells (Fas+GL7+B220+DAPI−) was significantly decreased in cDKO mice (Figure 7D right panel). Considering that cDKO mice had a decreased GC area and reduced GC B cells in the secondary response at day 28, we reasoned that the high TNP-specific IgG1 Ab titers we detected at day 28 in cDKO mice may reflect a delayed primary response to TNP-KLH. These results suggest that cDKO mice have a reduced capacity to form a specific primary and secondary immune response with GC formation.
WASP and N-WASP are required for the B-cell immune response to TNP-KLH. WT, WKO, and cDKO mice were immunized by IP injection of TNP-KLH in alum and boosted 3 weeks later with another dose of TNP-KLH. Anti-TNP IgM (A) and anti-TNP IgG1 (B) Ab titers were determined by ELISA. Test samples were corrected for background binding. (C) Spleen sections from immunized mice at day 28 were labeled to detect GCs (peanut agglutinin positive) and MOMA+ metallophilic macrophages to define the MZ. GC areas were quantified and indicated in arbitrary units. Original magnification was 10×. (D) Quantification of GC B cells (B220+DAPI-GL7+Fas+) from immunized mice at day 28 by flow cytometry. Note the marked reduction of GC B cells in cDKO mice compared with WT mice. Each group represents 8 mice. Black bar represents the mean value for the group. *P < .05 compared with WT. (E) Schematic model of how WASP and N-WASP activity regulate peripheral B-cell homeostasis.
WASP and N-WASP are required for the B-cell immune response to TNP-KLH. WT, WKO, and cDKO mice were immunized by IP injection of TNP-KLH in alum and boosted 3 weeks later with another dose of TNP-KLH. Anti-TNP IgM (A) and anti-TNP IgG1 (B) Ab titers were determined by ELISA. Test samples were corrected for background binding. (C) Spleen sections from immunized mice at day 28 were labeled to detect GCs (peanut agglutinin positive) and MOMA+ metallophilic macrophages to define the MZ. GC areas were quantified and indicated in arbitrary units. Original magnification was 10×. (D) Quantification of GC B cells (B220+DAPI-GL7+Fas+) from immunized mice at day 28 by flow cytometry. Note the marked reduction of GC B cells in cDKO mice compared with WT mice. Each group represents 8 mice. Black bar represents the mean value for the group. *P < .05 compared with WT. (E) Schematic model of how WASP and N-WASP activity regulate peripheral B-cell homeostasis.
Discussion
FO and MZ B cells are B-cell subsets with unique functions. FO B cells have a defined life span measured in weeks and have the ability to undergo affinity maturation in response to antigenic challenge to form long-lived, Ab-secreting plasma cells and memory cells. The newly formed MZ B cells migrate into the MZ, where they are retained and acquire self-renewing capacity with an unlimited life span. The cell-fate decision controlling MZ B-cell differentiation has been well characterized in mutant mouse models. There are 4 categories of proteins that govern MZ B-cell development: (1) proteins involved in BCR signaling strength, (2) proteins involved in BAFFR and NF-κB signaling, (3) Notch family proteins, and (4) proteins regulating integrin and chemokine activation.1 We and others have shown previously that WASP regulation of integrin and chemokine signaling is critical for MZ B-cell development.5,8 The specific signaling pathways leading to the formation of FO B cells are poorly defined. In the present study, we have addressed how cytoskeletal regulation by WASP and N-WASP regulate peripheral B-cell development. By specifically deleting these proteins in B cells, we show that the combined activity of WASP and N-WASP is required for the development and function of both MZ and FO B cells (Figure 7E).
Our results reveal a complex regulation of B-cell development and function by WASP and N-WASP. cDKO mice had no significant change in frequency of pro-B, pre-B, or immature B cells, suggesting that WASP and N-WASP are not required for the generation and expansion of early B-lineage progenitors, although we did not determine N-WASP quantity in cDKO pro-B cells directly because of their limited number. Likewise, cDKO mice had a normal number of T1 B cells in the spleen, suggesting that circulating T1 cells can enter into peripheral lymphoid organs in cDKO mice. In contrast, our results demonstrate profound abnormalities at later developmental stages. cDKO mice had diminished numbers of splenic FO B cells and, compared with WKO mice, the decrease in MZ precursor cells and MZ B cells was exacerbated in cDKO mice. Our data highlight the importance of cytoskeletal regulation in peripheral B-cell development, and are consistent with recent data from studies of mice lacking upstream signaling molecules of WASP family members. Mice that lack the small GTPases Rac1 and Rac2 and mice lacking the guanine exchange factors Vav1-3 undergo normal B-cell development in the BM while having a marked reduction of both FO and MZ B cells.23,24 A recent study demonstrated that Rac1−/−Rac2−/− transitional B cells fail to exit the red pulp to enter the white pulp of the spleen.25 This defect is partly explained by the decreased migratory response to chemokines required for entry into the white pulp cords, where the cells receive survival signals from BAFF and BCR signaling and develop into FO and MZ B cells.25,26 The phenotype of Rac1−/− Rac2−/− B cells is strikingly similar to that of the cDKO B cells studied herein, including decreased migratory and spreading responses. However, one important difference between Rac1−/− Rac2−/− and cDKO B cells is that the former have aberrant BCR and BAFFR signaling and therefore do not receive proper survival signals. In contrast, those cDKO B cells that enter into the white pulp are likely to receive BCR-dependent survival signals, because in the present study, cDKO B cells proliferated normally in response to BCR stimulation and showed no evidence of increased cell death after receptor stimulation. We propose that cDKO B cells have a reduced capacity to develop into FO and MZ B cells because of the markedly decreased migratory and spreading response. One unifying implication is that deletion of both WASP and N-WASP alters intrasplenic migration, preventing correct homing of T1 cells to the anatomical location for the development of MZ and FO B cells.
The MZ is critical for clearance of blood-borne pathogens.1 The development of MZ B cells depends on signals from the highly phagocytic MZ macrophages and on B cell–intrinsic signaling involving WASP8,27,28 and N-WASP (this study). In addition to reduced MZ macrophages, the cDKO mice also had reduced metallophilic macrophages. This differs from WKO mice, which had a normal number of metallophilic macrophages. This suggests that MZ B cells regulate the development and/or retention of these macrophages and that only when MZ B cells are much reduced in numbers (as in the cDKO mice) is the MZ rim of metallophilic macrophages disrupted. The metallophilic macrophages delineate the border between the MZ and the inner B-cell follicles and serve a role in antigen transport, at least in lymph nodes.29 It is possible that a breach in the MZ leads to altered uptake of blood-borne antigens. Artificial disruption of the MZ by deletion of the MZ and metallophilic macrophages using diphtheria toxin leads to reduced clearance of apoptotic cells and may be associated with the development of autoantibodies.30 We addressed the possibility that cDKO mice would have a breach in the MZ by examining the uptake of blood-borne TNP-Ficoll, and found that TNP-Ficoll was virtually absent from spleens of cDKO mice. cDKO mice failed to mount a specific IgM response to TNP-Ficoll because the TNP-reactive IgM serum titer in nonchallenged mice was markedly elevated. Increased serum titers of such “natural” IgM have been associated with low-affinity and potentially autoreactive IgM responses.31 In response to T cell–dependent antigenic challenge (ie, with TNP-KLH), cDKO mice had a reduced primary Ab response and a diminished capacity to form a well-defined GC in the spleen after secondary challenge. The GC is the site of B-cell affinity maturation that relies critically on the migratory and adhesive responses of B cells.32 B-cell localization to the GC dark and light zones is regulated by the chemokines CXCL12 and CXCL13, whereas the orphan G protein–coupled receptor EBI2 is critical for the retention of B cells in the outer follicle.33,34 Although beyond the scope of this study, it is possible that cDKO B cells have altered affinity maturation caused by decreased response to cues directing migration and adhesion within the GC reaction.
In conclusion, by studying cytoskeletal regulation in B cells, in the present study, we have identified WASP and N-WASP as key proteins in the transition from T1 cells to FO and MZ B cells. These observations provide novel insights into the critical regulation of the cell cytoskeleton for peripheral B-cell development and function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a postdoctoral fellowship from the Swedish Society for Medical Research and research grants from the Swedish Research Council to L.S.W. and by the National Institutes of Health (grants HL-59561 to S.B.S. and L.D.N., 2P30DK034854-26 and AI-50950 to S.B.S., DK-43351 to S.B.S. and C.T., and AI-076210 to C.T. and L.D.N.).
National Institutes of Health
Authorship
Contribution: L.S.W., L.D.N., and S.B.S. designed the research; L.S.W., C. Dahlberg, M.B., C.J.M., C. Detre, M.K., and M.A.E. performed the research; F.W.A., C.T., and L.D.N. contributed new reagents or analytical tools; L.S.W., C. Dahlberg, M.B., C.J.M., C. Detre, M.K., and S.B.S. analyzed the data; and L.S.W. and S.B.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott B. Snapper, MD, PhD, Harvard Medical School, Gastroenterology Division, Children's Hospital Boston, 300 Longwood Ave, Boston, MA 02115; e-mail: scott.snapper@childrens.harvard.edu; or Lisa S. Westerberg, PhD, Karolinska Institutet, Department of Medicine, Translational Immunology Unit L2:04, 171 76 Stockholm, Sweden; e-mail: lisa.westerberg@ki.se.

![Figure 4. WASP and N-WASP are dispensable for B-cell proliferation, but regulate B-cell spreading and migration. (A) Proliferation. Splenic B cells were stimulated for 48 hours with the indicated stimulus, followed by a 16-hour pulse with [3H]thymidine to determine the proliferative response. Bars represent mean values of cpm ([3H]thymidine) ± SD of triplicate wells from 1 of at least 3 independent experiments. (B) Spreading. Spreading of activated B cells was assessed on anti-CD44 Ab-coated surfaces. White arrowhead depicts the formation of long protrusions of spread B cells. (C) Graphs showing the average of relative numbers (± SD) of spread B cells in triplicate representative of 3 experiments. (D) Migration. Splenic B or T cells were allowed to migrate to CXCL12 for 3 hours using an in vitro chemotaxis chamber. Migrating cells were collected and enumerated by flow cytometry with reference beads. The percentage of migrating cells is shown as mean values ± SD of triplicate wells and data are representative of at least 3 experiments. *P < .05 compared with WT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/17/10.1182_blood-2010-09-308197/4/m_zh89991289740004.jpeg?Expires=1767709309&Signature=kyBg4uZwt4ez~x3GCoLjnAo2j8HqPJ6xkq9pTNOGzCpYghDUsBCMEgqxrKWo7zi2k9a~CnE8GX~JM3bvVv~XIrZX~X3g1bivYOiNIK1aeP66Bewth9Iusqi~AnAbR4ZqUV80EdNRwxsx02bOTLUKFZzf3-aAFtBi7yuyWSOzFCN9D9~PusQyoximWNbGBHYqcsbelY3h9L-c~wAYq6LmeR0ShIvSW91kBzroig4R4m1I27mzfPdhqRBnDnb~pWJmlx4XNYlghHN58egZWCDlN3-4eIgQ~wHUEWlHBU4zlFdeVIsYFlglLSFNNiSMfdfT6lid6VfwnxITVl5IyVR9Bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal