Relapse of acute myeloid leukemia (AML) is thought to reflect the failure of current therapies to adequately target leukemia stem cells (LSCs), the rare, resistant cells presumed responsible for maintenance of the leukemia and typically enriched in the CD34+CD38− cell population. Despite the considerable research on LSCs over the past 2 decades, the clinical significance of these cells remains uncertain. However, if clinically relevant, it is expected that LSCs would be enriched in minimal residual disease and predictive of relapse. CD34+ subpopulations from AML patients were analyzed by flow cytometry throughout treatment. Sorted cell populations were analyzed by fluorescence in situ hybridization for leukemia-specific cytogenetic abnormalities (when present) and by transplantation into immunodeficient mice to determine self-renewal capacity. Intermediate (int) levels of aldehyde dehydrogenase (ALDH) activity reliably distinguished leukemic CD34+CD38− cells capable of engrafting immunodeficient mice from residual normal hematopoietic stem cells that exhibited relatively higher ALDH activity. Minimal residual disease detected during complete remission was enriched for the CD34+CD38−ALDHint leukemic cells, and the presence of these cells after therapy highly correlated with subsequent clinical relapse. ALDH activity appears to distinguish normal from leukemic CD34+CD38− cells and identifies those AML cells associated with relapse.
Introduction
Although most patients with acute myeloid leukemia (AML) achieve complete remission (CR) after standard induction chemotherapy, the majority subsequently relapse and die of the disease.1,–3 A leukemia stem cell (LSC) paradigm may explain this failure of CR to reliably translate into cure. Leukemia appears to retain some semblance of the normal hematopoietic hierarchical structure (ie, rare LSCs with self-renewal capacity give rise to partially differentiated progeny that compose the bulk of the leukemia but possess only limited proliferative potential).4 It is theorized that existing therapies, although highly active against the leukemic bulk, often spare the hardier LSCs responsible for relapse.5,6
Since the 1994 report by Lapidot et al that only the rare AML cells characterized by a CD34+CD38− phenotype were capable of generating leukemia in immunodeficient mice,7 these putative LSCs have been the focus of considerable research. However, even in leukemia where the cancer stem cell (CSC) concept is perhaps best established, there is a paucity of data that LSCs are indeed responsible for disease resistance or relapse. Although it is generally accepted that CD34+CD38− cells are enriched for LSCs,7 this population is heterogeneous and includes both normal and leukemic cells. Moreover, recent data have challenged the CD34+CD38− phenotype of LSCs in AML, leading many investigators to advocate for a functional definition of LSCs: those leukemic cells capable of engrafting immunodeficient mice.8,,–11 However, this current “gold standard” for the identification of LSCs4,7 has proven to be somewhat elusive. A significant fraction of AML samples will not engraft mice, and the assay is cumbersome and often nonquantitative.12 In addition, the clinical implications of this assay are unclear.13
If CD34+CD38− leukemic cells are indeed clinically relevant, then minimal residual disease (MRD), any microscopic disease remaining during CR, should be relatively enriched for these cells; and their persistence after therapy should correlate with recurrence. Exploiting the similarities between LSCs and their normal counterparts,14 we recently used strategies established for the isolation of normal hematopoietic stem cells (HSCs)15,–17 in chronic myeloid leukemia (CML). We found that the CML CD34+CD38− fraction with high levels of aldehyde dehydrogenase (ALDH) activity was highly enriched for leukemic cells capable of engrafting immunodeficient mice.18 Accordingly, here we assess ALDH activity as a marker for clinically significant MRD in AML.
Methods
Human samples
Bone marrow was procured from a total of 27 AML patients (Table 1). All research samples represented excess bone marrow collected at diagnosis or during routine follow-up. Excess bone marrow from the harvests of 10 normal donors for allogeneic bone marrow transplantation was also studied. Specimens were collected between April 2008 and April 2011 by the Johns Hopkins Kimmel Cancer Center Specimen Accessioning Core. Appropriate informed consent was obtained from all patients and normal donors before specimen collection, in accordance with the Declaration of Helsinki and under a research protocol approved by the Johns Hopkins Institutional Review Board. Samples were obtained from 20 of the AML patients at initial diagnosis. An additional 7 patients who had already begun treatment at the time of first sample procurement were also studied. Initially, in an attempt to limit heterogeneity and ensure the presence of a detectable, leukemia-specific abnormality, specimens were restricted to patients with core-binding factor (CBF) AML. Later, the sample pool was expanded to all cases of AML except acute promyelocytic leukemia, which was excluded because of its uniquely favorable prognosis and distinct treatment approach.19 AML induction regimens consisted of cytarabine, daunorubicin, and etoposide20 or a clinical trial of flavopiridol, cytarabine, and mitoxantrone (FLAM).21 Consolidation therapy consisted of cytarabine plus daunorubicin,20 high-dose cytarabine, or FLAM.21 Patients who achieved morphologic CR with induction chemotherapy were followed until relapse or their last available bone marrow specimen. All analyzed research specimens are reported.
Patient clinical and demographic characteristics
| Patient no. . | Cytogenetics . | AML type . | Age, y . | Sex . | Race . | Outcome . | Other comments . |
|---|---|---|---|---|---|---|---|
| 1 | Complex, including Inv16 | De novo | 44 | Male | White | Relapsed | |
| 2 | Inv16 and +8 | Treatment-related | 64 | Female | White | CRp | |
| 3 | Inv16 and +22 | De novo | 50 | Female | White | CR | |
| 4 | Inv16 and +8 | De novo | 23 | Female | White | CR | |
| 5 | Inv16 | De novo | 58 | Female | Black | Relapsed | |
| 6 | Inv16 and FLT3 D835 (constitutional 47, XXY) | De novo | 40 | Male | White | Died | Died during induction |
| 7* | t(8;21) | Treatment-related | 51 | Female | White | Relapsed | |
| 8 | t(8;21) and FLT3 D835 | De novo | 54 | Male | White | Relapsed | Did not receive consolidation |
| 9 | t(8;21) | De novo | 24 | Male | Hispanic | Relapsed | |
| 10 | t(8;21) | De novo | 27 | Male | Asian | CR | |
| 11 | t(8;21) and del 12p | De novo | 49 | Male | White | CR | |
| 12 | +21 | Treatment-related MDS/AML | 64 | Male | White | Died | Died of infection while relapsing |
| 13 | +11 | CMML/AML | 54 | Male | White | CR | |
| 14* | Normal | De novo | 44 | Female | White | Relapsed | CR2 after allogeneic transplantation |
| 15* | Complex, including +21 | Treatment-related MDS/AML | 64 | Female | White | Relapsed | Probable constitutional +X |
| 16* | Complex, including del 7q | De novo | 34 | Female | Asian | CR | s/p allogeneic transplantation |
| 17 | Normal | De novo | 54 | Male | White | CRp | |
| 18 | Normal with FLT3 D835 | De novo | 69 | Male | White | Relapsed | 3/20 metaphases with +13 |
| 19* | t(8;21) and del Y | De novo | 49 | Male | White | CR | |
| 20* | t(8;21) | De novo | 55 | Male | Black | CR | |
| 21* | Normal | De novo | 48 | Male | White | CRi | |
| 22 | Inv16 and FLT3 D835 | De novo | 38 | Male | White | CR | |
| 23 | Normal | De novo | 61 | Male | Black | Primary Refractory | |
| 24 | Normal with FLT3 ITD | De novo | 21 | Female | White | Primary refractory | |
| 25 | Complex, including del 5 and del 7 | De novo | 57 | Male | White | Primary refractory | |
| 26 | t(9;11) and +8 | De novo | 58 | Female | Black | Relapsed | CD34− leukemia |
| 27 | +8, FLT3 ITD, and NPM1 | De novo | 58 | Female | Black | CR | CD34− leukemia |
| Patient no. . | Cytogenetics . | AML type . | Age, y . | Sex . | Race . | Outcome . | Other comments . |
|---|---|---|---|---|---|---|---|
| 1 | Complex, including Inv16 | De novo | 44 | Male | White | Relapsed | |
| 2 | Inv16 and +8 | Treatment-related | 64 | Female | White | CRp | |
| 3 | Inv16 and +22 | De novo | 50 | Female | White | CR | |
| 4 | Inv16 and +8 | De novo | 23 | Female | White | CR | |
| 5 | Inv16 | De novo | 58 | Female | Black | Relapsed | |
| 6 | Inv16 and FLT3 D835 (constitutional 47, XXY) | De novo | 40 | Male | White | Died | Died during induction |
| 7* | t(8;21) | Treatment-related | 51 | Female | White | Relapsed | |
| 8 | t(8;21) and FLT3 D835 | De novo | 54 | Male | White | Relapsed | Did not receive consolidation |
| 9 | t(8;21) | De novo | 24 | Male | Hispanic | Relapsed | |
| 10 | t(8;21) | De novo | 27 | Male | Asian | CR | |
| 11 | t(8;21) and del 12p | De novo | 49 | Male | White | CR | |
| 12 | +21 | Treatment-related MDS/AML | 64 | Male | White | Died | Died of infection while relapsing |
| 13 | +11 | CMML/AML | 54 | Male | White | CR | |
| 14* | Normal | De novo | 44 | Female | White | Relapsed | CR2 after allogeneic transplantation |
| 15* | Complex, including +21 | Treatment-related MDS/AML | 64 | Female | White | Relapsed | Probable constitutional +X |
| 16* | Complex, including del 7q | De novo | 34 | Female | Asian | CR | s/p allogeneic transplantation |
| 17 | Normal | De novo | 54 | Male | White | CRp | |
| 18 | Normal with FLT3 D835 | De novo | 69 | Male | White | Relapsed | 3/20 metaphases with +13 |
| 19* | t(8;21) and del Y | De novo | 49 | Male | White | CR | |
| 20* | t(8;21) | De novo | 55 | Male | Black | CR | |
| 21* | Normal | De novo | 48 | Male | White | CRi | |
| 22 | Inv16 and FLT3 D835 | De novo | 38 | Male | White | CR | |
| 23 | Normal | De novo | 61 | Male | Black | Primary Refractory | |
| 24 | Normal with FLT3 ITD | De novo | 21 | Female | White | Primary refractory | |
| 25 | Complex, including del 5 and del 7 | De novo | 57 | Male | White | Primary refractory | |
| 26 | t(9;11) and +8 | De novo | 58 | Female | Black | Relapsed | CD34− leukemia |
| 27 | +8, FLT3 ITD, and NPM1 | De novo | 58 | Female | Black | CR | CD34− leukemia |
CRp indicates CR with incomplete platelet recovery; CRi, CR with incomplete count recovery; MDS, myelodysplasia; S/P, status post; and CMML, chronic myelomonocytic leukemia.
No specimen from initial diagnosis.
Isolation of cells
CD34+ cell subsets were identified and isolated as we previously described.18 Briefly, mononuclear cells were isolated by density gradient centrifugation (Ficoll-Paque, GE Healthcare Life Sciences), and CD34+ cells were then selected via magnetic bead and column (Miltenyi Biotec). Samples were then viably cryopreserved in 90% FBS and 10% DMSO (Sigma-Aldrich) and stored until further use. After thawing and washing, cells were then stained with Aldefluor (Aldagen) to assess ALDH activity. Next, cells were labeled with monoclonal PE-conjugated anti-CD34 and allophycocyanin-conjugated anti-CD38 (BD Biosciences), then analyzed and sorted using a MoFlo cell sorter (Beckman Coulter) at the Johns Hopkins Bloomberg School of Public Health Flow Cytometry Core.
FISH
For those patients with cytogenetic abnormalities detectable by FISH, 250 to 1000 cell aliquots were sorted directly onto slides and then fixed with 3:1 methanol-glacial acetic acid (Sigma-Aldrich). All research and clinical FISH were performed and analyzed by the Johns Hopkins Kimmel Cancer Center Cytogenetics Core, using probes specific for the patient's known cytogenetic abnormality, per the manufacturer's guidelines (Abbot Molecular).
NOD/SCID-IL2Rγnull (NSG) mouse engraftment
NSG mice received 300 cGy irradiation and then were injected via tail vein with 103 to 105 cells of the CD34+ cell subpopulations obtained from a normal donor or AML patient, as we previously described.18 Mice were killed 3 to 4 months later, and their bone marrow was harvested. The harvested mouse bone marrow was treated with RBC lysis buffer (Sigma-Aldrich) and labeled with an allophycocyanin-conjugated monoclonal antibody against human CD45 (BD Biosciences). The human CD45+ population was sorted directly onto slides for analysis by FISH. All mouse research was performed under a protocol approved by the Johns Hopkins Animal Care and Use Committee and complied with National Institutes of Health and American Veterinary Medical Association guidelines.
Clinical data
All research samples were taken concurrently with clinical samples, which underwent review by an expert hematopathologist. Relevant clinical data were provided for each de-identified sample by the Johns Hopkins Kimmel Cancer Center Specimen Accessioning Core.
Statistical analysis
Means were compared between 2 groups using 2-sided Student t test and between multiple groups using ANOVA. Categorical data were compared using the Fisher exact test.
Results
ALDH activity distinguishes CD34+CD38− leukemic cells from their normal counterparts
Normal bone marrow CD34+CD38− cells consistently exhibited 2, nonoverlapping subpopulations by ALDH activity: one expressing low ALDH activity (CD34+CD38−ALDHlow) and another expressing high activity (CD34+CD38−ALDHhigh; Figure 1A). The normal marrow CD34+CD38−ALDHhigh cells constituted an average of 10% (range, 9%-12%) of the total CD34+ cells and 76% (range, 61%-85%) of the CD34+CD38− cells. As few as 1000 of these CD34+CD38−ALDHhigh cells generated engraftment when transplanted into NSG mice (data not shown).
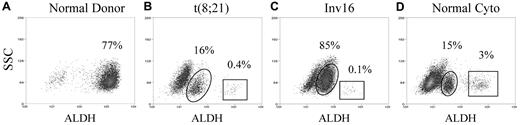
Staining patterns of a normal donor and diagnostic AML samples. Representative flow cytometric staining patterns of ALDH activity by side scatter (SSC) are displayed for CD34+CD38− cells isolated from: (A) a normal donor, (B) a patient (patient 9) with t(8;21) AML, (C) a patient (patient 1) with Inv16 AML, and (D) a patient (patient 17) with normal cytogenetic (cyto) AML. The AML samples, but not the normal marrows, contained a discrete CD34+CD38−ALDHint population (circled). In the patients with CBF AML, this CD34+CD38−ALDHint population was essentially completely leukemic by FISH. The CD34+CD38−ALDHlow population from the CBF AMLs was also almost entirely leukemic by FISH. In contrast, the small CD34+CD38−ALDHhigh populations (boxed) from CBF AML patients lacked the leukemia-specific FISH marker. The percentages of CD34+CD38− cells composed of the CD34+CD38−ALDHint and CD34+CD38−ALDHhigh populations are listed on each dot plot.
Staining patterns of a normal donor and diagnostic AML samples. Representative flow cytometric staining patterns of ALDH activity by side scatter (SSC) are displayed for CD34+CD38− cells isolated from: (A) a normal donor, (B) a patient (patient 9) with t(8;21) AML, (C) a patient (patient 1) with Inv16 AML, and (D) a patient (patient 17) with normal cytogenetic (cyto) AML. The AML samples, but not the normal marrows, contained a discrete CD34+CD38−ALDHint population (circled). In the patients with CBF AML, this CD34+CD38−ALDHint population was essentially completely leukemic by FISH. The CD34+CD38−ALDHlow population from the CBF AMLs was also almost entirely leukemic by FISH. In contrast, the small CD34+CD38−ALDHhigh populations (boxed) from CBF AML patients lacked the leukemia-specific FISH marker. The percentages of CD34+CD38− cells composed of the CD34+CD38−ALDHint and CD34+CD38−ALDHhigh populations are listed on each dot plot.
Initial AML analyses focused on patients with newly diagnosed CBF leukemias, as the FISH-detectable abnormalities allowed quantification of the percentage of leukemic cells in isolated populations. In contrast to the normals, the CD34+CD38− cells from all CBF AML patients exhibited 3 well-defined subpopulations by ALDH activity. In addition to the CD34+CD38−ALDHlow and CD34+CD38−ALDHhigh populations, the CBF AML samples contained a population with intermediate ALDH activity (CD34+CD38−ALDHint) that was not present in the normal donors (Figure 1B-C).
The CD34+CD38−ALDHhigh cells were rare in the newly diagnosed CBF AML patients, constituting an average of only 0.12% (range, 0.005%-0.5%, P < .001 vs the normal samples) of the total CD34+ cells and 1.24% (range, 0.03%-4.3%, P < .001 vs the normal samples) of the CD34+CD38− cells. This CD34+CD38−ALDHhigh population was essentially devoid of cells with the leukemia-specific cytogenetic abnormality (Table 2). Similar to those isolated from normal donors, as few as 1000 of these cells yielded normal human engraftment of NSG mice (Figure 2A; Table 3). Conversely, the CD34+CD38−ALDHlow and the CD34+CD38−ALDHint populations from newly diagnosed CBF AML patients were both virtually entirely leukemic by FISH (Table 2). As few as 1000 CD34+CD38−ALDHint cells produced leukemic engraftment of NSG mice (Figure 2B; Table 3); no engraftment was seen with 103 to 105 CD34+CD38−ALDHlow cells. The CD34+CD38− cells from other cytogenetic variants of AML, including those with normal cytogenetics, demonstrated similar staining patterns (Figure 1D) and (where applicable) FISH results.
Percentage of each sorted cell population positive for the leukemia-specific abnormality by FISH at diagnosis
| Patient no. . | Total CD34+ . | CD34+CD38− ALDHint . | CD34+CD38− ALDHhigh . |
|---|---|---|---|
| Inv16* | |||
| 1 | 96 | 96 | 2 |
| 2 | 96 | 98 | 4 |
| 3 | 81 | 92 | 3 |
| 4 | 62.5 | 88.2 | 1.8 |
| 5 | 99 | 98.5 | 0 |
| 6 | 99 | 99 | 0 |
| 22 | 87 | 98 | 1 |
| Average | 88.6 | 95.7 | 1.7 |
| Median | 96 | 98 | 1.8 |
| t(8;21)† | |||
| 8 | 90 | 91 | 1.3 |
| 9 | 97 | 98 | 0 |
| 10 | 98 | 97 | 0 |
| 11 | 99 | 99 | 0 |
| Average | 96 | 96.3 | 0.3 |
| Median | 97.5 | 97.5 | 0 |
| Total average | 91.3 | 95.9 | 1.2 |
| Total median | 96 | 98 | 1 |
| Patient no. . | Total CD34+ . | CD34+CD38− ALDHint . | CD34+CD38− ALDHhigh . |
|---|---|---|---|
| Inv16* | |||
| 1 | 96 | 96 | 2 |
| 2 | 96 | 98 | 4 |
| 3 | 81 | 92 | 3 |
| 4 | 62.5 | 88.2 | 1.8 |
| 5 | 99 | 98.5 | 0 |
| 6 | 99 | 99 | 0 |
| 22 | 87 | 98 | 1 |
| Average | 88.6 | 95.7 | 1.7 |
| Median | 96 | 98 | 1.8 |
| t(8;21)† | |||
| 8 | 90 | 91 | 1.3 |
| 9 | 97 | 98 | 0 |
| 10 | 98 | 97 | 0 |
| 11 | 99 | 99 | 0 |
| Average | 96 | 96.3 | 0.3 |
| Median | 97.5 | 97.5 | 0 |
| Total average | 91.3 | 95.9 | 1.2 |
| Total median | 96 | 98 | 1 |
The normal cutoff for Inv16 FISH is < 2.3%.
The normal cutoff for t(8;21) FISH is < 1.5%.
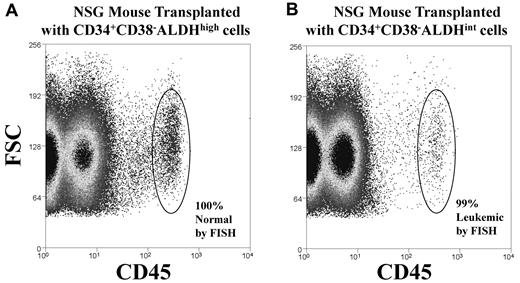
Mouse engraftment. CD34+CD38−ALDHhigh or CD34+CD38−ALDHint cells were isolated from the diagnostic sample of a patient (patient 4) with Inv16 AML and then transplanted into irradiated NSG mice. After 3 months, bone marrow was harvested from the transplanted mice, and engraftment was demonstrated by the presence of human CD45+ cells (circled), which were then isolated and analyzed by FISH for the Inv16 abnormality. Representative plots of CD45 versus forward scatter (FSC) are shown for mice transplanted with 1000 cells of: (A) CD34+CD38−ALDHhigh cells that generated normal (by FISH) human hematopoietic engraftment of mice or (B) CD34+CD38−ALDHint cells that produced leukemic (by FISH) engraftment of mice. Human chimerism data are shown in Table 3.
Mouse engraftment. CD34+CD38−ALDHhigh or CD34+CD38−ALDHint cells were isolated from the diagnostic sample of a patient (patient 4) with Inv16 AML and then transplanted into irradiated NSG mice. After 3 months, bone marrow was harvested from the transplanted mice, and engraftment was demonstrated by the presence of human CD45+ cells (circled), which were then isolated and analyzed by FISH for the Inv16 abnormality. Representative plots of CD45 versus forward scatter (FSC) are shown for mice transplanted with 1000 cells of: (A) CD34+CD38−ALDHhigh cells that generated normal (by FISH) human hematopoietic engraftment of mice or (B) CD34+CD38−ALDHint cells that produced leukemic (by FISH) engraftment of mice. Human chimerism data are shown in Table 3.
Mouse engraftment
| Dose, cells . | CD34+ total . | CD34+CD38−ALDHint . | CD34+CD38−ALDHhigh . | |||
|---|---|---|---|---|---|---|
| Chimerism . | FISH+ . | Chimerism . | FISH+ . | Chimerism . | FISH+ . | |
| 100 000 | 0.92 ± 0.46 | 95.5 | 1.21 ± 0.49 | 98.5 | ND | NA |
| 10 000 | 0.12 ± 0.06 | 98 | 0.19 ± 0.06 | 98.5 | 1.33 ± 0.78 | 4 |
| 1000 | 0 | NA | 0.14 ± 0.12 | 98 | 0.18 ± 0.16 | 0.5 |
| Dose, cells . | CD34+ total . | CD34+CD38−ALDHint . | CD34+CD38−ALDHhigh . | |||
|---|---|---|---|---|---|---|
| Chimerism . | FISH+ . | Chimerism . | FISH+ . | Chimerism . | FISH+ . | |
| 100 000 | 0.92 ± 0.46 | 95.5 | 1.21 ± 0.49 | 98.5 | ND | NA |
| 10 000 | 0.12 ± 0.06 | 98 | 0.19 ± 0.06 | 98.5 | 1.33 ± 0.78 | 4 |
| 1000 | 0 | NA | 0.14 ± 0.12 | 98 | 0.18 ± 0.16 | 0.5 |
Data are percentages (mean ± SEM) human CD45+ chimerism and the average percentage of those cells positive for leukemia by FISH.
ND indicates not done; and NA, not applicable.
This characteristic leukemic flow cytometric pattern was not seen in 4 of the 20 newly diagnosed AML patients. In 2 AML patients, most of the leukemic CD34+CD38− cells exhibited high ALDH activity, with no discernable separate population of normal CD34+CD38−ALDHhigh cells. One of these patients had normal cytogenetics with a FLT3 internal tandem duplication, and the other had complex cytogenetics that included deletions of chromosomes 5 and 7. Notably, both patients had primary refractory disease that ultimately proved fatal. In 2 additional patients, the diagnostic leukemic cytogenetic marker was present only in the CD34− cells, as has been previously described in a minority of AML cases.22 One of these patients had an 11q23 abnormality and has since relapsed within a year of diagnosis; the other has remained in CR for more than one year since diagnosis and 9 months since proceeding directly to allogeneic transplantation in first CR.
Presence of the CD34+CD38−ALDHint population after treatment predicts relapse
Of the 20 AML patients analyzed at diagnosis, 3 had primary refractory disease, one died during induction chemotherapy, one did not receive full induction or any consolidation chemotherapy because of medical complications, and 2 others had CD34− leukemia. Of the 13 patients with CD34+ leukemia who achieved morphologic CR after induction chemotherapy, follow-up samples were available in 9. An additional 7 AML patients who achieved CR, but in whom diagnostic samples were not available, were also followed: 2 starting after induction and 5 after consolidation therapy.
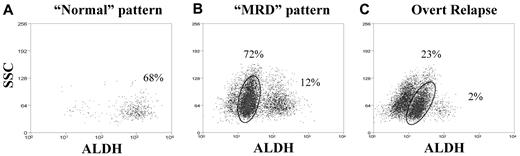
The CR samples exhibited 2 general patterns. Of the 8 patients analyzed in first morphologic CR after recovery from induction and before consolidation chemotherapy, 5 patients (Table 4) exhibited a “normal” pattern: a predominant CD34+CD38−ALDHhigh population and a smaller CD34+CD38−ALDHlow population, with no discernable CD34+CD38−ALDHint population (Figure 3A). Both CD34+CD38− subpopulations in these remission patients were normal by FISH. The 3 patients who have consistently exhibited this “normal” pattern remain in CR, with an average follow-up of 293 days (range, 185-370 days) since diagnosis. The other 2 patients ultimately relapsed; the CD34+CD38−ALDHint population was detected in both at the next follow-up interval while still in CR after consolidation chemotherapy. The remaining 3 patients (Table 4) exhibited an “MRD” pattern on recovery from induction, with a detectable CD34+CD38−ALDHint population, often without return of the CD34+CD38−ALDHhigh population to predominance (Figure 3B). The CD34+CD38−ALDHint population was greater than or equal to 85% leukemic by FISH in all 3 patients (Figure 4). Two of these 3 patients relapsed within 33 days of detection of the “MRD” pattern and subsequently died (Table 4). The third patient underwent allogeneic transplantation in first CR because of adverse risk cytogenetics (complex karyotype, including deletion 7q) and has remained in CR for more than 17 months.
Detection of the CD34+CD38−ALDHint population in available follow-up samples of AML patients who achieved morphologic CR
| Patient no. . | Postinduction/preconsolidation . | Time after consolidation . | Outcome . | |||||
|---|---|---|---|---|---|---|---|---|
| 0-6 mo . | 6-12 mo . | 12-18 mo . | 18-24 mo . | 24-36 mo . | 36-48 mo . | |||
| 1 | Absent | Present (0.25%) | Relapsed 34 days after detection | |||||
| 2 | Absent | Absent | Absent | Absent | CRp 688 days since diagnosis | |||
| 3 | Absent | Absent | CR 370 days since diagnosis | |||||
| 7 | Absent | Present (0.37%) | Relapsed 32 days after detection | |||||
| 10 | Absent | Absent | CR 323 days since diagnosis | |||||
| 11 | Absent | Absent | CR 691 days since diagnosis | |||||
| 12 | Present (0.26%) | Relapsed 25 days after detection | ||||||
| 13 | Absent | Absent | CR 185 days since diagnosis | |||||
| 14 | Present (0.25%) | Relapsed 62 days after detection | ||||||
| 15 | Present (0.17%) | Relapsed 33 days after detection | ||||||
| 16 | Present (0.04%) | CR 435 days after allogeneic HSCT | ||||||
| 17 | Absent | Absent | Absent | Absent | Absent | CRp 810 days since diagnosis | ||
| 18 | Absent | Present (0.03%) | Relapsed 81 days after detection | |||||
| 19 | Absent | Absent | CR 1482 days since diagnosis | |||||
| 20 | Absent | Absent | Absent | CR 497 days since diagnosis | ||||
| 21 | Absent | Absent | CRi 1335 days since diagnosis | |||||
| Patient no. . | Postinduction/preconsolidation . | Time after consolidation . | Outcome . | |||||
|---|---|---|---|---|---|---|---|---|
| 0-6 mo . | 6-12 mo . | 12-18 mo . | 18-24 mo . | 24-36 mo . | 36-48 mo . | |||
| 1 | Absent | Present (0.25%) | Relapsed 34 days after detection | |||||
| 2 | Absent | Absent | Absent | Absent | CRp 688 days since diagnosis | |||
| 3 | Absent | Absent | CR 370 days since diagnosis | |||||
| 7 | Absent | Present (0.37%) | Relapsed 32 days after detection | |||||
| 10 | Absent | Absent | CR 323 days since diagnosis | |||||
| 11 | Absent | Absent | CR 691 days since diagnosis | |||||
| 12 | Present (0.26%) | Relapsed 25 days after detection | ||||||
| 13 | Absent | Absent | CR 185 days since diagnosis | |||||
| 14 | Present (0.25%) | Relapsed 62 days after detection | ||||||
| 15 | Present (0.17%) | Relapsed 33 days after detection | ||||||
| 16 | Present (0.04%) | CR 435 days after allogeneic HSCT | ||||||
| 17 | Absent | Absent | Absent | Absent | Absent | CRp 810 days since diagnosis | ||
| 18 | Absent | Present (0.03%) | Relapsed 81 days after detection | |||||
| 19 | Absent | Absent | CR 1482 days since diagnosis | |||||
| 20 | Absent | Absent | Absent | CR 497 days since diagnosis | ||||
| 21 | Absent | Absent | CRi 1335 days since diagnosis | |||||
The percentage of the total mononuclear cells composed of the CD34+CD38−ALDHint population (when present) is indicated in parentheses.
CRp indicates CR with incomplete platelet recovery; and CRi, CR with incomplete count recovery.
Staining patterns after therapy. Representative examples of (A) “normal” and (B) “MRD” staining patterns, as well as (C) overt clinical relapse. CD34+CD38− cells are shown by ALDH versus side scatter (SSC). (A) An AML patient (patient 17) with normal cytogenetics, in durable CR exhibiting the “normal” pattern with no detectable CD34+CD38−ALDHint population. The comparison diagnostic pattern for this patient is shown in Figure 1D. (B) An AML patient (patient 1) with Inv16 AML, demonstrating the “MRD” pattern while still in CR; the circled CD34+CD38−ALDHint population was 95% leukemic by FISH. The comparison diagnostic pattern is shown in Figure 1C. (C) A patient (patient 9) with t(8;21) AML, in overt relapse resembling the original diagnostic pattern (shown in Figure 1B). The percentages of CD34+CD38− cells composed of the CD34+CD38−ALDHint and CD34+CD38−ALDHhigh populations are listed on each dot plot.
Staining patterns after therapy. Representative examples of (A) “normal” and (B) “MRD” staining patterns, as well as (C) overt clinical relapse. CD34+CD38− cells are shown by ALDH versus side scatter (SSC). (A) An AML patient (patient 17) with normal cytogenetics, in durable CR exhibiting the “normal” pattern with no detectable CD34+CD38−ALDHint population. The comparison diagnostic pattern for this patient is shown in Figure 1D. (B) An AML patient (patient 1) with Inv16 AML, demonstrating the “MRD” pattern while still in CR; the circled CD34+CD38−ALDHint population was 95% leukemic by FISH. The comparison diagnostic pattern is shown in Figure 1C. (C) A patient (patient 9) with t(8;21) AML, in overt relapse resembling the original diagnostic pattern (shown in Figure 1B). The percentages of CD34+CD38− cells composed of the CD34+CD38−ALDHint and CD34+CD38−ALDHhigh populations are listed on each dot plot.
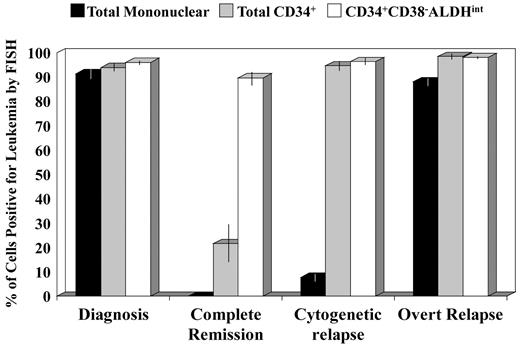
Percentages of cell populations positive for leukemia by FISH in each disease phase. Data are shown only for those patient samples with a FISH-detectable cytogenetic abnormality and a detectable CD34+CD38−ALDHint population. All 3 cell populations were highly leukemic at diagnosis. The CD34+CD38−ALDHint population was highly enriched for leukemic cells (≥ 85%) in all disease phases, including complete cytogenetic remission (P > .5). In contrast, the total CD34+ population contained only a minority of leukemic cells (average, 22%; P < .001 vs diagnosis) in complete cytogenetic remission (patients 1, 15, and 16) but rose to more than or equal to 95% leukemic with progression to cytogenetic (patients 5, 7, and 12), or overt (patients 1 and 7-9) relapse (P = .001 for differences in total CD34+ and in mononuclear cell FISH percentages across all 4 disease phases).
Percentages of cell populations positive for leukemia by FISH in each disease phase. Data are shown only for those patient samples with a FISH-detectable cytogenetic abnormality and a detectable CD34+CD38−ALDHint population. All 3 cell populations were highly leukemic at diagnosis. The CD34+CD38−ALDHint population was highly enriched for leukemic cells (≥ 85%) in all disease phases, including complete cytogenetic remission (P > .5). In contrast, the total CD34+ population contained only a minority of leukemic cells (average, 22%; P < .001 vs diagnosis) in complete cytogenetic remission (patients 1, 15, and 16) but rose to more than or equal to 95% leukemic with progression to cytogenetic (patients 5, 7, and 12), or overt (patients 1 and 7-9) relapse (P = .001 for differences in total CD34+ and in mononuclear cell FISH percentages across all 4 disease phases).
Eleven AML patients who achieved morphologic CR with induction chemotherapy have been followed since completion of consolidation chemotherapy (Table 4). Of the 7 patients with a consistently “normal” pattern after consolidation, none has yet relapsed, with an average duration of follow-up of 509 days (range, 185-810 days) since diagnosis. However, all 4 of the patients with the “MRD” pattern after consolidation have since relapsed (P = .003 vs those patients with a consistently “normal” pattern), at an average of 53 days (range, 32-81 days) after first detection of the “MRD” pattern (Table 4). Similar to the “MRD” cases after induction chemotherapy, the CD34+CD38−ALDHint population was overwhelmingly leukemic (≥ 95%) in those patients with FISH-detectable cytogenetic abnormalities (Figure 4). One of the relapsed patients converted to a “normal” pattern after reinduction and allogeneic transplantation and has remained in second CR for more than 1 year. The “MRD” pattern persisted in 2 other patients, who achieved a second CR after reinduction, both of whom have subsequently relapsed again. Of note, the CD34+CD38−ALDHint population could not be detected in an additional 2 patients, who have been in CR for more than 3 years but have been followed only since their second year after consolidation. Conversely, the CD34+CD38−ALDHint population was present in all 6 analyzed cases of overt clinical relapse (Figure 3C).
MRD after chemotherapy is enriched for leukemic CD34+CD38−ALDHint cells
Although the 3 patients in cytogenetic CR exhibiting the “MRD” pattern had no morphologic, karyotypic, or FISH evidence of disease in the unfractionated marrow cells, the leukemia-specific abnormality was still detectable in a minority (average, 22%; range, 8%-36%) of the total CD34+ cells and in the majority (average, 90%; range, 85%-95%) of the CD34+CD38−ALDHint cells (Figure 4). This leukemic CD34+CD38−ALDHint population, which represented only 0.15% (range, 0.04%-0.25%) of the total mononuclear cells, constituted an average of 34% (range, 9%-51%) of the total leukemic burden in these cytogenetic CR patients with the “MRD” pattern. In contrast, this population constituted only 2% (range, 0.3%-7%, P < .001 vs cytogenetic CR) of the total leukemic burden at diagnosis (Figure 5). Moreover, although the total leukemic burden decreased by more than 200-fold from diagnosis to cytogenetic CR (P < .001), the CD34+CD38−ALDHint population decreased by only 13-fold (P = .4; Figure 5).
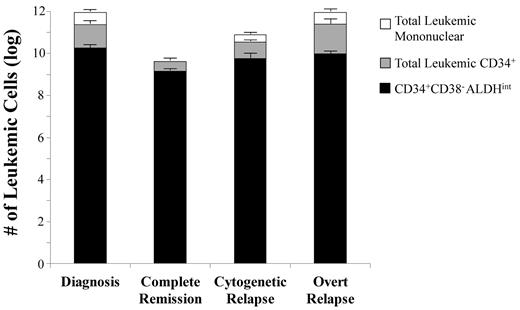
Composition of the total leukemic burden by cell populations in each disease phase. Data are shown only for those patient samples with a FISH-detectable cytogenetic abnormality and a detectable CD34+ CD38−ALDHint population. The total leukemic burden is assumed to be 1012 cells at diagnosis.37 Each vertical bar represents the total leukemic (mononuclear) cell burden (based on FISH) for each disease phase: initial diagnosis (patients 1-3, 5, 6, 8-13, and 22), cytogenetic CR with the “MRD” pattern (patients 1, 15, and 16), cytogenetic relapse (with FISH, but not morphologic, evidence of disease; patients 5, 7, and 12), and at overt relapse (patients 1 and 7-9). The CD34+ CD38−ALDHint cells represented an average of 2% (range, 0.3%-7%) of the total leukemic burden at initial diagnosis, 34% (range, 9%-51%) in cytogenetic CR, 8% (range, 2%-12%) in cytogenetic relapse, and 1% (range, 0.5%-2%) at overt relapse (P < .001). The CD34+ CD38−ALDHint population constituted an average of 8% (5%-13%) of the total leukemic CD34+ cells at initial diagnosis, 34% (range, 9%-51%) in cytogenetic CR (P < .001 vs initial diagnosis), 18% (range, 7%-37%) in cytogenetic relapse, and 7% (range, 2%-16%) in overt relapse. Although the total leukemic burden decreased by more than 2 logs from diagnosis to cytogenetic CR (P < .001), the absolute size CD34+ CD38−ALDHint population decreased only by 1 log (P = .4).
Composition of the total leukemic burden by cell populations in each disease phase. Data are shown only for those patient samples with a FISH-detectable cytogenetic abnormality and a detectable CD34+ CD38−ALDHint population. The total leukemic burden is assumed to be 1012 cells at diagnosis.37 Each vertical bar represents the total leukemic (mononuclear) cell burden (based on FISH) for each disease phase: initial diagnosis (patients 1-3, 5, 6, 8-13, and 22), cytogenetic CR with the “MRD” pattern (patients 1, 15, and 16), cytogenetic relapse (with FISH, but not morphologic, evidence of disease; patients 5, 7, and 12), and at overt relapse (patients 1 and 7-9). The CD34+ CD38−ALDHint cells represented an average of 2% (range, 0.3%-7%) of the total leukemic burden at initial diagnosis, 34% (range, 9%-51%) in cytogenetic CR, 8% (range, 2%-12%) in cytogenetic relapse, and 1% (range, 0.5%-2%) at overt relapse (P < .001). The CD34+ CD38−ALDHint population constituted an average of 8% (5%-13%) of the total leukemic CD34+ cells at initial diagnosis, 34% (range, 9%-51%) in cytogenetic CR (P < .001 vs initial diagnosis), 18% (range, 7%-37%) in cytogenetic relapse, and 7% (range, 2%-16%) in overt relapse. Although the total leukemic burden decreased by more than 2 logs from diagnosis to cytogenetic CR (P < .001), the absolute size CD34+ CD38−ALDHint population decreased only by 1 log (P = .4).
In the 3 patients with cytogenetic, but no morphologic, evidence of disease (ie, cytogenetic relapse), an average of 8% (range, 4.5%-9.5%) of the unfractionated marrow cells was leukemic by FISH compared with 96% (range, 94%-99%) of the CD34+CD38−ALDHint cells (P < .001, Figure 4). The relative size of the CD34+CD38−ALDHint fraction diminished in these early relapsing patients, representing an average of 8% (range, 2%-12%) of the total leukemic clone. At overt relapse, as at initial diagnosis, this population again composed only a small fraction (average, 1%; range, 0.5%-2%) of the total leukemic burden (Figure 5).
Discussion
Although a wealth of experimental data support the LSC model in AML (ie, tumor maintenance and resistance are restricted to rare cancer cells with stem cell features), there is little corroborating clinical evidence. Relapse could instead be the result of the total leukemic population's stochastic first-order kinetic response to drugs or of resistance emerging in any individual leukemic cell. This has led some to speculate whether CSCs might only represent laboratory artifacts with little clinical relevance.23,24 Compounding this controversy has been the difficulty in clinically identifying LSCs and assessing their response to therapy.
The fact that leukemic cells of various surface phenotypes are capable of engrafting immunodeficient mice with AML has led to a definition of LSCs based on their engraftment ability.8,,–11 However, the mouse engraftment assay may more accurately reflect the proliferative potential of the leukemic cells13 and/or their interaction with the mouse microenvironment,25 than it does their role in disease maintenance and relapse. Regardless of their tumorigenic potential in immunocompromised mice, those leukemic cells that persist after therapy (ie, MRD) are arguably the most clinically important.
Here we find that MRD was relatively enriched for the CD34+CD38−ALDHint cells. Although the total leukemic burden decreased by more than 2 logs in patients with detectable MRD after a cytogenetic CR to induction therapy, the CD34+CD38−ALDHint cells decreased by only 1 log, implying that these cells were more drug-resistant than the bulk leukemic cells (Figure 5). In addition, the CD34+CD38−ALDHint population was uniformly positive for the clonal leukemic cytogenetic marker (when assayable by FISH), was never present in normal donors, and produced leukemic engraftment when transplanted into immunodeficient mice. The percentage of these cells decreased as the leukemia recurred, consistent with dilution by their generated leukemic progeny (Figure 5). Importantly, the presence of these cells during CR was highly predictive of subsequent AML relapse, supporting their clinical relevance. Indeed, 6 of the 7 CR patients with the “MRD” pattern (ie, a detectable CD34+CD38−ALDHint fraction) ultimately relapsed; the lone exception underwent allogeneic transplantation in first CR. Furthermore, the CD34+CD38−ALDHint population disappeared from those patients who achieved durable CR.
Recent discoveries in the 5q− myelodysplastic syndrome demonstrated that the putative stem cell in that disease is more resistant than bulk or progenitor cells to lenalidomide.26 However, the CSCs remained detectable in all 5q− myelodysplastic syndrome patients studied (all of whom eventually relapsed), precluding any conclusion that eradication of the CSCs improved clinical outcomes. To our knowledge, the data reported here demonstrate, for the first time, that the detection of phenotypic LSCs correlates with clinical outcomes and suggest these cells may be responsible for AML relapse.
Like Pearce et al,16 we found that leukemic cells could be reliably separated from normal HSCs in most AML cases, based on relative differences in ALDH activity. The rare CD34+CD38−ALDHhigh cells at diagnosis phenotypically resembled normal HSCs and lacked leukemia-specific cytogenetic abnormalities; these cells generated normal human hematopoietic engraftment on transplantation into immunodeficient mice. In contrast, the CD34+CD38− cells with intermediate levels of ALDH appeared to represent a population of LSCs. This ability to phenotypically distinguish these cell populations offers the potential to simultaneously observe the efficacy of new therapies against putative LSCs and their toxicity against normal HSCs. It may also facilitate better identification of therapeutic targets and resistance mechanisms. Combining ALDH with other reported leukemia-specific markers, such as CD96,27 CD123,28 and C-type lectin-like molecule-1,29 may further enhance the sensitivity and specificity of LSC detection.
Interestingly, 2 of the primary refractory patients in this series had CD34+CD38− leukemic cells with similar ALDH levels to normal HSCs. We recently found that CML LSCs also exhibit ALDH activity comparable with normal HSCs.18 ALDH expression decreases with HSC differentiation,30,31 and it is possible that the LSCs in most cases of AML arise from a less primitive progenitor, as has recently been suggested.32 Alternatively, those AML cases with CD34+CD38−ALDHhigh leukemic cells may arise from more primitive cells, possibly translating to a poorer prognosis. Although this remains to be proven, it has been reported that high ALDH activity in AML blasts does correlate with poorer outcomes.33
Currently, cytogenetic and molecular aberrations are the best prognostic indicators for AML patients.2,34,35 However, these factors predict primarily for groups of patients and cannot prognosticate well for individual patients within any given risk strata. For example, CBF cytogenetic abnormalities are generally considered favorable; yet approximately half of these patients relapse.36 In contrast, the persistence of LSCs after therapy is a patient-specific variable and could serve as a powerful, individualized prognostic tool for patients. The detection of MRD appears to supply a lead time on the order of one to 3 months before overt clinical relapse, potentially affording sufficient time to prepare for allogeneic transplantation or enrollment on a clinical trial. Although this approach still requires validation in larger series of patients, it potentially provides a means to assess response at the level of the LSC and personalize care appropriately.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and normal donors who contributed research samples as well as the clinical and research staff at the Johns Hopkins Kimmel Cancer Center who assisted in specimen procurement.
This work was supported by the Leukemia & Lymphoma Society (Translational Research grant, R.J.J. and J.M.G.), National Institutes of Health (grants P01 CA15396, P01 CA070970, U01 CA70095, and 5T32 HL007525, R.J.J., S.J.S., J.E.K., and J.M.G.), the American Society of Hematology (Research Training Award for Fellows, J.M.G.), and the Maryland Stem Cell Research Fund (postdoctoral fellowship grant, J.M.G.).
National Institutes of Health
Authorship
Contribution: J.M.G. and R.J.J. designed the research and wrote the paper; J.M.G., B.N., H.Z., M.S.V., L.M., S.G., M.I.C., and B.P. performed the research; B.D.S., M.J.L., and J.E.K. contributed patients; and J.M.G., B.D.S., C.A.G., S.J.S., M.J.B., J.E.K., and R.J.J. analyzed the data.
Conflict-of-interest disclosure: R.J.J. and S.J.S. hold the patent for Aldefluor and, under a licensing agreement between Aldagen and the Johns Hopkins University, are entitled to a share of royalties received by the University. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. The remaining authors declare no competing financial interests.
Correspondence: Jonathan M. Gerber, Bunting-Blaustein Cancer Research Bldg, Rm 390, 1650 Orleans St, Baltimore, MD 21231; e-mail: jgerber2@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal