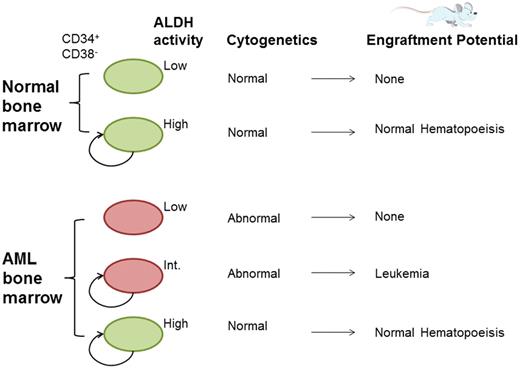
Normal bone marrow CD34+CD38− cells can be divided into 2 distinct populations based on ALDH activity (high and low), with the ability to engraft immunodeficient mice restricted to the ALDHhigh population. AML patient bone marrow contains an additional subpopulation with intermediate ALDH activity (ALDHint); this population contains cells that bear the diagnostic leukemic cytogenetic marker and transplant leukemia into immunodeficient mice.
Normal bone marrow CD34+CD38− cells can be divided into 2 distinct populations based on ALDH activity (high and low), with the ability to engraft immunodeficient mice restricted to the ALDHhigh population. AML patient bone marrow contains an additional subpopulation with intermediate ALDH activity (ALDHint); this population contains cells that bear the diagnostic leukemic cytogenetic marker and transplant leukemia into immunodeficient mice.
ALDH activity has been touted as a marker for normal and malignant stem cells, not only in the hematopoietic system2,,–5 but also in solid organs such as breast,6 colon,7 and ovary.8 Normal bone marrow CD34+CD38− cells can be subdivided into 2 nonoverlapping populations based on ALDH staining (high and low), with the ability to engraft immunodeficient mice restricted to the ALDHhigh population. Gerber et al now demonstrate that AML patient bone marrow contains an additional subpopulation with intermediate ALDH activity (ALDHint; see figure).1 This population is absent in normal control bone marrow. It is important to note that the ALDHint population is not necessarily a ubiquitous feature of AML; it was not present in 4 of the 20 newly diagnosed AML patients studied.
The ALDHint population fits the bill for the LSC compartment: as few as 1000 cells engraft leukemia into immunodeficient mice. The ALDHint population is uniformly positive for the clonal leukemic cytogenetic marker. Like the ALDHint population, cells within the ALDHlow population also contain the diagnostic leukemia cytogenetic marker, but in contrast to ALDHint cells cannot engraft immunodeficient mice. This suggests that cells contained within the ALDHlow population are the leukemic progeny of the ALDHint population.
The ALDHhigh population in AML patients is a reservoir of “normal” hematopoietic stem cells. This population is devoid of cytogenetic abnormalities and as few as 1000 cells engraft normal, nonleukemic hematopoiesis into immunodeficient mice, similar to the ALDHhigh population of normal bone marrow. Theoretically, on the basis of ALDH activity one could delicately separate out and collect normal hematopoietic stem cells amid a sea of leukemic cells.
Perhaps the most clinically relevant finding is that persistence of the aberrant ALDHint population after therapy was highly predictive of subsequent relapse. The authors followed 11 AML patients who achieved morphologic complete remission (CR) after induction chemotherapy. Of the 7 patients who consistently lacked the aberrant ALDHint population, none have relapsed (average duration of follow-up 509 days). In contrast, of 4 patients in whom the ALDHint population was detected, all 4 have subsequently relapsed. Perhaps detection of this aberrant population could be exploited as a measure of residual disease and could potentially be used to guide therapy.
ALDH activity has also been applied to investigate the ontogeny of the JAK2V617F clone in chronic myeloproliferative neoplasms (MPNs).9 In all 9 JAK2V617F-positive patients investigated, the ALDHhigh population was composed of cells bearing the JAK2V617F mutation, demonstrating that the mutation arises in a primitive hematopoietic progenitor. In the chronic phase of the disease the ALDHint population was absent, but subsequently appeared coincident with transformation to acute leukemia in 2 patients. This data further support the specificity of the ALDHint population to AML and its appearance as a herald of acute leukemia.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■