Abstract
We investigated whether TCRs restricted to the more ubiquitously expressed MHC class I molecules could be used to redirect human regulatory T cells (Tregs). Using a series of HLA-A2–restricted TCRs that recognize the same peptide-MHC class I complex (pMHC) with affinities varying up to 3500 fold, we observed that TCR affinity had no effect on the ability of the introduced TCRs to confer potent Ag-specific suppressive activity. Surprisingly, we found a naturally occurring, low-affinity MHC class I–restricted TCR specific for an NY-ESO-1 epitope that was unable to redirect a functional CD4 T-effector cell response could confer potent antigen-specific suppressive activity when expressed in Tregs and severely impair the expansion of highly functional HIV-1GAG–specific CD8 T cells expressing a high-affinity TCR. This suppressive activity was only observed when both Ags were presented by the same cell, and no suppression was observed when the target Ags were put in distinct cells. These studies underscore the clinical utility of using MHC class I–restricted TCRs to endow Tregs with specificity to control autoimmune disease and highlight the conditions in which this approach would have most therapeutic benefit.
Introduction
Regulatory T cells (Tregs), like all T cells, rely on the TCR to control their specificity and activity as regulators of aberrant immune responses. During positive selection in the thymus, Tregs seem to be more dependent on strong TCR signals compared with effector T cells (Teffs), because reduction of TCR signaling strength via mutations to the CD3 signaling apparatus leads to a preferential loss of Tregs and to the acquisition of severe autoimmunity.1 To date, it is unclear how TCR affinity affects Treg function in mature cells. This has become an important issue as several groups have reported success reprogramming the specificity of Tregs by introducing chimeric Ag receptors2 or TCRs.3–5 This approach would provide a way to rapidly generate therapeutic levels of Ag-specific Tregs,6 which in many murine-based studies have proven to be far superior to polyclonal Tregs in preventing and treating autoimmune diseases such as type 1 diabetes, multiple sclerosis, and arthritis.7 Moreover, many of the tissues targeted by autoimmune cells tend to lack the ability to express MHC class II. Therefore, endogenously and exogenously introduced MHC class II–restricted TCRs specific for an autoimmune Ag expressed in Tregs may fail to accumulate and become activated near the target tissue, potentially hindering the overall therapeutic effectiveness of Treg-based therapies. One way to overcome these limitations is to engineer Tregs to express MHC class I–restricted TCRs if they retain full suppressive activity in the absence of CD8.
It has been shown previously that tumor-specific, MHC class I–restricted TCRs that were engineered to be high-affinity variants could bypass the need for CD8 expression and confer function to CD4 T cells, suggesting that only high-affinity MHC class I–restricted TCRs would be functional in CD4+ Tregs.8 The use of high-affinity TCRs may be more advantageous because several studies have shown that augmented TCR affinity is correlated with improved Teff function8–12 ; however, in many cases, there was a maximal improvement in T-cell function that could not be improved by further augmentation of TCR affinity. Moreover, several TCRs with exceptionally high affinity for pMHC lost specificity, which shows that excessive enhancement of TCR affinity may be detrimental.8,11 These data suggest that for each TCR and for each therapeutic application, there is an optimal TCR affinity.13,14 In the present study, we investigated how TCR affinity affects Treg function in an effort to determine how to best deploy MHC class I–restricted TCRs for use in adoptive Treg therapy. Our studies show that in contrast to Teffs and contrary to our predictions and our previous studies,15 augmented TCR affinity does not affect or improve Treg function. Furthermore, because we failed to detect bystander suppression when the target Ag of the effector and Treg was expressed in distinct cells, our results suggest that Tregs expressing nonengineered, MHC class I–restricted TCRs are fully functional and therapeutically attractive as long as the Treg and Teff target the same cell.
Methods
Purification, stimulation, culture, and modification of primary human T cells
Cord blood was obtained from the Division of Maternal-Fetal Medicine at the University of Pennsylvania Hospital using an Institutional Review Board–approved protocol. Total CD4 T cells were isolated using the Human CD4+ T Cell Enrichment Cocktail (StemCell Technologies) according to the manufacturer's instructions. Tregs were isolated from purified CD4 T cells as follows: CD4 T cells were resuspended at up to 1 × 107 cells per 90 μL of MACS buffer, and 10 μL of anti-CD25 magnetic beads (Miltenyi Biotec) were added. After 20 minutes of incubation at 4°C, cells were washed once with MACS buffer and CD4+CD25+ cells were positively selected using magnetic MS columns (Miltenyi Biotec). Tregs were cultured in XVIVO 15 medium (Lonza) containing 10% heat-inactivated human AB serum (Valley Biomedical), 1% Glutamax (Invitrogen), Pen/Str (Invitrogen), 0.2% N-acetylcysteine (Ben Venue Labs), and human IL-2 (Aldesleukin; Chiron) at 0.5-0.75 × 106 cells/mL. Purified adult human T cells were obtained from the Human Immunology Core at the University of Pennsylvania under an Institutional Review Board–approved protocol. Declaration of Helsinki protocols were followed and donors gave written, informed consent.
Transduction and transfection of TCR genes into Tregs
Tregs were activated by adding CD3/28 beads at a cell:bead ratio of 1:3.16 Two days later, cells were transduced by adding high-titer lentiviral vectors to the cultured cells as described previously.17 Fresh medium was added to the cells on day 3 and twice weekly thereafter. On days 5-6, the magnetic beads were removed, and on the following day, transduction efficiency was determined by flow cytometry. Long-term Treg cultures were established as described previously18–20 using K562-based artificial APCs (aAPCs) that were lethally irradiated with 100 Gy, washed, resuspended in medium used for Treg cultures, and added to Tregs at a T cell:aAPC ratio of 2:1 to restimulate the Treg cultures. RNA transfection of the Tregs was performed by mixing 5 μg of in vitro–transcribed RNA (Invitrogen), encoding each TCR gene with 3 × 106 purified Tregs and electroporating as described previously.21
Soluble protein production and affinity measurement
Soluble high-affinity TCRs were produced as disulfide-linked heterodimeric TCRs, as described previously.22 We conducted surface plasmon resonance analysis of variant TCRs binding to biotin-tagged HLA A2-SL923 immobilized to a streptavidin-coated flow cell using a BIAcore 3000 or a BIAcore T100. An identical amount of HLA A2-NY-ESO-1 was immobilized onto one flow cell as a negative control, as described previously.10
Generation of aAPC
K562-based aAPC lines were generated as described previously.20 The dsRED SL9 lentiviral construct was generated by fusing dsRED to HIV-1GAG 54-102 and cloning this into pELPS,24 and the entire NY-ESO codon-optimized version of the NY-ESO-1 gene (NM_001327) was inserted into a pCLPS variant that contained an internal ribosome entry site green fluorescent protein element downstream of the NY-ESO-1 sequence.25
In vitro suppression assay
The CFSE-based suppression assay was performed as described previously.26 The bead-based assay was performed by mixing washed, expanded Tregs with various ratios of TCR-transduced Teffs. Treg-Teff mixtures were stimulated with aAPCs, and 5 days later the absolute number of CD8 T cells was determined by flow cytometry using CountBright absolute counting beads (Invitrogen) added to the FASC tubes along with anti-CD8 mAb and SL9 tetramer. Data were acquired on an LSR II flow cytometer (BD Biosciences) using FACSDiva software (BD Biosciences). The percent suppression was calculated using the formula: 1 − number of Teff divisions in suppressed condition/number of Teff divisions in unsuppressed condition × 100.
Results
Generation of MHC class I–restricted TCRs with various affinities for the same pMHC
To determine whether MHC class I–restricted TCRs could be used to effectively retarget human Tregs, we used a previously described HIV-1GAG–specific TCR that recognizes the peptide SLYNTVATL (SL9) in the context of HLA-A2. This particular TCR was useful for these studies because a high-affinity variant (a11/b6) was generated previously10 using a directed evolution process.27 To better understand how TCR affinity affects Treg function, we sought to expand the panel of TCRs that recognize this pMHC. By mixing and matching high-affinity and wild-type TCRα and TCRβ chains (awt/b6 and a11/bwt), we were able to generate TCRs that had affinities of 6.6 and 14.9nM, respectively, as determined by surface plasmon resonance (Table 1). Moreover, because the wild-type A2-SL9 awt/bwt TCR has the highest affinity of any naturally occurring human TCR described to date,10 we sought to obtain a TCR with a lower affinity that would be more representative of natural TCRs. We identified a TCR pair (a2/b1) that had a TCR affinity approximately 5-fold lower than that of the awt/bwt. After characterizing the relative affinity of each A2-SL9–specific TCR, we converted these soluble TCRs into full-length TCRs and inserted them into both lentiviral and RNA expression vectors.10 Because the awt/b6 and a11/bwt A2-SL9–specific TCRs had similar affinities, we chose to study only the awt/b6 TCR.
Affinity measurements of A2-SL9–specific TCRs
| TCR . | Kon, 1/Ms . | Koff, 1/s . | KD, nM . | T1/2 . |
|---|---|---|---|---|
| a2/b1 | 1.6 × 105 | 0.139 | 870 | 4.9 sec |
| aWT/bWT | 2.6 × 105 | 2.2 × 10−2 | 85 | 30 sec |
| a11/bWT | 7.4 × 104 | 1.1 × 10−3 | 14.9 | 10.5 min |
| aWT/b6 | 1.2 × 105 | 7.6 × 10−4 | 6.6 | 15.1 min |
| a11/b6 | 2.25 × 105 | 5.4 × 10−5 | 0.245 | 213.9 min |
| TCR . | Kon, 1/Ms . | Koff, 1/s . | KD, nM . | T1/2 . |
|---|---|---|---|---|
| a2/b1 | 1.6 × 105 | 0.139 | 870 | 4.9 sec |
| aWT/bWT | 2.6 × 105 | 2.2 × 10−2 | 85 | 30 sec |
| a11/bWT | 7.4 × 104 | 1.1 × 10−3 | 14.9 | 10.5 min |
| aWT/b6 | 1.2 × 105 | 7.6 × 10−4 | 6.6 | 15.1 min |
| a11/b6 | 2.25 × 105 | 5.4 × 10−5 | 0.245 | 213.9 min |
Kon indicates on rate; Koff, off rate; KD, affinity constant; and T1/2, half life.
A2-SL9–specific TCRs are able to redirect human CD4 Teff responses
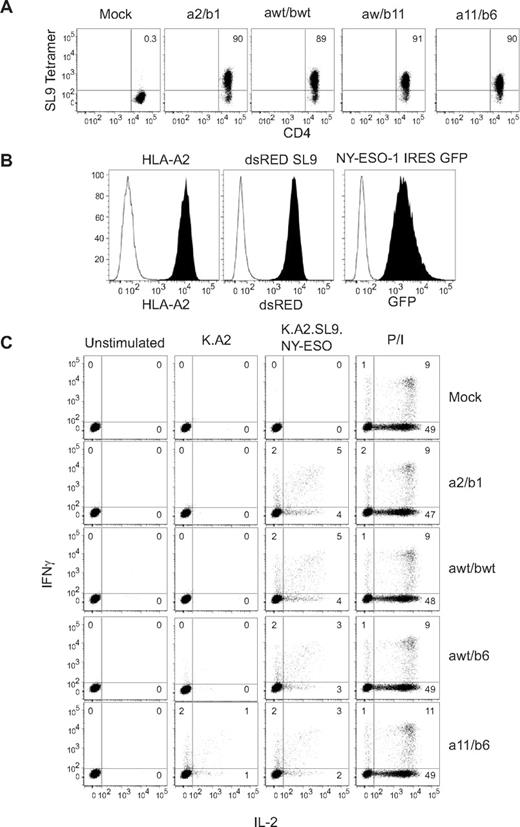
Because CD8 signaling via Lck recruitment has been shown to be important in generating MHC class I–restricted Ag-specific responses,28,29 we first investigated whether A2-SL9–specific TCRs could also function in CD4 T cells. We generated RNAs encoding each A2-SL9–specific TCR and transfected them into freshly isolated CD4 T cells. One day after transfection, TCR expression was evaluated by tetramer staining, and all A2-SL9 TCR transfected T-cell cultures were able to bind the A2-SL9–specific tetramer in a CD8-independent manner (Figure 1A). We also investigated whether these TCRs could redirect a functional CD4 Teff response by coculturing A2-SL9–specific TCR-transfected CD4 T cells with K562-based aAPCs30 expressing HLA-A2, the full-length NY-ESO-1 gene connected to green fluorescent protein via an internal ribosome entry site sequence, and a minigene that encoded the 50 amino acids that surround the SL9 epitope in the HIV-1GAG gene (Figure 1B), K.A2.SL9.NY-ESO-1. The NY-ESO-1 gene was added so that we could later determine the ability of redirected Tregs to suppress Teff that respond to a distinct Ag, allowing us to use the same aAPC to compare the ability of Tregs to suppress Teffs with the same or distinct specificities. Surprisingly, CD4 T cells that expressed the highest-affinity A2-SL9–specific TCR (a11/b6) produced IL-2 and IFNγ in response to the control aAPC lacking expression of the SL9 epitope (K.A2), and this response was augmented in the presence of aAPC expressing the SL9 Ag (K.A2.SL9.NY-ESO-1). The remaining A2-SL9–specific TCRs had minimal activity when incubated with the control K.A2 aAPCs and produced a polyfunctional response to K.A2.SL9.NY-ESO-1 aAPCs. These results demonstrate that both wild-type and most of the lower- and higher-affinity A2-SL9 TCR variants are functional in CD4 T cells and can redirect an Ag-specific Teff response.
A2-SL9–specific TCRs are able to redirect human CD4 Teff responses. (A) RNA encoding each of the A2-SL9–specific TCRs was transfected into resting primary human CD4 T cells and, after overnight culture, the ability to bind SL9 tetramer was measured by flow cytometry. (B) K562 cells were transduced with lentiviral vectors expressing HLA-A2, dsRED-SL9 minigene encoding, and full-length NYESO-1 linked to green fluorescent protein (GFP) via an internal ribosome entry site (IRES). (C) Cells described in panel A were mixed at a 1:1 ratio with aAPCs described in panel B. IFNγ and IL-2 production were measured by intracellular cytokine staining after 5 hours. As a positive control, T cells were also stimulated with 3 μg/mL of phorbol 12-myristate 13-acetate and 1 μg/mL of ionomycin (P/I). Data presented is representative of at least 3 independent experiments.
A2-SL9–specific TCRs are able to redirect human CD4 Teff responses. (A) RNA encoding each of the A2-SL9–specific TCRs was transfected into resting primary human CD4 T cells and, after overnight culture, the ability to bind SL9 tetramer was measured by flow cytometry. (B) K562 cells were transduced with lentiviral vectors expressing HLA-A2, dsRED-SL9 minigene encoding, and full-length NYESO-1 linked to green fluorescent protein (GFP) via an internal ribosome entry site (IRES). (C) Cells described in panel A were mixed at a 1:1 ratio with aAPCs described in panel B. IFNγ and IL-2 production were measured by intracellular cytokine staining after 5 hours. As a positive control, T cells were also stimulated with 3 μg/mL of phorbol 12-myristate 13-acetate and 1 μg/mL of ionomycin (P/I). Data presented is representative of at least 3 independent experiments.
TCR transduction does not alter the ability of Tregs to function in non-Ag–specific suppressive activity
Whereas RNA-engineered Tregs may have utility in providing short-term Ag-specific suppression, stable expression of the introduced TCRs by lentiviral mediated transduction may be required to maintain long-term tolerance.31 Compared with Teffs, human Tregs are hypoproliferative32 and therefore may be less susceptible to lentivirus-mediated transduction. By transducing each cell population on the optimal day (ie, day 1 for Teff and day 3 for Tregs), we found that Teffs were still slightly more transducible (Figure 2A; P = .0004 by paired t test, total of 5 independent experiments). Nonetheless, the overall level of Treg transduction was robust and should not limit the ability TCR-transduced Tregs to provide Ag-specific adoptive T-cell therapy. We also investigated whether TCR transduction altered the ability of the Tregs to suppress in a non-Ag–specific manner. We evaluated the ability of awt/b6 A2-SL9 TCR-transduced Tregs to function in a conventional Treg suppression assay in which expanded transduced or untransduced Tregs were mixed with CFSE-labeled PBMCs at various ratios, along with anti-CD3 mAb-coated beads. In agreement with our previous studies,18,19 expanded Tregs isolated from cord blood were highly suppressive regardless of whether they had been TCR transduced, maintaining greater than 90% suppression activity at a 8:1 Teff:Treg ratio in the experiment shown in Figure 2B and greater than 75% activity in all 3 replicate experiments, as shown in Figure 2C. The average suppression of polyclonal Tregs was 84% ± 11.7% and that of awt/b6 A2-SL9 TCR-transduced Tregs was 82% ± 8.4% at an 8:1 Teff:Treg ratio. This difference was not significant (P = .751). Therefore, TCR transduction does not alter the ability of Tregs to function in a non-Ag–specific suppressive assay.
TCR-transduction does not alter the ability of Tregs to function in a non-Ag–specific suppressive activity. (A) Primary human Tregs or CD4 T cells isolated from cord blood samples were stimulated with anti-CD3/28–coated beads for 3 or 1 day(s), respectively, and then either mock transduced or transduced with lentiviral vectors encoding the A2-SL9 TCR awt/b6. After 7-10 days of additional culture, transduction efficiency was determined by staining for the A2-SL9 Vβ-specific chain (Vβ5a), and the percentage of TCR transduced cells was determined by flow cytometry. (B) awt/b6 A2-SL9 TCR-transduced Tregs from panel A were cocultured with 1 × 105 CFSE-stained PBMCs at ratios ranging from 1:2 (1 Treg: 1 PBMC) to 1:32. Cells were stimulated with anti-CD3 Ab-coated beads at a ratio of 1 PBMC to 1 bead for 5 days, and then FACS analysis was performed to measure CFSE dilution of CD8 T cells. The percent suppression was calculated as described in the “In vitro suppression assay.” (C) Data were compiled from 3 independent experiments showing the percent suppression achieved at a 1:8 Treg:Teff ratio. Each symbol represents 1 experiment.
TCR-transduction does not alter the ability of Tregs to function in a non-Ag–specific suppressive activity. (A) Primary human Tregs or CD4 T cells isolated from cord blood samples were stimulated with anti-CD3/28–coated beads for 3 or 1 day(s), respectively, and then either mock transduced or transduced with lentiviral vectors encoding the A2-SL9 TCR awt/b6. After 7-10 days of additional culture, transduction efficiency was determined by staining for the A2-SL9 Vβ-specific chain (Vβ5a), and the percentage of TCR transduced cells was determined by flow cytometry. (B) awt/b6 A2-SL9 TCR-transduced Tregs from panel A were cocultured with 1 × 105 CFSE-stained PBMCs at ratios ranging from 1:2 (1 Treg: 1 PBMC) to 1:32. Cells were stimulated with anti-CD3 Ab-coated beads at a ratio of 1 PBMC to 1 bead for 5 days, and then FACS analysis was performed to measure CFSE dilution of CD8 T cells. The percent suppression was calculated as described in the “In vitro suppression assay.” (C) Data were compiled from 3 independent experiments showing the percent suppression achieved at a 1:8 Treg:Teff ratio. Each symbol represents 1 experiment.
MHC class I–restricted TCR-transduced Tregs can be expanded and enriched by aAPCs expressing cognate pMHC
We postulated that we could use aAPCs expressing HLA-A2, HIV-1GAG, and 4-1BBL (K.64.A2.SL9.4-1BBL) to restimulate, further expand, and enrich the Ag-specific Treg cultures. To test this, 4-1BBL was added to the aAPCs because we found previously that 4-1BB costimulation aided the expansion of cord blood–derived Tregs.18 We found that A2-SL9 TCR-transduced Tregs could be restimulated with aAPCs expressing HLA-A2 and the cognate Ag (Figure 3A). Moreover, we saw a significant enrichment of the A2-SL9 TCR-transduced Tregs from approximately 22% to 72% after 7 days of additional culture when K.64.A2.SL9. 4-1BBL, but not anti-CD3 Ab-coated K.64.A2.4.BBL aAPCs, were used to restimulate the Treg cultures (CD64 permits Ab loading onto aAPCs,20 as shown in Figure 3B). These Ag-specific, stimulated A2-SL9 TCR-transduced Tregs were restimulated again, cultured for an additional 10 days, and then their Treg phenotype was evaluated. We found that these long-term-cultured TCR-transduced Tregs (30 days of culture) retained a Treg phenotype, as indicated by their maintenance of Foxp3, CD27, and CD62-L expression (Figure 3C). Previously, we and others have found that expanded Tregs that lose CD27 and CD62-L expression also lose suppressive activity.26,33,34 In the present study, TCR-transduced Tregs maintained an anergic state, because they were unable produce IL-2 after phorbol 12-myristate 13-acetate + ionomycin stimulation (Figure 3D). Because cord blood by definition is limited as a source material of Tregs, multiple rounds of expansion may be required to obtain therapeutic numbers. Our data show that even though Tregs are less susceptible to lentiviral transduction, the use of Ag-expressing aAPCs would be an effective way to further expand highly functional Tregs while simultaneously enriching for a TCR-transduced population. These data also indicate that MHC class I–restricted TCRs can convey the necessary signals to promote the activation and expansion of CD4+ human Tregs.
MHC class I–restricted TCR-transduced Tregs can be expanded and enriched by aAPCs expressing cognate pMHC. (A) awt/b6 A2-SL9 TCR transduced and nontransduced Tregs were allowed to expand and after resting (on day 12), transduced Tregs were restimulated with K64.A2.SL9.41BBL and nontransduced Tregs were restimulated with K.64.A2.41BBL coated with anti-CD3 (OKT3) Ab. Population doublings were determined at the indicated time points. (B) Transduced Tregs (22% TCR positive) were restimulated with either Ag-specific stimulation provided by K.64.A2.SL9.41BBL or polyclonal stimulation provided by K.64.A2.41BBL coated with OKT3 Ab. The frequency of TCR-transduced Tregs was reevaluated by SL9 tetramer staining 7 days after restimulation. (C) A2-SL9 TCR-transduced Tregs or nontransduced Tregs from panel B were again restimulated using K.64.A2.SL9.41BBL or K.64.A2.41BBL coated with OKT3 Ab, respectively. After another 7-10 days of culture, transduced Tregs (black line) and nontransduced Tregs (gray shading) were stained for Foxp3, CD27, and CD62L. Isotype control staining is shown as a thin black line. (D) TCR-transduced Tregs, nontransduced Tregs, and CD4 T cells were expanded after CD3/28 bead stimulation. After resting, cells were treated with phorbol 12-myristate 13-acetate plus ionomycin for 6 hours and intracellular IL-2 was measured by flow cytometry. These data are representative of 3 independent experiments.
MHC class I–restricted TCR-transduced Tregs can be expanded and enriched by aAPCs expressing cognate pMHC. (A) awt/b6 A2-SL9 TCR transduced and nontransduced Tregs were allowed to expand and after resting (on day 12), transduced Tregs were restimulated with K64.A2.SL9.41BBL and nontransduced Tregs were restimulated with K.64.A2.41BBL coated with anti-CD3 (OKT3) Ab. Population doublings were determined at the indicated time points. (B) Transduced Tregs (22% TCR positive) were restimulated with either Ag-specific stimulation provided by K.64.A2.SL9.41BBL or polyclonal stimulation provided by K.64.A2.41BBL coated with OKT3 Ab. The frequency of TCR-transduced Tregs was reevaluated by SL9 tetramer staining 7 days after restimulation. (C) A2-SL9 TCR-transduced Tregs or nontransduced Tregs from panel B were again restimulated using K.64.A2.SL9.41BBL or K.64.A2.41BBL coated with OKT3 Ab, respectively. After another 7-10 days of culture, transduced Tregs (black line) and nontransduced Tregs (gray shading) were stained for Foxp3, CD27, and CD62L. Isotype control staining is shown as a thin black line. (D) TCR-transduced Tregs, nontransduced Tregs, and CD4 T cells were expanded after CD3/28 bead stimulation. After resting, cells were treated with phorbol 12-myristate 13-acetate plus ionomycin for 6 hours and intracellular IL-2 was measured by flow cytometry. These data are representative of 3 independent experiments.
Tregs transduced with MHC class I–restricted TCR can suppress in an Ag-specific manner
We investigated whether TCR-transduced Tregs could suppress in an Ag-specific manner. Because ex vivo–expanded, Ag-specific Tregs would likely be used to treat ongoing autoimmune diseases, we sought to determine whether Ag-specific Tregs could efficiently suppress previously primed Teffs. We isolated cord blood Tregs and either transduced them with awt/b6 A2-SL9 TCR or left them untransduced, and then expanded these populations for 13-18 days. The expanded Tregs were then mixed at various ratios with expanded adult CD8 Teffs that had been transduced with the awt/b6 A2-SL9 TCR and cocultured with K.64.A2.SL9.4-1BBL aAPCs. In preliminary studies, we found that expanded Teffs did not uniformly label with CFSE, which limited our ability to accurately measure cell division. Therefore, we developed a bead-based system that allowed us to quantitatively count the absolute number of A2-SL9–specific CD8 T cells present in the culture 5 days after restimulation. In pilot studies using freshly isolated Teffs, we obtained similar data using CFSE and bead methods for measuring Treg suppressive activity (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that these 2 assays for measuring suppressive activity are equivalent. We observed that highly rested, untransduced Tregs had limited ability to block Ag-specific CD8 T-cell expansion. In contrast, similarly highly rested Tregs that expressed an A2-SL9–specific TCR were able to potently suppress A2-SL9–specific T-cell expansion when present at a minimum Treg:Teff ratio of 1:8 (Figure 4A). We repeated this experiment with 4 cord blood samples and compiled the degree of A2-SL9–specific Teff suppression induced by the Tregs at a 1:4 Treg:Teff ratio. The TCR-engineered Tregs suppressed 57% ± 10% of the cell divisions observed, whereas the non-TCR-transduced Tregs could only suppress 15% ± 11% (Figure 4B, P = .001). These data indicate that MHC class I–restricted TCRs can promote Ag-specific suppression when introduced into human Tregs.
TCR affinity does not modulate Treg suppressive activity. (A-B) Freshly isolated cord blood Tregs were transduced with an A2-SL9–specific (awt/b6) TCR or mock-transduced and expanded for 12-18 days. Effector CD8 T cells derived from adult PBMCs were also transduced with the same A2-SL9–specific (awt/b6) TCR and cultured until they stopped expanding. Transduced or nontransduced Tregs were mixed with A2-SL9–specific CD8 T cells at the indicated ratios, along with K.A2.SL9.CD86.4-1BBL aAPCs. The ability of the polyclonal Tregs (▴) or A2-SL9–specific Tregs (■) to suppress the proliferation of the A2-SL9–specific CD8 T cells was measured using the bead-count assay described in supplemental Figure 1. Representative data are shown in panel A and composite data from 4 independent experiments are shown in panel B. (C) Suppressive activity of the A2-SL9 TCR-transduced Tregs was determined by mixing Tregs at the indicated ratios with CFSE-labeled CD8 T cells that had been transfected with RNA encoding the a2/b1 or the a11/b6 A2-SL9–specific TCR. These T-cell mixtures were stimulated with the aAPCs described in Figure 1B, and CFSE dilution was measured 5 days later by flow cytometry. The percent suppression was calculated as described in the “In vitro suppression assay.” A negative value indicates that more cell divisions were measured in these conditions relative to the control without Tregs.
TCR affinity does not modulate Treg suppressive activity. (A-B) Freshly isolated cord blood Tregs were transduced with an A2-SL9–specific (awt/b6) TCR or mock-transduced and expanded for 12-18 days. Effector CD8 T cells derived from adult PBMCs were also transduced with the same A2-SL9–specific (awt/b6) TCR and cultured until they stopped expanding. Transduced or nontransduced Tregs were mixed with A2-SL9–specific CD8 T cells at the indicated ratios, along with K.A2.SL9.CD86.4-1BBL aAPCs. The ability of the polyclonal Tregs (▴) or A2-SL9–specific Tregs (■) to suppress the proliferation of the A2-SL9–specific CD8 T cells was measured using the bead-count assay described in supplemental Figure 1. Representative data are shown in panel A and composite data from 4 independent experiments are shown in panel B. (C) Suppressive activity of the A2-SL9 TCR-transduced Tregs was determined by mixing Tregs at the indicated ratios with CFSE-labeled CD8 T cells that had been transfected with RNA encoding the a2/b1 or the a11/b6 A2-SL9–specific TCR. These T-cell mixtures were stimulated with the aAPCs described in Figure 1B, and CFSE dilution was measured 5 days later by flow cytometry. The percent suppression was calculated as described in the “In vitro suppression assay.” A negative value indicates that more cell divisions were measured in these conditions relative to the control without Tregs.
We have demonstrated previously that primary human CD8 T cells transduced with a high-affinity A2-SL9–specific TCR (a11/b6) displayed augmented effector functions, were able to control HIV-1 replication at low effector:target ratios, and could respond to cognate Ag in a highly polyfunctional manner.10 Therefore, we were interested in determining whether high-affinity TCRs could confer improved suppressive activity to TCR-transduced Ag-specific Tregs. We transduced the panel of A2-SL9–specific TCRs into freshly isolated cord blood Tregs and expanded these cells for 13-18 days until they stopped expanding and resumed a near resting cell volume, as described previously.19 To determine whether expanded, Ag-specific Tregs could also prevent the initial activation of potentially autoimmune T cells, we generated Ag-specific Teffs by electroporating RNAs encoding the lowest (a2/b1) or high-affinity (a11/b6) A2-SL9–specific TCR into freshly isolated, CFSE-labeled primary human CD8 T cells. A2-SL9 Ag-specific Tregs and Teffs were mixed in all pairwise combinations using various Treg:Teff ratios, and the CD8 T-cell proliferation in response to K.A2.SL9.NY-ESO-1 aAPCs was assessed by CFSE dilution (Figure 4C). In contrast to what we observed in Teffs,10 TCR affinity did not modulate the suppressive activity of Tregs. Tregs expressing the lowest-affinity TCRs were equally potent in blocking Ag-specific Teff expansion as those bearing the high-affinity TCRs. These data also indicate that TCR-transduced Tregs are equally adept at blocking ongoing and newly initiated Teff responses.
A MHC class I–restricted TCR that is unable to redirect CD4 T effector responses is capable of conferring robust Ag-specific suppression to Tregs
For most autoimmune diseases, the epitope(s) targeted by the immune system is unknown, and at present it is not logistically feasible to identify autoimmune target(s) and then develop a custom, Ag-specific therapy for each patient. Therefore, it would be desirable to develop Tregs that can become activated within a target tissue and suppress autoimmune cells of the same and of distinct specificities. To determine whether MHC class I–restricted TCR-transduced Tregs could mediate dominant tolerance,35 we used the previously described HLA-A2 restricted NY-ESO-1157-164 SLLMWITQC (A2-SC9) wild-type TCR, which has an affinity of 13-32μM8. Unlike what we observed with the A2-SL9–specific TCRs (Figure 1A), the A2-SC9 tetramer could not bind the CD4 T cells transfected (a 50:50 mixture of CD4 and CD8 T cells was transfected) with the A2-SC9 TCR despite high expression of the introduced Vβ chain (Figure 5A-D). This indicates that even though A2-SC9 TCR is well expressed in the CD4 T cells, the A2-SC9 tetramer has only a limited ability to bind to A2-SC9 TCR-expressing cells. For comparison, we performed a similar analysis with the lowest-affinity A2-SL9–specific TCR (a2/b1). As expected, we found that both the SL9-specific tetramer and the anti-Vβ5 Abs could recognize the TCR at similar frequencies (Figure 5E-H). Using the K.A2.SL9.NY-ESO-1 aAPCs (Figure 1B), we investigated whether A2-SC9 TCRs could redirect a CD4 Teff response. Consistent with previous results,11,13 we were unable to detect an Ag-specific, CD4 T-cell response to the NY-ESO-1 Ag. In this experiment, an equal number of CD8 T cells were mixed with the CD4 T cells, and we observed an NY-ESO-1 specific cytokine response in CD8 T cells, thereby demonstrating that the A2-SC9 effector responses are CD8 dependent (Figure 5I).
A MHC class I–restricted TCR that is unable to redirect CD4 T effector responses is capable of conferring robust Ag-specific suppression to Tregs. RNA encoding either the A2-SC9 specific TCR (C-D) or the A2-SL9 TCR (B-D) was transfected into an equal mixture of resting and purified CD4 and CD8 T cells and, after overnight culture, the ability to bind SC9 tetramer (A-C), introduced Vβ13 (B,D), SL9 tetramer (E,G), or introduced Vβ5 (F,H) was measured by flow cytometry using nontransfected cells stained with the same reagent to set the respective gates. (I) An equal mixture of CD4 and CD8 T cells were transfected with A2-SC9–specific TCR and incubated with the aAPCs described in Figure 1 or phorbol 12-myristate 13-acetate and ionomycin for 5 hours. The ability of these stimulated cells to produce IFNγ and IL-2 was measured by intracellular cytokine staining. Freshly isolated cord blood Tregs were isolated, and RNA transfected with nothing (polyclonal; J), A2-SL9 (K), or A2-SC9 (L). These Tregs were mixed at various ratios with CFSE-labeled CD8 T cells that had been transfected with awt/b6 A2-SL9–specific TCR. These T-cell mixtures were stimulated with aAPC as described in Figure 1B, and CFSE dilution was measured 5 days later by flow cytometry. The percent suppression was calculated as described in the “In vitro suppression assay.”
A MHC class I–restricted TCR that is unable to redirect CD4 T effector responses is capable of conferring robust Ag-specific suppression to Tregs. RNA encoding either the A2-SC9 specific TCR (C-D) or the A2-SL9 TCR (B-D) was transfected into an equal mixture of resting and purified CD4 and CD8 T cells and, after overnight culture, the ability to bind SC9 tetramer (A-C), introduced Vβ13 (B,D), SL9 tetramer (E,G), or introduced Vβ5 (F,H) was measured by flow cytometry using nontransfected cells stained with the same reagent to set the respective gates. (I) An equal mixture of CD4 and CD8 T cells were transfected with A2-SC9–specific TCR and incubated with the aAPCs described in Figure 1 or phorbol 12-myristate 13-acetate and ionomycin for 5 hours. The ability of these stimulated cells to produce IFNγ and IL-2 was measured by intracellular cytokine staining. Freshly isolated cord blood Tregs were isolated, and RNA transfected with nothing (polyclonal; J), A2-SL9 (K), or A2-SC9 (L). These Tregs were mixed at various ratios with CFSE-labeled CD8 T cells that had been transfected with awt/b6 A2-SL9–specific TCR. These T-cell mixtures were stimulated with aAPC as described in Figure 1B, and CFSE dilution was measured 5 days later by flow cytometry. The percent suppression was calculated as described in the “In vitro suppression assay.”
We also investigated whether Tregs expressing the A2-SC9 TCR were functional. We generated awt/b6 A2-SL9–specific effector CD8 T cells by RNA transfection and labeled them with CFSE. We mixed these Teffs with either polyclonal (Figure 5J), A2-SL9–specific (Figure 5K), or A2-SC9–specific (Figure 5L) Tregs at various ratios and determined the ability K.A2.SL9.NYESO-1 aAPCs to drive the expansion of the high-affinity A2-SL9–specific Teffs. The TCR-transfected Tregs bound the respective specific tetramer and Vβ Ab similar to the CD4 Teffs shown in Figure 5A-H (data not shown). Whereas the polyclonal Tregs could suppress when mixed at a 4:1 Teff:Treg ratio, this suppressive activity was not observed at ratios of 8:1 and below (Figure 5J). In contrast, Tregs expressing the same high-affinity awt/b6 A2-SL9 TCR as the Teffs were able to suppress Teff expansion even when diluted to a 32:1 Teff:Treg ratio (Figure 5K). Interestingly, Tregs expressing the A2-SC9 TCR, which was unable to redirect a CD4 Teff response (Figure 5I), showed much higher suppressive activity than polyclonal Tregs and as much activity as A2-SL9–specific Tregs (Figure 5L). These results indicate that nonengineered MHC class I–restricted TCRs can suppress highly potent CD8 T cells that recognize a distinct pMHC complex co-presented on the same target tissue as the Treg target Ag.
Target Ags must be expressed on the same cell to observe immunosuppression
We next investigated whether the target Ags of both the effectors and Tregs need to be presented by the same aAPC to observe bystander suppression or whether Tregs activated by one aAPC could suppress the Teffs activated by a different aAPC. We generated aAPCs that expressed HLA-A2 and the SL9 minigene (K.A2.SL9) and aAPCs that expressed HLA-A2 and the full-length NY-ESO-1 gene (K.A2.NY-ESO). Primary human CD8 T cells were then transfected with the wild-type A2-SL9 TCRs and CFSE labeled, cord blood–purified Tregs were transfected with the A2-SC9 (Figure 6A-D). When the A2-SC9 Tregs were mixed with A2-SL9 CD8 Teffs and activated by an equal mixture of K.A2.SL9 and K.A2.NY-ESO, we did not observe any Ag-specific suppression (Figure 6E-F). In contrast, as described in Figure 5, when the A2-SL9–expressing CD8 effectors were mixed with A2-SC9 Tregs and aAPCs that expressed both Ags (K.A2.SL9.NY-ESO-1), we observed potent, Ag-specific suppression of the A2-SL9 response by the A2-SC9 Tregs (Figure 6G-H). These data indicate that both Teffs and Tregs must be activated by the same aAPC to observe bystander suppression.
Target Ags must be expressed on the same cell to observe bystander immunosuppression. (A) RNA encoding the A2-SC9 TCRs was transfected into Tregs, and the ability of these cells to bind A2-SC9 tetramer was measured the following day by flow cytometry. (B) Same as panel A except TCR encoding RNA was not added before the transfection (mock control). (C) RNA encoding the A2-SL9 TCRs was transfected into a mixture of CD4 and CD8 Teffs, and the ability of these cells to bind A2-SL9 tetramer was measured the following day by flow cytometry. (D) Same as panel C except TCR encoding RNA was not added before the transfection (mock control). (E) A2-SL9 Teffs were mixed with the indicated ratios of mock-transfected, polyclonal Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9 and K.A2.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. (F) A2-SL9 Teffs were mixed with the indicated ratios of A2-SC9–transfected Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9 and K.A2.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. (G) A2-SL9 Teffs were mixed with the indicated ratios of mock-transfected, polyclonal Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. (H) A2-SL9 effectors were mixed with the indicated ratios of A2-SC9–transfected Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. A cartoon denoting the cell mixtures is displayed below each data panel. The percent suppression was calculated as described in “In vitro suppression assay.” A negative value indicates that more cell divisions were measured in these conditions relative to the control without Tregs.
Target Ags must be expressed on the same cell to observe bystander immunosuppression. (A) RNA encoding the A2-SC9 TCRs was transfected into Tregs, and the ability of these cells to bind A2-SC9 tetramer was measured the following day by flow cytometry. (B) Same as panel A except TCR encoding RNA was not added before the transfection (mock control). (C) RNA encoding the A2-SL9 TCRs was transfected into a mixture of CD4 and CD8 Teffs, and the ability of these cells to bind A2-SL9 tetramer was measured the following day by flow cytometry. (D) Same as panel C except TCR encoding RNA was not added before the transfection (mock control). (E) A2-SL9 Teffs were mixed with the indicated ratios of mock-transfected, polyclonal Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9 and K.A2.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. (F) A2-SL9 Teffs were mixed with the indicated ratios of A2-SC9–transfected Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9 and K.A2.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. (G) A2-SL9 Teffs were mixed with the indicated ratios of mock-transfected, polyclonal Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. (H) A2-SL9 effectors were mixed with the indicated ratios of A2-SC9–transfected Tregs. These T-cell mixtures were then incubated with an equal number of K.A2.SL9.NY-ESO aAPCs. The final ratio of T cells to aAPCs was 1. A cartoon denoting the cell mixtures is displayed below each data panel. The percent suppression was calculated as described in “In vitro suppression assay.” A negative value indicates that more cell divisions were measured in these conditions relative to the control without Tregs.
Discussion
A striking finding of our study is that a low-affinity, MHC class I–restricted TCR that was unable to provide Ag-specific Teff activity in the absence of CD8 was able to redirect the suppressive activity of Tregs as well as a high-affinity TCR that could confer Teff function to CD4 T cells. One interpretation of these data is that Tregs are less dependent on strong TCR signaling than are Teffs. There have been several studies showing that weaker TCR signals drive the differentiation of Teffs to Tregs35–39 ; however, the precise relationship between TCR affinity, the number of TCRs triggered, the duration of these signaling events, and the ability to generate functional Tregs is complicated, because a recent study showed that low amounts of a strong TCR agonist gave rise to Tregs that persisted the longest in vivo.40 Our studies demonstrate that whereas increased TCR affinity did not improve suppressive activity, as we observed previously for CD8 T-cell responses, increased affinity also did not impair suppressive activity. Therefore, whereas weak TCR signals may promote the differentiation of Tregs, our data indicate that strong TCR signals do not interfere with the function of fully differentiated natural Tregs.
Another possibility is that Tregs simply do not need CD4 to function, whereas most effector responses do. Injection of anti-CD4 blocking Ab resulted in the preferential accumulation of the Tregs,41 supporting the notion that Tregs are less dependent on CD4. Our studies and others3 using MHC class I–restricted TCRs to redirect Treg specificity provide further evidence that mature Tregs do not need CD4 signaling to function. The ability of Tregs to function in the absence of coreceptor (CD4) suggests that Tregs are not dependent on Lck activation for function.28 Previous studies have indicated that CD28-mediated recruitment of Lck is required for Treg development;42 however, a more recent study using a knock-in rather than a transgenic model could not completely replicate this finding.43 In any case, our studies examined the ability of mature Tregs to function in the absence of CD4-recruited Lck, whereas most other studies have examined differentiation of naive T cells to Tregs. It is unclear whether the signaling requirements to mediate suppressive activity for the development and differentiation of Tregs are the same. If Lck activation is dispensable for Treg but not Teff function, then targeting Lck activity could be an attractive target to shift the balance of immunity and tolerance.
Another key finding of our studies that has significant implications for the use of Ag-specific Tregs to treat autoimmune disease44 is that Tregs expressing the NY-ESO-1–specific TCR could suppress the A2-SL9 effectors as well as the A2-SL9 Tregs. These results suggest that bystander suppression can be as robust as Ag-specific suppression. Our findings show that the target Ag of both Tregs and Teffs must be expressed by the same cell, and this finding should be considered as Ag-specific Treg therapies are developed. This finding does not support earlier findings suggesting that Tregs could suppress effectors being activated by nearby APCs.45 Whereas there are many possible experimental differences between the present study and previous studies (eg, murine vs human, peptide vs natural processed Ag, Tregs from TCR transgenic mice vs TCR-transduced Tregs), the most likely explanation of this difference is that these previous studies preactivated Tregs before the initiation of the assay, whereas we initiated our experiments with resting Tregs. Therefore, the activation state of the Treg may influence its ability to function in a tissue-specific manner.
The results from the first in-human trial that used expanded Tregs to prevent GVHD demonstrate that isolating, expanding, and infusing adoptively transferred Tregs is both feasible and safe and, furthermore, there are hints that this type of therapy will be efficacious.46 The next series of challenges for the Treg cellular therapy field is to determine what kind and how many of a particular type of Treg should be used to effectively treat a particular disease state. For example, the rationale to use polyclonal Tregs to prevent or treat acute GVHD is strong.47 Tregs can be administered before disease initiation and at the time of transplantation and, because the exact target Ags are unknown in GVHD, the broad specificity of polyclonal Tregs is attractive. Autoimmune diseases such as type 1 diabetes and multiple sclerosis may present different challenges to Treg cellular therapy because in these, the autoimmune disease is generally well established before Treg therapy can be initiated. Moreover, these diseases are confined to a specific tissue, so therapies that can target this particular tissue are more likely to have therapeutic benefits and less likely to cause global immunosuppression.
There are many ways to generate Ag-specific Tregs to treat autoimmune disease. Approaches that isolate CD4+CD25+ T cells by tetramer technology and expand these cells in culture conditions that favor the generation of Tregs are attractive because there is no possibility that genotoxicity could be caused by integrating vectors48 ; however, significant issues related to functional exhaustion caused by extensive ex vivo culture and the uncertain stability of these suppressive cells once reintroduced in vivo detract from this approach. The use of chimeric Ag receptors (CARs) as a means to redirect Tregs to become activated at a site of autoimmune destruction is an attractive approach,2 although it requires the identification of a specific cell-surface marker that is well expressed in the tissue of interest and minimally expressed elsewhere. Moreover, it is not clear whether the signaling initiated by the linked costimulatory and CD3 ζ-chain motifs fully recapitulate what is needed to maintain natural Treg function and persistence. Lastly, whereas it may be possible to render CARs nonimmunogenic, this has not yet been accomplished.31 The use of TCRs to redirect Tregs to a specific tissue overcomes some of the shortcomings of using CARs, but may introduce others. MHC class II–restricted TCRs have been introduced into murine Tregs and these cells have successfully been used to treat an established arthritis model.4 The difficulty of using MHC class II–restricted TCRs to treat humans is that there is no predominant allele that is expressed within the human population that would force the development (and expense) of multiple MHC class II–restricted TCRs to enroll just enough patients to perform proof-of-principle testing in humans.
In the present study, we investigated whether cord blood–derived Tregs could be retargeted using MHC class I–restricted TCRs and, if so, how TCR affinity for cognate pMHC affects the suppressive activity of the engineered Tregs. Our results reveal that MHC class I–restricted TCRs can be used efficiently to retarget Tregs. Given the prevalence of HLA-A2 within the population, it is likely that this type of approach can be tested in the clinical setting with greater ease than MHC class II–restricted TCRs. In addition, because the inflamed tissue is more likely to express MHC class I than MHC class II molecules, Tregs expressing MHC class I TCRs might be more effective than MHC class II TCR-engineered Tregs, because they are more likely to accumulate and remain activated in the milieu of the autoimmune tissue. Our data indicate that there are multiple ways to engineer Tregs for adoptive T-cell therapy. In cases in which short-term suppression is desirable and potential genotoxicity from using an integrating vector is an unacceptable risk, we observed that RNA-engineered Tregs were quite effective in blocking potent T-cell responses. Given the improved safety profile and reduced expense of this approach,31 it may be attractive to try RNA transfection of TCRs first as a means to generate Ag-specific Tregs. If this fails to have therapeutic value, then the use of lentiviral vectors to provide stable, long-term TCR expression in the adoptively transferred Tregs may be required. In conclusion, these studies indicate that mature Tregs do not need particularly strong TCR signaling to control immune responses in an Ag-specific manner, and this should aid in the design and implementation of clinical trials to deliver Ag-specific Tregs to humans for the treatment of established autoimmune disease.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the JDRF (formerly known as the Juvenile Diabetes Research Foundation) Collaborative Center for Cell Therapy and the JDRF Center for Cord Blood Therapy for helpful suggestions and discussions; Julie Jadlowsky, Caitlin Baiducc, and Sunil Martin for proofreading the manuscript; Richard Carroll and Tatiana Golovina for helpful suggestions and advice; the University of Pennsylvania Center for AIDS Research (CFAR) and Cancer Center Immunology Core for providing primary human T cells; Sam Parry, Gwenn Danet, Martin Carroll, and the Stem Cell and Xenograft Core for providing cord blood samples; and the Flow Cytometry and Cell Sorting Facility.
This work was supported by JDRF (to J.L.R.) and by the National Institutes of Health (P01 CA 067493 to B.R.B.).
National Institutes of Health
Authorship
Contribution: G.P., L.Z., A.M., C.B.W., C.R.-O., N.L., A.D.B., J.G., and A.V. performed the research, analyzed the data, and edited the manuscript; Y.Z. contributed vital new reagents and edited the manuscript; B.R.B designed the research and edited the manuscript; B.K.J. designed the research, analyzed the data, and edited the manuscript; and J.L.R designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: N.L., J.G., A.V., and B.K.J are employees of Immunocore Ltd. A.D.B. is an employee of Adaptimmune Ltd. The remaining authors declare no competing financial interests.
Correspondence: James L. Riley, PhD, Department of Microbiology, Abramson Family Cancer Research Institute, Perelman School of Medicine, University of Pennsylvania, 556 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104-6160; e-mail: rileyj@exchange.upenn.edu.
References
Author notes
G.P. and L.Z. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal