Bosutinib, a dual Src/Abl tyrosine kinase inhibitor (TKI), has shown potent activity against chronic myeloid leukemia (CML). This phase 1/2 study evaluated the efficacy and safety of once-daily bosutinib 500 mg in leukemia patients after resistance/intolerance to imatinib. The current analysis included 118 patients with chronic-phase CML who had been pretreated with imatinib followed by dasatinib and/or nilotinib, with a median follow-up of 28.5 months. In this subpopulation, major cytogenetic response was attained by 32% of patients; complete cytogenetic response was attained by 24%, including in one of 3 patients treated with 3 prior TKIs. Complete hematologic response was achieved/maintained in 73% of patients. On-treatment transformation to accelerated/blast phase occurred in 5 patients. At 2 years, Kaplan-Meier–estimated progression-free survival was 73% and estimated overall survival was 83%. Responses were seen across Bcr-Abl mutations, including those associated with dasatinib and nilotinib resistance, except T315I. Bosutinib had an acceptable safety profile; treatment-emergent adverse events were primarily manageable grade 1/2 gastrointestinal events and rash. Grade 3/4 nonhematologic adverse events (> 2% of patients) included diarrhea (8%) and rash (4%). Bosutinib may offer a new treatment option for patients with chronic-phase CML after treatment with multiple TKIs. This trial was registered at www.clinicaltrials.gov as NCT00261846.
Introduction
Chronic myeloid leukemia (CML) is characterized by a Bcr-Abl tyrosine kinase fusion protein produced from the Philadelphia chromosome (Ph), which occurs as a result of reciprocal translocation between chromosomes 9 and 22.1 For the past 10 years, the tyrosine kinase inhibitor (TKI) imatinib has been the standard treatment of chronic phase (CP) CML based on multiple studies demonstrating efficacy and acceptable tolerability.2,3 However, some patients develop imatinib-resistant disease or intolerance to imatinib because of toxicities.4,5
The second-generation TKIs dasatinib and nilotinib have both demonstrated efficacy in patients with CP CML who had resistance or intolerance to imatinib, with more than half (53%-59%) of patients achieving a major cytogenetic response (MCyR).6,,,–10 Unfortunately, some patients also develop resistance or intolerance to dasatinib and/or nilotinib treatment.11 Few treatment options remain for patients previously treated with imatinib and dasatinib and/or nilotinib, and currently there are no approved therapies for this indication. Therefore, alternative treatments are needed for patients with CP CML after treatment with and resistance or intolerance to multiple TKIs.
Bosutinib (SKI-606) is an orally active, dual inhibitor of the Src and Abl tyrosine kinases, with minimal inhibitory activity against c-KIT or platelet-derived growth factor receptor.12,13 Previously reported data from a phase 1/2 study demonstrated substantial efficacy and acceptable tolerability in 288 patients with Ph+ CP CML who had been previously treated solely with imatinib and developed resistance or intolerance to imatinib.14 In this second-line setting after treatment with imatinib (median follow-up, 24.2 months), 53% of patients achieved a MCyR and 86% achieved or maintained a confirmed complete hematologic response (CHR), with responses observed across many Bcr-Abl kinase domain mutations, except for the T315I mutation. Bosutinib was also associated with an acceptable safety profile as second-line therapy, with mild or moderate gastrointestinal events and rash being the most commonly reported toxicities.14 The current analysis from the same phase 1/2 study focuses on the efficacy and safety of bosutinib after treatment with multiple TKIs (imatinib and dasatinib and/or nilotinib) in patients with Ph+ CP CML.
Methods
Patient population
The study design and eligibility criteria have been described previously.14 The current analysis included a study subpopulation of adults aged at least 18 years with a confirmed diagnosis of Ph+ CP CML; prior treatment with imatinib followed by dasatinib and/or nilotinib; an Eastern Cooperative Oncology Group Performance Status of 0 or 1; adequate bone marrow, hepatic, and renal function; no antiproliferative or antileukemia treatment within 7 days of bosutinib initiation (except hydroxyurea or anagrelide); and no allogeneic hematopoietic stem cell transplantation within 3 months.
All patients had received prior imatinib and developed either primary or acquired resistance or intolerance to imatinib and had also received and developed resistance or intolerance to prior dasatinib and/or nilotinib. For this analysis, resistance was defined as failure to achieve or maintain any of the following: hematologic improvement within 4 weeks, CHR after 12 weeks, any cytogenetic response by 24 weeks, or MCyR by 12 months.15 Acquired resistance was defined as loss of a MCyR or any hematologic response. Patients could also have resistance related to a Bcr-Abl mutation(s). Intolerance was defined as an inability to take the TKI because of drug-related grade 4 hematologic toxicity lasting more than 7 days, drug-related grade 3 or 4 nonhematologic toxicity, persistent grade 2 toxicity not responding to dose reduction and medical management, or loss of previously attained response on lower-dose TKI therapy with an inability to receive a higher dose because of drug-related toxicity at higher doses.
The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained for all patients before study participation, and the study protocol was approved by institutional review boards at each site.
Study design
This was a phase 1/2, open-label, multicenter, 2-part study. The phase 1 (part 1) dose-escalation study enrolled patients with CP CML (1 patient had accelerated phase [AP] CML) who were previously treated with only imatinib and had developed resistance to imatinib. The part 1 evaluation determined the part 2 starting dose of bosutinib 500 mg/day, despite not reaching a protocol-defined maximum tolerated dose.14 Part 2 of the study evaluated the efficacy and safety of bosutinib across multiple patient subpopulations, including those with CP CML who had been previously treated with imatinib and dasatinib and/or nilotinib. The primary analysis in the subpopulation of patients with CP CML previously treated with multiple TKIs was MCyR by 24 weeks.
Bosutinib was administered at a starting dose of 500 mg/day, and treatment continued until disease progression (transformation to AP or blast phase [BP] CML, or loss of previously attained response), unacceptable toxicity, or withdrawal of consent. Dose escalation to bosutinib 600 mg/day was permitted for lack of efficacy (CHR not reached by week 8 or complete cytogenetic response [CCyR] not reached by week 12) if no grade 3 or higher bosutinib-related adverse event (AE) had occurred. Doses could be held or lowered in 100-mg increments, as needed, based on severity and duration of treatment-related toxicities. Treatment was discontinued if a patient was unable to tolerate a dose of at least 300 mg/day.
Efficacy and safety assessments
All patients underwent baseline assessments within 14 days before registration,14 including sequencing of the Abl kinase domain to the first dose to identify baseline mutations. Sequencing was also performed at treatment completion to identify treatment-emergent Abl mutations.
Hematologic, cytogenetic, and molecular response criteria have been described previously.14 For hematologic response, patients must have either had an improvement from baseline to achieve a confirmed CHR or maintained a confirmed baseline CHR, with responses of at least 4 weeks in duration, with extramedullary involvement, and peripheral blood and/or bone marrow documentation.
Achievement of cytogenetic response required improvement from baseline, based on standard cytogenetics with at least 20 metaphases to determine the presence of Ph+ metaphases; if fewer than 20 metaphases were available for post-baseline assessments, FISH analysis of bone marrow aspirate with at least 200 cells for the presence of the Bcr-Abl fusion protein was used. CCyR required 0% Ph+ metaphases (< 1% positive cells by FISH), and partial cytogenetic response (PCyR) required 1% to 35% Ph+ metaphases; MCyR included both CCyR and PCyR. Complete blood counts with differential were performed at weeks 1, 2, 3, 4, 8, and 12; subsequently, both complete blood counts and cytogenetic assessments were performed at least every 3 months during the first 2 years, and every 6 months thereafter.
Molecular response was assessed at a central laboratory (Quest Diagnostics) using nonnested real-time PCR for the ratio of Bcr-Abl to Abl transcripts. Molecular responses were categorized as complete (CMR; undetectable Bcr-Abl transcript, with a PCR sensitivity of ≥ 5 log) or major (MMR; ≥ 3-log reduction from standardized baseline). Molecular analysis was performed monthly for the first 3 months, every 3 months through 2 years, and every 6 months thereafter; because of logistical constraints, patients enrolled in China, India, Russia, and South Africa could not be evaluated.
Disease progression included events of transformation from CP CML to AP or BP CML; white blood cell count that doubled over at least 1 month with a confirmed second count greater than 20 × 109/L; loss of confirmed CHR; or loss of MCyR with presence of Ph+ metaphases increased by at least 30%.
Incidence and severity of AEs were reported at each study visit through 30 days after the last dose of bosutinib. Physical examinations, vital signs, and laboratory tests were also performed routinely; a 12-lead electrocardiogram was obtained immediately before and after dosing on day 1, day 21, and at the end of treatment. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0.
Statistical analyses
Efficacy end points were summarized using response rates, CIs, and descriptive statistics, such as the Kaplan-Meier method; the study was not powered for comparative statistics between cohorts. Patients who had an adequate baseline assessment for a particular endpoint were evaluable for the respective response. Time to response was calculated from the start date of therapy to the first date of response (confirmed response for hematologic response and unconfirmed response for cytogenetic response). Duration of response was calculated from the first date of response to confirmed loss, progressive disease, or death. Progression-free survival (PFS) was calculated from the start date of therapy to the date of investigator-reported progression or death; progression (including transformation to AP or BP CML) was evaluated through 30 days after the last dose of study drug. Overall survival was calculated from the start date of therapy to the date of death, with patients censored at the last contact (survival was evaluated throughout the 2-year follow-up period after treatment discontinuation). For time-to-event endpoints except overall survival, patients were censored at the last follow-up visit for those not known to have the respective end point.
All patients who received at least 1 dose of bosutinib were included in the safety analysis. AEs were described both with and without regard to causality.
Results
Patients
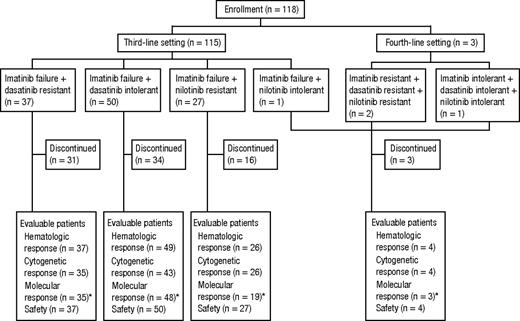
A total of 118 patients with CP CML after treatment with multiple TKIs were dosed in this study population between August 22, 2006, and March 19, 2010 (Figure 1). All patients were previously treated with imatinib; in addition, 37 patients had dasatinib resistance, 50 had dasatinib intolerance, 27 had nilotinib resistance, 1 had nilotinib intolerance, 2 had dasatinib and nilotinib resistance, and 1 had dasatinib and nilotinib intolerance. Demographic and baseline disease characteristics are summarized in Table 1. Twenty (17%) patients had Ph chromosome variants (n = 1) or additional karyotypic abnormalities (n = 19) at the time of screening, with abnormalities affecting chromosome 8 being the most common (n = 7 of 19). Bcr-Abl mutation status at baseline was available for 83 (70%) patients, and 39 (47%) of these had at least 1 mutation identified, with 9 (11%) harboring more than 1 mutation.
Disposition of patients. Part 2 of the study enrolled a total of 115 patients who previously received imatinib and either dasatinib or nilotinib and 3 patients who previously received all 3 TKIs. *Because of logistical constraints, patients from sites in China, India, Russia, and South Africa were not assessed for molecular response.
Disposition of patients. Part 2 of the study enrolled a total of 115 patients who previously received imatinib and either dasatinib or nilotinib and 3 patients who previously received all 3 TKIs. *Because of logistical constraints, patients from sites in China, India, Russia, and South Africa were not assessed for molecular response.
Baseline patient demographic and disease characteristics
| Characteristic . | IM + DAS resistant (n = 37) . | IM + DAS intolerant (n = 50) . | IM + NI resistant (n = 27) . | IM + DAS ± NI (n = 4)* . | Total (n = 118) . |
|---|---|---|---|---|---|
| Median age, y (range) | 54.0 (23-69) | 58.0 (25-79) | 52.0 (20-73) | 54.5 (31-62) | 56.0 (20-79) |
| Sex, n (%) | |||||
| Female | 23 (62) | 27 (54) | 13 (48) | 2 (50) | 65 (55) |
| Male | 14 (38) | 23 (46) | 14 (52) | 2 (50) | 53 (45) |
| Race, n (%) | |||||
| White | 27 (73) | 38 (76) | 17 (63) | 3 (75) | 85 (72) |
| Asian | 4 (11) | 9 (18) | 3 (11) | 0 | 16 (14) |
| Other | 6 (16) | 3 (6) | 7 (26) | 1 (25) | 17 (14) |
| Median duration of CML disease, y (range) | 7.5 (1.2-17.6) | 5.6 (0.6-18.3) | 5.9 (1.2-16.3) | 11.7 (2.2-11.9) | 6.7 (0.6-18.3) |
| ECOG Performance Status, n (%)† | |||||
| 0 | 28 (76) | 31 (62) | 25 (93) | 2 (50) | 86 (74) |
| 1 | 9 (24) | 18 (36) | 2 (7) | 2 (50) | 31 (26) |
| Median duration of prior therapy, (range) | |||||
| Imatinib, y | 2.6 (0.02-6.4) | 3.3 (0.1-6.6) | 2.5 (0.7-5.9) | 3.0 (1.4-6.4) | 2.7 (0.02-6.6) |
| Dasatinib, mo | 18.3 (1.7-47.9) | 17.3 (1.1-35.7) | 0 | 4.1 (1.3-6.9) | 17.7 (1.1-47.9) |
| Nilotinib, mo | 0 | 0 | 12.7 (1.7-38.9) | 5.4 (0.8-6.1) | 9.2 (0.8-38.9) |
| Additional prior therapies, n (%) | |||||
| Interferon | 25 (68) | 24 (48) | 10 (37) | 2 (50) | 61 (52) |
| Stem cell transplant | 2 (5) | 5 (10) | 0 | 2 (50) | 9 (8) |
| Characteristic . | IM + DAS resistant (n = 37) . | IM + DAS intolerant (n = 50) . | IM + NI resistant (n = 27) . | IM + DAS ± NI (n = 4)* . | Total (n = 118) . |
|---|---|---|---|---|---|
| Median age, y (range) | 54.0 (23-69) | 58.0 (25-79) | 52.0 (20-73) | 54.5 (31-62) | 56.0 (20-79) |
| Sex, n (%) | |||||
| Female | 23 (62) | 27 (54) | 13 (48) | 2 (50) | 65 (55) |
| Male | 14 (38) | 23 (46) | 14 (52) | 2 (50) | 53 (45) |
| Race, n (%) | |||||
| White | 27 (73) | 38 (76) | 17 (63) | 3 (75) | 85 (72) |
| Asian | 4 (11) | 9 (18) | 3 (11) | 0 | 16 (14) |
| Other | 6 (16) | 3 (6) | 7 (26) | 1 (25) | 17 (14) |
| Median duration of CML disease, y (range) | 7.5 (1.2-17.6) | 5.6 (0.6-18.3) | 5.9 (1.2-16.3) | 11.7 (2.2-11.9) | 6.7 (0.6-18.3) |
| ECOG Performance Status, n (%)† | |||||
| 0 | 28 (76) | 31 (62) | 25 (93) | 2 (50) | 86 (74) |
| 1 | 9 (24) | 18 (36) | 2 (7) | 2 (50) | 31 (26) |
| Median duration of prior therapy, (range) | |||||
| Imatinib, y | 2.6 (0.02-6.4) | 3.3 (0.1-6.6) | 2.5 (0.7-5.9) | 3.0 (1.4-6.4) | 2.7 (0.02-6.6) |
| Dasatinib, mo | 18.3 (1.7-47.9) | 17.3 (1.1-35.7) | 0 | 4.1 (1.3-6.9) | 17.7 (1.1-47.9) |
| Nilotinib, mo | 0 | 0 | 12.7 (1.7-38.9) | 5.4 (0.8-6.1) | 9.2 (0.8-38.9) |
| Additional prior therapies, n (%) | |||||
| Interferon | 25 (68) | 24 (48) | 10 (37) | 2 (50) | 61 (52) |
| Stem cell transplant | 2 (5) | 5 (10) | 0 | 2 (50) | 9 (8) |
IM indicates imatinib; DAS, dasatinib; NI, nilotinib; and ECOG, Eastern Cooperative Oncology Group.
Includes 3 patients who previously received all 3 inhibitors and 1 patient with NI intolerance.
ECOG Performance Status at baseline was missing for 1 patient with DAS intolerance.
As of the data cutoff on March 28, 2011, the median duration of follow-up was 28.5 months (range, 0.3-56.2 months). The median duration on bosutinib treatment was 8.3 months (range, 0.2-51.8 months) and varied between 7.3 months for dasatinib-resistant patients and 11.0 months for nilotinib-resistant patients. The median dose intensity was 478 mg/day (range, 185-563 mg/day). Dose interruptions were required for 70% of patients (dasatinib-resistant, 57%; dasatinib-intolerant, 82%; nilotinib-resistant, 67%; nilotinib-intolerant/previous treatment with all 3 TKIs, 75%). During the study, 20 (17%) patients had their dose of bosutinib escalated to 600 mg/day for lack of efficacy. As of the data cutoff, 29% of patients were still receiving bosutinib (dasatinib-resistant, 16%; dasatinib-intolerant, 32%; nilotinib-resistant, 41%; nilotinib-intolerant/previous treatment with all 3 TKIs, 25%); reasons for discontinuation of treatment are shown in Table 2.
Discontinuation from treatment
| Reason, n (%) . | IM + DAS resistant (n = 37) . | IM + DAS intolerant (n = 50) . | IM + NI resistant (n = 27) . | IM + DAS ± NI (n = 4)* . | Total (n = 118) . |
|---|---|---|---|---|---|
| Discontinued treatment | 31 (84) | 34 (68) | 16 (59) | 3 (75) | 84 (71) |
| Unsatisfactory response (efficacy) | 12 (32) | 7 (14) | 5 (19) | 1 (25) | 25 (21) |
| AE | 6 (16) | 15 (30) | 3 (11) | 0 | 24 (20) |
| Disease progression | 8 (22) | 4 (8) | 6 (22) | 2 (50) | 20 (17) |
| Death | 2 (5) | 2 (4) | 0 | 0 | 4 (3) |
| Patient request | 0 | 2 (4) | 1 (4) | 0 | 3 (3) |
| Lost to follow-up | 2 (5) | 0 | 0 | 0 | 2 (2) |
| Investigator request | 0 | 0 | 1 (4) | 0 | 1 (1) |
| Protocol violation | 0 | 1 (2) | 0 | 0 | 1 (1) |
| Other† | 1 (3) | 3 (6) | 0 | 0 | 4 (3) |
| Reason, n (%) . | IM + DAS resistant (n = 37) . | IM + DAS intolerant (n = 50) . | IM + NI resistant (n = 27) . | IM + DAS ± NI (n = 4)* . | Total (n = 118) . |
|---|---|---|---|---|---|
| Discontinued treatment | 31 (84) | 34 (68) | 16 (59) | 3 (75) | 84 (71) |
| Unsatisfactory response (efficacy) | 12 (32) | 7 (14) | 5 (19) | 1 (25) | 25 (21) |
| AE | 6 (16) | 15 (30) | 3 (11) | 0 | 24 (20) |
| Disease progression | 8 (22) | 4 (8) | 6 (22) | 2 (50) | 20 (17) |
| Death | 2 (5) | 2 (4) | 0 | 0 | 4 (3) |
| Patient request | 0 | 2 (4) | 1 (4) | 0 | 3 (3) |
| Lost to follow-up | 2 (5) | 0 | 0 | 0 | 2 (2) |
| Investigator request | 0 | 0 | 1 (4) | 0 | 1 (1) |
| Protocol violation | 0 | 1 (2) | 0 | 0 | 1 (1) |
| Other† | 1 (3) | 3 (6) | 0 | 0 | 4 (3) |
IM indicates imatinib; DAS, dasatinib; and NI, nilotinib.
Includes 3 patients who previously received all 3 inhibitors and 1 patient with NI intolerance.
Includes patient noncompliance (n = 2), lack of finances (n = 1), and started chemotherapy for gastric adenocarcinoma (n = 1).
Efficacy
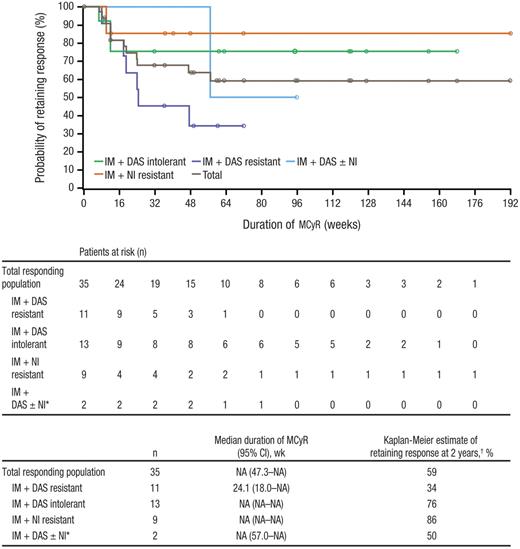
MCyR was attained by 32% (n = 35) of patients, with CCyR in 24% (n = 26) of patients, including one of 3 patients who were previously treated with all 3 TKIs; an additional 6% of patients achieved a minor cytogenetic response (Table 3). Of note, patients with CCyR or PCyR at baseline were considered nonresponders for this analysis despite potential maintenance of their response. When patients with a baseline CCyR or PCyR who maintained their response after baseline were included as responders in the analysis, the rates of MCyR and CCyR were 39% (n = 42) and 31% (n = 33), respectively. Median time to MCyR among responders was 12.4 weeks (range, 3.9-88.4 weeks). Kaplan-Meier median durations of MCyR and CCyR had not yet been reached overall and for most subpopulations, with overall estimates of 59% and 51% for the Kaplan-Meier probability of retaining MCyR and CCyR at 2 years, respectively (Figure 2). The Kaplan-Meier probability of retaining MCyR at 2 years was high among patients with nilotinib resistance (86%) and dasatinib intolerance (76%) but lower among those with dasatinib resistance (34%) and nilotinib intolerance/prior treatment with all 3 TKIs (50%; only 2 responders were included in this duration analysis). Of the 29 (27%; 95% CI, 19%-36%) patients who achieved MCyR by 24 weeks, 16 (55%) retained their response at 72 weeks and 9 (31%) retained their response at 96 weeks as of the data snapshot; 4 of these 29 patients have not yet reached their week 72 evaluation and are thus considered not to have retained their response at weeks 72 and 96.
Best cumulative response to bosutinib
| Response, n (%) . | IM + DAS resistant (n = 37) . | IM + DAS intolerant (n = 50) . | IM + NI resistant (n = 27) . | IM + DAS ± NI (n = 4)* . | Total (n = 118) . |
|---|---|---|---|---|---|
| Median follow-up, mo (range) | 20.0 (2.7-51.3) | 34.5 (0.3-56.2) | 23.0 (7.1-54.0) | 34.5 (22.8-40.0) | 28.5 (0.3-56.2) |
| Hematologic response† | |||||
| Evaluable patients | 37 | 49 | 26 | 4 | 116 |
| Complete response | 23 (62) | 39 (80) | 20 (77) | 3 (75) | 85 (73) |
| Hematologic response among patients with no baseline CHR | |||||
| Evaluable patients | 22 | 24 | 20 | 2 | 68 |
| Complete response | 11 (50) | 16 (67) | 15 (75) | 2 (100) | 44 (65) |
| Cytogenetic response‡ | |||||
| Evaluable patients | 35 | 43 | 26 | 4 | 108 |
| Major response | 11 (31) | 13 (30) | 9 (35) | 2 (50) | 35 (32) |
| Complete response | 5 (14) | 12 (28) | 7 (27) | 2 (50) | 26 (24) |
| Partial response | 6 (17) | 1 (2) | 2 (8) | 0 | 9 (8) |
| Minor response | 0 | 4 (9) | 2 (8) | 0 | 6 (6) |
| Molecular response§ | |||||
| Evaluable patients | 35 | 48 | 19 | 3 | 105 |
| Major response | 1 (3) | 12 (25) | 2 (11) | 1 (33) | 16 (15) |
| Complete response | 0 | 9 (19) | 2 (11) | 1 (33) | 12 (11) |
| Response, n (%) . | IM + DAS resistant (n = 37) . | IM + DAS intolerant (n = 50) . | IM + NI resistant (n = 27) . | IM + DAS ± NI (n = 4)* . | Total (n = 118) . |
|---|---|---|---|---|---|
| Median follow-up, mo (range) | 20.0 (2.7-51.3) | 34.5 (0.3-56.2) | 23.0 (7.1-54.0) | 34.5 (22.8-40.0) | 28.5 (0.3-56.2) |
| Hematologic response† | |||||
| Evaluable patients | 37 | 49 | 26 | 4 | 116 |
| Complete response | 23 (62) | 39 (80) | 20 (77) | 3 (75) | 85 (73) |
| Hematologic response among patients with no baseline CHR | |||||
| Evaluable patients | 22 | 24 | 20 | 2 | 68 |
| Complete response | 11 (50) | 16 (67) | 15 (75) | 2 (100) | 44 (65) |
| Cytogenetic response‡ | |||||
| Evaluable patients | 35 | 43 | 26 | 4 | 108 |
| Major response | 11 (31) | 13 (30) | 9 (35) | 2 (50) | 35 (32) |
| Complete response | 5 (14) | 12 (28) | 7 (27) | 2 (50) | 26 (24) |
| Partial response | 6 (17) | 1 (2) | 2 (8) | 0 | 9 (8) |
| Minor response | 0 | 4 (9) | 2 (8) | 0 | 6 (6) |
| Molecular response§ | |||||
| Evaluable patients | 35 | 48 | 19 | 3 | 105 |
| Major response | 1 (3) | 12 (25) | 2 (11) | 1 (33) | 16 (15) |
| Complete response | 0 | 9 (19) | 2 (11) | 1 (33) | 12 (11) |
IM indicates imatinib; DAS, dasatinib; and NI, nilotinib.
Includes 3 patients who previously received all 3 inhibitors and 1 patient with NI intolerance.
Evaluable patients had a baseline disease assessment. Patients with CHR at baseline were evaluable for hematologic response and were considered responders if they maintained their response at 2 consecutive post-baseline assessments ≥ 4 weeks apart.
Evaluable patients had a baseline disease assessment. Patients with CCyR at baseline were considered nonresponders for assessment of cytogenetic response.
Because of logistical constraints, patients from sites in China, India, Russia, and South Africa were not assessed for molecular response. MMR indicates ≥ 3 log reduction from standardized baseline Bcr-Abl:Abl ratio; and CMR, undetectable Bcr-Abl, with a sensitivity of ≥ 5 log. Molecular response was not assessed according to the International Scale.
Duration of MCyR while on bosutinib treatment. Duration of MCyR was based on the evaluable population; patients entering the study in cytogenetic remission (CCyR) were excluded. IM indicates imatinib; DAS, dasatinib; NI, nilotinib; and NA, not available. *Includes 2 patients: 1 patient who was nilotinib-intolerant and 1 who previously received all 3 inhibitors. †Probability of retaining response at 2 years was based on Kaplan-Meier estimates.
Duration of MCyR while on bosutinib treatment. Duration of MCyR was based on the evaluable population; patients entering the study in cytogenetic remission (CCyR) were excluded. IM indicates imatinib; DAS, dasatinib; NI, nilotinib; and NA, not available. *Includes 2 patients: 1 patient who was nilotinib-intolerant and 1 who previously received all 3 inhibitors. †Probability of retaining response at 2 years was based on Kaplan-Meier estimates.
A confirmed CHR was achieved or maintained by 85 (73%) patients. Of the 68 subjects who did not have a CHR at baseline, 44 (65%) achieved a confirmed CHR (Table 3). Of the 85 patients who achieved or maintained a confirmed CHR during the study, the Kaplan-Meier probability of retaining their confirmed CHR at 2 years was 67%, with a Kaplan-Meier median duration of CHR not yet reached.
Analysis of molecular response excluded those patients enrolled in countries (China, India, Russia, and South Africa) where molecular response was not assessed for logistical reasons. Among the remaining treated patients (n = 105), 16 (15%) achieved a MMR, including 12 (11%) with a CMR (Table 3). A total of 33 patients achieved a CCyR or maintained their baseline CCyR and were evaluable for a subgroup analysis of molecular response; of these, 16 (49%) achieved a MMR, including 12 (36%) with a CMR.
Among the 39 patients with known Bcr-Abl kinase domain mutations at baseline, the most common were F317L (n = 8), T315I (n = 7), G250E (n = 6), and Y253H (n = 6). CHR and MCyR were observed broadly across Bcr-Abl mutants, including those conferring clinical resistance to dasatinib (F317L) and nilotinib (Y253H, E255K/V, F359C/I/V; Table 4). Nine patients developed new mutations during treatment, including 8 patients who already had a baseline mutation and one patient who developed 2 emergent mutations; specific emergent mutations included V299L (n = 4), L248V (n = 2), T315I (n = 2), F359C (n = 1), and G250E (n = 1). Of the 9 patients with emergent mutations, one patient had 2 emergent mutations (G250E and V299L) and 8 had discontinued bosutinib because of progressive disease or unsatisfactory response.
Response by baseline mutation status
| Mutation status . | n . | Cumulative response, n/n evaluable (%) . | |
|---|---|---|---|
| CHR . | MCyR . | ||
| No mutation | 44 | 34/44 (77) | 15/43 (35) |
| Any mutation | 39 | 26/39 (67) | 11/35 (31) |
| > 1 mutation | 9 | 3/9 (33) | 2/9 (22) |
| Mutation type* | |||
| P-loop† | 14 | 9/14 (64) | 4/13 (31) |
| G250E | 6 | 3/6 | 0/5 |
| Y253H | 6 | 5/6 | 4/6 |
| E255K | 1 | 0/1 | 0/1 |
| E255V | 1 | 1/1 | 0/1 |
| Non–P-loop‡ | 29 | 18/29 (62) | 9/26 (35) |
| M244V | 3 | 3/3 | 2/3 |
| V299L | 2 | 1/2 | 0/2 |
| Q300R | 1 | 1/1 | 1/1 |
| T315I | 7 | 2/7 | 0/6 |
| F317L | 8 | 4/8 | 1/7 |
| N336S | 1 | 1/1 | 0/1 |
| M351T | 1 | 1/1 | 0/1 |
| F359C | 2 | 2/2 | 1/2 |
| F359I | 2 | 2/2 | 2/2 |
| F359V | 2 | 0/2 | 1/2 |
| L387F | 1 | 1/1 | 0/1 |
| H396R | 1 | 0/1 | 0/1 |
| E453A | 1 | 1/1 | 0/0 |
| C475V | 1 | 1/1 | 1/1 |
| F486S | 1 | 0/1 | 0/1 |
| Mutation status . | n . | Cumulative response, n/n evaluable (%) . | |
|---|---|---|---|
| CHR . | MCyR . | ||
| No mutation | 44 | 34/44 (77) | 15/43 (35) |
| Any mutation | 39 | 26/39 (67) | 11/35 (31) |
| > 1 mutation | 9 | 3/9 (33) | 2/9 (22) |
| Mutation type* | |||
| P-loop† | 14 | 9/14 (64) | 4/13 (31) |
| G250E | 6 | 3/6 | 0/5 |
| Y253H | 6 | 5/6 | 4/6 |
| E255K | 1 | 0/1 | 0/1 |
| E255V | 1 | 1/1 | 0/1 |
| Non–P-loop‡ | 29 | 18/29 (62) | 9/26 (35) |
| M244V | 3 | 3/3 | 2/3 |
| V299L | 2 | 1/2 | 0/2 |
| Q300R | 1 | 1/1 | 1/1 |
| T315I | 7 | 2/7 | 0/6 |
| F317L | 8 | 4/8 | 1/7 |
| N336S | 1 | 1/1 | 0/1 |
| M351T | 1 | 1/1 | 0/1 |
| F359C | 2 | 2/2 | 1/2 |
| F359I | 2 | 2/2 | 2/2 |
| F359V | 2 | 0/2 | 1/2 |
| L387F | 1 | 1/1 | 0/1 |
| H396R | 1 | 0/1 | 0/1 |
| E453A | 1 | 1/1 | 0/0 |
| C475V | 1 | 1/1 | 1/1 |
| F486S | 1 | 0/1 | 0/1 |
Patients with > 1 mutation may be counted in both the P-loop and non–P-loop categories.
P-loop mutations include those from positions 248 to 255.
Non–P-loop mutations include those from positions 244 to 247 and > 255.
Of the 20 patients who had their bosutinib dose escalated to 600 mg/day for lack of efficacy, 6 (30%) patients subsequently achieved a response with the higher dose, including 2 patients who achieved CHR, 3 patients who achieved a CCyR, and 1 patient who achieved a PCyR.
Safety and tolerability
The most common nonhematologic treatment-related AEs were gastrointestinal toxicities (ie, diarrhea [81%], nausea [43%], and vomiting [32%]; Table 5). Treatment-related grade 3 diarrhea was reported for 10 (8%) patients, and grade 3 vomiting was reported for 1 (1%) patient; no grade 4 severity of these events was reported. Gastrointestinal events typically had an early median time to onset (diarrhea, 1.5 days; nausea, 3.5 days; vomiting, 19.5 days) and frequently resolved (80%, 84%, and 94%, respectively) spontaneously or with supportive care and/or dose adjustments. The median duration of any event of diarrhea was 2.0 days, and the median duration of a grade 3 event of diarrhea was 7.0 days. Concomitant medication for management of diarrhea was received by 65% of patients (primarily loperamide, 59%) who experienced a diarrhea event. Three (3%) patients discontinued treatment because of gastrointestinal AEs, and diarrhea was not considered the primary reason for treatment discontinuation for any patient. Treatment-related pleural effusions were experienced by 9 (8%) patients; each had been previously exposed to dasatinib, and 7 of these 9 patients had a history of pleural effusions on prior treatments. One grade 3 treatment-related pleural effusion was reported; there were no grade 4 events. Notably, bosutinib treatment was associated with a low incidence (< 5% of patients) of treatment-related fluid retention, muscle spasms, myalgia, and cardiovascular events. One patient had an on-treatment QTcF interval increase of greater than 60 ms from baseline, although the event did not exceed 450 ms; the patient remains on treatment with no other grade 2 or higher QTcF events observed.
Treatment-related AEs occurring in ≥ 10% of patients
| AE . | All grades . | Grade 3/4 . |
|---|---|---|
| Diarrhea | 96 (81) | 10 (8) |
| Nausea | 51 (43) | 0 |
| Vomiting | 38 (32) | 1 (1) |
| Rash | 26 (22) | 5 (4) |
| Fatigue | 21 (18) | 1 (1) |
| Abdominal pain | 18 (15) | 0 |
| Headache | 16 (14) | 1 (1) |
| Upper abdominal pain | 15 (13) | 0 |
| Pruritus | 12 (10) | 1 (1) |
| AE . | All grades . | Grade 3/4 . |
|---|---|---|
| Diarrhea | 96 (81) | 10 (8) |
| Nausea | 51 (43) | 0 |
| Vomiting | 38 (32) | 1 (1) |
| Rash | 26 (22) | 5 (4) |
| Fatigue | 21 (18) | 1 (1) |
| Abdominal pain | 18 (15) | 0 |
| Headache | 16 (14) | 1 (1) |
| Upper abdominal pain | 15 (13) | 0 |
| Pruritus | 12 (10) | 1 (1) |
Data are n (%).
Grade 3/4 hematologic toxicities included thrombocytopenia (25%), neutropenia (19%), and anemia (8%; Table 6). Grade 3/4 neutropenia was associated with infection for only one patient. On-treatment grade 3/4 laboratory elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were experienced by 7% and 3% of patients, respectively (Table 6), of which only 1 (< 1%) patient experienced a grade 4 ALT or AST elevation. The median time to first elevated laboratory value of ALT and AST (any grade) was 21.5 and 29.0 days, respectively, and the median duration of a reported increase of ALT and AST AEs was 15.0 and 9.0 days. ALT and AST elevations rarely led to treatment discontinuation (3% and 1% of patients, respectively). Recovery from grade 3/4 laboratory elevations of ALT and AST to grade 0/1 severity took a median duration (cumulative) of 29.0 and 21.5 days, respectively.
Laboratory abnormalities reported by ≥ 20% of patients on therapy
| Laboratory abnormality . | At baseline . | On therapy . | ||
|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Anemia | 66 (56) | 1 (1) | 99 (84) | 10 (8) |
| Thrombocytopenia | 20 (17) | 1 (1) | 69 (58) | 30 (25) |
| Elevated ALT | 12 (10) | 0 | 58 (49) | 8 (7) |
| Neutropenia | 23 (19) | 4 (3) | 54 (46) | 23 (19) |
| Elevated creatinine | 13 (11) | 0 | 50 (42) | 1 (1) |
| Elevated AST | 15 (13) | 0 | 48 (41) | 4 (3) |
| Hypocalcemia | 8 (7) | 1 (1) | 44 (37) | 6 (5) |
| Elevated alkaline phosphatase | 12 (10) | 0 | 41 (35) | 0 |
| Hypophosphatemia | 8 (7) | 0 | 37 (31) | 3 (2) |
| Hyperglycemia | 15 (13) | 0 | 36 (31) | 1 (1) |
| Low bicarbonate | 4 (3) | 0 | 36 (31) | 0 |
| Hypermagnesemia | 11 (9) | 9 (8) | 34 (29) | 14 (12) |
| Increased PTT | 16 (14) | 0 | 30 (25) | 1 (1) |
| Elevated lipase | 7 (6) | 0 | 28 (24) | 8 (7) |
| Hyperkalemia | 3 (3) | 1 (1) | 27 (23) | 0 |
| Laboratory abnormality . | At baseline . | On therapy . | ||
|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Anemia | 66 (56) | 1 (1) | 99 (84) | 10 (8) |
| Thrombocytopenia | 20 (17) | 1 (1) | 69 (58) | 30 (25) |
| Elevated ALT | 12 (10) | 0 | 58 (49) | 8 (7) |
| Neutropenia | 23 (19) | 4 (3) | 54 (46) | 23 (19) |
| Elevated creatinine | 13 (11) | 0 | 50 (42) | 1 (1) |
| Elevated AST | 15 (13) | 0 | 48 (41) | 4 (3) |
| Hypocalcemia | 8 (7) | 1 (1) | 44 (37) | 6 (5) |
| Elevated alkaline phosphatase | 12 (10) | 0 | 41 (35) | 0 |
| Hypophosphatemia | 8 (7) | 0 | 37 (31) | 3 (2) |
| Hyperglycemia | 15 (13) | 0 | 36 (31) | 1 (1) |
| Low bicarbonate | 4 (3) | 0 | 36 (31) | 0 |
| Hypermagnesemia | 11 (9) | 9 (8) | 34 (29) | 14 (12) |
| Increased PTT | 16 (14) | 0 | 30 (25) | 1 (1) |
| Elevated lipase | 7 (6) | 0 | 28 (24) | 8 (7) |
| Hyperkalemia | 3 (3) | 1 (1) | 27 (23) | 0 |
Data are n (%).
PTT indicates partial thromboplastin time.
Dose interruptions and reductions, respectively, resulting from AEs were required for 63% and 48% of patients. Twenty-four (20%) patients discontinued bosutinib treatment because of AEs (Table 2), including 16% of dasatinib-resistant, 30% of dasatinib-intolerant, and 11% of nilotinib-resistant patients. AEs most frequently leading to discontinuation included thrombocytopenia (4%), neutropenia (3%), and elevated ALT (3%). Of note, 6 patients were in MCyR at the time that they discontinued treatment because of an AE.
Retrospective evaluation of cross-intolerance between bosutinib and dasatinib found that 11 of 50 (22%) patients with dasatinib intolerance experienced the same AE on bosutinib as a grade 3/4 event and 4 of 50 (8%) patients discontinued bosutinib because of the same AE (Table 7). The most common cross-intolerance events on bosutinib were hematologic events, with 8 of 20 (40%) patients with dasatinib intolerance related to myelosuppression experiencing grade 3/4 myelosuppression on bosutinib and 2 of 20 (10%) patients discontinuing bosutinib because of myelosuppression. Of the 19 patients with pleural effusions as the reason for dasatinib intolerance, only 2 patients experienced grade 3/4 pleural effusions on bosutinib (at days 597 and 967 of the study), and neither patient discontinued bosutinib because of a pleural effusion. No patient with dasatinib intolerance related to cardiovascular events, gastrointestinal events, musculoskeletal events, or skin disorders experienced the same toxicity as a grade 3/4 AE on bosutinib.
Cross-intolerance between bosutinib and prior dasatinib
| AE, n (%)* . | Dasatinib intolerant . | Grade 3/4 event . | Discontinued because of event . |
|---|---|---|---|
| Any AE | 50 | 11 (22) | 4 (8) |
| Hematologic events | 20 | 8 (40) | 2 (10) |
| Thrombocytopenia | 8 | 6 (75) | 1 (13) |
| Pancytopenia | 5 | 0 | 0 |
| Neutropenia | 4 | 4 (100) | 1 (25) |
| Hematotoxicity | 3 | 0 | 0 |
| Cardiovascular events | 3 | 0 | 1 (33) |
| Gastrointestinal events | 6 | 0 | 0 |
| Diarrhea | 3 | 0 | 0 |
| Musculoskeletal events | 4 | 0 | 0 |
| Respiratory events | 23 | 3 (13) | 1 (4) |
| Pleural effusion | 19 | 2 (11) | 0 |
| Dyspnea | 3 | 1 (33) | 1 (33) |
| Skin disorders | 5 | 0 | 0 |
| AE, n (%)* . | Dasatinib intolerant . | Grade 3/4 event . | Discontinued because of event . |
|---|---|---|---|
| Any AE | 50 | 11 (22) | 4 (8) |
| Hematologic events | 20 | 8 (40) | 2 (10) |
| Thrombocytopenia | 8 | 6 (75) | 1 (13) |
| Pancytopenia | 5 | 0 | 0 |
| Neutropenia | 4 | 4 (100) | 1 (25) |
| Hematotoxicity | 3 | 0 | 0 |
| Cardiovascular events | 3 | 0 | 1 (33) |
| Gastrointestinal events | 6 | 0 | 0 |
| Diarrhea | 3 | 0 | 0 |
| Musculoskeletal events | 4 | 0 | 0 |
| Respiratory events | 23 | 3 (13) | 1 (4) |
| Pleural effusion | 19 | 2 (11) | 0 |
| Dyspnea | 3 | 1 (33) | 1 (33) |
| Skin disorders | 5 | 0 | 0 |
Includes all AEs with ≥ 3 patients categorized as intolerant on prior dasatinib.
Clinical outcomes
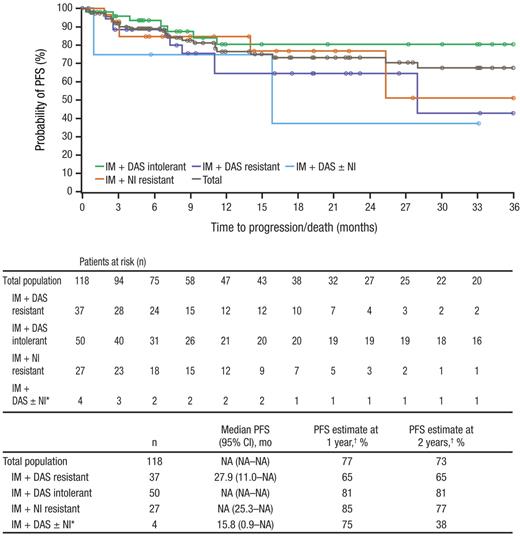
Confirmed transformation to AP CML while on treatment occurred in 5 (4%) patients; no patient transformed to BP CML. The Kaplan-Meier estimate of PFS among all treated patients at 1 year was 77% and at 2 years was 73%, with Kaplan-Meier median PFS not yet reached overall and for some subpopulations (Figure 3). Of clinical importance, among the 3 patients previously treated with all 3 currently approved TKIs, one patient with skin-related intolerance to imatinib, dasatinib, and nilotinib has remained on treatment and in CCyR and CMR for more than 33 months.
PFS while on bosutinib treatment. PFS is shown for all patients treated with bosutinib at a median follow-up of 28.5 months. Progression was determined by the investigator and defined as on-treatment transformation to accelerated or blast phase, loss of CHR, loss of MCyR with Philadelphia chromosome rate increased by 30%, doubling of white blood cell count to > 20 × 109/L, or death because of any cause within 30 days of the last study dose. IM indicates imatinib; DAS, dasatinib; NI, nilotinib; and NA, not available. *Includes 3 patients who previously received all 3 inhibitors and one patient with NI intolerance. †PFS rates at 1 and 2 years were based on Kaplan-Meier estimates.
PFS while on bosutinib treatment. PFS is shown for all patients treated with bosutinib at a median follow-up of 28.5 months. Progression was determined by the investigator and defined as on-treatment transformation to accelerated or blast phase, loss of CHR, loss of MCyR with Philadelphia chromosome rate increased by 30%, doubling of white blood cell count to > 20 × 109/L, or death because of any cause within 30 days of the last study dose. IM indicates imatinib; DAS, dasatinib; NI, nilotinib; and NA, not available. *Includes 3 patients who previously received all 3 inhibitors and one patient with NI intolerance. †PFS rates at 1 and 2 years were based on Kaplan-Meier estimates.
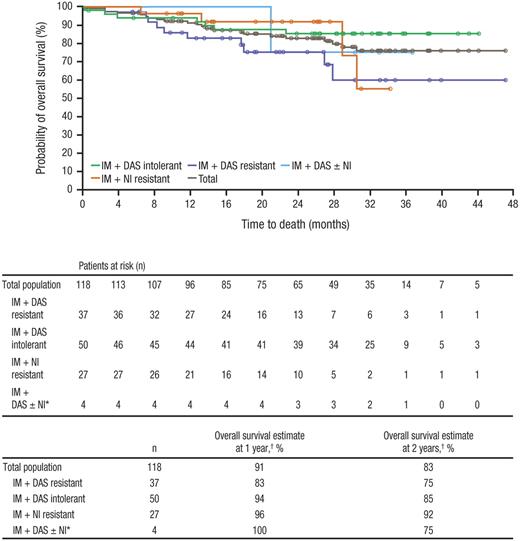
Overall, 22 deaths have been reported, with 6 deaths occurring during treatment or within 30 days of the last study medication dose; the remaining 16 patients died more than 30 days after discontinuing bosutinib (Table 8). Only one death was considered by the investigator to be the result of a treatment-related AE: gastrointestinal bleeding that occurred 78 days after treatment initiation in a patient with a history of gastritis and grade 3 thrombocytopenia, both of which were ongoing at the time of study entry. At 1 year, the Kaplan-Meier estimate of overall survival was 91% and at 2 years was 83%, with Kaplan-Meier median overall survival not yet reached (Figure 4).
Summary of study deaths
| Event (n = 118) . | n (%) . |
|---|---|
| Overall deaths | 22 (19) |
| Death within 30 days of last study dose | 6 (5) |
| Reason for death | |
| Disease progression | 10 (8) |
| AE unrelated to study drug* | 8 (7) |
| AE related to study drug† | 1 (1) |
| Other/unknown‡ | 3 (3) |
| Event (n = 118) . | n (%) . |
|---|---|
| Overall deaths | 22 (19) |
| Death within 30 days of last study dose | 6 (5) |
| Reason for death | |
| Disease progression | 10 (8) |
| AE unrelated to study drug* | 8 (7) |
| AE related to study drug† | 1 (1) |
| Other/unknown‡ | 3 (3) |
Causes of death (days since last dose of bosutinib) included: intracranial hemorrhage (174 days), internal hemorrhage (199 days), internal bleeding secondary to infarcted kidney (348 days), myocardial infarction (1 day), suspected acute myocardial infarction (13 days), respiratory insufficiency (278 days), respiratory failure (112 days), and pulmonary edema secondary to CML (371 days).
Gastrointestinal bleeding that occurred while on study treatment; the patient had a history of gastritis and grade 3 thrombocytopenia, both of which were ongoing at the time of study entry.
Causes of death (days since last dose of bosutinib) included: complications from grade 4 GVHD after cord blood transplant (581 days) and causes that remain unknown (n = 2; 509 and 618 days).
Overall survivalis shown for all patients treated with bosutinib at a median follow-up of 28.5 months. IM indicates imatinib; DAS, dasatinib; and NI, nilotinib. *Includes 3 patients who previously received all 3 inhibitors and 1 patient with NI intolerance. †Overall survival rates at 1 and 2 years were based on Kaplan-Meier estimates.
Overall survivalis shown for all patients treated with bosutinib at a median follow-up of 28.5 months. IM indicates imatinib; DAS, dasatinib; and NI, nilotinib. *Includes 3 patients who previously received all 3 inhibitors and 1 patient with NI intolerance. †Overall survival rates at 1 and 2 years were based on Kaplan-Meier estimates.
Discussion
Bosutinib demonstrated clinical activity and an acceptable safety profile in this study subpopulation of patients with CP CML who were previously treated with imatinib and dasatinib and/or nilotinib. This patient population has limited treatment options and clearly represents an area of unmet medical need for new approved therapies. There have been few published articles with dasatinib or nilotinib after failure of 2 prior TKIs in CML,16,–18 and the current analysis represents the largest analysis of a TKI after the failure of multiple prior TKIs in CML. Newer therapies, such as bosutinib, may represent a valid treatment option for some patients who have previously been treated with multiple TKIs for CML.
In this study, the rate of MCyR was 32% and the rate of confirmed CHR was 73% after a median follow-up time of 28.5 months. An additional 6% of evaluable patients achieved a minor cytogenetic response, which has been associated with prolonged survival in the second-line and greater settings.19 Further, responses were durable for most patient subpopulations, with overall median durations of MCyR and CHR not yet reached. The MCyR rate with bosutinib is similar, yet with longer median duration, compared with historical results from smaller studies of dasatinib or nilotinib used as third-line therapies. In a small study of 23 patients (primarily focused on AP/BP CML with only 4 CP CML patients) who received dasatinib after treatment with imatinib and nilotinib, one of 4 patients with CP CML achieved a CHR and a CCyR.17 A second study of 25 patients with CP CML who received either dasatinib or nilotinib as their third TKI reported a CHR in 19 (76%) patients and a MCyR in 8 (32%) patients.18 Among 39 patients with CP CML after treatment with imatinib and dasatinib, of which 26 (67%) patients were intolerant to dasatinib, nilotinib was associated with a CHR in 22 (79%) patients and MCyR in 16 (43%) patients.16 Typically, responses to dasatinib or nilotinib as the third TKI have been of short duration, with median failure-free survival of approximately 20 months.18 Patients with intolerance to prior therapy might be expected to have a better probability of response than those with resistance; in the current report, dasatinib-resistant patients had a trend for lower rates of CHR, CCyR, and MMR compared with dasatinib-intolerant patients.
Bosutinib also demonstrated durability of clinical outcomes among patients with CP CML who had previously received imatinib plus dasatinib and/or nilotinib, as previously outlined. Only 5 (4%) patients experienced confirmed transformation to AP CML during treatment, and no transformations to BP CML occurred. Kaplan-Meier estimates of PFS and overall survival at 2 years were both high (73% and 83%, respectively).
In addition, hematologic and cytogenetic responses to bosutinib were observed across Bcr-Abl kinase domain baseline mutations, except for the T315I mutation. Other baseline mutations associated with clinical resistance to dasatinib (F317L)20 and nilotinib (Y253H and F359C/I/V)21,22 were among those for which bosutinib demonstrated confirmed CHR (50%-83%) and MCyR (14%-67%), further supporting the observed benefit of bosutinib treatment in patients with resistance to dasatinib and/or nilotinib.
Bosutinib was associated with an acceptable safety profile. Treatment-emergent drug-related AEs were primarily manageable grade 1/2 gastrointestinal events (ie, diarrhea, nausea, vomiting) and rash, and were consistent with the AEs previously reported in patients treated only with prior imatinib.14 Cross-intolerance with dasatinib was infrequent, with 8% of patients discontinuing bosutinib because of the same AE attributed to their dasatinib intolerance. Bosutinib treatment was also notable for a low incidence of certain AEs that are common with other TKIs, such as pleural effusions, musculoskeletal events, and cardiac toxicities. Fluid retention has been associated with both dasatinib6,–8 and imatinib23,24 treatment, with rates of pleural effusion as high as 27% (6% with grade 3/4) reported for dasatinib.6,–8 In the current study, treatment-related pleural effusions of any grade were experienced by 9 (8%) patients, 7 of whom had a history of pleural effusions on prior dasatinib. Musculoskeletal events have been associated with imatinib treatment23,–25 but were infrequent with bosutinib. Only one patient experienced grade 2 or higher QTcF interval prolongation (> 60 ms increase from baseline) during bosutinib therapy, and limited dasatinib cross-intolerance related to cardiovascular events was seen. The rate of transaminase elevations was low, with on-treatment grade 3/4 ALT and AST elevations detected in 7% and 3% of patients, respectively, and 3 patients discontinuing bosutinib because of transaminase elevations. Although the incidence of on-therapy grade 3/4 hypermagnesemia appeared high (12%) in this analysis, the incidence of grade 3/4 events at baseline was also high (8%).
Hematologic toxicity was one of the most commonly reported reasons for dasatinib intolerance (n = 20), and some cross-intolerance was observed with bosutinib: 8 (40%) of these patients experienced a grade 3/4 hematologic cross-intolerance event on bosutinib and 2 (10%) discontinued bosutinib because of myelosuppression. However, the overall incidence of on-therapy grade 3/4 neutropenia (19%) and thrombocytopenia (25%) was relatively low. Further, these incidences compare favorably with the rates of these events reported in the literature for dasatinib (40%-63% and 32%-57%, respectively)6,–8 and are similar to rates reported with nilotinib (13%-31% and 20%-30%)9,10 as second-line therapy. Despite limited cross-intolerance between nilotinib and dasatinib, hematologic toxicity is the most commonly reported cause of cross-intolerance.26
Based on the presented data and the limited treatment options currently available for patients previously treated with 2 or more TKIs in CML, bosutinib may provide an effective therapeutic option for these patients. Investigation of bosutinib efficacy in the settings of newly diagnosed CP CML27 and AP or BP CML28 is ongoing.
Presented in part in abstract form at the Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2007; Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008; Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008; Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 7, 2010; Annual Congress of the European Hematology Association, Barcelona, Spain, June 11, 2010; Annual Meeting of the American Society of Hematology, Orlando, FL, December 7, 2010; Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 6, 2011; and Annual Congress of the European Hematology Association, London, United Kingdom, June 10, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Becker Hewes for assistance in the initiation and conduct of this study, and Dr Kimberly Brooks (SciFluent) for editorial/medical writing support that was funded by Pfizer Inc.
This study was sponsored by Wyeth Research, which was acquired by Pfizer Inc in October 2009. This work was supported by Pfizer Inc.
Authorship
Contribution: H.J.K., J.E.C., H.M.K., C.G.-P., and T.H.B. created and designed the study; H.M.K., J.E.C., M.B., D.-W.K., A.Z., and T.H.B. collected and assembled data; H.J.K., J.E.C., H.M.K., C.G.-P., A.C., N.B., E.L., V.K., and T.H.B. assisted in analysis and/or interpretation of the data; H.J.K., J.E.C., H.M.K., C.G.-P., M.B., D.-W.K., A.Z., and T.H.B. provided study materials and enrolled patients in the study; and all authors assisted in writing and critically revising the manuscript and gave final approval of the manuscript for publication.
Conflict-of-interest disclosure: H.J.K. and D.-W.K. have served as advisors for Pfizer Inc. J.E.C. has received research funding and served as an advisor for Ariad, Bristol-Myers Squibb, ChemGenex, Novartis, and Pfizer Inc; J.E.C. has also received research funding from Deciphera. H.M.K. has received research funding and served as an advisor for Pfizer Inc. C.G.-P. has received research funding and served as a consultant/advisor for Bristol-Myers Squibb and Pfizer Inc. A.C., N.B., E.L., and V.K. are employees of Pfizer Inc. A.C. additionally owns stock in Pfizer Inc. T.H.B. has received research funding from Novartis and has served as an advisor and received honoraria from Bristol-Myers Squibb, Novartis, and Pfizer Inc. The remaining authors declare no competing financial interests.
Correspondence: H. Jean Khoury, Winship Cancer Institute of Emory University, 1365 Clifton Rd NE, Suite C1152, Atlanta, GA 30322; e-mail: hkhoury@emory.edu.