Platelets store signaling molecules (eg, serotonin and ADP) within their granules. Transporters mediate accumulation of these molecules in platelet granules and, on platelet activation, their translocation across the plasma membrane. The balance between transporter-mediated uptake and elimination of signaling molecules and drugs in platelets determines their intracellular concentrations and effects. Several members of the 2 major transporter families, ATP-binding cassette (ABC) transporters and solute carriers (SLCs), have been identified in platelets. An example of an ABC transporter is MRP4 (ABCC4), which facilitates ADP accumulation in dense granules. MRP4 is a versatile transporter, and various additional functions have been proposed, notably lipid mediator release and a role in aspirin resistance. Several other ABC proteins have been detected in platelets with functions in glutathione and lipid homeostasis. The serotonin transporter (SERT, SLC6A4) in the platelet plasma membrane represents a well-characterized example of the SLC family. Moreover, recent experiments indicate expression of OATP2B1 (SLCO2B1), a high affinity transporter for certain statins, in platelets. Changes in transporter localization and expression can affect platelet function and drug sensitivity. This review summarizes available data on the physiologic and pharmacologic role of transporters in platelets.
Introduction
Platelets are derived from megakaryocytes. Despite being anucleate, they fulfill a multitude of functions. Platelets are not only key players in hemostasis and in vascular disease, they also contribute to inflammation, tumor angiogenesis, embryonic development, and immunologic responses.1,2 Platelets contain many biologically active molecules, such as factors triggering platelet aggregation, growth factors, and many other compounds, which are secreted on platelet activation. This function is highly dependent on their intracellular compartmentalization.
Platelets contain at least 3 types of intracellular granules, in which mediators are stored and concentrated, known as α, dense, and lysosomal granules.3 The α-granules mainly contain proteins critical to adhesion, such as von Willebrand factor, thrombospondin, and fibrinogen, as well as growth factors and protease inhibitors, clotting factors, and immunoglobulin G. Lysosomes hold a battery of hydrolytic enzymes, which are postulated to function in the elimination of circulating platelet aggregates and potentially also in host defense. Dense granules, such as lysosomes, are acidic organelles but, unlike the aforementioned organelles, contain extremely high concentrations of small molecules, especially ADP, ATP, serotonin, and calcium.3 Dense granule molecules participate in hemostasis in numerous ways, as ADP activates nearby platelets and serotonin causes vasoconstriction.
The biosynthesis of platelet-dense and α-granules is thought to occur in megakaryocytes through a common multivesicular intermediate body.4 However, the presence of plasma membrane proteins, such as GPIb, in the granule membranes suggests that these arise from both endogenous synthesis within the megakaryocyte as well as from fusion with endocytic vesicles during budding from the plasma membrane.5
Platelet-dense granules are absent (or greatly reduced), and/or their contents are significantly reduced in a heterogeneous group of congenital platelets defects called δ-storage pool deficiencies (δ-SPD).6 These are associated with a moderate bleeding tendency. The most severe δ-SPD is Hermansky-Pudlak syndrome, a rare autosomal recessive disorder in which oculocutaneous albinism, bleeding, and lysosomal ceroid storage result from defects of melanosomes, platelet-dense granules, and lysosomes.7,8 Mutations in a variety of genes have been identified as causes for Hermansky-Pudlak syndrome, for example, in genes known to function in vesicle trafficking and in the biogenesis of lysosome-related organelles.9
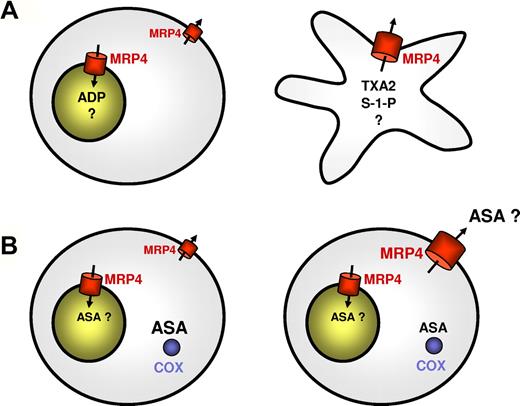
The accumulation of compounds, such as ADP, in high concentrations inside the dense granules points to their active transport. The identification of the ATP-binding cassette (ABC) protein ABCC4, better known as multidrug resistance protein 4 (MRP4), as a candidate transporter for adenine nucleotides in platelet-dense granules,10 represents a first step toward the elucidation of this important function of platelets. MRP4 is a markedly versatile transporter exhibiting a broad substrate specificity composed of a wide range of amphiphilic anions, including steroid conjugates and eicosanoids, as well as cyclic nucleotides and nucleotide analogues.11,12 Accordingly, several other tasks have been proposed for MRP4 in platelets taking into account that its localization can be shifted from granules to the plasma membrane on activation of the platelets and under certain pathophysiologic conditions.10,13,14 These include the release of lipid mediators15,16 as well as a role in aspirin resistance under certain conditions as in patients after coronary artery bypass graft surgery.14
Detailed experiments address the transport of serotonin in platelets, especially its transport across the plasma membrane. Serotonin, which is released into circulation mainly from the enterochromaffin cells in the gut, is rapidly taken up by platelets and stored in platelet-dense granules, which constitute almost all total body circulating serotonin.17 Platelets have been used as models of neuronal transport of serotonin and also of several amino acid transmitters for many years.18,19 Furthermore, studies of the secretion mechanisms in platelets have indicated major similarities to neuronal transmitter release despite the fact that these cells have different origins.20 Neurons also contain small dense core vesicles that contain small molecules, such as serotonin taken up and concentrated from intracellular or extracellular pools, which resemble in many features platelet-dense granules.
An important emerging concept is that platelets may function as “long-haul truckers” not only for endogenous biologically active substances, such as serotonin, but also for a variety of drugs. Although platelets are small in size (only 2.0-5.0 μm in diameter),3 they represent a relatively large compartment for drugs considering their high numbers (normal range, 150-450 × 109 cells/L blood). In adults (5 L blood), the whole body platelet volume, including one-third splenic sequestration, accounts to approximately 20 mL (10 fL/platelet). This potential function of platelets is not well characterized, although it seems to be of major importance. All systemic drugs reach their targets via the bloodstream and come into contact with platelets; thereby, they can either be concentrated and stored within or be excluded from platelets, depending on the presence of uptake and export transporters in the platelet plasma membrane. In some cases, platelets also house the target structures, as the cyclo-oxygenase (COX)–1 enzyme,14 which is inhibited by aspirin and other nonsteroidal anti-inflammatory drugs, or the 3-hydroxy-3-methylglutaryl coenzyme A reductase,21 which represents the target of statins. As a result of transporter-mediated uptake, drugs can act on the target structures inside the platelets and provoke therapeutically positive effects or negative side effects. On the other hand, elimination of drugs from the platelet by export pumps can limit the effect of the drug and lead to drug resistance. Thus, because of their various functions, platelet transporters can either serve as drug targets or as drug delivery systems, and these functions can be greatly altered in the setting of thrombocytopenia or with inherited or acquired platelet defects.
The purpose of this review is to examine the state of the art in knowledge about transporters important in platelet function as well as in pharmacotherapy.
Taxonomy of transporters
Two major protein superfamilies are involved in the transport of drugs as well as endogenous metabolites and signaling molecules: the ABC proteins with approximately 50 genes in humans grouped in 7 subfamilies,22,23 and the more than 300 solute carriers (SLCs) divided in 55 subfamilies.24,25 Members of both families have been identified in platelets (Table 1). In general, mammalian ABC transporters represent export pumps that bind and hydrolyse ATP, providing the energy for a unidirectional transport of a wide range of endogenous and exogenous compounds across membranes from the cytoplasm to the extracellular space or into cellular organelles, often against a concentration gradient.22,23 The typical structure of a functional (“full-size”) mammalian ABC transporter contains at least 2 clusters of usually 6 membrane-spanning segments as well as 2 nucleotide-binding domains (Figure 1A).22,23 In contrast, many SLC proteins function either by facilitating passive diffusion along the concentration gradient of the substrate (independently of energy input) or by cotransport and countertransport co-opting the concentration gradient of another solute. The structures of SLCs are often characterized by a cluster of 10 to 12 hydrophobic membrane-spanning segments (Figure 1B).24,25
Transporters identified in platelets
| Transporter . | Localization . | Proposed substrates . | Reference(s) . |
|---|---|---|---|
| ABC transporters | |||
| ABCA7 | Plasma membrane | Cholesterol, lipid mediators (?) | 29,52 |
| ABCB4 (MDR3) | Plasma membrane | Phospholipids, lipid mediators (?) | 29 |
| ABCC1 (MRP1) | Plasma membrane | Amphiphilic anions, glutathione, LTC4 | 29,49 |
| ABCC3 (MRP3) | Plasma membrane | Amphiphilic anions, LTC4 | 29 |
| ABCC4 (MRP4) | Dense granules, plasma membrane | Amphiphilic anions, purine nucleotide–based mediators; glutathione, lipid mediators | 10,14,15,29 |
| SLC transporters | |||
| SLC1A1 (EAAT3) | Plasma membrane | Glutamate and neutral amino acids | 19 |
| SLC6A4 (SERT) | Plasma membrane | Serotonin, monamine transmitter | 18,74,75 |
| SLC6A12 (BGT-1) | Plasma membrane | Betaine, GABA | 19 |
| SLC18A2 (VMAT2) | Dense granules | Serotonin, monamine transmitter | 85,–87 |
| SLC21/SLCO2B1 (OATP2B1) | Plasma membrane | Amphiphilic anions, statins | 21 |
| SLC23A2 (SVCT2) | Plasma membrane | Ascorbic acid | 88,89 |
| SLC35d3 | Dense tubular system (?) | Nucleotide sugars (?) | 90,91 |
| SLC44A2 (CTL-2) | Granules (?) | Choline (?) | 27,28 |
| Transporter . | Localization . | Proposed substrates . | Reference(s) . |
|---|---|---|---|
| ABC transporters | |||
| ABCA7 | Plasma membrane | Cholesterol, lipid mediators (?) | 29,52 |
| ABCB4 (MDR3) | Plasma membrane | Phospholipids, lipid mediators (?) | 29 |
| ABCC1 (MRP1) | Plasma membrane | Amphiphilic anions, glutathione, LTC4 | 29,49 |
| ABCC3 (MRP3) | Plasma membrane | Amphiphilic anions, LTC4 | 29 |
| ABCC4 (MRP4) | Dense granules, plasma membrane | Amphiphilic anions, purine nucleotide–based mediators; glutathione, lipid mediators | 10,14,15,29 |
| SLC transporters | |||
| SLC1A1 (EAAT3) | Plasma membrane | Glutamate and neutral amino acids | 19 |
| SLC6A4 (SERT) | Plasma membrane | Serotonin, monamine transmitter | 18,74,75 |
| SLC6A12 (BGT-1) | Plasma membrane | Betaine, GABA | 19 |
| SLC18A2 (VMAT2) | Dense granules | Serotonin, monamine transmitter | 85,–87 |
| SLC21/SLCO2B1 (OATP2B1) | Plasma membrane | Amphiphilic anions, statins | 21 |
| SLC23A2 (SVCT2) | Plasma membrane | Ascorbic acid | 88,89 |
| SLC35d3 | Dense tubular system (?) | Nucleotide sugars (?) | 90,91 |
| SLC44A2 (CTL-2) | Granules (?) | Choline (?) | 27,28 |
(?) indicates putative (unproven) substrate or localization.
Typical membrane topologies predicted for ABC and SLC transporters. (A) Structure of MRP4 (ABCC4) consisting of 2 clusters of 6 membrane-spanning segments and 2 regions containing nucleotide binding domains (NBDs). (B) Structure of the serotonin uptake transport (SERT, SLC6A2) exhibiting a cluster of 12 membrane-spanning segments.
Typical membrane topologies predicted for ABC and SLC transporters. (A) Structure of MRP4 (ABCC4) consisting of 2 clusters of 6 membrane-spanning segments and 2 regions containing nucleotide binding domains (NBDs). (B) Structure of the serotonin uptake transport (SERT, SLC6A2) exhibiting a cluster of 12 membrane-spanning segments.
The most prominent representative of the ABC proteins is ABCB1, better known as P-glycoprotein, which was originally identified by virtue of its ability to confer resistance to a range of structurally unrelated cytotoxic drugs in cancer cells (multidrug resistance). P-glycoprotein is expressed mainly in tissues with barrier function as in the gut or the blood-brain barrier,22 but not in platelets. Some features of P-glycoprotein, however, are shared by several members of the C-branch of the ABC family, the so-called MRPs.11 Members of this subfamily, such as MRP4, which is abundantly expressed in platelets, transport mainly amphiphilic anions, including many drugs or drug conjugates but also a number of endogenous signaling molecules, including arachidonate- and purine nucleotide-derived mediators.11 Other ABC transporters, especially of the A-branch, play an important role in the lipid homeostasis of cells, including platelets.26
The superfamily of SLCs is composed of differed types of carriers for a huge spectrum of substrates, including nutrients, such as inorganic ions, sugars, amino acids, nucleotides, and vitamins, as well as drugs. Subfamilies of SLC for which members have been characterized within platelets include: the oligospecific Na+- and Cl−-dependent monoamine neurotransmitter transporters (SLC6-family), with the serotonin transporter (SERT, SLC6A4) as a prominent member expressed in platelets; the vesicular amine transporter family (SLC18); the nucleoside-sugar transporter family (SLC35); as well as the multispecific organic anion transporting peptide family (SLC21/SLCO; Table 1). Members of the latter family have been recognized to play an important role in the distribution of certain drugs as statins. A member of this family, OATP2B1 (SLCO2B1), has also been characterized in platelets.21 Another SLC protein, the choline transporter-like protein 2 (CTL-2, SLC44A2) recently gained major attention in hematology when it was identified to carry the human neutrophil antigen (HNA)3a, one of the major antigens involved in transfusion-associated lung injury.27 This protein is also expressed in platelets.28
ABC transporters in platelets
Role of MRP4 (ABCC4) in platelets
The high concentration of ADP inside the platelet-dense granules (up to 0.6M) suggests the involvement of a primary active transport protein. A candidate protein mediating this active transport was found in MRP4. Besides its localization in the plasma membrane, MRP4 was demonstrated to be highly expressed in the membrane of dense granules (Figure 2A).10,13,14 Accordingly, an altered distribution of MRP4 was observed in platelets from a patient with Hermansky-Pudlak syndrome in which MRP4 was only detected in the plasma membrane because of the lack of dense granules.10 This intracellular localization distinguishes the MRP4 expression in platelets from that in other cell types, where it is localized mainly at the plasma membrane. It is conceivable that different splice variants of MRP4 are expressed in different tissues because at least 2 transcript variants of the human ABCC4/MRP4 gene exist that are predicted to encode proteins with distinct N-terminal extensions (reference sequences NM_005845.3 variant 1 and NM_001105515.1 shorter variant 2). Analysis of the MRP4 mRNA in platelets and mass spectrometry analysis of the protein,29 however, indicate that the platelet 190-kDa MRP4 glycoprotein corresponds to the long variant 1 expressed also in other tissues.30,31
Proposed functions of MRP4 in mediator storage and drug resistance depending on its localization. (A) Role of MRP4 in mediator storage and release in normal resting and activated platelets. In resting platelets, MRP4 is mainly present in the membrane of dense granules10,13,14 and mediates sequestration of mediators and possibly other compounds into these organelles (left panel); in activated platelets, MRP4 among other granule membrane proteins is inserted into the plasma membrane on granule exocytosis and may then contribute to the release of a variety of compounds, including de novo generated lipid mediators (right panel). (B) Proposed role of MRP4 in aspirin resistance. As suggested by Mattiello et al,14 aspirin effect on platelets is little related to MRP4-mediated aspirin transport in normal platelets, although MRP4 may sequestrate a part of the drug into dense granules (left panel). In patients after coronary artery bypass graft surgery, however, MRP4 is up-regulated on the plasma membrane already in resting platelets and mediates active extrusion of aspirin from the cells resulting in an insufficient intracellular COX-1 inhibition by this drug14,33 (right panel).
Proposed functions of MRP4 in mediator storage and drug resistance depending on its localization. (A) Role of MRP4 in mediator storage and release in normal resting and activated platelets. In resting platelets, MRP4 is mainly present in the membrane of dense granules10,13,14 and mediates sequestration of mediators and possibly other compounds into these organelles (left panel); in activated platelets, MRP4 among other granule membrane proteins is inserted into the plasma membrane on granule exocytosis and may then contribute to the release of a variety of compounds, including de novo generated lipid mediators (right panel). (B) Proposed role of MRP4 in aspirin resistance. As suggested by Mattiello et al,14 aspirin effect on platelets is little related to MRP4-mediated aspirin transport in normal platelets, although MRP4 may sequestrate a part of the drug into dense granules (left panel). In patients after coronary artery bypass graft surgery, however, MRP4 is up-regulated on the plasma membrane already in resting platelets and mediates active extrusion of aspirin from the cells resulting in an insufficient intracellular COX-1 inhibition by this drug14,33 (right panel).
The hypothesis that MRP4 is involved in the ADP storage in platelets is further supported by studies in patients with partial δ-SPDs, whose platelets are only deficient in ADP and not in serotonin.13 Based on the assumption that MRP4 is essential for the adenine nucleotide storage, one would expect that defects of MRP4 expression and localization are associated with decreased levels of adenine nucleotides in platelet-dense granules, but normal levels of other constituents, such as serotonin for which other transporters are involved. Two patients were identified who exhibited this rare phenotype of partial δ-SPD characterized by decreased platelet ADP content but normal serotonin levels. The MRP4 expression in the platelets of these patients was severely diminished.13 The underlying molecular defect leading to the diminished MRP4 expression in these platelets, however, has so far not been identified. A similar phenotype with selective adenine nucleotide deficiency was already previously observed in a dog model.32 Analyses of the MRP4 expression in platelets of patients with the more prevalent classic δ-SPD type, characterized by low adenine nucleotide and serotonin levels, revealed a different pattern. In these patients, MRP4 was found to be expressed in a quantitatively normal fashion, but its localization was significantly changed compared with normal platelets. In these patients, MRP4 seemed to be expressed only at the plasma membrane.13
Interestingly, such a shift in the intracellular localization of MRP4 seems to occur also under other pathologic conditions. Recently, Mattiello et al reported that, compared with platelets from healthy volunteers, platelets from patients undergoing coronary artery bypass graft surgery exhibit increased amounts of MRP4, which was localized preferentially at the plasma membrane.14 In these patients, suboptimal platelet inhibition by aspirin (so-called aspirin resistance) is particularly common, and Mattiello et al hypothesized that the up-regulated MRP4 increases active extrusion of aspirin from platelet cytosol, resulting in less effective COX-1 inhibition14,33 (Figure 2B). This assumption is based on the following observations: aspirin reduced uptake of Fluo-cAMP, a potential MRP4 substrate, into dense granules of normal platelets and is released after thrombin activation of platelets, indicating that aspirin itself is accumulated in dense granules. Platelets from coronary artery bypass graft patients showed a high expression of MRP4 whose in vitro inhibition by dipyridamole or MK-571 enhanced aspirin entrapment and increased its effect on COX-1. Platelets derived from megakaryocytes transfected with MRP4 siRNA exhibited higher aspirin entrapment.14 Further studies are required to elucidate the role of MRP4 in aspirin transport and in the interindividual variations of platelet inhibition by aspirin that are also often observed in patients with acute coronary syndrome or diabetes.33,34
When platelets are activated, granule integral membrane proteins become inserted into the plasma membrane on granule exocytosis.3 Thus, activated platelets exhibit an additional cohort of plasma membrane MRP4, which may alter platelet function by increased transport of various substrates. When inserted into the plasma membrane, MRP4 may also contribute to the export of a variety of lipid mediators, such as thromboxane A2 and leukotrienes (Figure 2A). In contrast to ADP and serotonin, these lipid mediators are supposed to be generated de novo on platelet activation. Because they exert their effects mainly extracellularly via interaction with membrane receptors, an efficient release from the cells is also required for these mediators.
It was first recognized for the 5-lipoxygenase product leukotriene C4 (LTC4) that its biosynthesis in leukocytes is followed by a distinct active export.35,36 MRP1 (ABCC1) was the first high-affinity transporter identified for LTC4,37 and its important role in LTC4 release from mast cells was confirmed in studies in Mrp1 knockout mice.38 ATP-dependent transport of this cysteinyl leukotriene was subsequently shown to be mediated also by other MRPs, including MRP3 (ABCC3)39 and MRP4.15 Although platelets lack 5-lipoxygenase activity, they contain LTC4 synthase40 and can produce LTC4 when supplied with LTA4 via transcellular mechanisms, and 3 LTC4 transporters, MRP1, MRP3, and MRP4, are expressed in platelets, which makes it likely that platelets play an important role in LTC4 homeostasis.
Primary prostaglandins (PGs) such as PGE2 and thromboxane A2, have been formerly assumed to diffuse passively from the cell, despite being poorly membrane permeable. Reid et al30 and Rius et al41 demonstrated, however, that prostaglandins, including thromboxane B2, are actively transported by MRP4 and that this transport is inhibited by a number of nonsteroidal anti-inflammatory drugs, such as ibuprofen and indomethacin.30
Another immune-modulating lipid mediator released by platelets on thrombin stimulation is sphingosine-1-phosphate (S-1-P). Platelet-derived S-1-P modulates the chemotaxis of monocytes and is involved in inflammatory processes.42 The release of S-1-P was found to depend on platelet thromboxane formation and activation of the thromboxane receptor.16 The actual release process probably also involves a member of the MRP family.16 It was suggested that MRP1 mediates S-1-P export in mast cells.43 Secretion of S-1-P from human platelets was blocked by MK571,16 a cysteinyl leukotriene analog, which was originally identified as a specific inhibitor for MRP137 but interferes also with MRP4.10,15 S-1-P excretion by platelets was also inhibited by dipyridamole and indomethacin,16 which are known to inhibit preferentially MRP4.10,30 This suggests that the release of S-1-P from platelets also involves members of the MRP family, preferentially MRP4.
Inserted in the plasma membrane, MRP4 may also extrude cyclic nucleotides, such as cAMP and cGMP,10,–12,44 which are both critical inhibitory intracellular second messengers regulating fundamental processes in platelets.45 The cellular levels of these mediators are controlled by the action of phosphodiesterases, which have been also suggested as targets in antiplatelet treatment,46 as well as by active secretion into the extracellular space, which may provide also extracellular purine-based mediators for paracrine functions.
From a therapeutic perspective, there are thus several aspects regarding MRP4 that provide reasons for its targeting in use of MRP4 inhibitors for antiplatelet therapy. MRP4 transport is inhibited by dipyridamole, which, however, has other effects, such as inhibition of phosphodiesterases.46 More specific (but as yet undeveloped) compounds would be a very interesting option to interfere selectively with platelet ADP storage and possibly also release of lipid mediators.
In addition to physiologic and pathophysiologic regulation, genetic variations may account for variable MRP4 function. The ABCC4/MRP4 gene is highly polymorphic with at least 25 nonsynonymous single nucleotide polymorphisms; however, the influence of these on MRP4 expression and function in vivo remains to be established.31 Interestingly, the ABCC4/MRP4 gene was one of the 68 genomic loci, which were identified as putative regulators of platelet formation in a recently published meta-analysis of genome-wide association studies for platelet count and volume.47
Mrp4(−/−) mice have been generated and characterized mainly with respect to their susceptibility toward nucleoside analogues.48 They exhibit no obvious bleeding tendency; however, extrapolating these observations to humans is problematic because of interspecies differences in the properties of the transporter and in the overall hemostatic process and compensatory mechanisms.
MRP1 (ABCC1) and MRP3 (ABCC3)
Reports on the expression of MRP1 in platelets had been controversial.15,49 The presence of MRP1 in platelet membranes could be proven by use of 2D-nanoLC-MS/MS,29 but in a significantly lower amount compared with MRP4. Both MRP1 and MRP4 are able to contribute to the transport of LTC4 as well as to platelet glutathione homeostasis because both MRPs mediate cotransport of a variety of endogenous and exogenous compounds with reduced glutathione.11,15,50 MRP1 exports in addition oxidized glutathione (glutathione disulfate).51 Another candidate transporter for LTC4 that is present in platelet membranes is MRP3, which also transports cysteinyl leukotrienes, although with a lower affinity as observed with MRP1.39 Interestingly, the expression of MRP3 and MRP4 increases during differentiation of hematopoietic progenitor cells toward megakaryocytes, suggesting that the expression of these proteins may be particularly important for platelet function.29 MRP1 and MRP3 are also known to confer resistance to a number of cytotoxic agents, such as etoposide.11 Thus, they may also play a role in resistance of platelets against toxins and can determine the action of several amphiphilic drugs on platelet function.
ABCA and ABCB transporters
Transcripts of several members of the ABCA family, such as ABCA3, ABCA4, ABCA6, ABCA7, and ABCA9, have been detected in human platelets.29 ABCA7 was shown to be preferentially expressed in platelets and localized at the plasma membrane of rat and human platelets.29,52 ABCA proteins are typically associated with lipid translocation processes in membranes. Two types of intramembrane lipid translocators can be defined: flippases, which translocate lipids from the outer leaflet to the inner leaflet of the membrane; and floppases, which mediate the reverse process (ie, translocation from the inner to the outer leaflet of the membrane bilayer).53 Such membrane remodeling processes also play an important role in platelet function, especially during secondary hemostasis. The flopping of phosphatidylserine from the inner to outer membrane leaflet during platelet activation, together with microparticle shedding, provides a catalytic surface for the assembly of coagulation complexes.54,55 There is a rare platelet defect, the Scott syndrome,56 which underscores the importance of this pathway. In these patients, although primary hemostatic function of platelets is intact, the platelets do not support the assembly of coagulation complexes because of defective scrambling of membrane phospholipids.56 In addition, there exists an inverse condition, the Stormorken syndrome, in which platelets constitutively expose phosphatidylserine on the external leaflet of their plasma membrane.57
Two protein classes are thought to be involved in membrane remodeling: a nonspecific and energy-independent family of so-called scramblases58 and ABC transporters, especially of the A- and B-branch. ABCA1, which is involved in cholesterol efflux from cells and in phagocytosis, and which is mutated in Tangier dyslipidemia (absence of high-density lipoprotein-cholesterol from plasma),59,60 has been considered also to play a role in Scott syndrome. This hypothesis is based on the observation that targeted deletion of the corresponding gene locus resulted in a phenotype evocative of partial Scott syndrome,61 and a mis-sense mutation in the ABCA1 gene was identified in a Scott syndrome patient.62 However, the expression of ABCA1 in platelets has not been established so far.
ABCA7, which has been identified in platelets, however, shares several features with ABCA1. ABCA7 also mediates the formation of high-density lipoprotein when exogenously transfected and expressed and was also reported to relate to the phagocytotic function of cells.63 Genetic variants of the ABCA7 gene have been found to be associated with Alzheimer disease.64 ABCB4, also known as MDR2/3 (Mdr2 in rodents, MDR3 in humans), represents another floppase identified in the platelet plasma membrane.29 This finding is surprising because the expression and function of MDR3 were thought to be limited to the canalicular membrane of hepatocytes.65 Here, MDR3 “flops” phosphatidylcholine from the inner to the outer leaflet of the canalicular membrane, thereby making this phospholipid available for extraction into the canalicular lumen by bile salts.66 Mutations in the ABCB4/MDR3 gene cause progressive familial intrahepatic cholestasis.66 However, so far none of the ABCA and ABCB transporters has been explicitly confirmed as a floppase for PS. The interaction of platelet microparticles with the endothelium and leukocytes in addition triggers inflammation. Thus, the lipid translocators in platelets may have an important role in thrombosis and in inflammation.
Besides their role in plasma membrane remodeling, MDR3 and ABCA proteins may be involved in the release of lipid mediators as lysophosphatidic acid and S1-P from platelets. Lysophosphatidic acid is a bioactive lipid that binds to cell surface G-protein-coupled receptors to regulate cell growth, differentiation, and development.67 In the cardiovascular system, lysophosphatidic acid alters the endothelial barrier function and is a weak platelet agonist.68,69 In addition, it was proposed that members of the ABCA subfamily are involved in the transport of S-1-P,52 especially ABCA7. Furthermore, ABCAs and MDR3 may function in the defense of platelets against highly lipophilic compounds.
SLC transporters in platelets
Serotonin transporters
Serotonin (5-hydroxytryptamine [5-HT]) is best known for its role as neurotransmitter involved in mood disorders.18 However, serotonin is also stored in platelets and, when released, promotes platelet aggregation via the serotonin receptor (5-HT2A) on platelets.17 Moreover, it exhibits strong vasoactive properties, possibly through stimulation of serotonin receptors on endothelial cells and through nitric oxide production.70 Recently, it was also shown that serotonin strongly induces extracellular matrix synthesis in interstitial fibroblasts and promotes tissue fibrosis.71
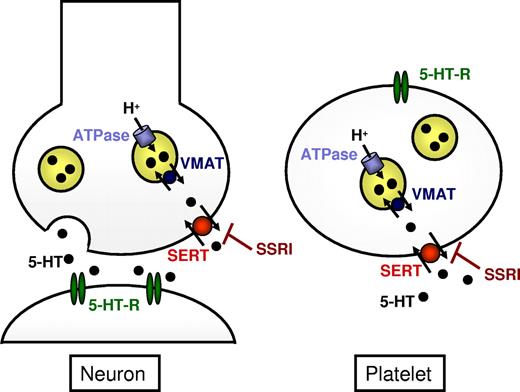
Serotonin storage in platelets results from a 2-step process: (1) the uptake across the platelet plasma membrane; and (2) the transport across the dense granule membrane. The uptake of serotonin into the platelet cytosol is mediated by SERT (also called 5-HTT). The cDNA of human SERT has been cloned, and the gene (SLC6A4) has been assigned to the human chromosome 17.72,73 Expression of SERT was characterized in different tissues, including brain and platelets. Thereby, the transport protein appears identical in brain and platelets.74 SERT belongs to the SLC6 gene family of Na+/Cl−-dependent neurotransmitter transporter proteins.75 The actual uptake process involves the binding of serotonin to its recognition site within the transporter and its transport across the membrane together with an Na+ ion. A second step involves the translocation of a K+ ion across the membrane to the outside of the cell. This requirement for K+ countertransport is unique for SERT within the SLC6 family.76 Because both tricyclic antidepressants and the newer selective serotonin reuptake inhibitors (SSRIs) bind to SERT and inhibit serotonin uptake, the importance of understanding the biochemical characteristics of this transporter has long been appreciated.18 SSRIs block the reuptake of serotonin into neurons as well as the uptake into platelets; thus, platelets were used as model for monitoring the effect of antidepressants.77,78
In addition, platelets exhibit uptake mechanisms for several amino acid transmitters, among them γ-aminobutyric acid, glutamate, aspartate, and glycine, and characteristics of the platelet uptake functions resemble those of the uptake in the central nervous system,19 qualifying platelets as a favored model system in neurology (Figure 3).
Transport and storage of monoamine mediators in neurons and platelets. SERT mediates reuptake of serotonin (5-HT) in neurons as well as uptake into platelets and is inhibited by tricyclic antidepressants and SSRIs.18,79 The storage in dense vesicles is supposed to be mediated by the VMAT (SLC18A).85,–87 The energy for this transport is provided by the function of the vacuolar H+-ATPase. 5-HT-R indicates serotonin receptor.
Transport and storage of monoamine mediators in neurons and platelets. SERT mediates reuptake of serotonin (5-HT) in neurons as well as uptake into platelets and is inhibited by tricyclic antidepressants and SSRIs.18,79 The storage in dense vesicles is supposed to be mediated by the VMAT (SLC18A).85,–87 The energy for this transport is provided by the function of the vacuolar H+-ATPase. 5-HT-R indicates serotonin receptor.
Prolonged intake of SSRIs can lead to a significant decrease in platelet serotonin.17,77 However, bleeding problems are rarely observed in SSRI-treated patients. This may be the result of the postulated prothrombotic disturbance in untreated depression accompanied with an increased cardiovascular risk.79 Although under normal conditions the bleeding risk induced by SSRIs is low, they can increase the risk for bleeding in major surgery.80 Serotonin promoter polymorphisms have been linked to major depressive disorders as well as to an increased risk of new cardiac events after acute myocardial infarction.81,–83 SERT knockout mice have been generated and are vital, but platelet function has not been studied in detail.84
Less is known about the transport of serotonin across the dense granule membrane, which is supposed to be mediated by a reserpine-sensitive vesicular monoamine transporter (VMAT, SLC18), which is also present in the secretory vesicles of monoaminergic neurons and neuroendocrine cells in the gut.85,86 This transport is driven by an electrochemical proton gradient across the vesicular membrane, which is generated by the vacuolar H+-ATPase and is potently inhibited by reserpine and tetrabenazine.85,86 In mammals, 2 closely related isoforms of the monoamine transporter, termed VMAT1 and VMAT2 (SLC18A1 and SLC18A2), respectively, have been identified, which are located on different vesicle subtypes.85,86 VMAT2 has been shown to be expressed mainly in synaptic dense core vesicles, which resemble platelet-dense granules.87 Both VMATs transport serotonin, dopamine, epinephrine, and norepinephrine but differ in their substrate preferences and affinities.
Transporters for nutrients and metabolic intermediates
Platelets, like other cells, need uptake transporters for nutrients. For example, platelets accumulate ascorbic acid through the expression of SVCT 2 (SLC23A2), a member of the Na+-dependent ascorbic acid transporter family (SLC23).88 Interestingly, platelets can compensate for fluctuations in ascorbate levels by modulating the expression of SVCT2 at the translational level.89 Platelets, although anucleate, contain RNA, some of which is translated into proteins, including transporters, according to requirements.89 SLC proteins also often transport metabolites from the cytosol into intracellular organelles. Interestingly, mutations in the Slc35d3 gene have been associated with platelet-dense granule defects in a murine model.90 Slc35d3 is a member of the SLC35 nucleotide sugar transporter family. Members of this family are characterized as antiporters, transporting nucleotide sugars from the cytosol into the lumen of the Golgi apparatus and/or the endoplasmic reticulum.91 In platelets, the dense tubular system represents residual endoplasmic reticulum of the parent megakaryocytes. However, the actual localization of this transporter in platelets as well as its role in platelet-dense granule dysfunction has not been further elucidated.
OATPs
Nutrients and drugs can share common transporters. The relevance of platelet uptake transporters for the distribution and action of drugs has been well demonstrated for statins. Available evidence supports the notion that the beneficial effects of statins on hypercholesterolemia-associated diseases, such as acute coronary syndrome, stroke, and atherosclerotic lesions, are attributable not only to their low-density lipoprotein-lowering effect but also to additional mechanisms of action.92 These “pleiotropic” effects include the stabilization of arterial plaques, normalization of endothelial functions, anti-inflammatory effects, and inhibition of platelet thrombus formation.93 On the molecular level, pleiotropic effects of statins have been attributed to the inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase in nonhepatic structures.94 This inhibition results in diminished biosynthesis of mevalonate, the precursor to cholesterol, and also to hydrophobic prenyl moieties, which are essential for proper sorting and function of several cell membrane-associated proteins, as receptors.95 If the modification of platelet function by statins proceeds via inhibition of platelet 3-hydroxy-3-methylglutaryl coenzyme A reductase, uptake of these drugs into platelets is a prerequisite. In the liver, the prime target organ for statins, uptake of statins, was shown to be mediated by transporters of the organic anion-transporting polypeptide (OATP/SLCO) family, mainly OATP1B1.96 A related transporter, OATP2B1, was detected in platelets and localized to the plasma membrane.21 OATP2B1 was shown before to transport atorvastatin and rosuvastatin as high-affinity substrates.97,98 Accordingly, an active transport of atorvastatin into platelets could be demonstrated, which was inhibited by the known OATB2B1 substrate estrone sulfate and vice versa.21 As a consequence of OATP2B1-mediated uptake of atorvastatin, a significant reduction of thrombin-induced Ca2+ mobilization in platelets was observed, which could be mechanistically explained by reduced prenylation of signal proteins. The effect was reversed by addition of mevalonate, the precursor to prenyl moieties as well as in presence of estrone sulfate, which competitively inhibits atorvastatin uptake.21
Besides statins, drugs and metabolites such as dehydroepiandrosterone-3-sulfate or estrone sulfate, which are described as OATP2B1 substrates, could be taken up into platelets by this transporter. For example, dehydroepiandrosterone-3-sulfate administration improves platelet superoxide dismutase activity, which protects cells against oxidative damage.99 The expression of OATP2B1 is probably only one example of how transporters in platelets influence effects of drugs, or vice versa, how drugs affect platelet biology. The list of drug uptake transporters in platelets is expected to grow rapidly, as this aspect of platelet transporters is addressed more intensively.
In conclusion, platelets contain a variety of transporters in their plasma membrane as well as within the membranes of their intracellular compartments (summarized in Figure 4). These proteins play a vital role in the storage and release of endogenous signaling molecules, implicating them as relevant structures for certain platelet function disorders as it has been shown for storage pool deficiencies. Platelets may serve also as vehicles that pick up compounds from various regions in the organism, including the gut, and transport them to their target organs. This would add new aspects to the role of platelets in many disorders, including host defense and immunology.100
Platelet transporters and their proposed functions at a glance. Platelets express a variety of SLC and ABC transporters, which are located in the plasma membrane as well as in the membranes of intracellular compartments,such as the dense (δ) and α (α) granules, and in other intracellular membrane systems, such as the dense tubular system (DTS; for details and references, see Table 1). They play a vital role in the uptake, sequestration, and release of mediators involved in platelet function. With respect to pharmacotherapy, the platelet represents a pharmacokinetic microcompartment, in which the interplay between uptake and elimination transporters determines intracellular drug concentrations.
Platelet transporters and their proposed functions at a glance. Platelets express a variety of SLC and ABC transporters, which are located in the plasma membrane as well as in the membranes of intracellular compartments,such as the dense (δ) and α (α) granules, and in other intracellular membrane systems, such as the dense tubular system (DTS; for details and references, see Table 1). They play a vital role in the uptake, sequestration, and release of mediators involved in platelet function. With respect to pharmacotherapy, the platelet represents a pharmacokinetic microcompartment, in which the interplay between uptake and elimination transporters determines intracellular drug concentrations.
Transporters are also potential new targets for pharmacologic inhibition of platelet function. Inhibiting a transporter instead of a receptor provides attractive options. On the one hand, the effects are long lasting and relatively robust even in patients with moderate compliance; and on the other hand, the drug effect can be counteracted immediately by transfusion of platelet concentrates, as the transfused platelets contain the substrates of the respective transporter. From a more pharmacologic point of view, platelets represent pharmacokinetic micro-compartments, in which the combination of uptake and elimination transport, possibly together with intracellular metabolism, determines pharmacokinetics of drugs. Transporters can be specifically used to direct antiplatelet drugs to target structures inside the platelets. Probably more important, many non-antiplatelet drugs may interfere with the physiologic substrates of these transporters in platelets and may in this way affect platelet function and modulate their hemostatic capacity. A better understanding of these mechanisms may help to recognize potential prothrombotic or prohemorrhagic effects of drugs. Finally, platelets may influence pharmacotherapy of possibly many drugs and accordingly contribute to interindividual variation in drug response. Therefore, a detailed understanding of the expression, function, and regulation of transporters in platelets will add to a better understanding of platelet biology and in many ways may result in improved therapeutic options.
Acknowledgments
The authors thank Theodore Warkentin (Department of Pathology and Molecular Medicine, McMaster University, Hamilton, ON) for expert proofreading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft, Germany (grants JE234/4-1 and SFB/TR19), and by the GANI-MED project (the Greifswald Approach to Individualized Medicine), funded by the Federal Ministry of Education and Research, Germany; and by the Center for Immune Reactions in Cardiovascular Disease, funded by the Federal Ministry of Education and Research, Germany.
Authorship
Contribution: G.J. wrote the paper and designed the figures; and A.G. and H.K.K. edited the manuscript and provided conceptual insights.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gabriele Jedlitschky, Institut für Pharmakologie, Center of Drug Absorption and Transport, Ernst-Moritz-Arndt-Universität Greifswald, D-17487 Greifswald, Germany; e-mail: jedlits@uni-greifswald.de; and Andreas Greinacher, Institut für Immunologie und Transfusionsmedizin, Ernst Moritz-Arndt-Universität Greifswald, 17487 Greifswald, Germany; e-mail: greinach@uni-greifswald.de.