Abstract
Memory T cells exhibit tremendous antigen specificity within the immune system and accumulate with age. Our studies reveal an antigen-independent expansion of memory, but not naive, CD8+ T cells after several immunotherapeutic regimens for cancer resulting in a distinctive phenotype. Signaling through T-cell receptors (TCRs) or CD3 in both mouse and human memory CD8+ T cells markedly up-regulated programmed death-1 (PD-1) and CD25 (IL-2 receptor α chain), and led to antigen-specific tumor cell killing. In contrast, exposure to cytokine alone in vitro or with immunotherapy in vivo did not up-regulate these markers but resulted in expanded memory CD8+ T cells expressing NKG2D, granzyme B, and possessing broadly lytic capabilities. Blockade of NKG2D in mice also resulted in significantly diminished antitumor effects after immunotherapy. Treatment of TCR-transgenic mice bearing nonantigen expressing tumors with immunotherapy still resulted in significant antitumor effects. Human melanoma tissue biopsies obtained from patients after topically applied immunodulatory treatment resulted in increased numbers of these CD8+ CD25− cells within the tumor site. These findings demonstrate that memory CD8+ T cells can express differential phenotypes indicative of adaptive or innate effectors based on the nature of the stimuli in a process conserved across species.
Introduction
Memory T cells represent an arm of the adaptive immune system that are long-lived, and capable of rapid antigen-specific responses. Memory T cells have been shown to have functional advantages more than naive T cells, as they develop more rapidly into cytolytic effector cells and produce greater amounts of cytokines after antigenic stimulation.1 Although T cells classically require T-cell receptor (TCR) engagement and proper costimulation for complete activation and proliferation, memory T cells have also been observed to proliferate in response to various cytokines during viral infections.2-6 These “bystander cells” proliferate and gain effector functions in response to the cytokine milieu produced during the course of viral and bacterial infections in mice and humans.7-10 Cytokines alone can induce this as a single dose of recombinant type-I interferon (IFN) resulting in a transient increase in the proliferation of CD8+, CD62L+CD44high memory T cells, which was independent of coligation of the TCRs.11 Such proliferation was not induced by the direct effects of type-I IFNs on CD8+ T cells, but was because of type-I IFN-driven production of secondary cytokines such as IL-15.4,12 Effector and memory CD8+ T cells express elevated levels of the receptors for IL-12 and IL-18, and secrete IFN-γ in response to stimulation with both cytokines,13 which suggests that other cytokine pathways can also induce their expansion. Similar to the secondary cytokine-driven proliferation observed after type-I IFN stimulation, IL-2, and toll-like receptor (TLR) agonists, that is, CpG and Poly:IC have also been described as having the capacity to induce bystander proliferation of CD8+CD44high T cells.12,14,15 The extent of antigen-specific proliferation versus bystander expansion has been the subject of considerable debate and may be contingent on the pathogen model and tissue examined.9,13,16 Cancer therapies that target the stimulation of the immune system via agonist antibodies, cytokine-based modalities, or TLR agonists have been shown to result in potent CD8+ T cell–mediated antitumor effects.17,18 We have previously shown that a combination immunotherapy consisting of an agonist CD40 antibody and IL-2 results in synergistic antitumor effects.19 Treatment of mice with other cytokine or TLR agonist combinations, such as CpGs and IL-15 or IL-2 and IL-12, also resulted in marked antitumor effects.18 In all of these models, the antitumor effects were associated with rapid, extensive CD8+ T-cell expansion. The antitumor effects were dependent on CD8+ T cells, the production of IFN-γ and IL-12, and the expression of Fas ligand (FasL), but were independent of CD4+ T cells, natural killer (NK) cells, and perforin expression.18,19 In the present study we sought to more completely characterize the CD8+ T cells expanded after cytokine immunotherapies and determine the role or need for antigen-specificity in their effector functions. Here we demonstrate that cytokine-mediated stimulation of memory CD8+ T cells results in antigen-nonspecific expansion, correlating with a unique phenotype that may also account for the dramatic antitumor effects observed as a cancer therapy, as well as providing insights as to their regulation.
Methods
Mice
C57BL/6 and BALB/c mice were purchased from the animal production area of the National Cancer Institute (NCI), or The Jackson Laboratory. Thymectomized mice received surgical thymectomy procedure by Charles River Laboratories at 6 to 8 weeks of age. OT-I C57BL/6 mice and wild-type (WT) controls were purchased from The Jackson Laboratory and were used at 8 to 24 weeks old. Mice were 8 to 16 weeks old and mouse studies were performed with the consent and approval of the University of Nevada–Reno, University of California, Davis, and NCI Institutional Animal Care and Use Committees.
Cell lines and reagents
Renca, B16, 3LL, P815, EL-4, and EG.7 cell lines (ATCC) were maintained in RF10 complete media (RF10c). RF10c was formulated as previously described.20,21 EG-7 cultures were supplemented with 0.1% G418 sulfate solution (Invitrogen). Anti–mouse CD40 (clone FGK115B3) was generated via ascites production as previously described. Recombinant human IL-2 (rhIL-2; TECIN Teceleukin) was provided by the NCI. Recombinant mouse IL-12 was purchased from Peprotech. Bromodeoxyuridine (BrdU) was purchased from BD Pharmingen and was used per the manufacturer's instructions.
Purified anti–mouse-CD3e (clone 145-2C11) and anti–mouse-CD28 (clone 37.51) were purchased from eBiosciences and used at 1 ug/mL and 5 ug/mL. The nondepleting, blocking anti-NKG2D (Clone CX5; Lewis Lanier, University of California, San Francisco) was administered intratumorally at 50 ug/dose concurrent with anti-CD40 and IL-2 treatment. Ovalbumin (OVA; Sigma-Aldrich) was diluted at a concentration of 5 mg/mL in D-PBS (Mediatech). Equal volumes (1:1) of OVA and incomplete Freunds adjuvant (Sigma-Aldrich) were subjected to water into oil emulsification. Two-hundred microliters of emulsions were immediately injected intraperitoneally into recipient WT or OT-I mice.
Treatment protocols
C57BL/6 and BALB/c mice were treated with agonist anti-CD40 antibody and recombinant human IL-2 (rhIL-2) as previously described.18 For studies where IL-12 was used as an alternative to anti-CD40 antibody, 0.5 ug of recombinant mouse IL-12 was administered for 5 consecutive days. rhIL-2 (106 IU) was administered in the IL-12 studies at the same time as the anti-CD40 and IL-2 regimen. Doses of anti-CD40 and rhIL-2 were reduced to 50 ug/dose and 3 × 105 IU/dose of anti-CD40 and IL-2 for the OT-I studies. In experiments involving BrdU, 1 mg BrdU in 0.1 mL D-PBS was injected intraperitoneally 24 hours before harvest. For in vivo blocking studies, 50 ug in 0.2 mL anti-NKG2D antibody in 0.2 mL D-PBS was injected intratumorally concurrent with anti-CD40/rhIL-2 therapy versus isotype. Renca cells (2 × 106 3LL, 1-2 × 106) were injected subcutaneously into strain-matched recipients and immunotherapy was initiated after tumors reached approximately 200 mm3 in volume. B16 cells (105) were injected subcutaneously into C57BL/6 and immunotherapy was initiated 4 days later. Tumor volumes were measured with calipers. In the intravenous Renca tumor model, 105 Renca cells were administered intravenously and immunotherapy was initiated 3 days later.
Flow cytometry and antibodies
Single cell suspensions were labeled with Fc Block (BD Pharmingen) and antibodies for 20 minutes, and then washed twice with staining buffer consisting of DPBS (Mediatech) and 1% FBS (Gemini Bio-Products). Samples were analyzed using a 3-color FACScan with Cell Quest Version 3.3 software (BD Biosciences), a 5-color FC 500/MPL (Beckman Coulter), a custom-configured LSRII with FACSDiva software (BD Biosciences), or a S1400 with Cellcapture Version 3 software (Stratedigm). The IntraPrep kit (Beckman Coulter) was used for granzyme staining, using the manufacturer's instructions. Data were analyzed using FlowJo Version 8 software (TreeStar). Anti–mouse antibodies included: PE-Cy7–conjugated anti-CD62L, FITC, PE, PE-Cy5, or APC-conjugated anti-CD25, APC-conjugated anti-CD44, PE or PE-Cy7–conjugated anti-NKG2D, FITC or PE-conjugated anti–PD-1, PE-conjugated anti-Vα2, APC-Cy7–conjugated anti-CD122 (eBioscience) FITC or APC-conjugated antiBrdU, FITC-conjugated anti-Vβ5.1/5.2, APC-conjugated anti-CD8, and APC-Cy7–conjugated anti-CD25 (BD Pharmingen). Pacific Blue–conjugated anti-CD44 (BioLegend), PE-TexasRed–conjugated anti-CD8, and PE-conjugated anti–human Granzyme B (Invitrogen).
Sorting
Splenocytes were harvested on day11. CD8+ T cells were enriched with nylon wool and sorted based on their expression for CD44 and CD8. The sorting was conducted using FACSAriaII.
Antibody-redirected lysis assay
Splenic CD8+ T cells were serially diluted in 96-well U bottom plates in RF10c media. P815 cells were labeled with 100uCi 51Cr (NEZ030S; Perkin Elmer) per 106 cells and incubated for 30 minutes with 10 ug/mL anti-CD3e. P815 targets (104) were added to each well and incubated at 37°C for 4 hours. Supernatants were removed, mixed 1:1 with scintillation fluid, and analyzed on a Wallac scintillation counter (Wallac). Total release was determined by adding 100 uL of 1× Triton ×-100 detergent (Sigma-Aldrich) to target cells. Specific release was calculated as: % lysis = 100%×(Experimental-Spontaneous)/(Maximum–Spontaneous)
EL4 and EG.7 killing assays
Spleens from treated and control OT-I mice were dissociated into single cell suspensions and serial dilutions of cells were incubated with 10451Cr-labeled EG.7 (OVA-EL4) or EL4 targets for 4 hours. Supernatants were removed and analysis/specific lysis was performed as in the antibody-redirect lysis assay.
Human melanoma study
The human melanoma study was approved by the Institutional Review Board at the Los Angeles Biomedical Center and the University of California, Los Angeles (UCLA), and was conducted under Good Clinical Practices guidelines at the Harbor-UCLA Medical Center and the UCLA Medical Center. This was a randomized, double-blind, placebo-controlled study to measure immunologic parameters only. No clinical outcomes were measured. Fourteen patients enrolled in the study after obtaining informed consents. Subjects were randomized in a 1:1 ratio to receive imiquimod 5% cream or vehicle cream (both supplied by 3M [later Graceway] Pharmaceuticals). Starting from day 0, subjects were instructed to apply the cream (250 mg) once daily more than the affected area for 14 days. On the day of surgery (day 14), subjects then underwent wide excision of the tumor and sentinel lymph node biopsy. Samples from the excised tumors were fixed in 10% formalin and processed with standard paraffin techniques.
Immunohistochemistry
Serial sections of skin from the paraffin-embedded blocks were obtained for immunohistochemistry (IHC) and evaluated for infiltration of immune cells. Sections were stained using the UltraVision LP Detection System HRP Polymer, and were stained for CD8 (Clone SP16) and CD25 (Clone 4C9), and were counterstained with methyl green (Thermo Fisher Scientific). All sides were coded and read in a blinded fashion. Images were captured with an Olympus BX4 microscope equipped with a Q-color 3 camera, using 4× numerical aperature objective lens. Magnification is specified in the figure legend. Images were processed for contrast and brightness using Adobe Photoshop C53.
Human and mouse T-cell cultures
Mouse splenocytes (2 × 106) were cultured in 6-well plates (Greiner) with anti-CD3/anti-CD28 or rhIL-2 for 3 days before analysis by flow cytometry. Human leukocytes were flushed from Leukopak filters (Delta Blood Bank). Lymphocytes were isolated using Lymphocyte Separation Medium (Mediatech), cultured at 106 cells/well in RF10c with 1 ug/mL PHA (Sigma-Aldrich) or rhIL-2, and analyzed by flow cytometry after 3 or 14 days.
Statistics
Statistical analysis was performed using Prism Version 4 (GraphPad Software). For analysis of 3 or more groups, the nonparametric ANOVA test was performed with the Bonferroni posttest. Analysis of differences between 2 normally distributed groups was performed using the Student t test. Nonparametric groups were analyzed with the Mann-Whitney test. Welch correction was applied to student t test datasets with significant differences in variance. Data were tested for normality and variance. A P value of < .05 was considered significant (*P < .05, **P < .01, ***P < .001).
Results
Memory CD8+ T cells markedly expand in vivo after successful systemic cancer immunotherapy regimens
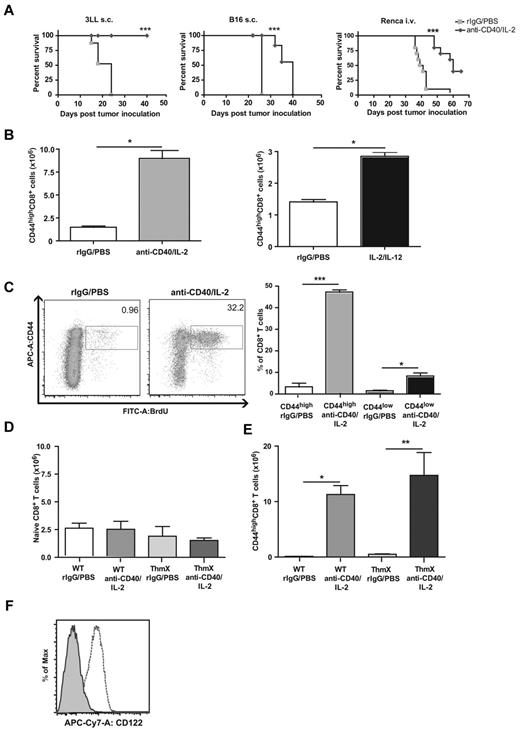
Consistent with previous studies, we treated mice with anti-CD40 plus IL-2 and observed significant antitumor effects in 3 different tumor models (Figure 1A). After treatment of mice with either anti-CD40/IL-2 or with IL-2/IL-12 combination regimens, we saw a tremendous expansion of memory phenotype CD44highCD8+ T cells, which dominated the response of both regimens, even in nontumor-bearing mice (Figure 1B). Assessment by BrdU uptake demonstrated that the CD44high memory, but not CD44low naive, CD8+ T cells were proliferating in response to treatment (Figure 1C). To determine whether only the memory cells were proliferating in the absence of naive T-cell conversion, thymectomized mice, which have a finite naive pool, were treated with immunotherapy. We observed that the total numbers of naive CD8+ T cells remained unchanged 11 days after immunotherapy in both the thymectomized and control mice (Figure 1D), and we observed that the effector/memory CD8+ T cells from both control and thymectomized mice significantly expanded 11 days after immunotherapy (Figure 1E). The data showed that only the memory pool was altered after immunotherapy as the total naive population was not changed. The selective expression of CD122 on the memory but not naive T cells is probably to give them an advantage in cytokine-only environments.22,23 Most CD44highCD8+ T cells expressed CD122, even in resting animals as opposed to the naive CD8+ T cells (Figure 1F). These results suggest that an antigen-nonspecific expansion of memory CD8+ T cells occurred after successful systemic immunotherapy.
Effects of immunotherapy regimens on memory CD8 T cell expansion and function in vivo. (A) Survival after anti-CD40 and IL-2 treatment of subcutaneously implanted 3LL (left), subcutaneously implanted B16 (middle), or intravenously injected Renca (right) tumor models. (B) Total numbers of splenic CD44highCD8+ T cells on day 11 of anti-CD40 and IL-2 (left) and 5 days after IL-2 and IL-12 (right) immunotherapy regimens in naive mice. (C) BrdU incorporation of CD44highCD8+ T cells in response to immunotherapy. Numbers in dot plots represent percentages of CD8+ T cells. (D) Percentage of naive CD8+ T cells in wildtype and thymectomized mice 11 days after anti-CD40 and IL-2 therapy. (E) Percentage of memory CD8+ T cells in WT and thymectomized mice on day 11 of anti-CD40 and IL-2 treatment. (F) Expression of CD122 on CD44high (dashed line) compared with CD44low CD8+ T cells (shaded) from resting C57BL/6 mice. Data are representative of at least 3 independent experiments (*P < .05, **P < .01, ***P < .001).
Effects of immunotherapy regimens on memory CD8 T cell expansion and function in vivo. (A) Survival after anti-CD40 and IL-2 treatment of subcutaneously implanted 3LL (left), subcutaneously implanted B16 (middle), or intravenously injected Renca (right) tumor models. (B) Total numbers of splenic CD44highCD8+ T cells on day 11 of anti-CD40 and IL-2 (left) and 5 days after IL-2 and IL-12 (right) immunotherapy regimens in naive mice. (C) BrdU incorporation of CD44highCD8+ T cells in response to immunotherapy. Numbers in dot plots represent percentages of CD8+ T cells. (D) Percentage of naive CD8+ T cells in wildtype and thymectomized mice 11 days after anti-CD40 and IL-2 therapy. (E) Percentage of memory CD8+ T cells in WT and thymectomized mice on day 11 of anti-CD40 and IL-2 treatment. (F) Expression of CD122 on CD44high (dashed line) compared with CD44low CD8+ T cells (shaded) from resting C57BL/6 mice. Data are representative of at least 3 independent experiments (*P < .05, **P < .01, ***P < .001).
Cytokine immunotherapy-expanded memory CD8+ T cells lack surface molecules indicative of recent TCR ligation, but up-regulate the expression of effector molecules
Because we have previously shown CD8+ T cells to be responsible for the antitumor effects,19 we next sought to further phenotypically characterize the expanded CD8 population. Interestingly, the expanded CD8+ T cells were negative for CD25, which is associated with TCR triggering, but a significant proportion of CD8+ T cells were NKG2D+ in both the lymph nodes and the spleen (Figure 2A-B). NKG2D+CD25−CD8+ memory T cells were the only population observed to expand after immunotherapy, whereas the percentage of memory CD8+ T cells exhibiting NKG2D+CD25+ or NKG2D− CD25+ phenotypes remained unchanged (Figure 2B).
Phenotype of cytokine induced CD8+ T cells in vivo and in vitro. (A) Gating schema and expression of NKG2D and CD25 on CD44highCD8+ T cells. (B) Percentages of CD25 and/or NKG2D expressing CD44highCD8+ populations in the spleen (left) and lymph node (right) on day 12 of anti-CD40 and IL-2 treatment. (C) Percentage of PD-1+ CD8+ T cells 12 days after anti-CD40 and IL-2 treatment in the spleen and lymph node. (D) Histograms depicting expression of NKG2D+ (left), PD-1+ (middle), and CD25+ (right) CD8+ T cells after anti-CD3 and anti-CD28 (solid line) or IL-2 (dashed line) stimulation compared with media alone (shaded). Data are representative of at least independent experiments (*P < .05, **P < .01, ***P < .001).
Phenotype of cytokine induced CD8+ T cells in vivo and in vitro. (A) Gating schema and expression of NKG2D and CD25 on CD44highCD8+ T cells. (B) Percentages of CD25 and/or NKG2D expressing CD44highCD8+ populations in the spleen (left) and lymph node (right) on day 12 of anti-CD40 and IL-2 treatment. (C) Percentage of PD-1+ CD8+ T cells 12 days after anti-CD40 and IL-2 treatment in the spleen and lymph node. (D) Histograms depicting expression of NKG2D+ (left), PD-1+ (middle), and CD25+ (right) CD8+ T cells after anti-CD3 and anti-CD28 (solid line) or IL-2 (dashed line) stimulation compared with media alone (shaded). Data are representative of at least independent experiments (*P < .05, **P < .01, ***P < .001).
Because the percentage of memory phenotype CD8 T cells expressing NKG2D in a naive animal is minimal (< 3%) and expands to such a great extent (> 30%) within 5 days after the start of therapy (data not shown), we hypothesized that the massive numbers of cells with this phenotype observed after immunotherapy were because of de novo up-regulation of NKG2D rather than expansion of a pre-existing population of NKG2D+ memory CD8+ T cells. To verify this, we sorted NKG2D(neg)CD25−CD8+CD44high T cells from congenic Ly5.1 mice to greater than 99% purity and adoptively transferred them into WT C57BL/6 mice. Two days after transfer, mice were treated as controls or with anti-CD40/IL-2 and harvested on day 11 to analyze for NKG2D phenotype. As hypothesized, CD45.1+CD8+ T cells from immunotherapy treated mice expanded and up-regulated NKG2D; ∼ 40% of CD45.1+CD8+ T cells were positive for NKG2D in the treated group compared with ∼ 3% in the controls (supplemental Figure 1A; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This calculated to a 240-fold expansion of CD45.1+CD8+CD44highNKG2D+ cells in the spleen and a 30-fold expansion in the lymph nodes (supplemental Figure 1B).
Lack of CD25 up-regulation suggested activation independent of TCR engagement. To further support this observation, we looked at expression of another molecule associated with TCR engagement, programmed death-1 (PD-1). PD-1 is highly expressed (> 50%) on both CD4+ and CD8+ T cells after TCR crosslinking24 and its prolonged expression both in vitro and in vivo during chronic inflammation has been correlated with immunosuppression and an exhausted phenotype.25-29 In addition to TCR engagement, PD-1 up-regulation has also been documented after stimulation with common γ-chain cytokines such as IL-2, although to a much lesser extent (∼ 15%-20%).30 Interestingly, signaling through PD-1 in the latter situation seemed to not interfere with cytokine-induced T-cell activation as PD-1–mediated inhibition of T-cell effector functions has been well documented to occur after TCR engagement. Consistent with cytokine-induced expression patterns, immunotherapy-expanded memory CD8+ T cells did not significantly up-regulate the TCR-dependent inhibitory molecule PD-1, a negative regulatory molecule in T-cell responses (Figure 2C).
To confirm that the phenotype of the expanded NKG2D+CD25−CD8+ T cells was consistent with one that is independent of TCR engagement, we performed in vitro studies comparing phenotypes of lymphocytes expanded under TCR-dependent or independent conditions. As expected, exposure of CD8+ T cells to anti-CD3/anti-CD28 versus IL-2 alone in vitro revealed the generation of cells with markedly different phenotypes; CD3 stimulation led to high levels of CD25 and PD-1 expression, whereas IL-2 stimulation alone did not, similar to what was observed with in vivo activated T cells. NKG2D up-regulation also occurred after cells were cultured in IL-2 (Figure 2D). These results indicate that cytokine-based stimulation results in a markedly different phenotype on the memory CD8+ T-cell population.
CD8 T cells displaying a TCR-independent activation phenotype are broadly lytic, present intratumorally, and recognize tumor targets in part through NKG2D
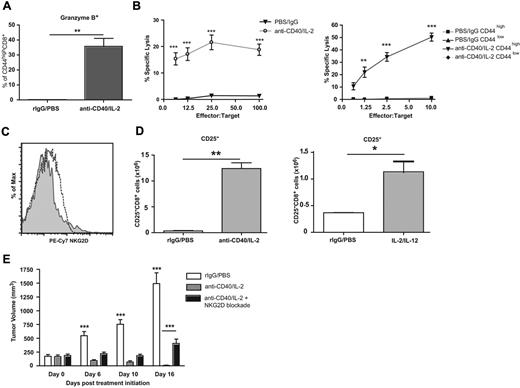
In addition to a TCR-independent activation phenotype, a large percentage of the expanded CD8+ T-cell population expressed granzyme B after immunotherapy (Figure 3A). To determine the lytic capabilities of CD8+ T cells after immunotherapy, we performed antibody-redirected lysis assays. The in vivo expanded CD8+ T cells were cytolytic toward tumor targets (Figure 3B). To confirm that the memory phenotype T cells were the effectors, we sorted CD44high and CD44lowCD8+ T cells of treated and untreated animals and performed the same cytolytic assay; only the CD44high CD8+ T cells of treated mice were cytolytic against tumor targets ex vivo (Figure 3B), which indicates that the memory and not naive population was affected after therapy.
Characterization of functional ability and tumor efficacy of immunotherapy expanded CD8+ T cells. (A) Granzyme B expression in CD44highCD8+ T cells on day 12 of anti-CD40 and IL-2 treatment. (B) Lytic ability of whole splenocytes (left) or sorted CD44high and CD44low CD8+ T cells (right) from anti-CD40 and IL-2 or control treated animals redirected against anti-CD3 labeled P815 targets. (C) NKG2D expression and (D) quantification of CD25 (pos) and (neg) CD8+ T cells from mice bearing orthotopic Renca tumors. (E) Tumor growth after immunotherapy concurrent with blockade of NKG2D in sc NKG2D ligand expressing Renca tumors. Data are representative of at least 3 independent experiments (*P < .05, **P < .01, ***P < .001).
Characterization of functional ability and tumor efficacy of immunotherapy expanded CD8+ T cells. (A) Granzyme B expression in CD44highCD8+ T cells on day 12 of anti-CD40 and IL-2 treatment. (B) Lytic ability of whole splenocytes (left) or sorted CD44high and CD44low CD8+ T cells (right) from anti-CD40 and IL-2 or control treated animals redirected against anti-CD3 labeled P815 targets. (C) NKG2D expression and (D) quantification of CD25 (pos) and (neg) CD8+ T cells from mice bearing orthotopic Renca tumors. (E) Tumor growth after immunotherapy concurrent with blockade of NKG2D in sc NKG2D ligand expressing Renca tumors. Data are representative of at least 3 independent experiments (*P < .05, **P < .01, ***P < .001).
To determine whether these cells were involved in antitumor responses, we next assessed their presence within the tumor itself. T cells of the same phenotype were found intratumorally using an orthotopic renal cell carcinoma model (Renca) after immunotherapeutic treatment with either anti-CD40/IL-2 or IL-2/IL-12, suggesting that these cells might play a role in the antitumor responses generated after cytokine immunotherapy (Figure 3C-D). We have previously demonstrated that the tumor regression induced by either anti-CD40/IL-2 or IL-2/IL-12 administration was dependent on CD8+ T cells.18,19,31,32 Thus, successful immunotherapy is associated with the increased presence of these expanded bystander CD25−CD8+ T cells within the tumor, as well as in the periphery.
Next, we sought to investigate how these bystander CD8+ T cells were recognizing tumors in vivo. A significant percentage of the CD44high CD8+ T cells expressed NKG2D after immunotherapy (Figure 2A-B). NKG2D ligation on cytokine-activated CD8+ T cells can induce MHC-unrestricted cytotoxicity.33 It is well known that NKG2D ligands are relatively absent from healthy tissues, but are up-regulated on many tumors or virally infected cells34-39 and NKG2D-deficient mice have an increased rate of developing certain tumors.40,41 The Renca tumor line has been documented to express various NKG2DL.42 To verify this, we confirmed the presence of the murine NKG2DL, Rae1-γ, and MULT1, by flow cytometry (supplemental Figure 2). We then treated mice bearing subcutaneous Renca tumors with anti-CD40/IL-2 with the concurrent administration of an NKG2D blocking antibody or a control antibody. Mice treated with immunotherapy alone had a marked and significant reduction in tumor growth and progression (Figure 3E), whereas blockade of NKG2D in mice receiving immunotherapy led to significant decreases in the efficacy of immunotherapy (Figure 3E). Complete abrogation of the antitumor effects was not observed, which suggests that other mechanisms may also play a role in the immunotherapeutic clearance of tumor cells. However, the data indicate that one mechanism by which the CD8+NKG2D+ T cells present within the tumor mediated their antitumor effects was through NKG2D.
Proliferation and lytic ability after immunotherapy occurs independently from TCR engagement
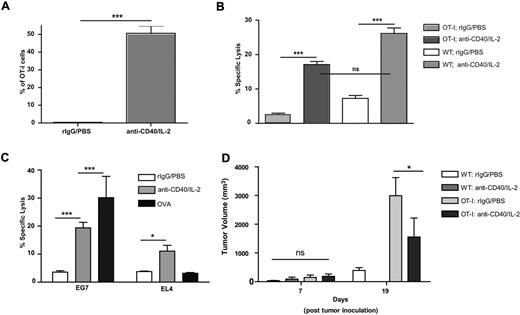
After correlating the phenotype of immunotherapy induced T cells in vivo with in vitro, cytokine activated CD8 T cells (Figure 2), we next sought to further delineate the phenotype of the CD8+ T cells after antigen-specific triggering versus cytokine stimulation in vivo. TCR-transgenic (TG) OT-I mice, > 95% of whose T-cell repertoire is specific for OVA, were immunized with ovalbumin or treated with immunotherapy. To confirm the purity of the transgenic mice, OT-I cells were gated using antibodies to the particular Vα and Vβ chain for the TCR transgene (Figure 4A). Although both treatments resulted in significant increases in the percentage of CD44highCD8+ (Figure 4B), only immunotherapy led to an increase in total CD44high numbers (Figure 4C). This phenomenon was not an artifact of the transgenic strain because after adoptive transfer of transgenic CD8+ T cells into WT recipients, immunotherapy resulted in a significant expansion of the CD44high population within the transferred cells in the absence of antigen as demonstrated by enhanced BrdU incorporation (Figure 4D). Thus in an antigen-specific model, the same CD8+ T cell can display differential phenotypes contingent on the stimulatory signal.
Phenotype of OT-I CD8+ T cells after cytokine immunotherapy versus immunization. (A) Expression of Vα2 and Vβ5.1/5.2 on peripheral blood CD8+ T cells from WT (left) and OT-I (right) mice. Numbers in the quadrants denote the percentages of CD8+ T cells expressing each marker. (B) Percentage and (C) numbers of CD44high expressing OT-I cells 11 days after anti-CD40 and IL-2 immunotherapy or OVA vaccination. (D) Dot plots depicting BrdU incorporation gated by CD44high expressing population in adoptively transferred OT-I cells after immunotherapy. Percentages of (E) CD25− and (F) PD-1 expression on CD44highOT-I cells from anti-CD40/IL-2 or OVA-vaccinated mice. (G) Percentage (left) and total numbers (right) of NKG2D+CD25− of CD44highOT-I cells on day 12 of anti-CD40 and IL-2 treatment. (H) Frequencies of NKG2D (left), PD-1 (middle), and CD25 (right) on OT-I cells after in vitro anti-CD3 and anti-CD28 (solid line) or IL-2 (dashed line) stimulations compared with media alone (shaded). Data are representative of at least 2 independent experiments (*P < .05, **P < .01, ***P < .001).
Phenotype of OT-I CD8+ T cells after cytokine immunotherapy versus immunization. (A) Expression of Vα2 and Vβ5.1/5.2 on peripheral blood CD8+ T cells from WT (left) and OT-I (right) mice. Numbers in the quadrants denote the percentages of CD8+ T cells expressing each marker. (B) Percentage and (C) numbers of CD44high expressing OT-I cells 11 days after anti-CD40 and IL-2 immunotherapy or OVA vaccination. (D) Dot plots depicting BrdU incorporation gated by CD44high expressing population in adoptively transferred OT-I cells after immunotherapy. Percentages of (E) CD25− and (F) PD-1 expression on CD44highOT-I cells from anti-CD40/IL-2 or OVA-vaccinated mice. (G) Percentage (left) and total numbers (right) of NKG2D+CD25− of CD44highOT-I cells on day 12 of anti-CD40 and IL-2 treatment. (H) Frequencies of NKG2D (left), PD-1 (middle), and CD25 (right) on OT-I cells after in vitro anti-CD3 and anti-CD28 (solid line) or IL-2 (dashed line) stimulations compared with media alone (shaded). Data are representative of at least 2 independent experiments (*P < .05, **P < .01, ***P < .001).
Similar to the in vitro T cell studies with WT mice, immunization of OT-I mice with OVA in vivo resulted in an increase in CD25+PD-1+ OT-I cells indicative of antigen-specific activation; however, after immunotherapy there were no increases in either CD25 or PD-1 on the OT-I cells (Figure 4E-F), but an increase in percentage and numbers of the NKG2D+CD44high population similar to WT (Figures 4G and 2B). In vitro studies demonstrated that OT-I CD8+ T cells paralleled the effects seen with WT-CD8+ cells (Figure 2D), as they also up-regulated CD25 and PD-1 after anti-CD3 plus anti-CD28 exposure to a much greater degree than IL-2 alone (Figure 4H).
Consistent with WT studies, after cytokine immunotherapy OT-I cells expressed increased amounts of granzyme B (Figure 5A) and were also lytically active (Figure 5B). When the T cells were assessed for lytic specificity, cells from mice immunized with OVA showed significantly increased lysis of only OVA-expressing tumor targets (EG7), whereas treatment with immunotherapy resulted in OT-I cells capable of lysing both OVA-expressing tumor cells (EG7) as well as the parental (EL4) OVA-negative cells (Figure 5C). To further confirm the nonspecific lytic ability of OT-I T cells after immunotherapy, we performed similar killing assays on control and anti-CD40/IL-2 treated WT and Rag2−/− OT-I TG mice in which all CD8+ T cells were specific for OVA. Indeed, immunotherapeutic treatment of both WT and TG mice resulted in EL-4 killing, whereas immunotherapy-treated TG cells were further able to lyse OVA-expressing cells to a greater extent indicating that TCR recognition remains intact (supplemental Figure 3A-B).
Functional analysis of OT-I cells after anti-CD40 and IL-2 treatment. (A) Granzyme B expression by OT-I T cells on day 12 day of anti-CD40 and IL-2 treatment. (B) Lytic ability (presented as percentage specific lysis at a 50:1 E/T ratio) of OT-I and WT mice on day 12 of anti-CD40 and IL-2 treatment. (C) Specific lysis of OVA-expressing EG7 and OVA-negative EL4 tumor lines after anti-CD40 and IL-2. Presented as percentage specific lysis at a 25:1 E/T ratio. (D) Growth of subcutaneous 3LL tumors in control and anti-CD40/IL-2-treated OT-I and WT mice compared. Data are representative of at least 2 independent experiments (*P < .05, **P < .01, ***P < .001).
Functional analysis of OT-I cells after anti-CD40 and IL-2 treatment. (A) Granzyme B expression by OT-I T cells on day 12 day of anti-CD40 and IL-2 treatment. (B) Lytic ability (presented as percentage specific lysis at a 50:1 E/T ratio) of OT-I and WT mice on day 12 of anti-CD40 and IL-2 treatment. (C) Specific lysis of OVA-expressing EG7 and OVA-negative EL4 tumor lines after anti-CD40 and IL-2. Presented as percentage specific lysis at a 25:1 E/T ratio. (D) Growth of subcutaneous 3LL tumors in control and anti-CD40/IL-2-treated OT-I and WT mice compared. Data are representative of at least 2 independent experiments (*P < .05, **P < .01, ***P < .001).
To determine whether antigen-nonspecific T cells were capable of mediating antitumor effects in vivo, OT-I mice bearing the OVA-negative 3LL lung carcinoma tumor were then treated with immunotherapy. The tumors grew significantly faster in OT-I control mice compared with WT recipients, which may be because of the total T cell number deficits reported in these mice43 as well as the absence of potential tumor-specific T-cell responses (Figure 5D). Nonetheless, we observed significant antitumor effects in the OT-I mice treated with cytokine immunotherapy, suggesting that antigen specificity (in that > 90% of the entire T-cell repertoire is directed to an antigen not present on the tumor) was not mandatory for antitumor effects of the T cells (Figure 5D). Together, these data demonstrate that antigen-specific triggering of CD8+ memory T cells by antigen or CD3 stimulation results in CD25 and PD-1 up-regulation and antigen-specific lytic potential, whereas cytokine stimulation alone results in CD8+ T cells that rapidly proliferate in an antigen-nonspecific manner, do not significantly up-regulate CD25 and PD-1, are broadly lytic, and capable of mediating antitumor effects in vivo.
Human memory CD8+ T cells show a phenotype similar to mouse T cells after antigen-nonspecific stimulation both in vitro and in vivo
We next sought to determine whether this expansion of CD25−CD8+ memory T cells also occurred in humans by analyzing tumor-infiltrating lymphocytes (TILs) from patients receiving an antigen-nonspecific immunotherapy. We assessed biopsies from melanoma patients receiving local treatment with the TLR7 agonist, imiquimod. Treatment was applied directly to the primary tumor for 14 days on a daily basis. The primary tumors were then biopsied and immunohistology used to determine the extent of T-cell infiltration and phenotype. Consistent with the mouse tumor studies, immunohistologic analyses indicated that immunotherapy resulted in the marked infiltration of CD8+CD25− T cells within the tumor site compared with vehicle-treated tumors (Figure 6A-B).
Human CD8 T cells exhibit similar phenotypic characteristics to mice after in vivo and in vitro antigen nonspecific stimulation. CD8 (purple) and CD25 (brown) expression in melanoma lesional biopsies after application of (A) vehicle or (B) 5% imiquimod cream daily for 14 days (40× magnification). (C) Dot plots depict gating schema and expression of HLA-DR and CD25 expression on human CD8+ T cells after 3 days of stimulation with PHA/IL-2 (TCR mimicking) or IL-2 alone in vitro. (D) Percentages of (TCR-dependent) CD25+HLA-DR+ (left) or PD-1+ (right) expressing CD45RO+CD8+ human T cells. (E) Percentage (left) and numbers (right) of CD25−HLA-DR+ (TCR-independently activated) human CD45RO+CD8+ T cells. (F) Percentages of CD25+ (left) and CD25− (right) human CD45RO+CD8+ T cells after 14 days of indicated stimulation. Data are representative of at least 2 independent experiments (*P < .05, **P < .01, ***P < .001).
Human CD8 T cells exhibit similar phenotypic characteristics to mice after in vivo and in vitro antigen nonspecific stimulation. CD8 (purple) and CD25 (brown) expression in melanoma lesional biopsies after application of (A) vehicle or (B) 5% imiquimod cream daily for 14 days (40× magnification). (C) Dot plots depict gating schema and expression of HLA-DR and CD25 expression on human CD8+ T cells after 3 days of stimulation with PHA/IL-2 (TCR mimicking) or IL-2 alone in vitro. (D) Percentages of (TCR-dependent) CD25+HLA-DR+ (left) or PD-1+ (right) expressing CD45RO+CD8+ human T cells. (E) Percentage (left) and numbers (right) of CD25−HLA-DR+ (TCR-independently activated) human CD45RO+CD8+ T cells. (F) Percentages of CD25+ (left) and CD25− (right) human CD45RO+CD8+ T cells after 14 days of indicated stimulation. Data are representative of at least 2 independent experiments (*P < .05, **P < .01, ***P < .001).
Similar to the murine studies, we then ascertained whether this phenotype could be generated in vitro. Human peripheral blood T cells were exposed to the TCR cross-linking mitogen, PHA, combined with IL-2, or with IL-2 alone in vitro for 3 days and analyzed for their phenotype. Consistent with what was observed in the mouse studies, mitogen exposure resulted in increased percentages of CD25+CD45RO+CD8+ T cells (Figure 6C-D) as well as up-regulation of PD-1 (Figure 6D) on the CD45RO+CD8+ T cells, whereas IL-2 alone resulted in increased expansion of the CD25−HLA-DR+CD45RO+CD8+ T-cell population (Figure 6E). In contrast to the mouse, NKG2D was constitutively expressed on human CD8+ T cells and therefore was not selectively up-regulated. Because the melanoma tumors were treated with imiquimod for 14 days before biopsy, it was important to ascertain whether CD25 might be down-regulated on T cells after prolonged engagement of their TCR. We therefore assessed the phenotype of the human T cells after prolonged TCR stimulation in vitro and observed that after 14 days of continuous exposure to PHA, T cells maintained an elevated expression of CD25 (Figure 6F). Interestingly, when the T cells were exposed to IL-2 alone for extended periods of time, an increased frequency of CD25+CD8+ T cells also emerged (Figure 6F). Similar to short-term assays, IL-2 alone led to nonspecific activation of memory CD8+ T cells as evidenced by increased expression of the activation antigen, HLA-DR, on the CD25−CD45RO+CD8 population even after 14 days (Figure 6H). These results demonstrate that antigen-nonspecific stimulation with IL-2 alone in vitro or with imiquimod in vivo resulted in the rapid expansion of the CD25−CD8+ T-cell population, whereas in vitro mitogen exposure resulted in marked expansion of the CD25+CD8+ T-cell population, similar to the mouse studies. Comparable up-regulation of CD25 and PD-1 were observed when human T cells were stimulated with anti-CD3/anti-CD28 (data not shown). These data, therefore, demonstrate that immunotherapeutic stimulation in both mice and humans results in the rapid antigen-nonspecific expansion of memory CD8+ T cells displaying a differential and unique phenotype compared with T cells in which TCRs are engaged.
Discussion
The findings presented herein demonstrate that memory CD8+ T cells can be a source of adaptive or innate effectors after cancer therapies depending whether TCR or cytokine-alone triggering has occurred, using a process remarkably conserved across the species. It is striking that both mouse and human CD8+ T cells exhibit a phenotypically similar pattern both in vitro and in vivo. Bystander expansion of memory CD8+ T cells has been well documented in mouse virus infection models, but has not been as established in cancer immunotherapy models nor has the phenotype been carefully examined. The success of various immunotherapeutic regimens such as TLR agonists or cytokine-based regimens with advanced tumor models may therefore lead to antigen nonspecific expansion of memory CD8+ T cells, which represent the primary effector mechanism.
To date, the majority of immunotherapeutic regimens used in cancer have focused on the goal of developing of tumor-specific responses by CD8+ T cells. This is highly desirable because it would allow for the generation of specific antitumor immunity and therefore be less probable to damage healthy tissues. However, because of the intrinsic nature of human cancers, the long-term efficacy of such an approach clinically may be limited because of the significant efforts of the tumor itself to avoid immune recognition and attack and the limited presence of sufficiently immunogenic tumor-associated and tumor-specific antigens. Down-regulation or loss of potential antigens present on the tumor, but not necessary for its survival, has occurred in numerous clinical scenarios and represents a significant hurdle with antigen-specific approaches.44,45 Our results suggest that memory T cells can exert antigen-nonspecific effects and these can be successfully exploited under the conditions of cytokine-based cancer immunotherapies.
The unique phenotype of these T cells has not been reported but fits with the concept of the “two-signal” model for T-cell activation. Memory T cells represent immune cells that have undergone at least 2 rounds of antigen selection (within the thymus and the periphery) and thus can be considered “safer” for antigen-nonspecific expansion. Memory cells express CD122 to a much greater extent than naive T cells, which accounts for their proliferative advantage for cytokine alone-driven processes. Furthermore, the lack of the high affinity IL-2 receptor complex on these bystander cells indicates that continuously high levels of cytokines are needed for their expansion and survival. In contrast, when encountering antigen, the up-regulation of CD25 gives the specific responders the advantage in a cytokine-limited environment for sustained responses. In addition to CD25, we also investigated expression of another molecule associated with TCR engagement, PD-1.24 PD-1 functions to suppress the immune response after activation25-27 and its prolonged expression such as occurs with chronic inflammation, has been correlated with an exhausted phenotype.28,29 In the context of cancer, the ligation of PD-1 on the surface of tumor-infiltrating T cells has been shown to induce anergy in those cells.46 Conversely, PD-1–deficient mice or mice treated with PD-L1 blocking antibodies demonstrate increased survival in an acute myeloid leukemia model.47 The cytokine-driven memory CD8+ T cells that we describe here did not significantly up-regulate PD-1, possibly rendering them less susceptible to the immunosuppressive environment created by tumor cells. It is important to note that this phenotype only applied to CD8+ T cells, as we have found that CD4+ T cells did indeed markedly up-regulate PD-1 after cytokine immunotherapy,20 indicating that the control of CD4+ T cells is perhaps more critical because of their role as producers of IL-2 and in driving the immune response. This also suggests that cytokine-alone expanded memory CD8+ T cells do not require PD-1 for their control as the cytokine environment itself controls the cells.
As the ligands for NKG2D are primarily present on stressed, proliferating, or malignant cells, the potential for autoreactivity would probably be less compared with tumor-associated determinants potentially present in healthy tissues. Our murine data suggest that the expanded memory T cells exhibit antigen-nonspecific behavior at least partly because of NKG2D. NKG2D signaling on activated memory CD8+ T cells has been found to exclusively trigger perforin-dependent tumor killing33,42 which may be the dominant killing mechanism that occurs in the absence of TCR ligation.
We observed significant antitumor effects in the TCR TG OT-I mice bearing a tumor not expressing the cognate antigen after immunotherapy. The data demonstrate that the expansion of these antigen-nonspecific memory CD8+ T cells leads to significant antitumor effects. Although systemic administration of high amounts of cytokines potentially increases the likelihood of systemic toxicities, perhaps because of these expanded nonspecific killer cells, the generation of MHC-unrestricted killers may prove highly beneficial for the treatment of less immunogenic tumors. It is, therefore, possible to envision a therapeutic approach where both antigen-specific and antigen-nonspecific approaches are combined to allow for maximal antitumor effects and our data suggest the effectors can be delineated based on phenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cherish Agutos and Ruth Gault for assistance in the preparation of this paper, and Kenneth Hunter and Lisbeth Welniak for helpful discussions.
This work was supported by National Institutes of Health (NIH) grant CA095572. L.L.L. is an American Cancer Society Professor and supported by NIH grants AI068129 and CA72669.
National Institutes of Health
Authorship
Contribution: J.K.T. and D.E.C.W. performed experiments, analyzed data, and assisted in writing of the paper; M.N.B. and G.D.S. assisted with experiments and revisions of the paper; K.L.A. performed experiments on thymectomized mice; E.A. assisted with human in vitro experiments; J.M.W. performed IL2/IL12 studies and provided experimental oversight; K.W.B. and N.C. provided human melanoma lesional biopsies for IHC staining; D.L.L. assisted with experimental design and writing of the paper; L.L.L. provided NKG2D blocking antibody; and R.H.W., B.R.B., D.R., and W.J.M. assisted in experimental oversight, analysis of data, and assisted in writing of the paper.
Conflict of-interest-disclosure: The authors declare no competing financial interests.
Correspondence: William J. Murphy, Professor and Vice Chair for Research, Depts of Dermatology and Internal Medicine, University of California, Davis School of Medicine, 2921 Stockton Blvd, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.
References
Author notes
J.K.T. and D.E.C.W. contributed equally to this work.