Abstract
Mutations in the coding region of telomerase complex genes can result in accelerated telomere attrition and human disease. Manifestations of telomere disease include the bone marrow failure syndromes dyskeratosis congenita and aplastic anemia, acute myeloid leukemia, liver cirrhosis, and pulmonary fibrosis. Here, we describe a mutation in the CCAAT box (GCAAT) of the TERC gene promoter in a family in which multiple members had typical features of telomeropathy. The genetic alteration in this critical regulatory sequence resulted in reduced reporter gene activity and absent binding of transcription factor NF-Y, likely responsible for reduced TERC levels, decreased telomerase activity, and short telomeres. This is the first description of a pathogenic mutation in the highly conserved CCAAT box and the first instance of a mutation in the promoter region of TERC producing a telomeropathy. We propose that current mutation-screening strategies should include gene promoter regions for the diagnosis of telomere diseases. This clinical trial was registered at www.clinicaltrials.gov as #NCT00071045.
Introduction
Telomeres, the structures that cap the ends of linear chromosomes, consist in vertebrates of hundreds to thousands of TTAGGG repeats. To prevent critical telomere shortening, highly proliferative cells express telomerase (encoded by TERT), a reverse transcriptase that adds TTAGGG repeats to telomeres, with TERC as its RNA template. Constitutional loss-of-function mutations in telomerase complex genes result in deficient telomere maintenance and accelerated telomere attrition. Telomere disease in humans manifests clinically as a spectrum of the BM failure syndromes dyskeratosis congenita and aplastic anemia (AA), acute myeloid leukemia, cirrhosis, and pulmonary fibrosis.1 Sequencing strategies for the diagnosis of telomere disease are based on screening exons and their flanking regions of telomerase genes for mutations. Pathogenic mutations in the promoter region of TERT or TERC have not been firmly established. Here, we report a mutation in the CCAAT box of the TERC promoter region that leads to telomere disease.
Methods
Patients and control subjects
Diagnosis of AA was performed as described previously.2 Blood samples were collected after written informed consent was obtained in accordance with the Declaration of Helsinki, according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, protocol 04-H-0012 (www.clinicaltrials.gov #NCT00071045).
Sequence analysis and telomere length measurement
A TERC promoter/gene region (−1661 through +502; +1 is defined as the transcriptional start site of TERC) was amplified by PCR on leukocyte genomic DNA (10 ng), followed by bidirectional sequencing. A total of 378 people served as control subjects. Telomere length was measured as described previously.3,4
Gel shift assay
The gel shift assay was performed with the LightShift Chemiluminescent EMSA kit (Pierce) with unlabeled (competitors) or 5′-biotin end-labeled (probes) oligonucleotide duplexes (wild-type or mutant), which covered the TERC-CCAAT box and adjacent regions (nucleotide −63 through −39). Anti–NF-YA antibody (Rockland Immunochemicals) was used for supershift assay. DNA-protein complexes were analyzed by PAGE and the Chemiluminescent Nucleic Acid Detection Module (Pierce).
Plasmid construction, transfection, and luciferase activity assays
Plasmids carrying wild-type or mutant TERC promoter regions (starting at positions −798, −436, −272, −107, and −42; ending at a position +62) were constructed based on the pGL4.18[luc2P/Neo] luciferase vector (Promega). After plasmid transfection into HEK293T or HeLa cells (2.5 × 105), luciferase activity was measured with the Dual-Glo Luciferase Assay System (Promega).
RT-PCR for TERC expression
TERC expression levels were determined on total RNA isolated from PBMCs or skin fibroblasts with the RNeasy Mini Kit (QIAGEN). RT-PCR for actin and TERC was performed with the OneStep RT-PCR Kit (QIAGEN) and custom-made primers and probes.
Results and discussion
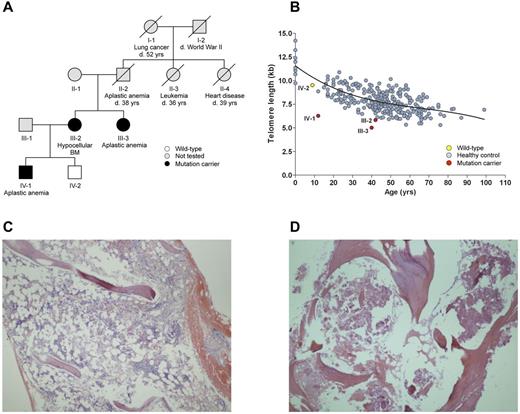
Telomere disease was suspected in our 39-year-old white index patient (III-3) who was diagnosed with AA and had a family history of AA and leukemia (Figure 1A). Her peripheral blood leukocyte telomere length was 5.4 kb, which is very short compared with age-matched healthy control subjects (Figure 1B). Her sister (III-2), who had normal blood cell counts but a severely hypocellular BM (Figure 1C), and her nephew (IV-1), diagnosed with AA at age 11 years, also had very short leukocyte telomeres, but an unaffected nephew (IV-2) had normal telomere length (Figure 1B). Neither the proband, her sister, or affected nephew showed classic mucocutaneous stigmata of dyskeratosis congenita. No mutations were found in the coding region of TERC, TERT, or exon 6a of TINF2. However, the proband, her sister, and her affected nephew, but not the unaffected nephew, carried a heterozygous mutation in the CCAAT box positioned at −58 to −54 (CCAAT > GCAAT) of the TERC core promoter region. The mutation was absent in 378 healthy subjects of various ethnic backgrounds. A −714 C insertion, present in 16.7% of healthy control subjects,5 was found in the index patient, her sister, and her affected nephew on the same allele as the −58C>G. The remaining upstream region of the TERC promoter was wild type to base −1661.
Pedigree, telomere length, and BM histology. (A) Pedigree of proband (III-3). Individuals II-2 and II-3 are suspected mutation carriers. Slashed symbols indicate deceased (d.) individuals. Neither the proband, her sister, or affected nephew showed abnormal pigmentation of the skin, nail dystrophy, or oral leukoplakia, nor was there evidence of pulmonary or immunologic problems, growth retardation, developmental delay, or microcephaly. During cholecystectomy, an enlarged liver was noticed in III-2; however, this finding was not further evaluated after surgery. (B) Blood leukocyte telomere length (in kilobases) as a function of age. Mutation carriers have very short telomeres in peripheral blood leukocytes. The curve marks the 50th percentile of telomere length for control subjects derived from 298 healthy National Institutes of Health blood bank donors. A sample's telomere length was expressed as a telomere to single-copy gene (T/S) ratio, which was converted to kilobases. BM biopsy showed profound hypocellularity in the proband (III-3; C) and hypocellular BM and eosinophilic ground substance in her sister (III-2; D).
Pedigree, telomere length, and BM histology. (A) Pedigree of proband (III-3). Individuals II-2 and II-3 are suspected mutation carriers. Slashed symbols indicate deceased (d.) individuals. Neither the proband, her sister, or affected nephew showed abnormal pigmentation of the skin, nail dystrophy, or oral leukoplakia, nor was there evidence of pulmonary or immunologic problems, growth retardation, developmental delay, or microcephaly. During cholecystectomy, an enlarged liver was noticed in III-2; however, this finding was not further evaluated after surgery. (B) Blood leukocyte telomere length (in kilobases) as a function of age. Mutation carriers have very short telomeres in peripheral blood leukocytes. The curve marks the 50th percentile of telomere length for control subjects derived from 298 healthy National Institutes of Health blood bank donors. A sample's telomere length was expressed as a telomere to single-copy gene (T/S) ratio, which was converted to kilobases. BM biopsy showed profound hypocellularity in the proband (III-3; C) and hypocellular BM and eosinophilic ground substance in her sister (III-2; D).
The CCAAT box is frequently present in promoter regions of RNA polymerase II–transcribed genes, located preferentially −60 to −100 nucleotides from the transcriptional start site.6 NF-Y, a nuclear protein composed of NF-YA, NF-YB, and NF-YC, binds with high affinity to the CCAAT box.7 Binding of NF-Y to the CCAAT box of the TERC promoter region is crucial for TERC promoter activity.8,9
In humans, a few CCAAT box mutations have been reported, but none that clearly cause disease. In hereditary persistence of fetal hemoglobin, a benign condition that benefits sickle cell anemia and β-thalassemia patients, mutations in the CCAAT boxes of the HBG1 and HBG2 promoters have been reported.10-13 In β-thalassemia intermedia, a mutation in the CCAAT box of the HBB promoter was described, but no meaningful functional data supported the hypothesis that this mutation was pathogenic.14
A TERC promoter mutation (−99C>G) was present in a patient with paroxysmal nocturnal hemoglobinuria15 and in a patient with myelodysplastic syndrome.16 Telomere lengths in these patients were not reported, and functional analyses were inconclusive. The paroxysmal nocturnal hemoglobinuria and myelodysplastic syndrome phenotypes were not explained by this mutation.
To elucidate the effect of the −58C>G mutation in the CCAAT box on TERC promoter function, we performed gel shift and reporter gene assays and determined TERC expression in primary cells. The gel shift assay showed a shifted band of wild-type biotinylated probe, which spanned bases −63 through −39 of the TERC promoter region; addition of anti-NF-YA antibody resulted in supershift of the band. Mutant probe did not bind to NF-Y (Figure 2A). Although wild-type unlabeled oligonucleotide out-competed binding of wild-type probe to NF-Y, mutant unlabeled oligonucleotide did not (Figure 2B). To investigate the effect of loss of NF-Y binding to the mutant box on TERC promoter activity, we generated wild-type and mutant TERC promoter–luciferase reporter plasmids of different lengths and transiently transfected HEK293T cells with these constructs. The relative luciferase activity of mutant constructs was decreased 3.5- to 9-fold compared with the wild-type construct (Figure 2C). Transfection of HeLa cells resulted in comparable patterns (Figure 2D). Cotransfection of wild-type and mutant constructs in a 1:1 ratio in HEK293T cells reduced luciferase activity by approximately 2-fold compared with the wild-type construct alone (data not shown). TERC expression was determined by RT-PCR in PBMCs and was lower in affected individuals III-2 and IV-1 than in unaffected individual IV-2 (TERC/actin relative expression ± SEM: 0.00536 ± 0.00130, 0.619 ± 0.107, and 1.00 ± 0.232, respectively). TERC expression also was lower in skin fibroblasts from the proband than in an unrelated healthy subject (0.781 ± 0.0517 and 1.00 ± 0.0402, respectively). However, these results should be interpreted cautiously, because TERC expression is highly variable within individuals.17
Functional analysis of wild-type and mutant CCAAT boxes of the TERC promoter. (A) Gel shift and supershift assays. Gel shift assay was performed with HeLa nuclear extract with wild-type probe (wt, 5′-Bio/cttggccaatccgtgcggtcgg-3′), a mutant probe (mt1, 5′-Bio/cttgggcaatccgtgcggtcgg-3′), and additional mutant control probes (mt2, 5′-Bio/cttggagtctccgtgcggtcgg-3′; mt3, 5′-Bio/cttggccattccgtgcggtcgg-3′); bold and underlined letters indicate mutated nucleotides. Anti-NF-YA antibody was used for supershift assay. Arrows show bands that were shifted and supershifted with the wt probe but not with the mt1 probe (−58C>G) or additional mutant control probes (mt2 and mt3). (B) A 200-fold molar excess of unlabeled mutant competitor (mt1, mt2, or mt3) did not compete with wild-type binding, whereas wild-type competitor did (indicated with an arrow). Mutant promoter activity in HEK293T (C) or HeLa cells (D) was reduced compared with wild-type in reporter gene assays. Depicted on the x-axis is the 5′ position from the transcriptional start site of TERC for each TERC promoter-luciferase construct; each construct ended at nucleotide +69. Luciferase activity is reported as relative fold increase compared with the empty vector pGL4.18[luc2P/Neo] (given an arbitrary value of 1), normalized to protein concentration. Results shown are means of 3 (HEK293T) or 1 (HeLa) independent experiment performed in duplicate. Error bars indicate SEM. Detailed methods that include sequences of primers and probes used in the present study will be provided on request.
Functional analysis of wild-type and mutant CCAAT boxes of the TERC promoter. (A) Gel shift and supershift assays. Gel shift assay was performed with HeLa nuclear extract with wild-type probe (wt, 5′-Bio/cttggccaatccgtgcggtcgg-3′), a mutant probe (mt1, 5′-Bio/cttgggcaatccgtgcggtcgg-3′), and additional mutant control probes (mt2, 5′-Bio/cttggagtctccgtgcggtcgg-3′; mt3, 5′-Bio/cttggccattccgtgcggtcgg-3′); bold and underlined letters indicate mutated nucleotides. Anti-NF-YA antibody was used for supershift assay. Arrows show bands that were shifted and supershifted with the wt probe but not with the mt1 probe (−58C>G) or additional mutant control probes (mt2 and mt3). (B) A 200-fold molar excess of unlabeled mutant competitor (mt1, mt2, or mt3) did not compete with wild-type binding, whereas wild-type competitor did (indicated with an arrow). Mutant promoter activity in HEK293T (C) or HeLa cells (D) was reduced compared with wild-type in reporter gene assays. Depicted on the x-axis is the 5′ position from the transcriptional start site of TERC for each TERC promoter-luciferase construct; each construct ended at nucleotide +69. Luciferase activity is reported as relative fold increase compared with the empty vector pGL4.18[luc2P/Neo] (given an arbitrary value of 1), normalized to protein concentration. Results shown are means of 3 (HEK293T) or 1 (HeLa) independent experiment performed in duplicate. Error bars indicate SEM. Detailed methods that include sequences of primers and probes used in the present study will be provided on request.
In conclusion, we show absent binding of NF-Y and reduced reporter gene activity with a mutant CCAAT box (GCAAT) in vitro, which suggests that the CCAAT-box disruption may lead to reduced TERC levels, lower telomerase activity, short telomeres, and human telomere disease. We believe that the terms telomere disease or telomeropathy are more adequate than dyskeratosis congenita to describe the phenotype observed in this family. First, these terms are descriptive of the underlying molecular defect. Second, telomeropathies are a large spectrum of phenotypes, from no clinical manifestations to macrocytosis, AA, pulmonary fibrosis, or the more severe phenotype in infancy Revesz syndrome, all with different prognoses. We prefer to reserve the term dyskeratosis congenita for patients with the classic clinical presentation and not for all subjects with a telomerase mutation.
We provide the first example of a TERC promoter mutation producing a telomeropathy and the first instance of a mutation in a CCAAT box that is causative in human disease. Mutation-screening strategies for the diagnosis of telomere diseases should include promoter regions of major genes related to telomere biology. Similar strategies may also be helpful for marrow failure syndromes such as Diamond-Blackfan anemia or Shwachman-Diamond syndrome, because with current approaches, a mutation can be identified only in approximately half of inherited marrow failure cases.18
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr J. E. Decker and Dr T. Winkler for kindly providing TERC expression data in fibroblasts.
This research was supported in part by the National Institutes of Health (National Heart, Lung, and Blood Institute) Intramural Research Program. A.M.A. was supported by the KiKa Foundation, Amstelveen, The Netherlands, and the René Vogels Foundation, Oirschot, The Netherlands.
National Institutes of Health
Authorship
Contribution: A.M.A., S.K., R.T.C., and N.S.Y. conceived and designed the experiments, analyzed the data, and wrote the paper; A.M.A., S.K., and R.T.C. performed the experiments; and M.M.v.d.H.E., V.H.J.v.d.V., R.T.C., and N.S.Y. contributed reagents, materials and analysis tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neal S. Young, MD, 10 Center Dr, Bldg 10-CRC/Room 3-5140, Hematology Branch, NHLBI, NIH, Bethesda, MD 20892; e-mail: youngns@mail.nih.gov.

![Figure 2. Functional analysis of wild-type and mutant CCAAT boxes of the TERC promoter. (A) Gel shift and supershift assays. Gel shift assay was performed with HeLa nuclear extract with wild-type probe (wt, 5′-Bio/cttggccaatccgtgcggtcgg-3′), a mutant probe (mt1, 5′-Bio/cttgggcaatccgtgcggtcgg-3′), and additional mutant control probes (mt2, 5′-Bio/cttggagtctccgtgcggtcgg-3′; mt3, 5′-Bio/cttggccattccgtgcggtcgg-3′); bold and underlined letters indicate mutated nucleotides. Anti-NF-YA antibody was used for supershift assay. Arrows show bands that were shifted and supershifted with the wt probe but not with the mt1 probe (−58C>G) or additional mutant control probes (mt2 and mt3). (B) A 200-fold molar excess of unlabeled mutant competitor (mt1, mt2, or mt3) did not compete with wild-type binding, whereas wild-type competitor did (indicated with an arrow). Mutant promoter activity in HEK293T (C) or HeLa cells (D) was reduced compared with wild-type in reporter gene assays. Depicted on the x-axis is the 5′ position from the transcriptional start site of TERC for each TERC promoter-luciferase construct; each construct ended at nucleotide +69. Luciferase activity is reported as relative fold increase compared with the empty vector pGL4.18[luc2P/Neo] (given an arbitrary value of 1), normalized to protein concentration. Results shown are means of 3 (HEK293T) or 1 (HeLa) independent experiment performed in duplicate. Error bars indicate SEM. Detailed methods that include sequences of primers and probes used in the present study will be provided on request.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-10-383182/4/m_zh89991288440002.jpeg?Expires=1763721682&Signature=V37CtAQMwt4iXIz1d9cwbOicQ47BmUK08eLrCT4je49d3Fl5nDM9xDbHJPcZuq9Of0Pf0axEtmh2BqPs~kUT4JPbpn6jhApZ8G5MswblF5uJX-m3ynica7xaIWNzH-LClaNh3avu37rYuhtmhMSFf8tkudCG1CXpKQtEVsooMdcodrR7r3umkNH7xAQzHCBt22orGMmKzhzCk~Poz4XiWx9l0cWrZDQ2oss0-egNIYeTw80B2utOpKg63hJo23A1gos3Pl7Wr64o-lQPKELSUentwQkNIAoWzPzaKzpNmUYE3BCy2exNfK3-qnWSAW2EcFxdRUosdzT6QuYSlphI0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal