Abstract
In previous work we transferred a human factor IX–encoding adeno-associated viral vector (AAV) into skeletal muscle of men with severe hemophilia B. Biopsy of injected muscle up to 1 year after vector injection showed evidence of gene transfer by Southern blot and of protein expression by IHC and immunofluorescent staining. Although the procedure appeared safe, circulating F.IX levels remained subtherapeutic (< 1%). Recently, we obtained muscle tissue from a subject injected 10 years earlier who died of causes unrelated to gene transfer. Using Western blot, IHC, and immunofluorescent staining, we show persistent factor IX expression in injected muscle tissue. F.IX transcripts were detected in injected skeletal muscle using RT-PCR, and isolated whole genomic DNA tested positive for the presence of the transferred AAV vector sequence. This is the longest reported transgene expression to date from a parenterally administered AAV vector, with broad implications for the future of muscle-directed gene transfer.
Introduction
Hemophilia B is an X-linked bleeding disorder resulting from a mutation in the factor IX (F.IX) gene. For those with severe hemophilia (< 1% normal circulating F.IX), bleeding into joints and potentially fatal bleeds into critical closed spaces can be largely prevented by maintaining circulating F.IX above 1% through periodic infusion of F.IX concentrates. Gene transfer raises the possibility of sustained levels of F.IX after a single vector injection. Although an ideal target organ for gene transfer of a secreted protein is the liver, many with hemophilia have liver disease because of hepatitis B and/or C infection. Skeletal muscle is a potential alternative target tissue for those that may not be candidates for liver-directed gene transfer. Indeed, adeno-associated viral vector (AAV)–mediated gene transfer of an encoded transgene1-5 and encoded F.IX6-9 to skeletal muscle was successful in multiple animal models before human administration. Although the first human parenteral administration of AAV was safe and led to muscle fiber expression of F.IX,10-12 circulating levels remained subtherapeutic because of limitations on dose per injection site (required, based on animal studies, to avoid antibody (Ab) formation to factor IX) and limitations on reasonable numbers of injections.13 Here we describe skeletal muscle expression of F.IX protein and RNA 10 years after AAV-F.IX gene transfer, the longest duration of transgene expression from an AAV vector observed in humans to date.
Methods
Case history
Subject F was a 43-year-old white male with severe hemophilia B (F.IX < 1%), and a history of a hemophilic pseudotumor but no F.IX inhibitor, when he underwent intramuscular AAV vector administration into the vastus lateralis bilaterally.11 He was enrolled in the mid-dose cohort, 1 of 8 subjects injected at skeletal muscle sites in the upper and/or lower extremities with AAV-F.IX at doses of 1.5 × 1012 vg/site, with numbers of sites ranging from 10 to 90, based on dose cohort. No short- or long-term vector-related side effects were observed, but F.IX levels did not rise to > 1% and there was no change in levels of F.IX concentrate use (twice weekly prophylactic infusion). Nine years later, because of serious limitations of daily activities, he underwent surgery for the longstanding abdominal/retroperitoneal pseudotumor. After months of postoperative complications, he died from sepsis. Permission for autopsy including studies of vector-injected muscle and liver was granted.
Protein extraction and Western blot
After approval by the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee, we injected mice in the right quadriceps with AAV2-CMV-hFIX, with left quadriceps serving as a negative control, and harvested muscle 3 weeks later. Frozen mouse and human muscle were ground and lysed, and protein concentrations determined by Coomassie Plus (Pierce). Samples were run on gels, transferred to polyvinylidene difluoride membrane via iBlot (Invitrogen), and incubated in 1:1000 anti-F.IX-HRP Ab (Cedarlane Laboratories) 2 hours at room temperature (RT). Blots were washed and developed with Amersham ECL plus (GE Healthcare).
IHC and IF
Immunoperoxidase IHC.
Slides were treated with Background Buster (Innovex), rinsed with 0.1M TTBS, then incubated in 1:200 rabbit anti-F.IX Ab (Sigma-Aldrich) overnight at 4°C. Slides were rinsed with Tween 20 Tris-buffered saline (TTBS) and incubated with HRP-polymer conjugated anti–rabbit Ab (SuperPicTure Polymer Detection Kit; Invitrogen) 30 minutes at RT, developed with diaminobenzidine chromogen (SuperPicTure kit), and counterstained with hematoxylin.
Immunofluorescence (IF).
Slides were treated with Fc receptor blocker (Innovex) and rinsed with TTBS. Primary Ab was applied as described in IHC, incubated in 1:200 Alexa 488 anti–rabbit Ab (Invitrogen) 30 minutes at RT, rinsed with TTBS, and mounted with SlowFade Gold with DAPI (4',6-diamidino-2-phenylindole; Invitrogen).
RNA extraction, RT PCR, F.IX cDNA PCR
RNA was extracted by TRIzol/chloroform extraction, isolated, and eluted from RNEasy columns (QIAGEN). We generated cDNA using the high-capacity reverse transcription kit (Applied Biosystems), and amplified human F.IX cDNA with 2 primer pairs. First set: forward 5′-TGGCGGCAGTTGCAAGGATGAC-3′, reverse 5′-TGGCACTGCTGGTTCACAGGAC-3′. Second set: forward 5′-GCTCACCCGTGCTGAGACTGTT-3′, reverse 5′-GGTTCGTCCAGTTCCAGAAGGGC-3′.
gDNA extraction, AAV vector gDNA PCR
We extracted whole gDNA with the MasterPure DNA Purification Kit (Epicentre). We amplified vector gDNA with primers yielding a 242-bp fragment: forward 5′-CCAAAGGTTATGCAGCGCGTG-3′, reverse 5′-TACTTACCAACCTGCGTGCTGGC.
Results and discussion
Evidence of F.IX protein expression in injected muscle 10 years after gene transfer
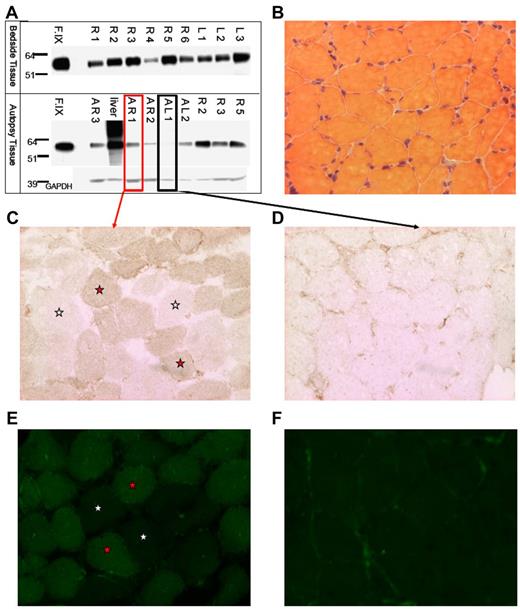
We isolated muscle tissue from the vastus lateralis of both lower extremities of subject F, who died from complications unrelated to AAV gene transfer. Intradermal injections of India ink served as landmarks for injection sites. We isolated total protein from muscle and Western-blotted for human F.IX protein (Figure 1A). F.IX protein was detected in most muscle samples analyzed. We sectioned both highly and weakly positive muscle samples from autopsy (AR1 and AL1, respectively, Figure 1A), and assayed muscle F.IX expression by immunoperoxidase and IF staining. Muscle histology was normal by H&E staining (Figure 1B). Sections were incubated with an anti-F.IX primary Ab followed by either an HRP-conjugated or fluorescently labeled secondary Ab. In sections from the strongly positive Western blot sample AR1, we saw F.IX-positive muscle fibers in a mosaic-form pattern (positive and negative fibers in a checkerboard pattern) as previously reported for AAV2 muscle transfer,11,12 by both immunohistochemical (Figure 1C) and immunofluorescent staining (Figure 1E). As a control, sample AL1, weakly positive by Western blot, showed minimal staining, in a primarily extracellular pattern (Figure 1D,F), as we had previously described. Together with Western blot detection of F.IX, positive muscle fiber staining by 2 histologic methods confirms expression of F.IX in skeletal muscle 10 years after gene transfer.
Evidence of F.IXprotein in subject Fmuscle. (A) Western blot for human F.IX on protein extracted from tissue removed at bedside on patient expiry (top half) and at autopsy (bottom half); A indicates autopsy; R, right leg; L, left leg; F.IX, Benefix recombinant hF.IX control. (B) H&E stain of patient-injected muscle showing normal histology. (C-D) F.IX IHC of strongly F.IX-positive (C, same sample boxed in red from panel A) and very weakly positive (D, same sample boxed in black from panel A) tissue sections of injected patient muscle. (C-F) Magnification: ×20; red stars indicate F.IX-expressing muscle fibers; and white stars, no F.IX production. (E-F) F.IX IF of strongly F.IX-positive (E, same sample boxed in red from panel A) and very weakly positive (F, same sample boxed in black from panel A) sections of injected patient muscle.
Evidence of F.IXprotein in subject Fmuscle. (A) Western blot for human F.IX on protein extracted from tissue removed at bedside on patient expiry (top half) and at autopsy (bottom half); A indicates autopsy; R, right leg; L, left leg; F.IX, Benefix recombinant hF.IX control. (B) H&E stain of patient-injected muscle showing normal histology. (C-D) F.IX IHC of strongly F.IX-positive (C, same sample boxed in red from panel A) and very weakly positive (D, same sample boxed in black from panel A) tissue sections of injected patient muscle. (C-F) Magnification: ×20; red stars indicate F.IX-expressing muscle fibers; and white stars, no F.IX production. (E-F) F.IX IF of strongly F.IX-positive (E, same sample boxed in red from panel A) and very weakly positive (F, same sample boxed in black from panel A) sections of injected patient muscle.
Evidence of F.IX RNA expression and AAV vector DNA persistence 10 years after gene transfer
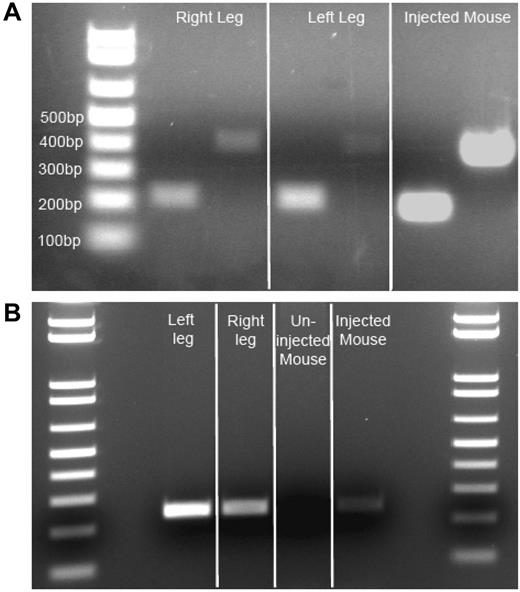
Isolated injected muscle RNA from autopsy samples was reverse transcribed to obtain cDNA. Using 2 different primer pairs, we amplified F.IX cDNA from both muscle cDNA samples, as well as from vector-injected mouse control cDNA (Figure 2A), but were unable to detect F.IX cDNA in uninjected human skeletal muscle (data not shown). Positive samples showed amplification of F.IX bands of 216 and 408 bp (injected human muscle and injected mouse muscle cDNA positive control). This remarkable finding indicated ongoing transcription from an AAV2 vector 10 years after administration. After transcription and translation had been verified, we isolated genomic DNA from subject and control mouse muscle, and from subject liver, to amplify vector DNA. We amplified a 242-bp product primed from the CMV promoter into the exon 1/intron 1 boundary of the transgene cassette of the vector genome from both left and right vastus lateralis of the subject, and from the positive mouse control, while the contralateral uninjected mouse quadriceps was negative (Figure 2B). Subject liver DNA also yielded a faint 242-bp band (data not shown). The gel bands were isolated and sequenced to confirm that the amplicon carried the predicted sequence of the AAV vector.
Evidence of F.IX RNA, AAV vector DNA insubject Fmuscle. (A) Evidence of active transcription of F.IX RNA. Two independent primer pair PCR amplifications (216-bp and 408-bp amplicons) specific for human F.IX cDNA on patient muscle cDNA derived from isolated RNA. Three-week post-AAV2-CMV-F.IX–injected mouse (C57BL/6) muscle RNA-derived cDNA serves as a positive control. (B) Evidence of AAV vector genome in patient muscle. Primers amplifying a 242-bp genomic DNA segment from the CMV promoter of the vector to the exon 1/intron 1 junction of the transgene cassette reveal persistence of vector in injected patient muscle DNA. Three-week post-AAV2-CMV-F.IX–injected quadriceps mouse muscle genomic DNA serves as a positive control, while the contralateral uninjected quadriceps muscle serves as a negative mouse control.
Evidence of F.IX RNA, AAV vector DNA insubject Fmuscle. (A) Evidence of active transcription of F.IX RNA. Two independent primer pair PCR amplifications (216-bp and 408-bp amplicons) specific for human F.IX cDNA on patient muscle cDNA derived from isolated RNA. Three-week post-AAV2-CMV-F.IX–injected mouse (C57BL/6) muscle RNA-derived cDNA serves as a positive control. (B) Evidence of AAV vector genome in patient muscle. Primers amplifying a 242-bp genomic DNA segment from the CMV promoter of the vector to the exon 1/intron 1 junction of the transgene cassette reveal persistence of vector in injected patient muscle DNA. Three-week post-AAV2-CMV-F.IX–injected quadriceps mouse muscle genomic DNA serves as a positive control, while the contralateral uninjected quadriceps muscle serves as a negative mouse control.
These data suggest AAV2-mediated gene transfer to human skeletal muscle persists and is transcriptionally and translationally active for a period of up to 10 years. Although concerns regarding risk of an immune response to the transgene product, based on studies in animals, limited the dose per injection site, and thus the ability to achieve therapeutic efficacy, these data do establish that AAV2-mediated gene transfer to human skeletal muscle can persist for up to a decade. The use of alternate serotypes14-16 and/or delivery techniques that can perfuse muscle more extensively17-19 may improve therapeutic efficacy for this approach.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Drs Denis Phichith and Mustafa Naci Yazicioglu for technical assistance, and Drs Charles Esmon and Florea Lupu for laboratory assistance at the Oklahoma Medical Research Foundation.
This work was supported by the National Institutes of Health (HL64190, K.A.H.; and T32-HL07439, G.B.), Howard Hughes Medical Institute, and the Center for Cellular and Molecular Therapeutics at Children's Hospital of Philadelphia.
National Institutes of Health
Howard Hughes Funding
Authorship
Contribution: G.B. collected samples, designed and performed experiments, and wrote the manuscript; G.M.P. communicated with patient's family, assured regulatory measures were met, provided clinical input, and edited the manuscript; A.R. sectioned and stained patient muscle for F.IX IHC and IF; A.W.F. performed the injections; S.M.H. and F.M. coordinated acquisition and delivery of patient samples; F.M. edited the manuscript; and K.A.H. designed experiments, edited the manuscript, and was the sponsor of the clinical trial.
Conflict-of-interest disclosure: G.M.P. and K.A.H. are inventors on several patents of AAV vector delivery to muscle. K.A.H. has waived all financial interest in a patent related to AAV2-F.IX. The remaining authors declare no competing financial interests.
Correspondence: Katherine A. High, Children's Hospital of Philadelphia, 3501 Civic Center Blvd, Colket Translational Research Bldg Ste 5000, Philadelphia, PA 19104; e-mail: high@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal