Abstract
The endothelial protein C receptor (EPCR) plays an important role in cardiovascular disease by binding protein C/activated protein C (APC). EPCR structure contains a hydrophobic groove filled with an unknown phospholipid needed to perform its function. It has not been established whether lipid exchange takes place in EPCR as a regulatory mechanism of its activity. Our objective was to identify this phospholipid and to explore the possibility of lipid exchange as a regulatory mechanism of EPCR activity driven by the endothelially expressed secretory group V phospholipase A2 (sPLA2-V). We identified phosphatidylcholine (PCh) as the major phospholipid bound to human soluble EPCR (sEPCR). PCh in EPCR could be exchanged for lysophosphatidylcholine (lysoPCh) and platelet activating factor (PAF). Remarkably, lysoPCh and PAF impaired the protein C binding ability of sEPCR. Inhibition of sPLA2-V, responsible for lysoPCh and PAF generation, improved APC binding to endothelial cells. EPCR-dependent protein C activation and APC antiapoptotic effect were thus significantly enhanced. In contrast, endothelial cell supplementation with sPLA2-V inhibited both APC generation and its antiapoptotic effects. We conclude that APC generation and function can be modulated by changes in phospholipid occupancy of its endothelial cell receptor.
Introduction
The endothelial cell protein C/activated protein C receptor (EPCR) is a transmembrane glycoprotein that is abundantly expressed on the surface of the endothelium of the large vessels.1 EPCR binds protein C, activated protein C (APC), and factor VII/VIIa (FVII/VIIa) with high affinity.2,3 On EPCR binding on the endothelial surface, protein C activation by the thrombin-thrombomodulin complex is notably increased and factor Xa-dependent FVII activation is reduced.4,5 EPCR is a cornerstone molecule in the protein C system, which plays an important role in cardiovascular pathologies, such as thrombosis, coronary artery disease, and stroke,6-8 and is involved in mechanisms of vascular biology, such as inflammation and endothelial barrier protection related to sepsis, lung function, and metastasis.9,10
Previous structural studies showed that EPCR folds into 2 α-chains which, together with a β-sheet platform, form a hydrophobic groove. This is occupied by a phospholipid, which has not yet been identified but is necessary to preserve the ligand binding properties of EPCR.11 One of the aims of the present study was to identify the exact nature of this phospholipid. Because EPCR shares significant homology with CD1d and MHC class I–like molecules, which behave as lipid antigen-presenting molecules,12 we speculated that the EPCR hydrophobic pocket could be filled with more than one phospholipid. We provide evidence that fully active EPCR accommodates phosphatidylcholine (PCh) within its hydrophobic groove and that, interestingly, secretory group V phospholipase A2 (sPLA2-V) generates bioactive lipids: lysophosphatidylcholine (lysoPCh) and platelet-activating factor (PAF), which notably impair the ability of EPCR to interact with protein C and FVII.
Methods
An expanded version is available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All animal experiments and human sample withdrawal were approved by the Ethics Committee of the University of Navarra.
Materials
Protein G, phosphomolybdic acid, n-octyl-β-d-glucopyranoside, liver bovine phosphatidylethanolamine (PE) [molecular weight (MW) = 768 Da, which is a mixture of PE 18:0 and PE 20:4], L-α-lysophosphatidylcholine (L-α-lysoPCh; MW = 505 Da, which is a mixture of lysoPCh 16:0, lysoPCh 18:0, and lysoPCh 18:1), PAF, sphingosine 1 phosphate (S1P), staurosporine, and TNF-α were from Sigma-Aldrich. Liver bovine L-α-phosphatidylcholine (L-α-PCh; MW = 786 Da, which is a mixture of PCh 18:0 and PCh 18:2) and egg yolk PCh (MW = 760 Da, which is a mixture of PCh 16:0 and PCh 18:1), were from Avanti Polar Lipids. Anti-EPCR mAbs RCR-2 and RCR-252 were kindly provided by Dr K. Fukudome (Saga Medical School, Saga, Japan). APC, protein C, and thrombin were from Enzyme Research Laboratories. FVIIa was from Novo Nordisk. Lepirudin was from Behringwerke AG. Chromogenic substrate S-2366 was from Chromogenix. sPLA2-V was from Abcam. Manoalide was from Santa Cruz Biotechnology. Mouse anti–human sPLA2-V and rabbit anti–mouse sPLA2-V Abs were from Cayman. Anti–human thrombomodulin was from American Diagnostica. Anti–rabbit Dako Envision+ System-HRP was from Dako North America. Biotinylated Phe-Pro-Arg chloromethylketone (PPACK-b) was from Calbiochem. Streptavidin AlexaFluor-647 conjugate and annexin V–Alexa-647 were from Invitrogen. Actinomycin D was from BD Biosciences. HPTLC silica plates were purchased from Merck.
Recombinant proteins expression and purification
Human and murine yeast-derived sEPCR as well as human mammalian (HEK-293) cell–derived sEPCR were expressed and purified as described.13-15
Purification of endothelium-derived and spontaneously shed sEPCR
A total of 7 L of supernatant from EA.hy926 cell culture was collected, concentrated and dialyzed against 10mM HEPES, pH 7.4, and supplemented with 150mM NaCl (HBS). The purification of sEPCR was carried out by 2-step affinity chromatography (Äkta FPLC; GE Healthcare) using RCR-2 and RCR-252 mAbs immobilized on 2 NHS columns (Hi-Trap; GE Healthcare).
Lipid extraction from sEPCR variants and subsequent lipid identification
The lipid fraction of sEPCR was extracted for subsequent analysis by thin layer chromatography (TLC) and mass spectrometry analysis.
Chloroform and methanol were used to extract the lipid for TLC analysis. The dried lipid was resuspended in 50 μL of pure chloroform, and TLC analysis was performed.
The lipid fraction for mass spectrometry analysis was obtained using Triton X-100 and 1-butanol/DIPE. The extracted lipid was subjected to mass spectrometry analysis. Linked-scan tandem quadrupole mass spectrometry was performed for detection of phospholipids using a Waters Q-TOF-Micro quadrupole mass spectrometer (Waters) in the nanospray mode with fused silica nanospray capillaries (New Objectives). Fragments of the parental ions were detected in the positive ion mode.
Lipid extraction for subsequent analysis of sEPCR function
n-octyl-β-d-Glucopyranoside was used to extract the lipid from the yeast-derived sEPCR. The delipidated sEPCR was subsequently used for surface plasmon resonance (SPR) and fluorescence spectroscopy analyses.
Assessment of the interaction between lipids and sEPCR by fluorescence spectroscopy analysis
The binding of PCh, lysoPCh, PAF, and S1P to human delipidated sEPCR was studied by measuring the changes in the intrinsic fluorescence of delipidated sEPCR on incubation with increasing amounts of the lipids. The data were used to calculate the dissociation constant (KD) for each lipid.
Assessment of the interaction between lysoPCh or PAF and sEPCR by mass spectrometry
Each lipid was incubated with sEPCR at 10-fold excess. Once the unbound lipid was removed by gel exclusion chromatography, the lipid fraction of the eluted protein was extracted and analyzed by mass spectrometry.
Effect of sPLA2-V on sEPCR
sEPCR at 2.38μM was incubated with 0.57μM sPLA2-V for 2 hours, and the APC binding capacity of EPCR was assessed by SPR.
Biomolecular interaction analyses by SPR
All interaction experiments were performed by SPR technology using a BIAcore X Biosensor (GE Healthcare) basically as previously described.3
To compare the binding of APC to different sEPCR variants and to sEPCR preincubated with sPLA2-V, we captured these variants through the anti-EPCR RCR-2 mAb immobilized on the surface of a CM5 sensor chip (GE Healthcare) by the amine coupling method in a Biacore X Biosensor (GE Healthcare). APC, protein C, or FVIIa was injected under no mass transport limitation condition. A 10-seconds injection of 85% (volume/volume) phosphoric acid was injected to regenerate the RCR-2 surface.
Kinetic experiments were performed capturing unmodified or delipidated sEPCR forms on RCR-2 and injecting a range of concentrations of APC or FVIIa. Mass transport limitation effect was not detectable under these conditions. The remaining analyte after each injection was eliminated by injecting EDTA. Kinetic parameters were calculated using BIAevaluation Version 3.2RC1 software (GE Healthcare).
Because we experienced problems with capturing PAF-regenerated sEPCR, we decided to reverse the system. APC-PPACK-b was captured onto the FC2 of a streptavidin chip (GE Healthcare). PAF-loaded sEPCR was subsequently injected on both FC1 and FC2, and the interaction was monitored.
Flow cytometric experiments
All the flow cytometric experiments were performed in a FACSCalibur (BD Biosciences). The binding of Alexa-647–conjugated APC (APC-PP*) to human aortic endothelial cells (HAECs; Lonza Switzerland) or endothelium-derived EA.hy926 cells (kindly provided by C. J. Edgell, University of North Carolina, Chapel Hill, NC), was studied as described after incubating them with manoalide or sPLA2-V.3 The expression of EPCR, thrombomodulin, and sPLA2-V on the surface of cells was assessed using anti–human EPCR RCR-2, anti–human thrombomodulin, and anti–human sPLA2-V mAbs, respectively. Apoptosis of staurosporine-stimulated endothelial cells was assessed by incubating them with fluorescently labeled annexin V and actinomycin D.
Activation of protein C on the endothelial surface
The effect of manoalide and sPLA2-V on APC generation by thrombin on the surface of HAECs or EA.hy926 was studied as previously described.13
Silencing sPLA2-V expression in EA.hy926 cells
Lentiviral pLKO.1-puro plasmids containing short hairpins for sPLA2-V (clones NM_000929.1-243s1c1, NM_000929.1-375s1c1, NM_000929.1-486s1c1, NM_000929.1-507s1c1, and NM_000929.1-386s1c1) were obtained from shRNA Mission (Sigma-Aldrich), and infective particles were obtained according to the manufacturer's instructions. EA.hy926 cells were transduced and cell populations selected using puromycin. The sPLA2-V silencing was evaluated by flow cytometry as mentioned in “Flow cytometric experiments.” However, because the baseline expression of sPLA2-V was very low and assessing silencing success would therefore be difficult, we decided to incubate first the lentiviral transfected EA.hy926 cells with 0.5μM PMA to increase sPLA2-V expression sufficiently to allow us to compare silencing and choose the cells that best fit our purposes.
Immunohistochemical assessment of sPLA2-V expression in tissues
Sections from carotid arteries from mice subjected to a laser injury-induced thrombosis16 and from human left atrial appendages were analyzed for the presence of sPLA2-V using an anti–sPLA2-V Ab followed by the anti–rabbit Dako Envision+ System-HRP, and for the presence of fibrin deposits using Masson trichrome staining.
Results
PCh is the phospholipid located in the hydrophobic pocket of EPCR
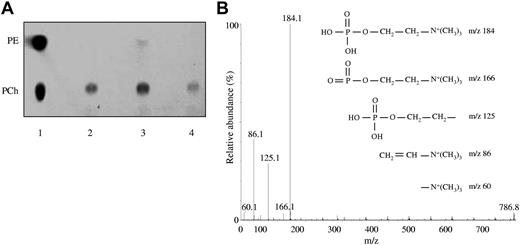
The lipid fraction extracted from yeast-expressed human sEPCR migrated in the TLC plate as a single spot exactly as PCh standard did. The same result was obtained using human sEPCR produced in mammalian cells, thus suggesting that the lipid naturally occupying the pocket of human sEPCR is PCh (Figure 1A). Furthermore, when the experiment was performed using sEPCR spontaneously shed from human endothelial cells, we obtained the same result (supplemental Figure 1). The experiment performed with murine sEPCR revealed a major spot corresponding to PCh and a minor one that migrates like PE.
Identification of the phospholipid bound to EPCR. (A) Lane 1 indicates that a mixture of PE and PCh was loaded as standard; lane 2, lipid fraction extracted from yeast-produced sEPCR; lane 3, lipid fraction extracted from yeast-produced murine sEPCR; and lane 4, lipid fraction from sEPCR produced in a mammalian expression system. (B) MS/MS fragmentation analysis of the lipid fraction from yeast-produced sEPCR. The peak at m/z 786 matches up with commercial PCh. The spectrum generated by fragmentation of the molecule at m/z 786 fits properly with commercial PCh. The different PCh polar group fragmentation products and their respective m/z are started.
Identification of the phospholipid bound to EPCR. (A) Lane 1 indicates that a mixture of PE and PCh was loaded as standard; lane 2, lipid fraction extracted from yeast-produced sEPCR; lane 3, lipid fraction extracted from yeast-produced murine sEPCR; and lane 4, lipid fraction from sEPCR produced in a mammalian expression system. (B) MS/MS fragmentation analysis of the lipid fraction from yeast-produced sEPCR. The peak at m/z 786 matches up with commercial PCh. The spectrum generated by fragmentation of the molecule at m/z 786 fits properly with commercial PCh. The different PCh polar group fragmentation products and their respective m/z are started.
To further analyze the nature of the EPCR lipid content, the sEPCR organic extract was subjected to mass spectrometry analysis, which showed a peak at m/z 786, whose fragmentation yielded a pattern of peaks that are characteristic of the PCh polar group (Figure 1B). Commercially available PCh (MW = 786 Da) rendered the same results, as did the lipid extracted from murine sEPCR. Finally, 2 peaks corresponding to 2 PCh molecules of different MW (786 and 758 Da) were obtained by testing human sEPCR produced in mammalian cells, and both of them exhibited the characteristic peak pattern of the PCh polar group fragmentation. Collectively, these analyses demonstrate that the main lipid molecule bound to human EPCR is PCh.
PCh is essential for EPCR to interact with its ligands
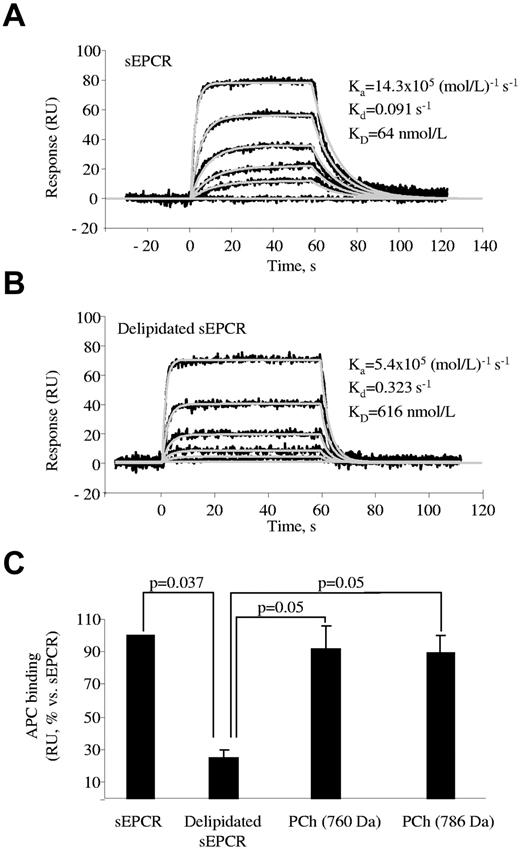
We used SPR technology to analyze whether PCh was necessary to preserve the ability of sEPCR to interact with APC. The affinity of EPCR for APC was approximately 10 times lower when PCh was removed from sEPCR (KD = 64 ± 8nM and 616 ± 156nM with and without PCh, respectively, n = 3; Figure 2A-B), a result that is consistent with previous findings.11 The ability of APC to bind to delipidated sEPCR was fully recovered after incubation with PCh (Figure 2C). It is noteworthy that the KD was 62 ± 27nM (n = 3), which is very similar to that obtained with nonmanipulated sEPCR. Delipidated sEPCR also showed a reduced ability to bind to FVIIa (KD = 83 ± 16 and 625 ± 147nM with and without PCh, respectively).
SPR analysis of the influence of PCh on the APC binding to sEPCR. sEPCR was captured on a CM5 chip through RCR-2 mAb. (A) Binding of APC (0, 3, 9, 27, 81, and 243nM) to sEPCR. (B) Binding of APC (0, 9, 27, 81, 243, and 729nM) to delipidated sEPCR. In both cases, a representative experiment of 3 independent repeats is shown. Black lines represent experimental data; and gray lines, fittings to a Langmuir 1:1 kinetic model. (C) Binding at equilibrium of 100nM APC to sEPCR, delipidated sEPCR, and delipidated sEPCR reconstituted with 760 and 786 Da MW PCh. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons.
SPR analysis of the influence of PCh on the APC binding to sEPCR. sEPCR was captured on a CM5 chip through RCR-2 mAb. (A) Binding of APC (0, 3, 9, 27, 81, and 243nM) to sEPCR. (B) Binding of APC (0, 9, 27, 81, 243, and 729nM) to delipidated sEPCR. In both cases, a representative experiment of 3 independent repeats is shown. Black lines represent experimental data; and gray lines, fittings to a Langmuir 1:1 kinetic model. (C) Binding at equilibrium of 100nM APC to sEPCR, delipidated sEPCR, and delipidated sEPCR reconstituted with 760 and 786 Da MW PCh. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons.
lysoPCh and PAF but not S1P can locate in the hydrophobic pocket of EPCR
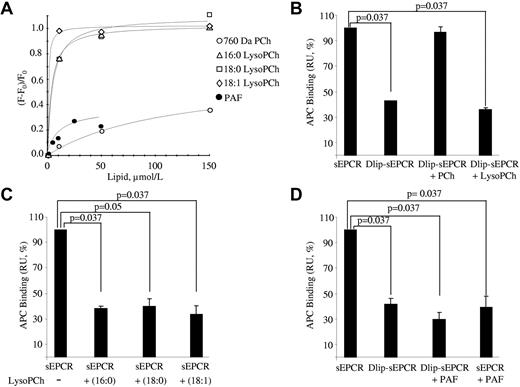
Because lysoPCh is derived from PCh and preserves the structure of the latter except that it lacks one of the fatty acid groups (supplemental Figure 2), we speculated that EPCR could accommodate it. For this reason, we used fluorescence spectroscopy to monitor whether the intrinsic fluorescence of delipidated sEPCR changed on incubation with lysoPCh. As can be seen, the 3 lysoPCh forms tested did modify the intrinsic fluorescence in a dose-dependent manner (Figure 3A). As a reference, changes in the intrinsic fluorescence of delipidated sEPCR were also monitored on PCh (MW = 760 Da) addition. Interestingly, delipidated sEPCR interacted more strongly with all lysoPCh forms than with PCh, as shown by the KD obtained for each condition: 7.5 ± 8.5, 3.6 ± 1.0, 1.1 ± 0.2, and 108.4 ± 54.5μM for lysoPCh 16:0, 18:0, 18:1, and PCh, respectively. Bearing in mind the similarity of PAF and, to a lesser extent, S1P to PCh and lysoPCh (supplemental Figure 2), we wondered whether EPCR could accommodate either of these molecules. S1P did not modify the intrinsic fluorescence of delipidated sEPCR. However, PAF did modify it, suggesting there was an interaction. The affinity was again better than that obtained with PCh (KD = 16.1 ± 12.0μM).
Binding of lysoPCh to sEPCR. (A) Delipidated sEPCR was incubated with increasing amounts of 16:0, 18:0, and 18:1 lysoPCh, 760 Da MW PCh, and PAF, and the change in the sEPCR intrinsic fluorescence was registered. A representative experiment is shown for each lipid. The absolute changes in fluorescence on addition of LPC 16:0, LPC 18:0, LPC 18:1, PC, and PAF were 1.22, 1.05, 1.23, 0.31, and 0.4, respectively. (B) SPR analysis of the binding of APC to delipidated sEPCR reconstituted with PCh or lysoPCh. sEPCR or delipidated sEPCR was captured on a CM5 chip through RCR-2 mAb. Binding at equilibrium of 100nM APC to sEPCR and to delipidated sEPCR preincubated or not with PCh or lysoPCh is shown. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. Dlip-sEPCR indicates delipidated sEPCR. (C) SPR analysis of the binding of APC to sEPCR reconstituted with lysoPCh. sEPCR was captured on a CM5 chip through RCR-2 mAb. Binding at equilibrium of 100nM APC to sEPCR preincubated or not with different species of lysoPCh is shown. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. (D) SPR analysis of the binding of APC to sEPCR reconstituted with PAF. APC-PPACK-b was captured onto an SA chip. Binding at equilibrium of 100nM sEPCR, delipidated sEPCR, PAF-relipidated sEPCR, and sEPCR preincubated with PAF are shown. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. Dlip-sEPCR indicates delipidated sEPCR.
Binding of lysoPCh to sEPCR. (A) Delipidated sEPCR was incubated with increasing amounts of 16:0, 18:0, and 18:1 lysoPCh, 760 Da MW PCh, and PAF, and the change in the sEPCR intrinsic fluorescence was registered. A representative experiment is shown for each lipid. The absolute changes in fluorescence on addition of LPC 16:0, LPC 18:0, LPC 18:1, PC, and PAF were 1.22, 1.05, 1.23, 0.31, and 0.4, respectively. (B) SPR analysis of the binding of APC to delipidated sEPCR reconstituted with PCh or lysoPCh. sEPCR or delipidated sEPCR was captured on a CM5 chip through RCR-2 mAb. Binding at equilibrium of 100nM APC to sEPCR and to delipidated sEPCR preincubated or not with PCh or lysoPCh is shown. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. Dlip-sEPCR indicates delipidated sEPCR. (C) SPR analysis of the binding of APC to sEPCR reconstituted with lysoPCh. sEPCR was captured on a CM5 chip through RCR-2 mAb. Binding at equilibrium of 100nM APC to sEPCR preincubated or not with different species of lysoPCh is shown. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. (D) SPR analysis of the binding of APC to sEPCR reconstituted with PAF. APC-PPACK-b was captured onto an SA chip. Binding at equilibrium of 100nM sEPCR, delipidated sEPCR, PAF-relipidated sEPCR, and sEPCR preincubated with PAF are shown. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. Dlip-sEPCR indicates delipidated sEPCR.
To gain further evidence regarding the interaction between sEPCR and lysoPCh or PAF, we incubated delipidated sEPCR with lysoPCh (a mixture of 16:0 and 18:0) or PAF, removed the excess of lipid by gel exclusion chromatography, and extracted the lipid fraction bound to the purified protein to subject it to mass spectrometry analysis. Peaks were obtained at both m/z 496 and 524 in the case of lysoPCh, which correspond to the MW of the lysoPCh molecules used in the experiment. Furthermore, the fragmentation spectrum was identical to that obtained with pure lysoPCh 16:0 or 18:0. These results confirm that lysoPCh does bind to EPCR (supplemental Figure 3). In the case of PAF, we detected a peak at m/z 524, which corresponds to the molecular weight of PAF, and the fragmentation spectrum was identical to that obtained with pure PAF (supplemental Figure 4).
lysoPCh and PAF displace PCh from the EPCR pocket and impair APC binding
The finding that these “one-armed” phospholipids bind to EPCR prompted us to determine whether the presence of this lipid could alter EPCR interaction with APC. We incubated delipidated sEPCR with lysoPCh and observed that, unlike PCh, lysoPCh did not restore the ability of delipidated sEPCR to bind to APC (Figure 3B). Indeed, the affinity for APC was notably worse in the case of lysoPCh-reconstituted sEPCR (KD = 268 ± 21.6nM; n = 3). Finding out whether lysoPCh was able to displace PCh from the EPCR hydrophobic groove thus became a particularly appealing aim. For this reason, we incubated sEPCR (note that in this case sEPCR still retains its natural phospholipid) with a 100-molar excess of lysoPCh 16:0, 18:0, or 18:1. Then we captured this sEPCR with RCR-2 mAb immobilized on an SPR sensor chip surface to check its ability to bind to APC. The binding was again notably reduced (Figure 3C). Furthermore, the impairment of affinity for APC varied in a lysoPCh dose-dependent manner (supplemental Figure 5A). These findings not only provide further evidence that lysoPCh decreases the EPCR ligand interaction properties but interestingly suggest that lysoPCh is able to actively displace the original lipid from the hydrophobic pocket.
We also examined the effect of PAF on the sEPCR ability to bind to APC. We demonstrated that the APC binding ability of delipidated sEPCR was not recovered on PAF loading (KD = 50.6 ± 55.8μM vs 707 ± 179nM for PAF-reconstituted sEPCR and nonmanipulated sEPCR, respectively; n = 3). The same result (ie, reduced binding) was observed when sEPCR was preincubated with 100-molar excess PAF and subsequently tested with immobilized APC (Figure 3D). Again, the decrease in affinity for APC varied in a PAF dose-dependent manner (supplemental Figure 5B).
The SPR and spectroscopy experiments were performed with yeast-expressed sEPCR. When sEPCR expressed in mammalian cells was used the results were similar (supplemental Figure 6).
The binding of APC to EPCR on endothelial cells is modulated by the action of sPLA2-V
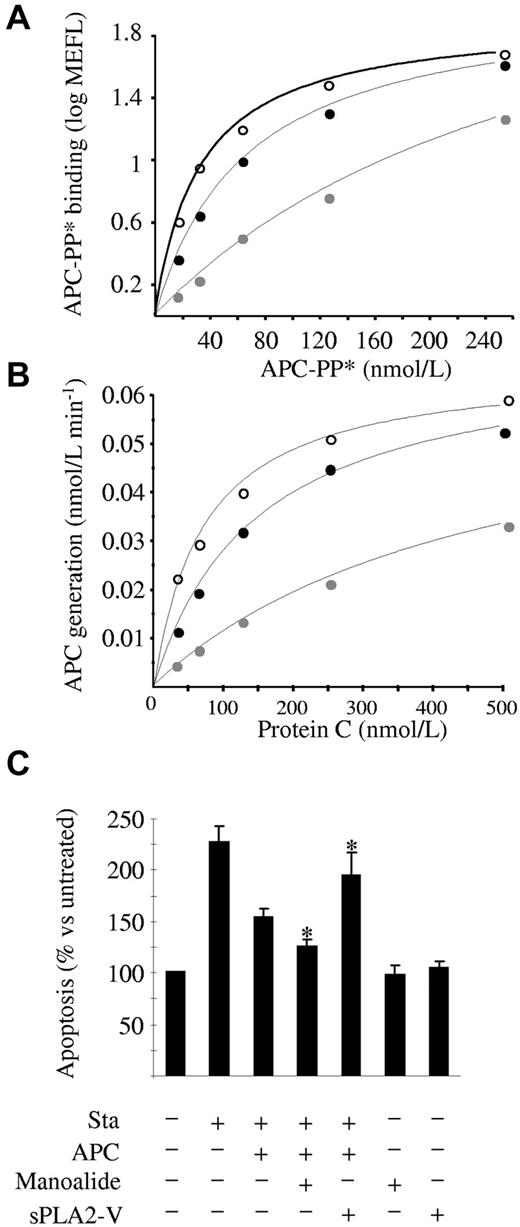
It was worthwhile at this point to examine whether lysoPCh and PAF were also able to bind to EPCR on the cell surface and thus decrease its APC binding efficiency. For this purpose, we incubated HAECs or EA.hy 926 cells with lysoPCh or PAF for 24 and 48 hours. Flow cytometric analyses showed no change in the APC binding capacity (data not shown), suggesting that EPCR on the endothelial surface cannot change its original lipid by exogenously supplied lysoPCh or PAF, in line with experiments performed with transfectants expressing wild-type CD1d molecules and that showed only a limited ability to present exogenously added LPC.17 However, whether in situ-produced lysoPCh and PAF were able to interact with cell surface EPCR still remained unanswered. lysoPCh and PAF are originated by the action of sPLA2-V, which is constitutively expressed by endothelial cells and increased by inflammatory stimuli, such as TNF-α, VEGF, and atherosclerosis.18-20 Remarkably, when sEPCR was incubated with sPLA2-V, sEPCR binding to APC significantly decreased (100% vs 45.2% ± 2.1%, P = .015). Therefore, once we had confirmed that sPLA2-V was expressed in HAECs and EA.hy926 (supplemental Figure 7), we focused on modulating its activity and checking APC binding. Interestingly, the blockade of sPLA2-V activity by its inhibitor manoalide increased APC binding to HAECs (KD = 71.6 ± 11.1 and 32.7 ± 1.9nM, P = .05, n = 3, in the absence or presence of manoalide, respectively). We used 0.5μM manoalide, which was the lowest concentration with the maximum effect (data not shown). Accordingly, the exogenous addition of sPLA2-V decreased APC binding (KD = 330.5 ± 112.6nM, n = 3, P = .05 vs control, and P = .027 vs manoalide group; Figure 4A). We incubated the cells with sPLA2-V for 2 hours because this was the time at which the maximum effect was observed (data not shown). Neither manoalide nor sPLA2-V modified the EPCR expression on the cell surface (supplemental Figure 8). We performed similar experiments using the EA.hy926 cell line and obtained identical results: manoalide significantly enhanced APC binding, whereas pretreatment with sPLA2-V significantly decreased it, an effect that was reversed by manoalide (supplemental Figures 9 and 10). Therefore, we suggest that lysoPCh and PAF generated on the cell surface can be accommodated within EPCR and that sPLA2-V is a biologic modulator of the EPCR function on the surface of the endothelial cells.
Effect of sPLA2-V on EPCR function on endothelial cells. (A) APC binding to HAECs. Cells were incubated with increasing amounts of APC-PP* after 48-hour pretreatment with 0.5μM manoalide (○), 2-hour pretreatment with 20 μg/mL sPLA2-V (gray circle), or no pretreatment (●). APC-PP* binding was assessed by flow cytometry. A representative experiment of 3 independent repeats is shown. MEFL indicates molecules of equivalent fluorescein. (B) Protein C activation on HAECs. Increasing amounts of protein C were incubated with thrombin for 30 minutes in the presence of HAECs, 48 hours pretreated with 0.5μM manoalide (○), 2 hours pretreated with 20 μg/mL sPLA2-V (gray circle), or nonpretreated (●). The amount of APC generated was measured with the chromogenic substrate S-2366. A representative experiment of 3 independent repeats is shown. (C) Inhibitory effect of APC on staurosporine-induced apoptosis in HAECs. Cells were pretreated with manoalide or sPLA2-V as in panels A and B, and then supplemented with 50nM APC for 4 hours after which apoptosis was induced with 10μM staurosporine for 60 minutes. Apoptosis was estimated by assessing the number of cells positive for annexin V–Alexa 647 binding by flow cytometry. A total of 6.6% ± 1.1% of the untreated cells were found to be apoptotic, and this percentage increased to 15.1% ± 1.0% after staurosporine incubation. For clarity purposes, results refer to the untreated cells, whose apoptotic rate was considered 100%. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. Sta indicates staurosporine. *P < .05 versus the staurosporine + APC group.
Effect of sPLA2-V on EPCR function on endothelial cells. (A) APC binding to HAECs. Cells were incubated with increasing amounts of APC-PP* after 48-hour pretreatment with 0.5μM manoalide (○), 2-hour pretreatment with 20 μg/mL sPLA2-V (gray circle), or no pretreatment (●). APC-PP* binding was assessed by flow cytometry. A representative experiment of 3 independent repeats is shown. MEFL indicates molecules of equivalent fluorescein. (B) Protein C activation on HAECs. Increasing amounts of protein C were incubated with thrombin for 30 minutes in the presence of HAECs, 48 hours pretreated with 0.5μM manoalide (○), 2 hours pretreated with 20 μg/mL sPLA2-V (gray circle), or nonpretreated (●). The amount of APC generated was measured with the chromogenic substrate S-2366. A representative experiment of 3 independent repeats is shown. (C) Inhibitory effect of APC on staurosporine-induced apoptosis in HAECs. Cells were pretreated with manoalide or sPLA2-V as in panels A and B, and then supplemented with 50nM APC for 4 hours after which apoptosis was induced with 10μM staurosporine for 60 minutes. Apoptosis was estimated by assessing the number of cells positive for annexin V–Alexa 647 binding by flow cytometry. A total of 6.6% ± 1.1% of the untreated cells were found to be apoptotic, and this percentage increased to 15.1% ± 1.0% after staurosporine incubation. For clarity purposes, results refer to the untreated cells, whose apoptotic rate was considered 100%. Data are mean ± SD of 3 independent experiments. Mann-Whitney U test was used for statistical comparisons. Sta indicates staurosporine. *P < .05 versus the staurosporine + APC group.
EPCR ability to increase APC generation on endothelial cells is modulated through lipid generation by sPLA2-V
Because sPLA2-V influences the binding of APC to EPCR on endothelial cells and protein C binds to EPCR similarly to APC (ie, through the Gla domain), we speculated that modulating sPLA2-V activity would influence the EPCR-dependent activation of protein C by thrombin on the endothelial surface. As expected, activation of protein C on HAECs was significantly increased in the presence of manoalide and significantly reduced on exogenous sPLA2-V supplementation (Figure 4B). Flow cytometric experiments showed that thrombomodulin expression on the surface of the cells was not influenced by modulation of sPLA2-V activity (supplemental Figure 8). The fact that variations in APC generation were the result of differences in the Km (control: 136.1 ± 24.5nM; manoalide: 99.6 ± 9.1nM, P = .05 vs control; sPLA2-V: 526.3 ± 173.7nM, P = .037 vs control) rather than in the Vmax (control: 0.070 ± 0.008nM min−1; manoalide: 0.078 ± 0.003nM min−1; sPLA2-V: 0.068 ± 0.007nM min−1) is consistent with an impairment of protein C binding to EPCR in the presence of sPLA2-V. We obtained similar results using EA.hy926 cells and were able to reverse the effect of sPLA2-V through manoalide supplementation (supplemental Figures 9 and 10). In addition, when we silenced the sPLA2-V expression in EA.hy926, the thrombin-dependent APC generation on the cell surface increased 2-fold, a result consistent with an improvement in the EPCR ligand ability and which therefore further substantiates the theory that sPLA2-V modulates EPCR function in cells (supplemental Figure 11). Thus, the anticoagulant activity of EPCR on endothelial cells could be modulated by sPLA2-V.
EPCR-dependent antiapoptotic effect of APC on endothelial cells is modulated through lipid generation by sPLA-2V
APC is known to exert numerous cellular effects through EPCR binding. Among them, the prevention of the staurosporine-induced apoptosis of endothelial cells has been well characterized.21 We induced apoptosis in HAECs with staurosporine and observed that the antiapoptotic effect of APC was significantly increased up to 125% ± 15% (P = .037) on preincubation with manoalide (Figure 4C). Accordingly, supplementation with exogenous sPLA2-V significantly decreased the antiapoptotic effect of APC down to 46.6% ± 20.7% (P = .037; Figure 4C). Under the conditions used in the experiments, sPLA2-V neither increased the apoptosis of the cells nor decreased PAR-1 expression (supplemental Figure 12). Similar results were also obtainedwithEA.hy926 cells (supplemental Figure 9). These results suggest that the inhibitory effect of sPLA2-V goes beyond the antithrombotic nature of the protein C/APC-EPCR interaction, as it also reduces its cytoprotective properties on endothelial cells.
sPLA2-V is present in thrombi formed in vivo
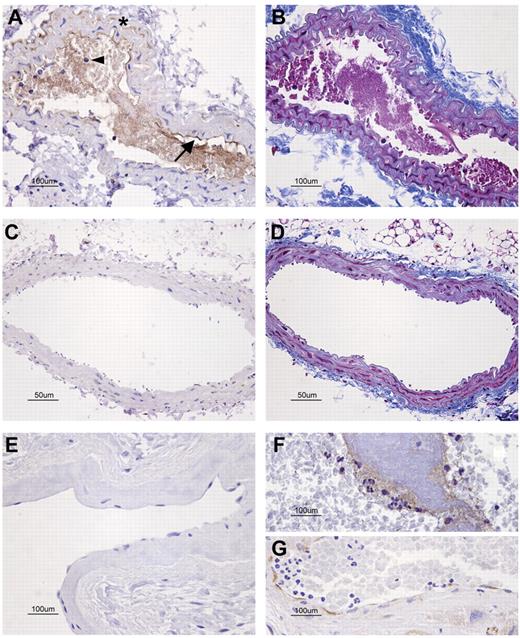
Keeping in mind the notable effect of sPLA2-V on EPCR function, we speculated that its modulation could play a role in thrombosis. For this reason, we studied the expression of sPLA2-V in the carotid arteries of mice within the area close to the thrombus induced by laser injury. Although we detected neither sPLA2-V nor fibrin in the noninjured carotid arteries (Figure 5C-D), sPLA2-V expression was abundant in the laser-injured ones: within the thrombi, in the neutrophils trapped, and on the endothelial surfaces where thrombi were attached (Figure 5A-B). Interestingly, we also detected positive sPLA2-V staining in a human thrombus formed in the left atrium of a patient with atrial fibrillation as well as on the endocardial surface nearby (Figure 5F-G), whereas we detected no staining in the left atrial endocardium of a donor free of cardiac abnormalities (Figure 5E).
Immunohistochemical detection of sPLA2-V in sections of injured vessels with an attached thrombus. (A-D) Carotid artery sections of a mouse subjected to left carotid artery laser injury model. Rabbit anti–mouse sPLA2-V Ab (A,C) or Masson trichrome for fibrin detection (B,D) was used. (A) sPLA2-V was present in the injured carotid artery: in endothelial cells (arrow), neutrophils (arrowhead), vascular smooth muscle cells (*), and around fibrin in the lumen. (B) The soft purple staining in the lumen of the injured carotid artery revealed the presence of extensive fibrin deposits. (C) There was no sPLA2-V in the noninjured carotid artery. (D) Fibrin was not detected in the noninjured artery. (E-G) The presence of sPLA2-V was also studied in human left atrial tissue sections. (E) sPLA2-V was absent in the atrial sample of a patient in sinus rhythm. (F-G) sPLA2-V was detected around the fibrin thrombus and along the endothelial lining in the atrial section corresponding to a patient in atrial fibrillation. The microscope was a Nikon Eclipe 80i (Nikon Instruments Inc) equipped with a DXM1200F digital camera (Nikon). Panels A, B, and E through G were captured with a 40×/0.75 NA objective (Plan Fluor; Nikon). Panels C and D were captured with a 20×/0.50 NA objective (Plan Fluor; Nikon). All images were acquired using Nikon ACT-1 Version 2.63 software.
Immunohistochemical detection of sPLA2-V in sections of injured vessels with an attached thrombus. (A-D) Carotid artery sections of a mouse subjected to left carotid artery laser injury model. Rabbit anti–mouse sPLA2-V Ab (A,C) or Masson trichrome for fibrin detection (B,D) was used. (A) sPLA2-V was present in the injured carotid artery: in endothelial cells (arrow), neutrophils (arrowhead), vascular smooth muscle cells (*), and around fibrin in the lumen. (B) The soft purple staining in the lumen of the injured carotid artery revealed the presence of extensive fibrin deposits. (C) There was no sPLA2-V in the noninjured carotid artery. (D) Fibrin was not detected in the noninjured artery. (E-G) The presence of sPLA2-V was also studied in human left atrial tissue sections. (E) sPLA2-V was absent in the atrial sample of a patient in sinus rhythm. (F-G) sPLA2-V was detected around the fibrin thrombus and along the endothelial lining in the atrial section corresponding to a patient in atrial fibrillation. The microscope was a Nikon Eclipe 80i (Nikon Instruments Inc) equipped with a DXM1200F digital camera (Nikon). Panels A, B, and E through G were captured with a 40×/0.75 NA objective (Plan Fluor; Nikon). Panels C and D were captured with a 20×/0.50 NA objective (Plan Fluor; Nikon). All images were acquired using Nikon ACT-1 Version 2.63 software.
Discussion
EPCR improves the efficiency of protein C activation and is involved in cell signaling mechanisms resulting in antiapoptotic, anti-inflammatory, and endothelial barrier protective effects.4,9 When the crystal structure of EPCR was solved, a phospholipid inside its hydrophobic groove was found. However, the nature of this phospholipid remained to be elucidated.11 We provide enough evidence to claim that the major lipid located within the hydrophobic pocket of EPCR is PCh. It is interesting to note that, in line with previous studies,11 its removal caused a remarkable decrease in APC affinity, which was restored on PCh supplementation. A similar finding was obtained with FVIIa, thus confirming the important role played by the phospholipid in ligand binding.3 Our results are consistent with those obtained with the highly homologous CD1d, which is also loaded with PCh in its hydrophobic groove. Furthermore, a recent in silico simulation suggested a relationship between the phospholipid binding to EPCR and the size of the surface of interaction with protein C, which would be reduced (ie, ligand binding would be worsened) in the absence of the phospholipid.22
Lipid exchange occurs in CD1d and was anticipated for EPCR in the in silico study.22 Among bioactive lipids, lysoPCh exhibits a high structural homology with PCh because it is the product of an enzymatic process that removes one of the fatty acids from the latter. Furthermore, lysoPCh binds to CD1d.17,23 When incubated with delipidated EPCR, lysoPCh dose-dependently modified its intrinsic fluorescence. Moreover, the mass spectrometry pattern of the lipid fraction extracted from the reconstituted EPCR fully corresponded to lysoPCh. Thus, EPCR is able to accommodate lysoPCh within its hydrophobic groove. Finally, the binding, according to the KD, was stronger than that observed with PCh and, it should be noted, remarkably impaired the affinity of EPCR for APC and FVIIa (supplemental Discussion). These findings fully conform to the in silico simulation.22 PAF is a bioactive lipid structurally similar to lysoPCh, also generated by endothelial cells, and was able to displace PCh and bind to EPCR as well.
sPLA2-V is the main source of lysoPCh in the endothelium. sPLA2-V is secreted by the endothelial cells and generates lysoPCh by releasing a fatty acid from PCh.24 A subsequent modification can convert lysoPCh into PAF. Interestingly, we observed that sPLA2-V was able to induce a reduction in the ligand binding ability of sEPCR, thus anticipating that the enzyme could be involved in the modulation of EPCR function. To approach this topic, we moved to the cellular context and studied what happened with EPCR when the sPLA2-V activity was modulated. Remarkably, an increase in the EPCR-dependent binding of APC was observed when the PLA2 inhibitor manoalide had been previously incubated with endothelial cells. This effect had to be the result of sPLA2-V inhibition: although manoalide also inhibits sPLA2-IIa and X, the former is not present in resting endothelial cells18-20 and the latter is not present either, as we showed (supplemental Figure 7). In addition, when sPLA2-V expression was specifically inhibited by shRNA, the EPCR activity also increased. Accordingly, APC binding was notably reduced when endothelial cells were supplemented with sPLA2-V. Thus, SPR and cellular experiments suggest that sPLA2-V modulates the EPCR function. The fact that direct addition to cells of lysoPCh and PAF was unable to alter the EPCR ligand binding ability is intriguing. It could be that direct supplementation with lipids induced micelle formation, thus precluding any lipid exchange. It could also be that intracellular trafficking of the receptor would be needed for direct lipid exchange, in agreement with a similar mechanism that has recently been demonstrated for the highly homologous CD1d.25 Collectively, these findings underline the relevant role that sPLA2-V may play as a modulator of EPCR function in vivo.
As anticipated, the changes in the functionality of EPCR through sPLA2-V had relevant biologic consequences: the activation of protein C by thrombin and the antiapoptotic effect of APC were impaired as the sPLA2-V activity was increased and vice versa. Thus, a new role for sPLA2-V as a down-regulator of EPCR function can be proposed, which would be especially important in scenarios of inflammation, in which its endothelial expression is enhanced. Because APC is one of the cornerstones of the cross-talk between inflammation and coagulation, it is interesting that we detected sPLA2-V in the endothelium in situations of injury leading to thrombosis (ie, murine carotid artery), whereas it was not detected in noninjured vessels. Finally, the presence of sPLA2-V in the left atrial wall, with an attached thrombus, of a patient with atrial fibrillation, suggests that our observations can be applied to humans.
Our findings can be clinically relevant. Overexpression of sPLA2-V would not only reduce the amount of APC generated but would also impair the APC-dependent cell signaling through the EPCR-PAR-1 axis, thus reducing the APC-associated benefit in a variety of settings as endothelial barrier protection, apoptosis reduction, metastasis prevention, and others.
In conclusion, we have demonstrated that the major lipid bound to the hydrophobic groove of EPCR is PCh. PCh can be replaced by sPLA2-V–produced lysoPCh or PAF. As a consequence, the affinity of EPCR for protein C/APC and factor VII/VIIa decreases, which leads to a reduction in the endothelial capacity to generate APC and in the APC-evoked cell protective mechanisms. We suggest that a new prothrombotic/proinflammatory role can be attributed to sPLA2-V, which emerges as a promising therapeutic target. Its modulation may help to take greater advantage of the anticoagulant and cytoprotective actions of the APC-EPCR axis.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Eva Molina and Maider Esparza for their excellent technical assistance, Gregorio Rábago for tissue samples, and Fernando Corrales and Carmen Miqueo from the Proteomics facility of Centre for Applied Medical Research, a member of ProteoRed-ISCIII, for their assistance in performing mass spectrometry experiments.
This work was supported by the Unión Temporal de Empresas project Centre for Applied Medical Research, Instituto de Salud Carlos III (PI08/1349, PI10/01432 and Red Temática de Investigación RECAVA RD06/0014/0008), and Health Department, Gobierno de Navarra (15/09). J.L.-S. was supported by a fellowship from the Education Department, Gobierno de Navarra. J.C. was supported by a grant from the Sociedad Española de Trombosis y Hemostasia.
Authorship
Contribution: J.L.-S., C.P., R.M., and J.H. designed research and wrote the manuscript; J.L.-S., C.P., M.A., J.C., I.T., and S.E.V. performed research; C.T.E. contributed vital new reagents; and J.L.-S., C.P., C.T.E., R.M., I.T., and J.H. analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.L.-S. is Department of Biochemistry and Molecular Biology, University of Chicago, Chicago, IL.
Correspondence: José Hermida, Laboratory of Thrombosis and Haemostasis, Division of Cardiovascular Sciences, Centre for Applied Medical Research, University of Navarra.Avenida Pío XII 55, 31008 Pamplona, Spain; e-mail: jhermida@unav.es.
References
Author notes
J.L.-S. and C.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal