In its relatively short history, novel insights on the endothelial protein C receptor (EPCR) have provided time-after-time fertile grounds for research on the interactions between coagulation factors and the vasculature. Yet again EPCR provides an opportunity to explore new frontiers, as López-Sagaseta and colleagues in this issue of Blood discover a novel lipid-based regulatory mechanism for EPCR that suggests its involvement in vascular disease may be more intricate than previously appreciated.1
EPCR was identified as an endothelial receptor for protein C, capable of enhancing the activation of protein C by the thrombin-thrombomodulin complex.2 Subsequently, direct cytoprotective effects of activated protein C (APC) on cells were found to require EPCR and a homologous EPCR-dependent cellular pathway for factor VII(a) has been suggested.3 These functions in physiologically and therapeutically relevant pathways, such as the cytoprotective protein C pathway and a novel factor VII(a) cellular pathway, earned EPCR its reputation as a cytoprotective receptor. Now López-Sagaseta and colleagues provide yet another dimension to EPCR as they describe a mechanism by which an endothelial-derived phospholipase can modify or “encrypt” EPCR to lose its ability to bind APC, thereby rendering EPCR unable to mediate APC's direct cytoprotective activities on cells.1 These findings conceptualize a novel phenomenon of “cellular APC resistance” similar to the well-known “anticoagulant APC resistance” associated with an increased risk for venous thrombosis.3,4 Although more work needs to be done to establish (patho)physiologic relevance of “cellular APC resistance,” López-Sagaseta et al provide intriguing hints as to its potential involvement in cardiovascular disease.
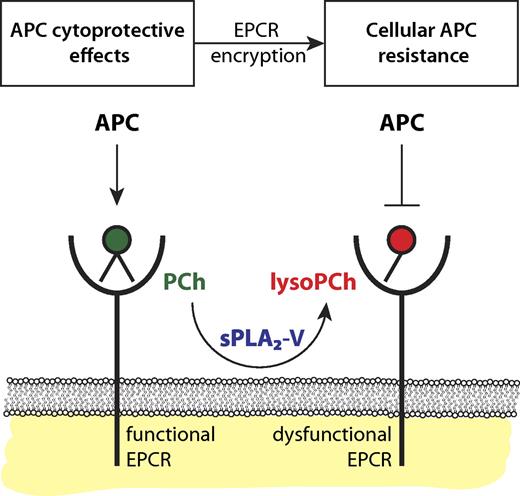
Encryption of EPCR by sPLA2-V induces cellular APC resistance. Functional EPCR loaded with a phosphatidylcholine (PCh) in its hydrophobic groove (green) promotes the generation of APC by the thrombin-thrombomodulin complex and supports APC's direct cytoprotective effects on cells. Secreted phospholipase A2 group V (sPLA2-V) changes the PCh in EPCR for a lysoPCh. This lysoPCh-loaded EPCR (red), or encrypted EPCR, is dysfunctional with regard to protein C and APC binding. Thus, sPLA2-V modified EPCR fails to promote APC generation by the thrombin-thrombomodulin complex and abrogates APC's direct cytoprotective effects on cells thereby inducing cellular APC resistance with potential implications for the susceptibility to thrombosis and inflammation.
Encryption of EPCR by sPLA2-V induces cellular APC resistance. Functional EPCR loaded with a phosphatidylcholine (PCh) in its hydrophobic groove (green) promotes the generation of APC by the thrombin-thrombomodulin complex and supports APC's direct cytoprotective effects on cells. Secreted phospholipase A2 group V (sPLA2-V) changes the PCh in EPCR for a lysoPCh. This lysoPCh-loaded EPCR (red), or encrypted EPCR, is dysfunctional with regard to protein C and APC binding. Thus, sPLA2-V modified EPCR fails to promote APC generation by the thrombin-thrombomodulin complex and abrogates APC's direct cytoprotective effects on cells thereby inducing cellular APC resistance with potential implications for the susceptibility to thrombosis and inflammation.
The EPCR crystal structure revealed the presence of a phospholipid buried in its hydrophobic groove that helps stabilize the interactive surface of EPCR for interactions with the GLA-domain of APC.5 The current paper demonstrates that not only phosphatidylcholine (PCh) but also PCh metabolites, such as lysoPCh and platelet-activating factor (PAF), can occupy EPCR's hydrophobic groove, although resulting in an EPCR with reduced affinity for APC.1 This raises the immediate question of whether other lipids can incorporate in EPCR as well. Insights garnered from EPCR's homology to CD1 lipid antigen-presenting molecules, especially CD1d, support the idea that they might. Various phospholipids and sphingolipids were found to occupy the hydrophobic groove of CD1d.6 Not only can CD1d incorporate different lipids, the type of lipid has been implicated to contribute to a biased cytokine response of natural killer T cells, as the lipid head group protruding from the hydrophobic binding groove participates in the CD1d interactive surface with the T cell receptor.6,7 Future studies will have to determine whether these observations for CD1d will also be true for EPCR.
As a branch of the large phospholipase family, phospholipases A2 (PLA2s) hydrolyze phospholipids at the sn-2 position producing free fatty acids and lysophospholipids. Most PLA2s are expressed intracellulary but some secreted PLA2s (sPLA2s) circulate in plasma and are best known for their proatherogenic properties and participation in inflammation via the generation of bioactive lipid mediators.8 Extending their observation that lysoPCh and PAF-loaded EPCR resulted in diminished APC binding, López-Sagaseta et al found that sPLA2 group V (sPLA2-V) can modify the EPCR lipid, as incubation of endothelial cells or purified EPCR with sPLA2-V resulted in diminished APC binding and inhibition of EPCR-dependent antiapoptotic effects of APC on cells.1 This suggests a model in which EPCR encryption by lipid-editing enzymes results in cellular APC resistance (see figure). This model may have important implications for thrombotic and inflammatory vascular disease because EPCR inactivation in vivo, either genetically or induced by blocking antibodies, aggravates and increases susceptibility to thrombotic and inflammatory disease.9
What counter measures can cells employ to restore EPCR's cytoprotective functions when inflammatory mediators up-regulate sPLA2s to induce cellular APC resistance? Perhaps EPCR shedding induced by inflammatory mediators should be viewed as a protective measure to rid cells of encrypted EPCR. Answers to this and many other questions remain unclear at present. What is clear is that the intriguing observations by López-Sagaseta and colleagues will stimulate innovative thinking on the regulation of EPCR function in vascular disease.
Obviously one should also be cautious in the interpretation of these results. Purified systems and in vitro cell culture do not represent a physiologic environment. Plasma contains a broad spectrum of lipid-rich lipoprotein particles, abundant lipid carrier proteins, and a host of other factors that all could potentially negate the reported effects on EPCR. Thus, the implications of EPCR lipid editing, EPCR encryption, and cellular APC resistance for vascular disease remain to be determined. Notwithstanding, sPLA2s are expressed abundantly in atherosclerotic lesions and a variety of inflammatory conditions. In addition, the link between plasma levels of sPLA2 and cardiovascular disease seems consistent with a potential role of sPLA2s in inducing EPCR lipid modifications and possible contributions thereof to cardiovascular disease.8,10 Should such links become more tangible then evaluation of sPLA2 inhibitors as a therapeutic strategy to boost the endogenous protein C anticoagulant and cytoprotective pathways might become worthwhile.10
As is often the case with new discoveries that push the frontiers of our knowledge, they raise more questions than they answer. Certainly, the tantalizing observations by López-Sagaseta and colleagues raise many new questions, but most importantly, they stimulate the conceptualization of new basic research with the potential of translation into novel therapeutic approaches to combat thrombosis and vascular diseases.
Conflict-of-interest disclosure. The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal