Abstract
In recent years, advances in the humanized mouse system have led to significantly increased levels of human hematopoietic stem cell (HSC) engraftment. The remaining limitations in human HSC engraftment and function include lymphoid-skewed differentiation and inefficient myeloid development in the recipients. Limited human HSC function may partially be attributed to the inability of the host mouse microenvironment to provide sufficient support to human hematopoiesis. To address this problem, we created membrane-bound human stem cell factor (SCF)/KIT ligand (KL)–expressing NOD/SCID/IL2rgKO (hSCF Tg NSG) mice. hSCF Tg NSG recipients of human HSCs showed higher levels of both human CD45+ cell engraftment and human CD45+CD33+ myeloid development compared with NSG recipients. Expression of hSCF/hKL accelerated the differentiation of the human granulocyte lineage cells in the recipient bone marrow. Human mast cells were identified in bone marrow, spleen, and gastrointestinal tissues of the hSCF Tg NSG recipients. This novel in vivo humanized mouse model demonstrates the essential role of membrane-bound hSCF in human myeloid development. Moreover, the hSCF Tg NSG humanized recipients may facilitate investigation of in vivo differentiation, migration, function, and pathology of human mast cells.
Introduction
The humanized mouse system, a xenogeneic transplantation and engraftment model for human hematopoietic stem cells (HSCs) and peripheral blood (PB) mononuclear cells (MNCs), facilitates the investigation of human hematopoietic and immune systems in vivo.1,2 Since the pioneering work using SCID-hu3 and Hu-PBL-SCID models,4 investigators have attempted to better recapitulate human biology in mice across xenogeneic immunologic barriers. Recently, the introduction of targeted null mutations of immune-related genes, such as Rag1, Rag2, Il2rg, or Prf1 in recipient mice, has improved engraftment levels of human CD45+ leukocytes.2,5-9 However, limitations remain in the ability of the host mouse hematopoietic microenvironment to support human hematopoiesis. The impaired development of human T-lymphoid and myeloid lineage cells compared with human B-lymphoid lineage cells in NOD/SCID and other immune-compromised mice may be the result of the lack of appropriate microenvironmental support. The recently created human leukocyte antigen (HLA) class I expressing immune-compromised NOD/SCID/IL2r γ null (NSG) mice partially addresses this issue for human T-cell development. Human CD8+ T cells developing within these recipients of transplanted human HSCs exhibited cytokine production and cytotoxicity in an HLA-restricted manner.10-12
To create a hematopoietic microenvironment more suitable for human myeloid development, we developed a new immune-compromised mouse strain that expresses human membrane bound stem cell factor (SCF) under the control of the phosphoglycerate kinase (PGK) promoter (hSCF Tg NSG). Using hSCF Tg NSG mice as recipients of human HSCs, we aimed to clarify the role of membrane-bound form of SCF in supporting the engraftment of human hematopoietic cells and influencing the differentiation of the human myeloid lineage in the recipient mouse BM, spleen, and other organs. Here we show nearly complete human hematopoietic chimerism in the BM of hSCF Tg NSG recipients. In the BM of these recipients, human granulocytes accounted for the majority of engrafted human cells reflecting the physiologic human BM status. In addition to the development of immature and mature granulocytes, c-Kit+ human mast cells differentiated efficiently in BM, spleen, and mucosal tissues. The hSCF Tg NSG mice, by supporting efficient human myeloid development including mast cells, may serve as a novel platform for in vivo investigation of human mast cell development and allergic responses.
Methods
Mice
NOD.Cg-Prkdcscid IL2rgtm1Wjl (NSG) mice and NOD.Cg-Prkdcscid IL2rgtm1Wjl Tg(PGK1-KITLG*220)441Daw/J, abbreviated as hSCF Tg NSG mice, were generated at The Jackson Laboratory. The human membrane-bound SCF transgene driven by the human PGK promoter was backcrossed more than 10 generations from the original C3H/HeJ strain background13 onto the NSG strain. All the mice were bred and maintained at The Jackson Laboratory and animal facility at RIKEN RCAI under defined flora according to guidelines established and approved by the Institutional Animal Committees at each respective institution.
Purification and transplantation of human HSCs
All experiments were performed with authorization from the Institutional Review Board for Human Research at RIKEN RCAI. Cord blood (CB) samples were first processed for isolation of MNCs using LSM lymphocyte separation medium (MP Biomedicals). CB MNCs were then enriched for human CD34+ cells using anti–human CD34 microbeads (Miltenyi Biotec) and sorted for 7-AAD− lineage (hCD3/hCD4/hCD8/hCD19/hCD56)−CD34+CD38− HSCs using FACSAria (BD Biosciences). To achieve high purity of donor HSCs, doublets were excluded by analysis of forward scatter (FSC)–height/FSC-width and side scatter (SSC)-height/SSC-width. Purity of each sorted sample was higher than 95%. Newborn (within 2 days of birth) hSCF Tg and non-Tg NSG recipients received 150 cGy total body irradiation using a 137Cs-source irradiator, followed by intravenous injection of 5 × 102 to 5.3 × 104 sorted HSCs via the facial vein.
Analysis of human cell engraftment by flow cytometry
The recipient PB harvested from the retro-orbital plexus was evaluated for human hematopoietic engraftment every 3 to 4 weeks starting at 4 to 6 weeks after transplantation. After lysis of erythrocytes, cells were stained with anti-hCD45, anti-msCD45, anti-hCD3, anti-hCD19, anti-hCD33, and anti-hCD56 to determine human hematopoietic chimerism and to analyze cell lineages engrafted in the recipients. At 8 to 35 weeks after transplantation, the recipients were killed and single-cell suspensions of BM and spleen were analyzed using flow cytometry. Antibodies used for flow cytometry are specified in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The labeled cells were analyzed using FACSCantoII or FACSAria (BD Biosciences).
Morphologic analysis of cytospin specimens
Cytospin specimens of FACS-purified human myeloid cells were prepared with a Shandon Cytospin 4 cytocentrifuge (Thermo Electric) using standard procedures. To identify nuclear and cytoplasmic characteristics of each myeloid cell, cytospin specimens were stained with 100% May-Grünwald solution (Merck) for 3 minutes, followed by 50% May-Grünwald solution in phosphate buffer (Merck) for additional 5 minutes, and then with 5% Giemsa solution (Merck) in phosphate buffer for 15 minutes. All staining procedures were performed at room temperature. Light microscopy was performed with Zeiss Axiovert 200 (Carl Zeiss).
Microarray analysis
Purified hCD45+CD33+c-Kit−CD203c−HLA-DR− granulocytes and hCD45+CD33+ c-Kit−CD203c−HLA-DR+CD14+ monocytes from BM of 4 hSCF Tg NSG recipients and 3 non-Tg NSG recipients as well as neutrophils and monocytes from 2 healthy persons were evaluated using Human Genome U133 plus Version 2.0 GeneChips (Affymetrix). Total RNA was extracted with TRIzol (Invitrogen) from more than 104 sorted cells and amplified to cDNA using the Ovation Pico WTA System (Nugen). Biotinylated cDNA was synthesized with Two-Cycle Target Labeling Kit (Affymetrix). Microarray data were analyzed using the Bioconductor package (Bioconductor; http://www.bioconductor.org). The signal intensities of the probe sets were normalized using the GC-RMA program (Bioconductor). The RankProd program was used to select differentially expressed genes with a cutoff P value of less than .01 and an estimated false-positive rate of less than 0.05.14 Gene annotation was obtained from Ingenuity Pathway Analysis and Gene Ontology Annotation databases (Ingenuity systems, http://www.ingenuity.com; Gene Ontology Annotation, http://www.ebi.ac.up/GOA). For differentially transcribed genes, GO term enrichment analysis was performed according to a method described by Draghici et al15 with a correction of multiple testing using false discovery rate.16 Eventually, GO terms with the false discovery rate-corrected P value < .05 were selected as functionally enriched terms. Raw data for microarray data are accessible at the RefDIC database (http://refdic.rcai.riken.jp) under the following accession numbers: RSM06616, RSM06617, RSM06618, RSM06620, RSM06621, RSM06622, RSM06623, RSM06633, RSM06642, RSM06648, RSM06665, RSM06667, RSM06668, RSM06669, RSM06670, RSM08241, and RSM08243. Differences in expression levels were considered significant if P is < .05 using Kruskal-Wallis, Wilcoxon-Mann-Whitney, or Student t test in KaleidaGraph (Synergy Software).
IHC and immunofluorescence imaging
Thin (∼ 5-μm) sections prepared from paraformaldehyde-fixed paraffin-embedded tissues were stained with H&E using standard procedures. Immunohistochemistry (IHC) and immunofluorescence labeling were performed using standard procedures. Antibodies used for IHC and immunofluorescence labeling were mouse anti–human mast cell tryptase monocolonal antibody (Dako North America, clone AA1), mouse anti–human CD45 monoclonal antibody (Dako North America, clone 2B11+PD7/26), rabbit anti–human CD117 monoclonal antibody (Epitomics, clone YR145), and rabbit anti–human CD14 polyclonal antibody (Atlas Antibodies). Light microscopy was performed using an Axiovert 200 (Carl Zeiss). For quantification of tryptase+ cell frequency, 3 high-power fields from 3 different recipients were examined using AutoMeasure module of AxioVision software (Release 4, Carl Zeiss). Confocal microscopy was performed using a LSM710 equipped with C-APOCHROMAT 40×/1.2 (Carl Zeiss).
Results
Human hematopoietic repopulation is enhanced in hSCF Tg NSG recipients
The humanized mouse model system has served as a tool to investigate human hematopoiesis, immunity, and diseases in vivo. However, one of the major limitations in the system is that the microenvironment supporting human hematopoiesis and immunity is primarily of mouse origin. In the present study, we created a strain of NSG mice expressing membrane-bound human SCF to analyze the role of the BM microenvironment in human hematopoietic lineage determination and development.
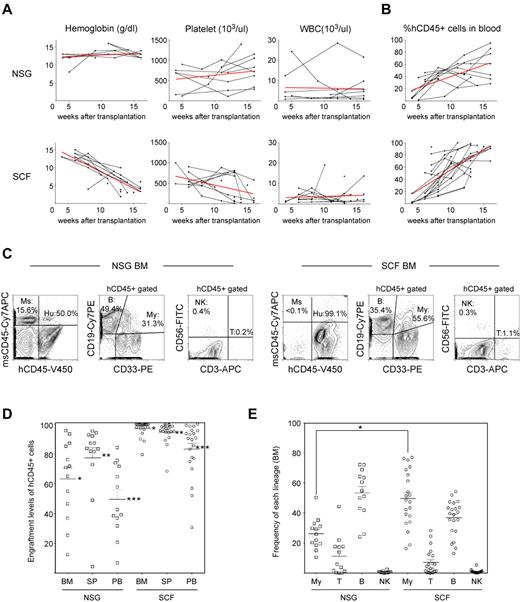
c-Kit, the receptor for SCF, is expressed at lower levels in human CB Lin−CD34+CD38− early HSCs and at high levels in mast cells.17-19 For reconstitution of human myeloid and lymphoid cells, 5 × 102 to 5.3 × 104 FACS-purified CB Lin−CD34+CD38− HSCs were transplanted into newborn sublethally irradiated (1.5 Gy) hSCF Tg NSG mice and into non-Tg NSG controls (Table 1). To determine the kinetics of human hematopoietic chimerism in the recipient circulation, we performed flow cytometric analysis of PB every 3 to 4 weeks starting at 4 to 6 weeks after transplantation. During long-term observation, all the 21 hSCF Tg NSG recipient mice became moribund at 8 to 35 weeks after transplantation. Complete blood count analysis demonstrated reduced erythrocyte hemoglobin concentration in the PB of hSCF Tg NSG recipients compared with non-Tg NSG recipients (Figure 1A). Anemia in hSCF Tg NSG recipients was not associated with abnormalities in mean corpuscular volume, mean corpuscular hemoglobin, or mean corpuscular hemoglobin concentration (supplemental Figure 1). The suppression of host erythropoiesis in the hSCF Tg NSG recipients was related to the irradiation and engraftment of the human HSCs because unmanipulated nontransplanted hSCF Tg NSG mice did not develop anemia (supplemental Figure 1). Impaired mouse erythropoiesis in these engrafted hSCF Tg NSG mice was associated with rapid expansion of hCD45+ hematopoietic cells compared with non-Tg NSG recipients (Figure 1B).
Summary of hSCF Tg NSG and non-Tg NSG recipients analyzed
| Recipient ID . | CB ID . | Graft dose . | Survival, wks . | CBC at time of death . | % chimerism at time of death . | % of CD45+ in BM . | % of CD33+ in BM . | % of CD45+ in BM . | % chimerism of erythroid cells in BM . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC, × 103/L . | RBC, × 104/μL . | Hemoglobin, g/dL . | Hematocrit, % . | Platelets, ×103/μL . | PB . | BM . | Spleen . | CD33+ . | CD3+ . | CD19+ . | CD3− CD56+ . | CD117+ CD203c+ . | HLA DR− . | HLA DR+ . | CD117+ CD203c+ . | HLA DR− . | HLA DR+ . | |||||
| N1-1 | 1 | 5000 | 21 | 1.1 | 500 | 10.0 | 35.0 | 550 | 75.5 | 93.3 | 95.3 | 31.1 | 1.2 | 50.2 | 0.3 | 0.9 | 51.5 | 47.6 | 0.3 | 16.0 | 14.8 | NA |
| N1-2 | 1 | 5000 | 16 | 1.4 | 730 | 13.0 | 44.0 | 1100 | 42.6 | 76.1 | 72.9 | 35.2 | 0.1 | 54.8 | 0.4 | 0.7 | 39.6 | 59.7 | 0.2 | 13.9 | 21.0 | NA |
| N1-3 | 1 | 5000 | 24 | NA | NA | NA | NA | NA | 20.7 | 12.6 | 4.4 | 32.9 | 3.8 | 60.7 | 0.0 | 4.4 | 37.9 | 57.7 | 1.0 | 12.5 | 19.0 | 3.1 |
| S1-1 | 1 | 5000 | 23 | 4.1 | 220 | 6.7 | 15.6 | 150 | 99.1 | 99.7 | 94.4 | 77.2 | 24.5 | 19.2 | 0.5 | 3.9 | 80.6 | 15.5 | 3.0 | 62.2 | 11.9 | NA |
| S1-2 | 1 | 5000 | 20 | 6.8 | 220 | 5.0 | 18.0 | 30 | 83.2 | 99.4 | 97.7 | 75.5 | 2.6 | 28.4 | 0.3 | 6.7 | 68.7 | 24.6 | 5.1 | 51.8 | 18.6 | 36.3 |
| S1-3 | 1 | 5000 | 21 | 0.7 | 320 | 7.0 | 25.0 | 330 | 76.9 | 99.7 | 95.8 | 70.9 | 1.5 | 16.6 | 0.2 | 14.6 | 50.0 | 35.4 | 10.4 | 35.5 | 25.1 | 0.0 |
| S1-4 | 1 | 500 | 26 | 0.5 | 150 | 3.0 | 11.0 | 250 | 30.7 | 97.0 | 86.3 | 69.0 | 2.0 | 47.5 | 0.1 | 6.9 | 68.6 | 24.5 | 4.8 | 47.3 | 16.9 | NA |
| N2-1 | 2 | 10 000 | 35 | 0.8 | 390 | 8.0 | 30.0 | 110 | 31.7 | 54.9 | 87.1 | 50.3 | 21.8 | 25.8 | 2.4 | 19.1 | 7.4 | 73.5 | 9.6 | 3.7 | 37.0 | 11.5 |
| S2-1 | 2 | 10 000 | 16 | 1.3 | 186 | 4.1 | 13.2 | 583 | 90.7 | 96.6 | 99.3 | 18.7 | 3.2 | 40.6 | 1.2 | 34.4 | 18.0 | 47.6 | 6.4 | 3.4 | 8.9 | NA |
| S3-1 | 3 | 10 000 | 13 | 0.2 | 215 | 3.9 | 12.6 | 793 | 52.0 | 97.8 | 90.9 | 54.2 | 0.0 | 42.8 | 0.7 | 19.0 | 44.6 | 36.4 | 10.3 | 24.2 | 19.7 | NA |
| S3-2 | 3 | 10 000 | 15 | 2.2 | 161 | 3.1 | 9.8 | 458 | 93.6 | 99.6 | 98.2 | 61.5 | 4.4 | 20.4 | 1.4 | 7.3 | 60.5 | 32.2 | 4.5 | 37.2 | 19.8 | NA |
| S4-1 | 4 | 10 000 | 13 | 0.5 | 97 | 1.9 | 5.1 | 6 | 98.6 | 100.0 | 98.2 | 52.0 | 1.2 | 39.8 | 0.3 | 2.7 | 81.9 | 15.4 | 1.4 | 42.6 | 8.0 | 27.2 |
| N5-1 | 5 | 12 000 | 35 | 3.1 | 310 | 7.0 | 25.0 | 550 | 6.8 | 36.8 | 48.9 | 29.9 | 17.5 | 41.9 | NA | 22.6 | 7.0 | 70.4 | 6.8 | 2.1 | 21.1 | 5.8 |
| N6-1 | 6 | 14 000 | 23 | 2.1 | 520 | 10.0 | 34.0 | 620 | 57.6 | 65.0 | 83.6 | 29.3 | 17.6 | 43.1 | 1.6 | 3.3 | 45.1 | 51.6 | 1.0 | 13.2 | 15.1 | 7.4 |
| N6-2 | 6 | 14 000 | 21 | 1.6 | 680 | 12.0 | 40.0 | 660 | 64.6 | 25.3 | 81.5 | 10.4 | 17.2 | 64.4 | 0.5 | 33.9 | 17.5 | 48.6 | 3.5 | 1.8 | 5.1 | 1.4 |
| N6-3 | 6 | 14 000 | 24 | 2.3 | 490 | 10.0 | 33.0 | 80 | 73.8 | 71.8 | 92.8 | 18.5 | 13.9 | 51.1 | 0.8 | 10.6 | 8.5 | 81.4 | 2.0 | 1.6 | 15.1 | 12.8 |
| S6-1 | 6 | 14 000 | 16 | 2.2 | 236 | 4.5 | 15.5 | 33 | 70.0 | 88.5 | 94.7 | 39.4 | 5.8 | 43.6 | 0.7 | 68.9 | 8.1 | 23.0 | 27.1 | 3.2 | 9.1 | 33.1 |
| S6-2 | 6 | 14 000 | 14 | 4.6 | 270 | 6.0 | 21.0 | 108 | 85.4 | 90.0 | 95.2 | 33.3 | 11.6 | 46.8 | 0.9 | 36.5 | 16.0 | 47.5 | 12.2 | 5.3 | 15.8 | 5.8 |
| S7-1 | 7 | 15 000 | 16 | 4.9 | 293 | 6.1 | 19.4 | 132 | 93.6 | 99.7 | 98.8 | 76.5 | 1.4 | 42.3 | 0.6 | 1.6 | 80.1 | 18.3 | 1.2 | 61.3 | 14.0 | NA |
| S8-1 | 8 | 16 000 | 13 | 1.0 | 183 | 3.3 | 9.4 | 78 | 98.6 | 100.0 | 99.7 | 16.2 | 19.9 | 52.1 | 0.9 | 25.1 | 1.0 | 73.9 | 4.1 | 0.2 | 12.0 | NA |
| S8-2 | 8 | 16 000 | 11 | 1.0 | 376 | 5.6 | 18.4 | 568 | 89.5 | 99.6 | 98.5 | 48.6 | 0.2 | 54.5 | 0.6 | 4.9 | 42.0 | 53.1 | 2.4 | 20.4 | 25.8 | NA |
| N9-1 | 9 | 18 000 | 20 | 2.7 | 650 | 13.0 | 42.0 | 130 | 71.6 | 87.2 | 92.0 | 15.0 | 1.7 | 72.4 | 0.4 | 1.4 | 24.7 | 74.0 | 0.2 | 3.7 | 11.1 | 9.8 |
| S9-1 | 9 | 18 000 | 16 | 1.4 | 164 | 3.4 | 10.6 | 80 | 90.4 | 99.9 | 97.0 | 51.4 | 0.2 | 35.1 | 0.2 | 2.4 | 54.9 | 42.7 | 1.2 | 28.2 | 21.9 | 25.5 |
| N10-1 | 10 | 20 000 | 19 | 1.0 | 660 | 12.0 | 42.0 | 77 | 38.4 | 46.5 | 87.1 | 25.9 | 44.4 | 24.1 | 0.8 | 3.9 | 6.2 | 89.9 | 1.0 | 1.6 | 23.3 | 12.0 |
| N10-2 | 10 | 20 000 | 14 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| N10-3 | 10 | 20 000 | 12 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| S10-1 | 10 | 20 000 | 19 | 2.5 | 292 | 6.1 | 20.9 | 6 | 95.5 | 99.7 | 97.3 | 42.3 | 14.4 | 35.5 | 0.9 | 5.7 | 28.8 | 65.5 | 2.4 | 12.2 | 27.7 | 4.3 |
| S10-2 | 10 | 20 000 | 14 | 1.5 | 170 | 3.0 | 11.0 | 30 | 95.8 | 99.7 | 89.3 | 27.3 | 28.4 | 28.0 | 5.4 | 18.6 | 19.8 | 61.6 | 5.1 | 5.4 | 16.8 | 17.1 |
| N11-1 | 11 | 36 000 | 20 | 25.1 | 470 | 9.0 | 31.0 | 240 | 84.1 | 70.7 | 94.0 | 19.5 | 5.9 | 63.6 | 0.7 | 3.8 | 17.8 | 78.4 | 0.7 | 3.5 | 15.3 | 42.3 |
| S11-1 | 11 | 36 000 | 10 | 19.2 | 420 | 8.0 | 27.0 | 410 | 77.5 | 98.0 | 95.3 | 34.0 | 0.2 | 43.5 | 0.5 | 4.5 | 34.6 | 60.9 | 1.5 | 11.8 | 20.7 | 58.5 |
| N12-1 | 12 | 53 000 | 8 | NA | NA | NA | NA | NA | 36.9 | 95.6 | 83.3 | 20.3 | 0.0 | 72.2 | 0.2 | NA | NA | NA | NA | NA | NA | NA |
| S12-1 | 12 | 53 000 | 11 | NA | NA | NA | NA | NA | 100.0 | 100.0 | 99.9 | 43.5 | 6.5 | 39.1 | 1.5 | NA | NA | NA | NA | NA | NA | NA |
| S12-2 | 12 | 53 000 | 8 | NA | NA | NA | NA | NA | 93.1 | 100.0 | NA | 65.6 | 0.2 | 13.2 | NA | 1.6 | 83.7 | 14.7 | 1.0 | 54.9 | 9.6 | NA |
| S12-3 | 12 | 53 000 | 13 | 13.6 | 211 | 3.9 | 10.9 | 165 | 57.0 | 79.5 | 68.3 | 47.5 | 12.5 | 35.7 | 2.2 | 8.8 | 59.9 | 31.3 | 4.2 | 28.5 | 14.9 | NA |
| N13-1 | 13 | 17 000 | 20 | 6.4 | 720 | 13.0 | 45.0 | 470 | 39.2 | 85.0 | 81.8 | 21.8 | 0.0 | 69.0 | 0.2 | 0.4 | 46.1 | 53.5 | 0.1 | 10.0 | 11.7 | 7.6 |
| S13-1 | 13 | 17 000 | 20 | 2.8 | 290 | 7.0 | 27.0 | 510 | 74.9 | 93.7 | 94.5 | 38.1 | 6.0 | 47.8 | 0.4 | 26.6 | 31.3 | 42.1 | 10.1 | 11.9 | 16.0 | 48.4 |
| Recipient ID . | CB ID . | Graft dose . | Survival, wks . | CBC at time of death . | % chimerism at time of death . | % of CD45+ in BM . | % of CD33+ in BM . | % of CD45+ in BM . | % chimerism of erythroid cells in BM . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC, × 103/L . | RBC, × 104/μL . | Hemoglobin, g/dL . | Hematocrit, % . | Platelets, ×103/μL . | PB . | BM . | Spleen . | CD33+ . | CD3+ . | CD19+ . | CD3− CD56+ . | CD117+ CD203c+ . | HLA DR− . | HLA DR+ . | CD117+ CD203c+ . | HLA DR− . | HLA DR+ . | |||||
| N1-1 | 1 | 5000 | 21 | 1.1 | 500 | 10.0 | 35.0 | 550 | 75.5 | 93.3 | 95.3 | 31.1 | 1.2 | 50.2 | 0.3 | 0.9 | 51.5 | 47.6 | 0.3 | 16.0 | 14.8 | NA |
| N1-2 | 1 | 5000 | 16 | 1.4 | 730 | 13.0 | 44.0 | 1100 | 42.6 | 76.1 | 72.9 | 35.2 | 0.1 | 54.8 | 0.4 | 0.7 | 39.6 | 59.7 | 0.2 | 13.9 | 21.0 | NA |
| N1-3 | 1 | 5000 | 24 | NA | NA | NA | NA | NA | 20.7 | 12.6 | 4.4 | 32.9 | 3.8 | 60.7 | 0.0 | 4.4 | 37.9 | 57.7 | 1.0 | 12.5 | 19.0 | 3.1 |
| S1-1 | 1 | 5000 | 23 | 4.1 | 220 | 6.7 | 15.6 | 150 | 99.1 | 99.7 | 94.4 | 77.2 | 24.5 | 19.2 | 0.5 | 3.9 | 80.6 | 15.5 | 3.0 | 62.2 | 11.9 | NA |
| S1-2 | 1 | 5000 | 20 | 6.8 | 220 | 5.0 | 18.0 | 30 | 83.2 | 99.4 | 97.7 | 75.5 | 2.6 | 28.4 | 0.3 | 6.7 | 68.7 | 24.6 | 5.1 | 51.8 | 18.6 | 36.3 |
| S1-3 | 1 | 5000 | 21 | 0.7 | 320 | 7.0 | 25.0 | 330 | 76.9 | 99.7 | 95.8 | 70.9 | 1.5 | 16.6 | 0.2 | 14.6 | 50.0 | 35.4 | 10.4 | 35.5 | 25.1 | 0.0 |
| S1-4 | 1 | 500 | 26 | 0.5 | 150 | 3.0 | 11.0 | 250 | 30.7 | 97.0 | 86.3 | 69.0 | 2.0 | 47.5 | 0.1 | 6.9 | 68.6 | 24.5 | 4.8 | 47.3 | 16.9 | NA |
| N2-1 | 2 | 10 000 | 35 | 0.8 | 390 | 8.0 | 30.0 | 110 | 31.7 | 54.9 | 87.1 | 50.3 | 21.8 | 25.8 | 2.4 | 19.1 | 7.4 | 73.5 | 9.6 | 3.7 | 37.0 | 11.5 |
| S2-1 | 2 | 10 000 | 16 | 1.3 | 186 | 4.1 | 13.2 | 583 | 90.7 | 96.6 | 99.3 | 18.7 | 3.2 | 40.6 | 1.2 | 34.4 | 18.0 | 47.6 | 6.4 | 3.4 | 8.9 | NA |
| S3-1 | 3 | 10 000 | 13 | 0.2 | 215 | 3.9 | 12.6 | 793 | 52.0 | 97.8 | 90.9 | 54.2 | 0.0 | 42.8 | 0.7 | 19.0 | 44.6 | 36.4 | 10.3 | 24.2 | 19.7 | NA |
| S3-2 | 3 | 10 000 | 15 | 2.2 | 161 | 3.1 | 9.8 | 458 | 93.6 | 99.6 | 98.2 | 61.5 | 4.4 | 20.4 | 1.4 | 7.3 | 60.5 | 32.2 | 4.5 | 37.2 | 19.8 | NA |
| S4-1 | 4 | 10 000 | 13 | 0.5 | 97 | 1.9 | 5.1 | 6 | 98.6 | 100.0 | 98.2 | 52.0 | 1.2 | 39.8 | 0.3 | 2.7 | 81.9 | 15.4 | 1.4 | 42.6 | 8.0 | 27.2 |
| N5-1 | 5 | 12 000 | 35 | 3.1 | 310 | 7.0 | 25.0 | 550 | 6.8 | 36.8 | 48.9 | 29.9 | 17.5 | 41.9 | NA | 22.6 | 7.0 | 70.4 | 6.8 | 2.1 | 21.1 | 5.8 |
| N6-1 | 6 | 14 000 | 23 | 2.1 | 520 | 10.0 | 34.0 | 620 | 57.6 | 65.0 | 83.6 | 29.3 | 17.6 | 43.1 | 1.6 | 3.3 | 45.1 | 51.6 | 1.0 | 13.2 | 15.1 | 7.4 |
| N6-2 | 6 | 14 000 | 21 | 1.6 | 680 | 12.0 | 40.0 | 660 | 64.6 | 25.3 | 81.5 | 10.4 | 17.2 | 64.4 | 0.5 | 33.9 | 17.5 | 48.6 | 3.5 | 1.8 | 5.1 | 1.4 |
| N6-3 | 6 | 14 000 | 24 | 2.3 | 490 | 10.0 | 33.0 | 80 | 73.8 | 71.8 | 92.8 | 18.5 | 13.9 | 51.1 | 0.8 | 10.6 | 8.5 | 81.4 | 2.0 | 1.6 | 15.1 | 12.8 |
| S6-1 | 6 | 14 000 | 16 | 2.2 | 236 | 4.5 | 15.5 | 33 | 70.0 | 88.5 | 94.7 | 39.4 | 5.8 | 43.6 | 0.7 | 68.9 | 8.1 | 23.0 | 27.1 | 3.2 | 9.1 | 33.1 |
| S6-2 | 6 | 14 000 | 14 | 4.6 | 270 | 6.0 | 21.0 | 108 | 85.4 | 90.0 | 95.2 | 33.3 | 11.6 | 46.8 | 0.9 | 36.5 | 16.0 | 47.5 | 12.2 | 5.3 | 15.8 | 5.8 |
| S7-1 | 7 | 15 000 | 16 | 4.9 | 293 | 6.1 | 19.4 | 132 | 93.6 | 99.7 | 98.8 | 76.5 | 1.4 | 42.3 | 0.6 | 1.6 | 80.1 | 18.3 | 1.2 | 61.3 | 14.0 | NA |
| S8-1 | 8 | 16 000 | 13 | 1.0 | 183 | 3.3 | 9.4 | 78 | 98.6 | 100.0 | 99.7 | 16.2 | 19.9 | 52.1 | 0.9 | 25.1 | 1.0 | 73.9 | 4.1 | 0.2 | 12.0 | NA |
| S8-2 | 8 | 16 000 | 11 | 1.0 | 376 | 5.6 | 18.4 | 568 | 89.5 | 99.6 | 98.5 | 48.6 | 0.2 | 54.5 | 0.6 | 4.9 | 42.0 | 53.1 | 2.4 | 20.4 | 25.8 | NA |
| N9-1 | 9 | 18 000 | 20 | 2.7 | 650 | 13.0 | 42.0 | 130 | 71.6 | 87.2 | 92.0 | 15.0 | 1.7 | 72.4 | 0.4 | 1.4 | 24.7 | 74.0 | 0.2 | 3.7 | 11.1 | 9.8 |
| S9-1 | 9 | 18 000 | 16 | 1.4 | 164 | 3.4 | 10.6 | 80 | 90.4 | 99.9 | 97.0 | 51.4 | 0.2 | 35.1 | 0.2 | 2.4 | 54.9 | 42.7 | 1.2 | 28.2 | 21.9 | 25.5 |
| N10-1 | 10 | 20 000 | 19 | 1.0 | 660 | 12.0 | 42.0 | 77 | 38.4 | 46.5 | 87.1 | 25.9 | 44.4 | 24.1 | 0.8 | 3.9 | 6.2 | 89.9 | 1.0 | 1.6 | 23.3 | 12.0 |
| N10-2 | 10 | 20 000 | 14 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| N10-3 | 10 | 20 000 | 12 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| S10-1 | 10 | 20 000 | 19 | 2.5 | 292 | 6.1 | 20.9 | 6 | 95.5 | 99.7 | 97.3 | 42.3 | 14.4 | 35.5 | 0.9 | 5.7 | 28.8 | 65.5 | 2.4 | 12.2 | 27.7 | 4.3 |
| S10-2 | 10 | 20 000 | 14 | 1.5 | 170 | 3.0 | 11.0 | 30 | 95.8 | 99.7 | 89.3 | 27.3 | 28.4 | 28.0 | 5.4 | 18.6 | 19.8 | 61.6 | 5.1 | 5.4 | 16.8 | 17.1 |
| N11-1 | 11 | 36 000 | 20 | 25.1 | 470 | 9.0 | 31.0 | 240 | 84.1 | 70.7 | 94.0 | 19.5 | 5.9 | 63.6 | 0.7 | 3.8 | 17.8 | 78.4 | 0.7 | 3.5 | 15.3 | 42.3 |
| S11-1 | 11 | 36 000 | 10 | 19.2 | 420 | 8.0 | 27.0 | 410 | 77.5 | 98.0 | 95.3 | 34.0 | 0.2 | 43.5 | 0.5 | 4.5 | 34.6 | 60.9 | 1.5 | 11.8 | 20.7 | 58.5 |
| N12-1 | 12 | 53 000 | 8 | NA | NA | NA | NA | NA | 36.9 | 95.6 | 83.3 | 20.3 | 0.0 | 72.2 | 0.2 | NA | NA | NA | NA | NA | NA | NA |
| S12-1 | 12 | 53 000 | 11 | NA | NA | NA | NA | NA | 100.0 | 100.0 | 99.9 | 43.5 | 6.5 | 39.1 | 1.5 | NA | NA | NA | NA | NA | NA | NA |
| S12-2 | 12 | 53 000 | 8 | NA | NA | NA | NA | NA | 93.1 | 100.0 | NA | 65.6 | 0.2 | 13.2 | NA | 1.6 | 83.7 | 14.7 | 1.0 | 54.9 | 9.6 | NA |
| S12-3 | 12 | 53 000 | 13 | 13.6 | 211 | 3.9 | 10.9 | 165 | 57.0 | 79.5 | 68.3 | 47.5 | 12.5 | 35.7 | 2.2 | 8.8 | 59.9 | 31.3 | 4.2 | 28.5 | 14.9 | NA |
| N13-1 | 13 | 17 000 | 20 | 6.4 | 720 | 13.0 | 45.0 | 470 | 39.2 | 85.0 | 81.8 | 21.8 | 0.0 | 69.0 | 0.2 | 0.4 | 46.1 | 53.5 | 0.1 | 10.0 | 11.7 | 7.6 |
| S13-1 | 13 | 17 000 | 20 | 2.8 | 290 | 7.0 | 27.0 | 510 | 74.9 | 93.7 | 94.5 | 38.1 | 6.0 | 47.8 | 0.4 | 26.6 | 31.3 | 42.1 | 10.1 | 11.9 | 16.0 | 48.4 |
A total of 21 human HSC-engrafted hSCFTg NSG (S) recipients and 15 human HSC-engrafted non-Tg NSG (N) recipients were created.
WBC indicates white blood cell count; RBC, red blood cell count; and NA, not applicable.
Human hematopoietic engraftment is enhanced in hSCF Tg NSG recipients. (A) hSCF Tg NSG recipients developed progressive anemia as evidenced by reduced hemoglobin concentration compared with non-Tg NSG mice transplanted with human HSCs from the same donor source. (B) Human CD45+ chimerism was analyzed over time in PB of hSCF Tg and non-Tg NSG recipients. (C) Representative flow cytometric contour plots demonstrating the presence of human CD45+ cells, CD19+ B cells, CD33+ myeloid cells, CD3+ T cells, and CD56+CD3− NK cells in recipient BM. (D) At the time of death, engraftment levels of human CD45+ cells in the BM, spleen, and PB of hSCF Tg NSG recipients were significantly higher compared with non-Tg NSG controls (BM: hSCF Tg n = 21, non-Tg n = 13, P < .0001; spleen: hSCF Tg n = 21, non-Tg n = 13, P = .0065; PB: hSCF Tg n = 21, non-Tg n = 13, P < .0001). (E) In hSCF Tg NSG recipient BM, significantly greater human CD33+ myeloid lineage development was observed (hSCF Tg n = 21, non-Tg n = 13, P = .0002).
Human hematopoietic engraftment is enhanced in hSCF Tg NSG recipients. (A) hSCF Tg NSG recipients developed progressive anemia as evidenced by reduced hemoglobin concentration compared with non-Tg NSG mice transplanted with human HSCs from the same donor source. (B) Human CD45+ chimerism was analyzed over time in PB of hSCF Tg and non-Tg NSG recipients. (C) Representative flow cytometric contour plots demonstrating the presence of human CD45+ cells, CD19+ B cells, CD33+ myeloid cells, CD3+ T cells, and CD56+CD3− NK cells in recipient BM. (D) At the time of death, engraftment levels of human CD45+ cells in the BM, spleen, and PB of hSCF Tg NSG recipients were significantly higher compared with non-Tg NSG controls (BM: hSCF Tg n = 21, non-Tg n = 13, P < .0001; spleen: hSCF Tg n = 21, non-Tg n = 13, P = .0065; PB: hSCF Tg n = 21, non-Tg n = 13, P < .0001). (E) In hSCF Tg NSG recipient BM, significantly greater human CD33+ myeloid lineage development was observed (hSCF Tg n = 21, non-Tg n = 13, P = .0002).
At 8 to 35 weeks after transplantation, individual hSCF Tg NSG recipients were analyzed to determine levels of reconstitution of human hematopoiesis and immunity in the BM, spleen, and PB. At the time of necropsy, we did not observe any gross macroscopic abnormalities in these recipients. We performed flow cytometric analysis to evaluate the engraftment levels of human CD45+ cells (calculated as % hCD45+ cells relative to total numbers of mouse and human CD45+ cells in the nucleated cell gate). Engraftment levels of human CD45+ leukocytes in the BM, spleen, and PB were significantly higher in hSCF Tg NSG recipients (mean ± SEM; 97.1% ± 1.1%, 94.5% ± 1.6%, and 83.1% ± 3.9%, respectively; n = 21) compared with engrafted non-Tg NSG recipients (63.1% ± 7.3%, 77.3% ± 6.9%, and 49.5% ± 6.5%, respectively; n = 13; P < .0001, P = .0065, and P < .0001 by 2-tailed t test, respectively; Figure 1C-D). Compared with enhanced engraftment of human leukocytes in the recipient BM, development of human erythroid precursors was not significantly different in hSCF Tg NSG recipients compared with non-Tg NSG recipients (hSCFTg: 25.6% ± 6.1%; n = 10 and non-Tg NSG controls: 11.4% ± 3.6%; n = 10; P = .0601; supplemental Figure 2). We next analyzed the development of human lymphoid and myeloid cells in the engrafted human CD45+ hematopoietic cell populations by flow cytometry using monoclonal antibodies against hCD3, hCD19, hCD56, and hCD33 along with anti–human and anti–mouse CD45 antibodies (Figure 1C,E). Although there was recipient-to-recipient variability, the frequency of human CD33+ myeloid cells within the total human CD45+ population was significantly higher in the hSCF Tg NSG recipient BM than in the non-Tg NSG recipient BM and constituted the majority of human hematopoietic cells (hSCF Tg: 49.7% ± 4.0%; n = 21 and non-Tg NSG controls: 26.2% ± 2.9%; n = 13; P = .0002 by 2-tailed t test). In contrast, in non-Tg NSG recipients, the majority of human hematopoietic cells in the BM were B cells (53.3% ± 4.5%; n = 13), consistent with previous reports.5,9,20 These findings demonstrate that the expression of membrane-bound hSCF in BM microenvironment results in significantly more efficient engraftment of human HSCs as well as enhanced development of human CD33+ myeloid cells from the engrafted human HSCs.
Human myeloid lineage development in hSCF Tg NSG recipients
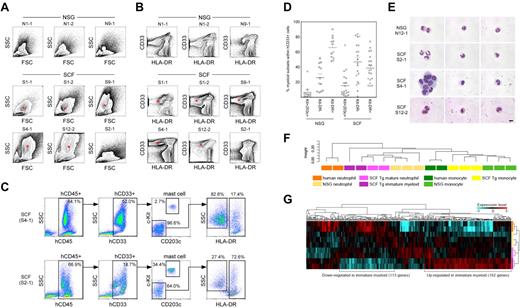
Next, we examined the development of human myeloid subsets in the human membrane-bound SCF-expressing BM microenvironment. Flow cytometric scatter plots demonstrate the development of the SSC high granulocyte fraction in hSCF Tg NSG recipients, which correlate with CD33+HLA-DR− cells (Figure 2A-B). To quantify the frequencies of different myeloid subsets in these recipients, we first identified CD33+c-Kit+CD203c+ mature human mast cells among human CD45+ cells. Next, we identified CD33+HLA-DR− human granulocyte lineage cells and human CD33+HLA-DR+ antigen-presenting cells (APCs) among human CD45+ cells excluding mature mast cells (Figure 2C).
HLA-DR-negative human myeloid cells predominate in hSCF Tg NSG recipient BM. (A) Flow cytometric contour plots demonstrating FSC and SSC characteristics of 6 hSCF Tg NSG recipient BM (S1-1, S1-2, S9-1, S4-1, S12-2, and S2-1) and 3 non-Tg NSG recipient BM (N1-1, N1-2, and N9-1). Polymorphonuclear myeloid cells (red asterisks) are present at high frequencies in hSCF Tg NSG recipient BM. (B) Flow cytometry contour plots demonstrating hCD33 and HLA-DR expression in the same recipients as shown in panel A. Consistent with their FSC and SSC characteristics, hSCF Tg NSG recipient BM contained a prominent CD33+HLA-DR− granulocyte population (red asterisks; N1-1, killed at 21 weeks; N1-2, killed at 16 weeks; N9-1, killed at 20 weeks; S1-1, killed at 23 weeks; S1-2, killed at 20 weeks; S9-1, killed at 16 weeks; S4-1, killed at 13 weeks; S12-2, killed at 8 weeks; and S2-1, killed at 16 weeks). (C) Representative flow cytometric scatter plots of hSCF Tg NSG recipient BM demonstrating the identification of human c-Kit+CD203c+ mast cells within the hCD33+ fraction and HLA-DR−SSChigh granulocytes and HLA-DR+SSClow APCs within the c-Kit−CD203c− fraction (S4-1, killed at 13 weeks; and S2-1, killed at 16 weeks). (D) Frequencies of human c-Kit+CD203c+ mast cells, CD33+HLA-DR− granulocytes, and CD33+HLA-DR+ APCs within the total hCD45+hCD33+ myeloid cell population in the BM of hSCF Tg and non-Tg NSG recipients. Numbers of cells in the granulocyte/neutrophil fraction were significantly higher in hSCF Tg NSG recipient BM (hSCF Tg n = 20, non-Tg n = 12, P = .0001). (E) CD33+HLA-DR− cells from hSCF Tg and non-Tg NSG recipient BM were FACS-purified and examined by MGG staining. In 9 of 13 hSCF Tg recipients (S4-1 and S12-2 shown as representative), immature myeloid cells composed the majority of cells in this fraction. In 4 of 13 hSCF Tg recipients (S2-1 shown as representative) and 4 of 5 non-Tg NSG recipients (N12-1 shown as representative), mature neutrophils (band and segmented forms) were observed (N12-1, killed at 8 weeks; S2-1, killed at 16 weeks; S4-1, killed at 13 weeks; and S12-2, killed at 8 weeks). (F-G) Global transcriptional profiles of FACS-purified CD33+cKit−CD203−HLA-DR− granulocytes and CD33+c-Kit−CD203c−HLA-DR+CD14+ monocytes derived from hSCF Tg NSG and non-Tg NSG recipient BM as well as human CD16+ neutrophils and CD14+ monocytes were compared. (F) Unsupervised clustering for each group is shown. (G) The expression heatmap demonstrates genes that are significantly under- and over-represented in each population.
HLA-DR-negative human myeloid cells predominate in hSCF Tg NSG recipient BM. (A) Flow cytometric contour plots demonstrating FSC and SSC characteristics of 6 hSCF Tg NSG recipient BM (S1-1, S1-2, S9-1, S4-1, S12-2, and S2-1) and 3 non-Tg NSG recipient BM (N1-1, N1-2, and N9-1). Polymorphonuclear myeloid cells (red asterisks) are present at high frequencies in hSCF Tg NSG recipient BM. (B) Flow cytometry contour plots demonstrating hCD33 and HLA-DR expression in the same recipients as shown in panel A. Consistent with their FSC and SSC characteristics, hSCF Tg NSG recipient BM contained a prominent CD33+HLA-DR− granulocyte population (red asterisks; N1-1, killed at 21 weeks; N1-2, killed at 16 weeks; N9-1, killed at 20 weeks; S1-1, killed at 23 weeks; S1-2, killed at 20 weeks; S9-1, killed at 16 weeks; S4-1, killed at 13 weeks; S12-2, killed at 8 weeks; and S2-1, killed at 16 weeks). (C) Representative flow cytometric scatter plots of hSCF Tg NSG recipient BM demonstrating the identification of human c-Kit+CD203c+ mast cells within the hCD33+ fraction and HLA-DR−SSChigh granulocytes and HLA-DR+SSClow APCs within the c-Kit−CD203c− fraction (S4-1, killed at 13 weeks; and S2-1, killed at 16 weeks). (D) Frequencies of human c-Kit+CD203c+ mast cells, CD33+HLA-DR− granulocytes, and CD33+HLA-DR+ APCs within the total hCD45+hCD33+ myeloid cell population in the BM of hSCF Tg and non-Tg NSG recipients. Numbers of cells in the granulocyte/neutrophil fraction were significantly higher in hSCF Tg NSG recipient BM (hSCF Tg n = 20, non-Tg n = 12, P = .0001). (E) CD33+HLA-DR− cells from hSCF Tg and non-Tg NSG recipient BM were FACS-purified and examined by MGG staining. In 9 of 13 hSCF Tg recipients (S4-1 and S12-2 shown as representative), immature myeloid cells composed the majority of cells in this fraction. In 4 of 13 hSCF Tg recipients (S2-1 shown as representative) and 4 of 5 non-Tg NSG recipients (N12-1 shown as representative), mature neutrophils (band and segmented forms) were observed (N12-1, killed at 8 weeks; S2-1, killed at 16 weeks; S4-1, killed at 13 weeks; and S12-2, killed at 8 weeks). (F-G) Global transcriptional profiles of FACS-purified CD33+cKit−CD203−HLA-DR− granulocytes and CD33+c-Kit−CD203c−HLA-DR+CD14+ monocytes derived from hSCF Tg NSG and non-Tg NSG recipient BM as well as human CD16+ neutrophils and CD14+ monocytes were compared. (F) Unsupervised clustering for each group is shown. (G) The expression heatmap demonstrates genes that are significantly under- and over-represented in each population.
In the hSCF Tg NSG recipient BM, there were increased percentages of CD33+HLA-DR− granulocytes and decreased percentages of CD33+HLA-DR+ APCs compared with non-Tg NSG controls (hSCF Tg: 46.7% ± 5.9% and 38.3% ± 4.0%, respectively; n = 20 and non-Tg NSG controls: 25.8% ± 5.0% and 65.5% ± 4.1%, respectively; n = 12; P = .0204 and P = .0001 by 2-tailed t test, respectively; Figure 2D). In the BM of 11 of 20 hSCF Tg NSG recipients examined, c-Kit−CD203c−HLA-DR− SSChigh granulocytes accounted for the highest frequency of total human myeloid cells (Figure 2D; Table 1). To examine the morphologic features of the human granulocytes developing in the hSCF Tg recipients, we carried out May-Grünwald-Giemsa (MGG) staining using cytospin specimens of FACS-purified CD33+c-Kit−CD203c−HLA-DR− cells from BM of hSCF Tg and non-Tg NSG recipients. In 9 of 13 hSCF Tg recipient BM cells examined, the majority of myeloid cells showed the morphology of immature granulocytes with large nuclear-to-cytoplasmic ratio and nuclei with few lobulations, largely consisting of myelocytes and metamyelocytes (S4-1 and S12-2 shown as representative in Figure 2E). In 4 of 13 hSCF Tg recipients examined and in 4 of 5 non-Tg NSG recipients, mature segmented neutrophils were present in the sorted CD33+c-Kit−CD203c−HLA-DR− granulocyte population (N12-1 and S2-1 shown as representative in Figure 2E). These findings indicate that both by quantitative and morphologic examinations, human granulocytic cells with various degrees of maturity predominate among the CD33+ myeloid cells developing within the hSCF Tg NSG recipients. To examine the myeloid differentiation capacity of hematopoietic stem and progenitor populations in a functional manner, we performed a colony-forming cell assay using CD34+CD38− and CD34+CD38+ cells derived from BM of hSCF Tg NSG recipients and non-Tg NSG recipients. In both cell populations, myeloid and erythroid colony formation were similar between hSCF Tg NSG and non-Tg NSG recipient BM (supplemental Figure 3).
We then analyzed global transcriptional profiles of CD33+HLA-DR−c-Kit−CD203c− granulocytes from hSCF Tg NSG recipient BM (n = 4), non-Tg NSG recipient BM (n = 3), and primary human BM (n = 2). Additional control samples included BM monocytes from hSCF Tg recipients (n = 3), non-Tg NSG recipient BM monocytes (n = 3), and primary human BM monocytes (n = 2). Unsupervised clustering demonstrated a clear segregation of transcriptional profiles between granulocytes and monocytes regardless of the source. This suggests that human granulocytes and monocytes in humanized mouse undergo a distinct differentiation process similar to their counterparts in human BM (Figure 2F). We next examined whether there were any differences in gene expression within 3 distinct granulocyte sources (hSCF Tg recipient BM, non-Tg NSG recipient BM, and primary human BM neutrophils; Figure 2G). As seen in the heatmap representation, we found clusters of genes differentially transcribed in the distinct sources of granulocytes (Figure 2G). Multiple genes associated with transcriptional regulation were included in the genes up-regulated in human immature granulocytes derived from the BM of hSCF Tg NSG mice, suggesting that these cells are more actively cycling and proliferating compared with mature granulocytes from the BM of hSCF Tg NSG and non-Tg NSG mice and primary human BM neutrophils (Figure 2G; supplemental Tables 1 and 2).
Development of human mast cells in hSCF Tg NSG recipients
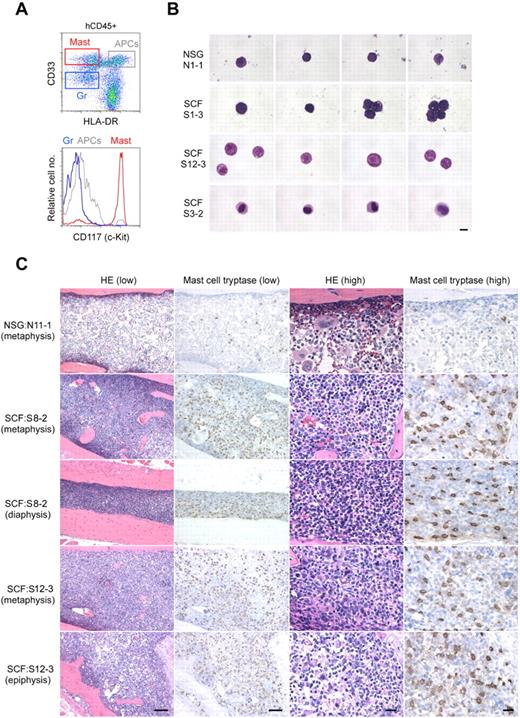
We next investigated the development of human mast cells in the membrane-bound hSCF-expressing NSG mice. Overall, the frequencies of cKit+CD203c+ cells within total BM CD33+ myeloid cells were similar between hSCF Tg NSG and non-Tg NSG recipients when excluding 2 non-Tg NSG recipients observed for more than 8 months (P = .1439 by 2-tailed t test; Figure 2D; Table 1). In 7 of 20 hSCF Tg NSG recipients, compared with one of 10 non-Tg NSG recipients, the frequency of cKit+CD203c+ cells in BM CD33+ cells was greater than 15% (Figure 2D; Table 1). When these cKit+CD203c+ cells were FACS-purified and examined by MGG staining, their morphology was consistent with mast cells with various degrees of cytoplasmic granulation (Figure 3A-B). Histologic examination of H&E-stained bone sections showed increased cellularity in hSCF Tg NSG recipients compared with non-Tg NSG recipients (Figure 3C). We then performed IHC staining for mast cell tryptase to identify human mast cells in the BM. Consistent with the quantitative analysis by flow cytometry, tryptase+ cells were abundantly observed in the hSCF Tg NSG recipients compared with non-Tg NSG recipients (Figure 3C). This does not reflect an increase in mouse mast cells because nearly all nucleated hematopoietic cells in the hSCF Tg NSG recipients are of human origin (Figure 1D). The same sections were further subjected to the immunofluorescence staining followed by confocal imaging demonstrating that these are mast cells and not CD14+ monocytes (supplemental Figure 4).
Human mast cell development in hSCF Tg NSG recipient BM. (A) Representative flow cytometric scatter plot and histogram demonstrating the identification of human CD45+CD33+CD117+ mast cells. (B) FACS-sorted hCD45+CD33+CD117+CD203c+ human mast cells from a representative non-Tg NSG recipient BM (N1-1, 0.9% human mast cells within hCD45+CD33+ population) and hSCF Tg NSG recipient BM (S1-3, 14.6%; S12-3, 8.8%; and S3-2, 7.3% human mast cells within the hCD45+CD33+ population) were examined by MGG staining (N1-1, killed at 21 weeks; S1-3, killed at 21 weeks; S12-3, killed at 13 weeks; and S3-2, killed at 15 weeks). (C) H&E- and anti-mast cell tryptase antibody–stained bone sections demonstrate hypercellular BM with high frequency of tryptase+ human mast cells in hSCF Tg NSG recipients. Non-Tg NSG recipient: N11-1, 70.7% hCD45+. hSCF Tg NSG recipients: S8-2, 99.6%; and S12-3, 79.5% hCD45+ (N11-1, killed at 20 weeks; S8-2, killed at 11 weeks; and S12-3, killed at 13 weeks).
Human mast cell development in hSCF Tg NSG recipient BM. (A) Representative flow cytometric scatter plot and histogram demonstrating the identification of human CD45+CD33+CD117+ mast cells. (B) FACS-sorted hCD45+CD33+CD117+CD203c+ human mast cells from a representative non-Tg NSG recipient BM (N1-1, 0.9% human mast cells within hCD45+CD33+ population) and hSCF Tg NSG recipient BM (S1-3, 14.6%; S12-3, 8.8%; and S3-2, 7.3% human mast cells within the hCD45+CD33+ population) were examined by MGG staining (N1-1, killed at 21 weeks; S1-3, killed at 21 weeks; S12-3, killed at 13 weeks; and S3-2, killed at 15 weeks). (C) H&E- and anti-mast cell tryptase antibody–stained bone sections demonstrate hypercellular BM with high frequency of tryptase+ human mast cells in hSCF Tg NSG recipients. Non-Tg NSG recipient: N11-1, 70.7% hCD45+. hSCF Tg NSG recipients: S8-2, 99.6%; and S12-3, 79.5% hCD45+ (N11-1, killed at 20 weeks; S8-2, killed at 11 weeks; and S12-3, killed at 13 weeks).
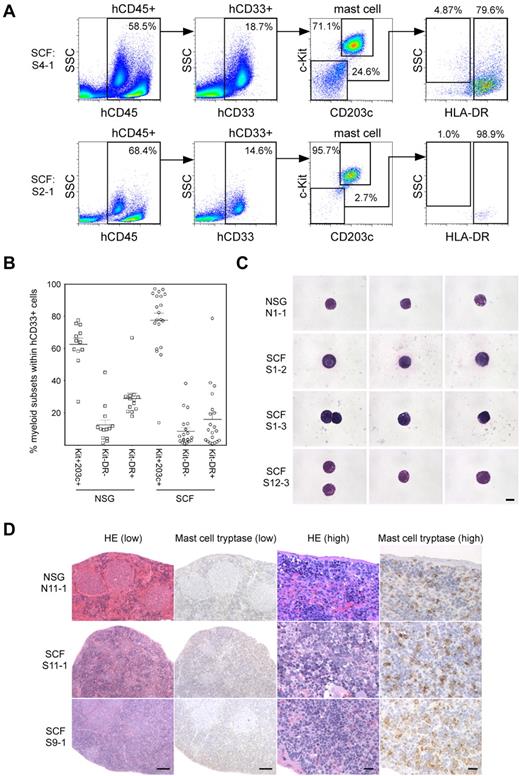
Mast cell progenitors and mature mast cells reside in high frequencies in the spleen of normal immunocompetent mice.21 We next examined the spleen of human HSC-engrafted hSCF Tg NSG recipients. Human CD33highc-Kit+CD203c+ mast cells accounted for the highest frequency among total hCD45+hCD33+ myeloid cells in the spleen of both hSCF Tg NSG and non-Tg NSG HSC-engrafted recipients (Figure 4A-B). However, the frequencies of human mast cells in the myeloid cell population were significantly higher in hSCF Tg NSG recipients than in non-Tg NSG controls (hSCF Tg: 77.4% ± 4.5%; n = 20 and non-Tg NSG controls: 62.5% ± 3.9%; n = 12; P = .0304 by 2-tailed t test; Figure 4B). These human cells with surface expression phenotype of mast cells also showed morphologic features of mature mast cells (Figure 4C). Mast cell tryptase IHC staining confirmed the presence of human mast cells within the recipient spleen (Figure 4D). These findings indicate that the expression of membrane-bound human SCF in the recipient mouse microenvironment enhances development of human mast cells from transplanted human HSCs within hematopoietic organs, such as the BM and spleen, consistent with the activation of c-Kit signaling.
Human mast cell development in hSCF Tg NSG recipient spleen. (A) Human mast cell development is enhanced in hSCF Tg NSG recipient spleens (S4-1, killed at 13 weeks; and S2-1, killed at 16 weeks). (B) Frequencies of human c-Kit+CD203c+ mast cells, CD33+HLA-DR− granulocyte population, and CD33+HLA-DR+ APCs within total hCD45+hCD33+ myeloid cells in the spleens of hSCF Tg and non-Tg NSG recipients. Human mast cell development in the spleen was significantly greater in the hSCF Tg NSG recipients (hSCF Tg: n = 20, non-Tg NSG: n = 12, P = .0304). (C) FACS-sorted hCD45+CD33+CD117+CD203c+ human mast cells from a representative non-Tg NSG recipient spleen (N1-1, 59.3% human mast cells within hCD45+CD33+ population) and hSCF Tg NSG recipient spleen (S1-2, 85.7%; S1-3, 77.7%; and S12-3, 56.1% human mast cells within hCD45+CD33+ population) were examined by MGG staining (N1-1, killed at 21 weeks; S1-2, killed at 20 weeks; S1-3, killed at 21 weeks; and S12-3, killed at 13 weeks). (D) H&E- and anti–mast cell tryptase antibody–stained spleen sections demonstrating the presence of human mast cells in non-Tg NSG recipients and hSCF Tg NSG recipients (non-Tg NSG recipient: N11-1, 94.0% hCD45+; hSCF Tg NSG recipients: S11-1, 95.3%; and S9-1, 97.0% hCD45+; N11-1, killed at 20 weeks; S11-1, killed at 10 weeks; and S9-1, killed at 16 weeks).
Human mast cell development in hSCF Tg NSG recipient spleen. (A) Human mast cell development is enhanced in hSCF Tg NSG recipient spleens (S4-1, killed at 13 weeks; and S2-1, killed at 16 weeks). (B) Frequencies of human c-Kit+CD203c+ mast cells, CD33+HLA-DR− granulocyte population, and CD33+HLA-DR+ APCs within total hCD45+hCD33+ myeloid cells in the spleens of hSCF Tg and non-Tg NSG recipients. Human mast cell development in the spleen was significantly greater in the hSCF Tg NSG recipients (hSCF Tg: n = 20, non-Tg NSG: n = 12, P = .0304). (C) FACS-sorted hCD45+CD33+CD117+CD203c+ human mast cells from a representative non-Tg NSG recipient spleen (N1-1, 59.3% human mast cells within hCD45+CD33+ population) and hSCF Tg NSG recipient spleen (S1-2, 85.7%; S1-3, 77.7%; and S12-3, 56.1% human mast cells within hCD45+CD33+ population) were examined by MGG staining (N1-1, killed at 21 weeks; S1-2, killed at 20 weeks; S1-3, killed at 21 weeks; and S12-3, killed at 13 weeks). (D) H&E- and anti–mast cell tryptase antibody–stained spleen sections demonstrating the presence of human mast cells in non-Tg NSG recipients and hSCF Tg NSG recipients (non-Tg NSG recipient: N11-1, 94.0% hCD45+; hSCF Tg NSG recipients: S11-1, 95.3%; and S9-1, 97.0% hCD45+; N11-1, killed at 20 weeks; S11-1, killed at 10 weeks; and S9-1, killed at 16 weeks).
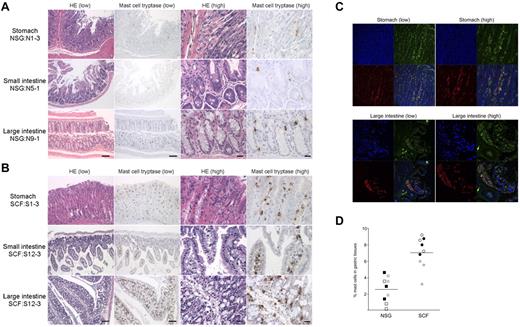
Next, we investigated whether the transgenic expression of hSCF results in the efficient development of mucosal tissue-type human mast cells in respiratory and gastrointestinal mucosal layers, as well as in hematopoietic organs. For this purpose, we performed IHC staining of human tryptase-expressing mast cells in the lung, stomach, small intestine, and large intestine in hSCF Tg NSG and non-Tg NSG recipients. In the hSCF Tg NSG recipient lungs, human tryptase-positive mast cells were identified within cellular infiltrates (supplemental Figure 5). In tissue sections from the stomach, small intestine, and large intestine, human tryptase-positive mast cells were present in both hSCF Tg NSG and non-Tg NSG recipients (Figure 5A-B). The mast cell tryptase+ cells in gastrointestinal tissues of hSCF Tg mice were further examined by immunofluorescence microscopy using anti–human CD45 and anti–human-c-Kit antibodies. We found the presence of hCD45+c-Kit+ cells in the gastric tissues of the hSCF Tg recipients consistent with IHC staining for mast cell tryptase (Figure 5C). Because gastric tissue is one of the major sites of mast cell populations in humans and mice, we quantified human mast cells in the gastric tissue of hSCF Tg and non-Tg NSG recipients transplanted with human HSCs. IHC staining for human mast cell tryptase followed by quantification of tryptase+ cells demonstrated the presence of human mast cells in gastric tissues of hSCF Tg NSG recipients (7.01% ± 0.63%, 3 sites per recipient analyzed in 3 mice) compared with non-Tg NSG recipients (2.53% ± 0.53%, 3 sites per recipient analyzed in 3 mice; P < .0001 by 2-tailed t test; Figure 5D). Collectively, transgenic expression of human membrane-bound SCF influences human myeloid development and mast cell development in hematopoietic organs and mucosal tissues along with the achievement of high chimerism of human hematopoietic cells in hematopoietic organs.
Human mast cell development in hSCF Tg NSG recipient stomach, small intestine, and large intestine. H&E- and anti–mast cell tryptase antibody–stained sections of (A) non-Tg NSG recipient stomach (NSG control, N1-3), small intestine (N5-1), and large intestine (N9-1) and (B) hSCF Tg NSG recipient stomach (S1-3), small intestine (S12-3), and large intestine (S12-3) demonstrating the presence of human mast cells (N1-3, killed at 24 weeks; N5-1, killed at 35 weeks; N9-1, killed at 20 weeks; S1-3, killed at 21 weeks; and S12-3, killed at 13 weeks). (C) Confocal immunofluorescence images of hSCF Tg stomach (S1-9) demonstrate human CD45+ (green) and human CD117+ (red) mast cells. (D) Frequencies of tryptase+ cells were quantified by sampling 3 areas each from hSCF Tg (n = 3) and non-Tg (n = 3) NSG recipients: hSCF Tg NSG recipients, 7.0% ± 0.6%; and non-Tg NSG recipients, 2.5% ± 0.5% (P < .0001 by 2-tailed t test).
Human mast cell development in hSCF Tg NSG recipient stomach, small intestine, and large intestine. H&E- and anti–mast cell tryptase antibody–stained sections of (A) non-Tg NSG recipient stomach (NSG control, N1-3), small intestine (N5-1), and large intestine (N9-1) and (B) hSCF Tg NSG recipient stomach (S1-3), small intestine (S12-3), and large intestine (S12-3) demonstrating the presence of human mast cells (N1-3, killed at 24 weeks; N5-1, killed at 35 weeks; N9-1, killed at 20 weeks; S1-3, killed at 21 weeks; and S12-3, killed at 13 weeks). (C) Confocal immunofluorescence images of hSCF Tg stomach (S1-9) demonstrate human CD45+ (green) and human CD117+ (red) mast cells. (D) Frequencies of tryptase+ cells were quantified by sampling 3 areas each from hSCF Tg (n = 3) and non-Tg (n = 3) NSG recipients: hSCF Tg NSG recipients, 7.0% ± 0.6%; and non-Tg NSG recipients, 2.5% ± 0.5% (P < .0001 by 2-tailed t test).
Discussion
A supportive microenvironment is essential for hematopoietic and immune system homeostasis. Critical roles played by various niches in the maintenance of cell cycle quiescence and self-renewal capacity of HSCs have been demonstrated, and the thymic microenvironment is critical for T-cell education.22,23 However, despite significant progress over the last decade, the stromal microenvironment within the humanized mouse is predominately of mouse origin. Although several key molecules, such as SDF1, are cross-reactive between human and mouse, a humanized microenvironment is required both to further improve human hematopoietic development in the recipients and to investigate in vivo the interactions between hematopoietic cells and their microenvironment.
In the present study, we humanized membrane-bound stem cell factor [SCF = KIT ligand (KL)] using the construct and mouse strain created by Majumdar et al.13 Toksoz et al reported that human membrane-bound SCF expressed by mouse stromal cells efficiently supports long-term human hematopoiesis in vitro.24 The importance of SCF interaction with cKit+ HSCs and mast cell progenitors in murine hematopoiesis is highlighted by the hematopoietic abnormalities in mice with Kitl and Kit mutations.25-27 In human hematopoiesis, SCF-cKit signaling is critical for the maintenance of stem and progenitor cell activities.28 Human SCF/KL has been shown to drive cell-cycle entry by primitive hematopoietic cells in vitro.29 Both long-term colony-initiation and colony-forming capacities are expanded ex vivo by cytokine supplementation that includes SCF/KL.30-32 Therefore, to elucidate the role of membrane-bound human SCF in differentiation, proliferation, and maturation of human hematopoiesis in vivo, we created a novel NSG mouse strain that can support the engraftment of human HSCs and express hSCF in microenvironment. In hSCF Tg NSG recipients transplanted with human HSCs, the engraftment levels of human CD45+ cells were significantly higher compared with non-Tg NSG controls. Majumdar et al reported that human SCF binds mouse c-Kit receptor but that the binding affinity is weaker compared with the binding affinity of human SCF to human c-Kit.13 Therefore, the significant improvement of human hematopoietic chimerism could be attributed to preferential binding of human SCF to human HSCs instead of murine c-Kit+ HSCs resulting in accelerated signaling through c-Kit in human HSCs and by impaired or attenuated support of mouse HSCs.1,33,34 Presumably because of the competition between human and mouse hematopoietic stem or myeloid/erythroid progenitor cells, we observed diminished mouse erythrocyte hemoglobin concentration with normal range of mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration in all the 21 hSCF Tg NSG recipients but not in any of the non-Tg NSG recipients or nontransplanted hSCF Tg NSG adults. In addition to the greatly increased levels of human hematopoietic repopulation, we identified significant differences in human hematopoietic differentiation in hSCF Tg NSG recipients compared with non-Tg NSG recipients. Namely, there were substantially increased levels of human myeloid differentiation from HSCs in the hSCF Tg NSG mice, whereas human B cells accounted for the greatest population in the BM of non-Tg NSG mice. Because normal human BM contains myeloid cells at a relatively high frequency (36.2%-62.2%),35 human SCF may be important in recapitulating human BM myelopoiesis in immunodeficient mice. In addition, membrane-bound human SCF may exert distinct effects on human myeloid development in the BM and in the spleen. In the BM of hSCF Tg recipients, the majority of human myeloid cells were c-Kit−CD203c−HLA-DR− granulocytes. Among these granulocytes, myeloid cells at various levels of maturity were identified, with myelocytes and metamyelocytes predominating in the majority of hSCF Tg NSG recipients. Because immature cells were more prominent in hSCF Tg NSG recipients compared with non-Tg NSG recipients, we performed microarray analysis to identify transcriptional signature specific to the immature human granulocytes that developed in the hSCF Tg NSG mice. Approximately 300 genes were differentially transcribed in the immature granulocytes in hSCF Tg NSG recipients compared with the mature granulocytes in non-Tg NSG recipients. Some of the up-regulated genes were associated with cell cycle or metabolism.
In several hSCF Tg NSG recipients, human mast cells composed the greatest subfraction among engrafted human myeloid cells. In the spleens of hSCF Tg NSG-engrafted mice, human mast cells were present at the highest frequency among the myeloid lineage developed in the recipients. MGG staining revealed both mature and immature mast cells in hSCF Tg NSG recipient BM. Human mast cells were identified not only in hematopoietic organs but also in lung, gastric tissue, and intestinal tissues of hSCF Tg NSG recipients. Aberrant expression of CD30 and CD25 on mast cells is associated with systemic mastocytosis and other mast cell disorders.36,37 We did not find significantly up-regulated expression of these antigens in the mast cells derived from BM or spleen of hSCF Tg NSG recipients.
To date, several mouse strains have been developed for supporting normal and malignant human hematopoietic cell engraftment and normal myeloid cell differentiation using Il2rgnull immune-compromised mice (supplemental Table 3).5,6,8,9,20,38-41 Among these, human thrombopoietin knock-in Rag2null Il2rgnull mice were reported to support both human hematopoietic engraftment and myeloid differentiation in the BM. Both SCF and thrombopoietin exhibit species specificity between humans and mouse in supporting HSCs and myeloid cells in both species. These approaches focusing on the 2 distinct molecules based on the 2 immune-compromised mouse backgrounds will allow us to investigate human hematopoiesis and immunity from stem cells to myeloid progenitors to mature myeloid effecter cells in vivo. Altogether, the newly created hSCF Tg NSG mouse model engrafted with purified human HSCs will facilitate the in vivo understanding of human hematopoietic hierarchy and mast cell biology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Williams for providing C3H/HeJ mice carrying the human SCF transgene and Dr Kodo and his staff at the Tokyo Cord Blood Bank for their generous assistance in processing and providing cord blood samples.
This work was supported by the National Institutes of Health (research grants HL077642, CA34196, AI46629, UO1 AI073871, DK089572), the University of Massachusetts Center for AIDS Research (P30 AI042845), the Juvenile Diabetes Foundation, International, the Helmsley Foundation, USAMRIID (L.D.S.), Project for Developing Innovation Systems, Program for Fostering Regional Innovation (O.O.), Takeda Science Foundation and Ministry of Education, Culture, Sports, Science and Technology, Japan (F.I.), and the Japan Society for the Promotion of Science through the Funding Program for Next Generation World-Leading Researchers (NEXT Program), initiated by the Council for Science and Technology Policy.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S. Takagi, S. Tanaka, T.H., and S.M. performed the experiments; A.H., T.W., and O.O. performed and analyzed the microarray experiments; J.K., H. Kiyono, H. Koseki, O.O., T.S., and S. Taniguchi participated in discussions on research planning and analysis of results; and S. Takagi, Y.S., L.D.S., and F.I. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fumihiko Ishikawa, Research Unit for Human Disease Models, RIKEN Research Center for Allergy and Immunology, 1-7-22 Suehiro-cho Tsurumi-ku, Yokohama, Kanagawa, Japan 230-0045; e-mail: f_ishika@rcai.riken.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal