Abstract
Small-molecule drugs that target the B-cell antigen receptor (BCR) signalosome show clinical efficacy in the treatment of B-cell non-Hodgkin lymphoma. These agents, including the Bruton tyrosine kinase (BTK) inhibitor PCI-32765, display an unexpected response in patients with chronic lymphocytic leukemia (CLL): a rapid and sustained reduction of lymphadenopathy accompanied by transient lymphocytosis, which is reversible upon temporary drug deprivation. We hypothesized that this clinical response reflects impaired integrin-mediated adhesion and/or migration. Here, we show that PCI-32765 strongly inhibits BCR-controlled signaling and integrin α4β1-mediated adhesion to fibronectin and VCAM-1 of lymphoma cell lines and primary CLL cells. Furthermore, PCI-32765 also inhibits CXCL12-, CXCL13-, and CCL19-induced signaling, adhesion, and migration of primary CLL cells. Our data indicate that inhibition of BTK by PCI-32765 overcomes BCR- and chemokine-controlled integrin-mediated retention and homing of malignant B cells in their growth- and survival-supporting lymph node and bone marrow microenvironment, which results in clinically evident CLL regression.
Introduction
Chronic lymphocytic leukemia (CLL), the most common adult leukemia, is an incurable malignancy of mature B lymphocytes characterized by the accumulation of resting malignant B cells in peripheral blood and the presence of proliferating malignant B cells in the lymph nodes (LN), spleen, and bone marrow (BM).1-3 It is well established that the tumor microenvironment plays a major role in the pathogenesis of CLL: various cytokines, chemokines, and adhesion molecules provided within the LN, spleen, and BM microenvironment, as well as signaling by the B-cell antigen receptor (BCR), play a critical role in the localization, growth, survival, and drug resistance of CLL cells.2-9
Because either tonic, chronic, or antigen-driven BCR signaling is involved in the pathogenesis of most types of B-cell malignancies, the BCR signalosome provides a rational therapeutic target, including for CLL.9 Regarding selectivity and clinical safety, Bruton tyrosine kinase (BTK) is a particularly promising target: it is a key component of the BCR signaling pathway, is only critical for B cells, and loss of BTK function is not lethal (eg, X-linked agammaglobulinemia patients and Btk-deficient mice).10 Indeed, the selective, potent, orally administered, and irreversible small-molecule BTK-inhibitor PCI-3276511 shows promising clinical activity in phase 1 and 2 studies in B-cell non-Hodgkin lymphoma, including complete or partial remission in a significant proportion of enrolled patients with diffuse large B-cell lymphoma, mantle cell lymphoma, and CLL.12-14
Interestingly, the CLL patients display an unexpected clinical response on treatment with PCI-32765: a rapid (within days) and sustained reduction of lymphadenopathy is accompanied by transient lymphocytosis, which is reversible upon temporary deprivation of the drug13 (R. Advani, J.J.B., and N. Fowler, unpublished observations, 2010). Notably, some of the other efficacious small-molecule drugs that target the BCR signaling pathway, that is, inhibitors of SYK (R788/R406) and PI3K (CAL-101), show a similar response in clinical trials with CLL.15,16 On the basis of our previous studies on B-cell adhesion and migration,17-19 we hypothesized that this clinical response reflects attenuated microenvironment retention and homing of the CLL cells because of impaired BCR- or chemokine-controlled integrin-mediated adhesion or migration.
Methods
Namalwa, Daudi, L363, or primary patient CLL cells, pretreated for 1 hour with 1μM PCI-32765, were allowed to adhere to either fibronectin- or VCAM-1–coated 96-well plates in the presence of anti-IgM, phorbol 12-myristate 13-acetate (PMA), or chemokines (CXCL12, CXCL13, or CCL19), or were allowed to migrate toward these chemokines in VCAM-1–coated transwells, essentially as described previously.18,20 For further details and other methods, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
This study was conducted and approved by the Academic Medical Center Medical Committee on Human Experimentation. Informed consent was obtained in accordance with the Declaration of Helsinki.
Results and discussion
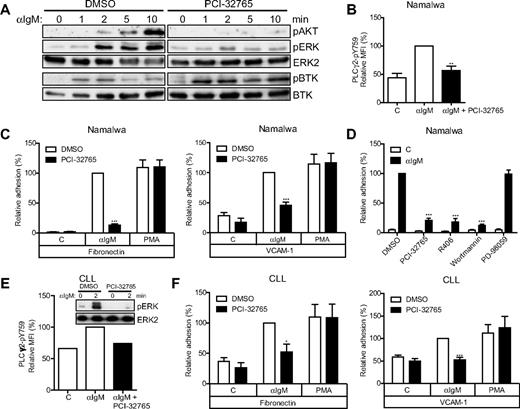
In line with the reported BTK specificity of PCI-32765,11,21 in the Burkitt lymphoma cell line Namalwa, we observed concentration-dependent inhibition by PCI-32765 of anti-IgM–induced phosphorylation of protein kinase B (PKB/AKT), ERK, and the BTK substrate site Y759 of phospholipase C-γ2 (PLC-γ2), whereas phosphorylation of the activating LYN/SYK substrate site Y551 of BTK itself was actually augmented, which suggests inhibition of BTK-mediated negative feedback (Figure 1A-B; supplemental Figure 1A). Moreover, anti-IgM–induced adhesion to the extracellular matrix component fibronectin and the cellular adhesion molecule VCAM-1, which is mediated by integrin α4β1,18 was almost completely abolished by PCI-32765 treatment (Figure 1C; supplemental Figure 1B). In contrast, integrin-mediated adhesion induced by PMA, which activates protein kinase C downstream of BTK, was not affected (Figure 1C). This demonstrates that the observed inhibitory effect of PCI-32765 on BCR-controlled integrin activation was specific and not caused by general cellular insensitivity or toxicity. Targeting of PI3K (wortmannin) or SYK (R406), but not MEK (PD-98059), also abolished BCR-controlled integrin-mediated adhesion (Figure 1D).
PCI-32765 abrogates BCR-controlled signaling and adhesion. (A) Namalwa cells pretreated with 1μM PCI-32765 were stimulated with αIgM and immunoblotted for phosphorylated (p) AKT, ERK, and BTK (pY551). Total ERK2 and BTK were used as loading controls. The blots are representative of 4 independent experiments. (B) Namalwa cells pretreated with PCI-32765 were stimulated with αIgM, and phosphorylated phospholipase C-γ2 (PLCγ2-pY759) was measured by flow cytometry (n = 6). (C) Namalwa cells pretreated with PCI-32765 were stimulated with αIgM or PMA and allowed to adhere to fibronectin-coated (n = 13) or VCAM-1–coated (n = 8) surfaces. (D) Namalwa cells pretreated with PCI-32765 (BTK inhibitor), R406 (SYK inhibitor), wortmannin (PI3K inhibitor), or PD-98059 (MEK inhibitor) were stimulated with αIgM and allowed to adhere to fibronectin-coated surfaces (n = 7). (E) Primary CLL cells (patient 898) pretreated with 1μM PCI-32765 were stimulated with αIgM and immunoblotted for pERK. Total ERK2 was used as loading control. Phosphorylated PLCγ2 (pY759) was measured by flow cytometry from the same patient sample. (F) CLL cells pretreated with PCI-32765 were stimulated with αIgM or PMA and allowed to adhere to fibronectin-coated (n = 5 patients) or VCAM-1–coated (n = 6 patients) surfaces. Graphs are presented as normalized mean + SEM (100% = stimulated cells without inhibitors). C indicates control (unstimulated); and MFI, mean fluorescence intensity. *P < .05; **P < .01; ***P < .001.
PCI-32765 abrogates BCR-controlled signaling and adhesion. (A) Namalwa cells pretreated with 1μM PCI-32765 were stimulated with αIgM and immunoblotted for phosphorylated (p) AKT, ERK, and BTK (pY551). Total ERK2 and BTK were used as loading controls. The blots are representative of 4 independent experiments. (B) Namalwa cells pretreated with PCI-32765 were stimulated with αIgM, and phosphorylated phospholipase C-γ2 (PLCγ2-pY759) was measured by flow cytometry (n = 6). (C) Namalwa cells pretreated with PCI-32765 were stimulated with αIgM or PMA and allowed to adhere to fibronectin-coated (n = 13) or VCAM-1–coated (n = 8) surfaces. (D) Namalwa cells pretreated with PCI-32765 (BTK inhibitor), R406 (SYK inhibitor), wortmannin (PI3K inhibitor), or PD-98059 (MEK inhibitor) were stimulated with αIgM and allowed to adhere to fibronectin-coated surfaces (n = 7). (E) Primary CLL cells (patient 898) pretreated with 1μM PCI-32765 were stimulated with αIgM and immunoblotted for pERK. Total ERK2 was used as loading control. Phosphorylated PLCγ2 (pY759) was measured by flow cytometry from the same patient sample. (F) CLL cells pretreated with PCI-32765 were stimulated with αIgM or PMA and allowed to adhere to fibronectin-coated (n = 5 patients) or VCAM-1–coated (n = 6 patients) surfaces. Graphs are presented as normalized mean + SEM (100% = stimulated cells without inhibitors). C indicates control (unstimulated); and MFI, mean fluorescence intensity. *P < .05; **P < .01; ***P < .001.
Screening a panel of CLL patients revealed that CLL cells obtained from the majority (ie, 77%) of patients with a germline unmutated BCR, which have a worse prognosis,2,3,9 could be further stimulated to adhere to either VCAM-1 or fibronectin by anti-IgM treatment, in contrast to the mutated BCR subgroup (supplemental Table 1). This most likely reflects the relatively anergic state of the mutated CLL cells with regard to BCR signaling,22 as supported by their prominent adhesion in response to PMA and chemokines (supplemental Table 1). Importantly, in all unmutated CLL anti-IgM responders, apart from inhibition of BCR signaling (Figure 1E), PCI-32765 strongly inhibited the anti-IgM–stimulated integrin-mediated adhesion to fibronectin (mean inhibition ∼ 75%) and VCAM-1 (mean inhibition ∼ 100%; Figure 1F). PMA-stimulated adhesion was unaffected (Figure 1F), which again excluded cellular toxicity and demonstrated the specificity of PCI-32765, also in CLL cells.
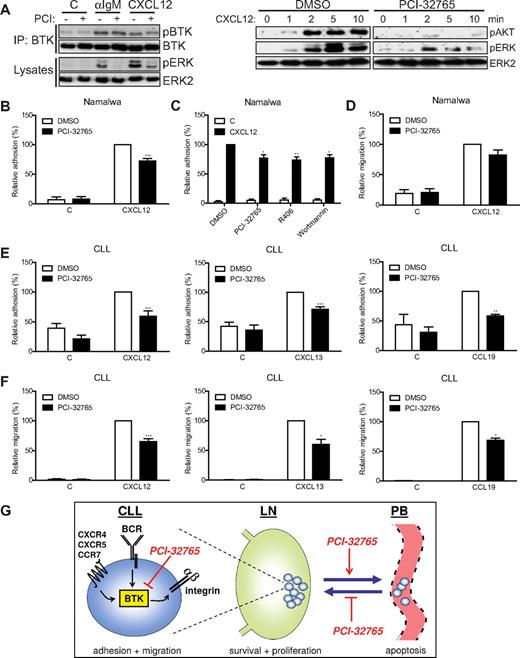
Next, we addressed the possible effect of PCI-32765 on chemokine responses. The chemokine CXCL12 also induced phosphorylation of the activating LYN/SYK substrate site Y551 of BTK (Figure 2A). Apart from concentration-dependent inhibition of CXCL12-induced phosphorylation of AKT and ERK, but not BTK (Figure 2A; supplemental Figure 1A), CXCL12-induced adhesion to VCAM-1 was inhibited in part by treatment of the Namalwa cells with PCI-32765 (Figure 2B). A similar reduction was observed with the SYK inhibitor R406 and the PI3K inhibitor wortmannin (Figure 2C). CXCL12-induced signaling and adhesion of BTK-negative L363 myeloma cells was not affected, which demonstrates the BTK specificity of the PCI-32765 effect (supplemental Figures 1A and 2A). Furthermore, migration of Namalwa cells toward CXCL12 was consistently but not significantly inhibited by PCI-32765 (Figure 2D), whereas prominent inhibition was observed in Daudi B cells (supplemental Figure 2B-C).
PCI-32765 inhibits chemokine-induced signaling, adhesion and migration. (A) Namalwa cells pretreated with 1μM PCI-32765 were stimulated with αIgM or CXCL12, and total BTK was immunoprecipitated and subsequently immunoblotted for phosphorylated (p) BTK. Total BTK was used as loading control. Cell lysates were immunoblotted for pAKT and pERK. Total ERK2 was used as loading control. The blots are representative of 4 independent experiments. (B) Namalwa cells pretreated with PCI-32765 were allowed to adhere to surfaces coated with both VCAM-1 and CXCL12 (n = 10). (C) Namalwa cells pretreated with PCI-32765 (BTK inhibitor), R406 (SYK inhibitor), or wortmannin (PI3K inhibitor) were allowed to adhere to surfaces coated with both VCAM-1 and CXCL12 (n = 5). (D) Namalwa cells pretreated with PCI-32765 were allowed to migrate toward CXCL12 on VCAM-1–coated transwells (n = 7). (E) CLL cells pretreated with 1μM PCI-32765 were allowed to adhere to surfaces coated with both VCAM-1 and either CXCL12 (n = 7 patients), CXCL13 (n = 5 patients), or CCL19 (n = 3 patients). (F) CLL cells pretreated with 1μM PCI-32765 were allowed to migrate toward CXCL12 (n = 6 patients), CXCL13 (n = 4 patients), or CCL19 (n = 3 patients) on VCAM-1–coated transwells. (G) Inhibition of BTK by PCI-32765 impairs BCR-controlled integrin-mediated adhesion and chemokine (CXCL12, CXCL13, and CCL19)–induced adhesion and migration of CLL cells. Consequently, PCI-32765 overcomes BCR- and chemokine-controlled integrin-mediated retention of CLL cells in their growth- and survival-supporting LN and BM microenvironment, which results in their egress from these protective niches into the circulation (peripheral blood), and will prevent chemokine-driven homing into these niches, resulting in CLL regression. Graphs are presented as normalized mean + SEM (100% = stimulated cells without inhibitors). IP indicates immunoprecipitation; C, control (absence of chemokines); and PB, peripheral blood. *P < .05; **P < .01; ***P < .001.
PCI-32765 inhibits chemokine-induced signaling, adhesion and migration. (A) Namalwa cells pretreated with 1μM PCI-32765 were stimulated with αIgM or CXCL12, and total BTK was immunoprecipitated and subsequently immunoblotted for phosphorylated (p) BTK. Total BTK was used as loading control. Cell lysates were immunoblotted for pAKT and pERK. Total ERK2 was used as loading control. The blots are representative of 4 independent experiments. (B) Namalwa cells pretreated with PCI-32765 were allowed to adhere to surfaces coated with both VCAM-1 and CXCL12 (n = 10). (C) Namalwa cells pretreated with PCI-32765 (BTK inhibitor), R406 (SYK inhibitor), or wortmannin (PI3K inhibitor) were allowed to adhere to surfaces coated with both VCAM-1 and CXCL12 (n = 5). (D) Namalwa cells pretreated with PCI-32765 were allowed to migrate toward CXCL12 on VCAM-1–coated transwells (n = 7). (E) CLL cells pretreated with 1μM PCI-32765 were allowed to adhere to surfaces coated with both VCAM-1 and either CXCL12 (n = 7 patients), CXCL13 (n = 5 patients), or CCL19 (n = 3 patients). (F) CLL cells pretreated with 1μM PCI-32765 were allowed to migrate toward CXCL12 (n = 6 patients), CXCL13 (n = 4 patients), or CCL19 (n = 3 patients) on VCAM-1–coated transwells. (G) Inhibition of BTK by PCI-32765 impairs BCR-controlled integrin-mediated adhesion and chemokine (CXCL12, CXCL13, and CCL19)–induced adhesion and migration of CLL cells. Consequently, PCI-32765 overcomes BCR- and chemokine-controlled integrin-mediated retention of CLL cells in their growth- and survival-supporting LN and BM microenvironment, which results in their egress from these protective niches into the circulation (peripheral blood), and will prevent chemokine-driven homing into these niches, resulting in CLL regression. Graphs are presented as normalized mean + SEM (100% = stimulated cells without inhibitors). IP indicates immunoprecipitation; C, control (absence of chemokines); and PB, peripheral blood. *P < .05; **P < .01; ***P < .001.
Finally, we studied the effect of PCI-32765 on the response of primary CLL cells to CXCL12, CXCL13, and CCL19, the major chemokines involved in homing and retention of CLL cells in the LN and BM microenvironment.5 As far as determined, the CLL cells of all patients expressed the corresponding receptors, that is, CXCR4, CXCR5, and CCR7 (supplemental Figure 3A), and the CLL cells of most patients showed enhanced adhesion or migration in response to these chemokines, irrespective of the IgHV mutation status (supplemental Table 1). PCI-32765 inhibited chemokine-induced signaling (supplemental Figure 3B), and without exception, strongly inhibited CXCL12-, CXCL13-, and CCL19-induced adhesion of CLL cells (Figure 2E). Furthermore, without compromising cell viability (supplemental Figure 4), PCI-32765 partially inhibited (30%-40%) migration of the CLL cells toward these chemokines (Figure 2F).
Taken together, the present data demonstrate that inhibition of BTK by PCI-32765 impairs BCR-controlled adhesion and chemokine-controlled adhesion and migration of CLL cells. These findings in human primary CLL cells are corroborated by previous studies in which we observed impaired BCR-controlled adhesion and reduced CXCL12-/CXCL13-controlled adhesion, migration, and LN homing of Btk-deficient chicken or murine B cells.18,19 The present results nicely explain the observed clinical response of CLL patients treated with PCI-3276512-14 : Impaired BCR- and chemokine-controlled retention of malignant cells in the BM and LN will cause the observed reduced lymphadenopathy and (transient) lymphocytosis, which deprives the cells of critical microenvironmental growth and survival signals, resulting in tumor regression, whereas temporary drug deprivation restores chemokine-driven LN homing and retention of CLL cells (Figure 2G). In accordance, shortly after PCI-32765 treatment of the patients, most circulating CLL cells displayed low CXCR4 expression (supplemental Figure 5), characteristic of LN- and BM-derived CLL cells.5,8 Likewise, because targeting SYK and PI3K also inhibits BCR- and CXCL12-controlled adhesion (Figures 1D and 2C), as well as CXCL12-induced migration,6,23,24 this may also explain the observed lymphocytosis in clinical trials of CLL patients with the SYK inhibitor R788/fostamatinib (the R406 prodrug) and the PI3K-δ inhibitor CAL-101.15,16
These novel insights regarding drugs that target the BCR (and chemokine receptor) signalosome may not only provide support for their further use as monotherapy, exploiting the microenvironment dependence as the Achilles' heel of CLL, but also for their exploration as rational combination therapy. Once the malignant B cells egress from their protective niches into circulation, they may become more accessible and vulnerable to chemotherapy and antibody therapy (eg, rituximab). This would make a promising and highly efficacious combination therapy, possibly resulting in greater benefit for CLL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Robbert Hoogeboom for providing some of the CLL patient samples.
This work was supported by Pharmacyclics Inc.
Authorship
Contribution: M.F.M.d.R. designed the research, performed experiments, analyzed the data, designed the figures, and wrote the manuscript; A.K. performed experiments; C.R.G. and E.E. provided CLL patient samples and data and reviewed the manuscript; B.Y.C. performed experiments and analyzed data; J.J.B. designed the research and reviewed the manuscript; S.T.P cosupervised the study and reviewed the manuscript; and M.S. designed the research, supervised the study, analyzed the data, and wrote and revised the manuscript.
Conflict-of-interest disclosure: B.Y.C. and J.J.B. are employees of Pharmacyclics Inc and have a financial interest in PCI-32765. M.S. has received research support from Pharmacyclics Inc. The remaining authors declare no competing financial interests.
Correspondence: Marcel Spaargaren, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: marcel.spaargaren@amc.uva.nl.
References
Author notes
S.T.P. and M.S. share senior authorship.