Abstract
Exposure to ambient particulate matter (PM) air pollution has been reported to trigger inflammation and thrombosis. However, molecular mechanisms underlying the modulation of coagulation pathways in PM-induced thrombosis remain largely unknown. We report here that Sirt1, a member of class III histone deacetylase, controls lung inflammation and coagulation after PM exposure. Sirt1 knock-out mice exhibited aggravated lung vascular leakage and inflammation after PM exposure, which was correlated with increased NF-κB acetylation and activation. Furthermore, Sirt1 knock-out mice were highly susceptible to PM-induced lung coagulation as demonstrated by increased fibrin formation. The increased fibrin formation was associated with reduced tissue factor pathway inhibitor (TFPI) expression and increased plasminogen activator inhibitor-1 (PAI-1) activity in the lungs, thus favoring elevated coagulation and disrupted fibrinolysis responses. Thrombomodulin (TM), a central player of the anticoagulant protein C system, is regulated by Kruppel-like factor 2 (KLF2) at the transcriptional level. Our data show that PM exposure led to decreased lung KLF2 and TM expression in wild-type mice, and lung KLF2 and TM protein levels were further decreased in Sirt1 knock-out mice. Importantly, Sirt1 gene delivery inhibited TM and KLF2 down-regulation and reduced lung coagulation after PM exposure. Collectively, our studies indicate that Sirt1 functions as a suppressor of coagulation after particulate matter exposure.
Introduction
Exposure to ambient particulate matter (PM) air pollution has been associated with impaired pulmonary function and increased cardiovascular disease-related mortality including inflammation and thrombosis.1-5 Acute exposure to increased ambient fine particulate matter < 2.5 μm in diameter (PM2.5) was estimated to cause the premature deaths of tens of thousands of people each year in the United States.6
Urban particulate matter (UPM) PM2.5, one of the major air pollutants closely monitored by the US Environmental Protection Agency (EPA), is a complex mixture of substances that are directly emitted into the air from dust, soot, smoke, and combustion.6 To protect public health, the EPA issued the current 24-hour PM2.5 standard at 35 μg/m3.6 Despite the enormous health problems caused by PM2.5 exposure, there have been few studies performed to explore the molecular mechanisms of PM2.5-induced lung vascular dysfunction. PM2.5 can reach deep in the lung to the small airway and alveoli,6 and directly cause lung inflammation and injury.7-13 The pulmonary complications may lead to detrimental effects on other organs, particularly cardiovascular dysfunction such as systemic microvascular dysfunction and thrombosis.1,2,4,5,13 A recent study demonstrated that short-term UPM exposure triggered the activation of primary hemostasis in healthy mice, with no substantial secondary hemostasis activation, leading to arterial but not venous thrombosis.5 Whereas another study showed that short-term UPM exposure may cause activation of coagulation responses and fibrin formation in the lung.14
Tissue factor pathway inhibitor (TFPI) and plasminogen activator inhibitor-1 (PAI-1) play important roles in coagulation and fibrinolysis cascades, respectively. TFPI is an inhibitor of tissue factor (TF)–mediated coagulation responses.15 PAI-1, on the other hand, is a major regulator of fibrinolysis, which functions by inhibiting the activity of tissue plasminogen activator (t-PA).16 PAI-1 inhibition of t-PA activity may lead to excessive fibrin formation. Thrombomodulin (TM), a transmembrane protein predominantly expressed in endothelial cells, is a major component of the TM/protein C anticoagulant system.17 TM binds to thrombin to suppress thrombin activity and alter thrombin substrate specificity to generate activated protein C (APC).17 APC then degrades coagulation factors to inhibit thrombin formation.17 TM also has anti-inflammatory activity.18-20 TM is highly expressed in lung endothelial cells.20 TF/TFPI, t-PA/PAI-1, and TM/protein C systems are known to play distinct regulatory roles in coagulation and fibrinolysis pathways. The molecular mechanisms underlying their regulation after PM exposure, nevertheless, remain elusive.
Sirt1, a ubiquitously expressed deacetylase of the sirtuin (class III histone deacetylase) family,21 regulates a wide variety of cellular responses including cell proliferation, apoptosis, and inflammatory responses by protein deacetylation.21-26 NF-κB, a transcription factor that regulates inflammatory responses, is an endogenous substrate for Sirt1.27-30 It has been reported that Sirt1 deacetylates NF-κB, thereby inhibiting its activity in several in vitro and in vivo systems.27-30 NF-κB activation has been reported to suppress TM expression,31,32 implying that Sirt1 may be involved in protection against both inflammation and coagulation.
Sirt1 down-regulation has been associated with the development of age-related diseases including cardiovascular diseases, diabetes, neurodegeneration, and inflammation.21-26 Interestingly, Sirt1 expression in the lung is reduced in the elderly who are known to be particularly susceptible to PM-induced cardiopulmonary mortality.24,33 In this study, we investigated the role of Sirt1 in PM-induced lung coagulation.
Methods
Reagents
Tamoxifen was purchased from Sigma-Aldrich. Rat anti–mouse CD31 antibody was a product of BD Biosciences. Fibrin monoclonal antibody specific for β-chain of fibrinogen was obtained from American Diagnostica. Mouse plasmin was obtained from HTI. Thrombomodulin antibody was purchased from Santa Cruz Biotechnology. Rabbit anti–mouse KLF2 antibody was obtained from Millipore. Rabbit anti–mouse NF-κB/p65, acetyl-NF-κB/p65, histone H3, and Sirt1 antibodies were obtained from Cell Signaling. DyLight 488 goat anti–mouse IgG, DyLight 488 donkey anti–rabbit IgG, and DyLight 594 goat anti–rat IgG secondary antibody were from Biolegend.
Mice
Mice were housed in cages with access to food and water in a temperature-controlled room with a 12-hour dark/light cycle. All experiments and animal care procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler. Sirt1 floxed mice (Sirt1flox/flox) have been previously described.34 Sirt1flox/flox mice and transgenic UBC-Cre-ERT2 mice (The Jackson Laboratory) were crossed to generate Sirt1flox/flox/UBC-Cre-ERT2 mice. To induce Cre-mediated deletion of Sirt1 exon 4, Sirt1flox/flox/UBC-Cre-ERT2 mice, 6 to 7 weeks after the birth, were given tamoxifen (100 mg/Kg body weight) by intraperitoneal injection daily for 5 days to induce nuclear translocation of Cre recombinase as described in our previous study.35 Ten days after tamoxifen treatment, sex and age matched Sirt1−/− and Sirt1+/+ littermates were used in the studies. Wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory. Eight- to 10-week-old female mice were used in the study.
PM2.5 exposure
In vivo gene delivery
Sirt1 gene delivery to mouse lungs was conducted as previously described using the polyethylenimine (PEI) reagent (Fermentas).37 PEI is a nonviral, nonliposomal gene delivery reagent that can be used at a nontoxic level with a small quantity of DNA. PEI reagent has been shown to provide highly efficient and nontoxic gene delivery to mouse lungs including lung interstitial and endothelial tissues.37 Sirt1 plasmid (pAdTrack-Sirt1) and control plasmid (pAdTrack-CMV) were obtained from Addgene. Briefly, 5 μg of DNA was dissolved in 100 μL sterile PBS containing 5% glucose. PEI, 1.8 μL diluted in 50 μL of 5% glucose solution, was added to 100 μL DNA solution and vortexed immediately. The DNA mixture was incubated for 10 minutes at room temperature and given to mice by tail vein injection. Forty-eight hours after Sirt1 DNA delivery, mice were exposed to PBS or PM2.5 by intranasal instillation.
Collection of mouse lung samples
Mice were anesthetized before the collection of lung samples. Five minutes before the end of the experiments, 400 U heparin was injected intravenously to prevent postmortem clotting. Lungs were then perfused with Ca2+/Mg2+-free PBS through right ventricle. Lungs were excised and homogenized in an ice-cold homogenization buffer (Ca2+/Mg2+-free PBS supplemented with protease inhibitor cocktail, plus 15 U/mL heparin). For some experiments, bronchoalveolar lavage (BAL) fluids were collected without lung perfusion. BAL fluids were used for cytokines and albumin ELISA assay as previously described.38
Immunofluorescence, histology, ELISA, and immunoblotting assays
Cryosections were prepared for use in immunofluorescence staining as previously described.31 Briefly, after fixation, lung sections were permeabilized, blocked, and incubated with antibodies against Sirt1 CD31, fibrin, or thrombomodulin, followed by fluorescence-conjugated secondary antibodies. Images were captured at room temperature using Nikon Elipse TE-2000-U microscope with a 40×/1.30 NA objective and NIS Elements software (Nikon). For histology study, lungs were inflated with 4% paraformaldehyde and fixed. Paraffin-embedded lung tissues were cut to 5 μm sections for H&E staining. BAL albumin, TNF-α, and IL-6 levels were determined by ELISA. Lung nuclear protein was extracted as previously described.39 Nuclear NF-κB DNA binding activity was measured using the TransAM NF-κB Transcription Factor Assay Kit from Active Motif as previously described.38,39 Blood plasma and lung PAI-1 levels were determined using the PAI-1 ELISA Kit from Cell Sciences. Protein expression was analyzed by immunoblotting.38,40 The fibrin polymers in samples were detected using fibrinogen β-chain–specific antibody as previously described.40,41 Briefly, aliquots of lung homogenates were incubated with 0.1 U plasmin at 37°C for 4 hours with shaking to release fibrin monomers. The samples were then centrifuged and supernatants were collected to examine the levels of fibrin formation by immunoblotting assay as previously described.40 Blots were scanned and analyzed using Quantity One Version 4.6.2 (Bio-Rad).
Cell counting in BAL fluids
Cell counting in BAL fluids was performed by flow cytometry. Cells were Fc blocked by CD16/32 antibody on ice for 10 minutes and then stained with fluorescence-labeled Gr-1 antibody. After washing, cells were resuspended in PBS and counted by flow cytometry as previously described.38 Neutrophils were gated as Gr-1high cells.
Statistical analysis
Data were analyzed and expressed as mean ± SEM. Student t test was used for all studies unless otherwise stated. Statistical significance was assigned to P values < .05.
Results
Conditional Sirt1 knock-out mice
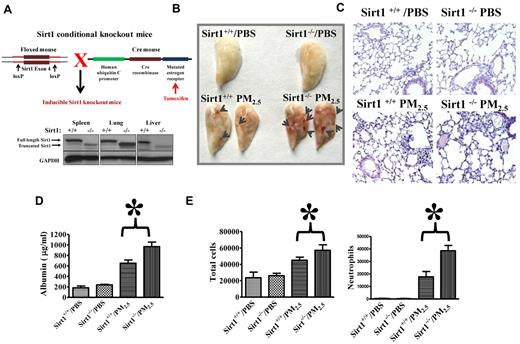
Inducible Sirt1 knock-out mice were used in the study. It was previously reported that deletion of the floxed exon 4 encoding 51 amino acids of the conserved Sirt1 catalytic domain generated a nonfunctional truncated Sirt1 protein.34,42 Sirt1flox/flox mice with or without Cre expression were used to generate Sirt1−/− or Sirt1+/+ mice as described in “Mice.” Sirt1 deletion was confirmed by immunoblotting. As previously reported,34 no full-length Sirt1 protein, but a nonfunctional truncated Sirt1 was detected in the tissues of Sirt1−/− mice after tamoxifen injection. In contrast, Sirt1+/+ littermates showed normal full-length Sirt1 expression (Figure 1A).
Aggravated lung injury and coagulation in Sirt1 knock-out mice after PM2.5 exposure. (A) To generate inducible Sirt1 knock-out mice, floxed Sirt1 mice containing a loxP-flanked Sirt1 exon 4 were bred with UBC-Cre-ERT2 mice. Tamoxifen-inducible and Cre-mediated recombination resulted in deletion of Sirt1 exon 4. Inducible knock-out mice were given tamoxifen by intraperitoneal injection. Ten days after tamoxifen treatment, spleen, lung, and liver were collected. Tissue homogenates were subjected to immunoblotting to confirm Sirt1 deletion. (B) Sirt1−/− and Sirt1+/+ littermate mice were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were perfused with PBS via the right ventricle to remove blood from the vascular bed of the lung. Lung images show that PM2.5 exposure led to blood clot formation (arrows). Images are representative of 6 mice in each experimental group. (C) Histologic examination by H&E staining shows increased leukocyte infiltration and blood clot formation in the lungs of Sirt1−/− mice after PM2.5 exposure. Images are representative of 4 mice in each experimental group. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, BAL fluids were collected. (D) BAL albumin levels were assessed by ELISA. (E) Total cells and neutrophils in the BAL fluids were counted by flow cytometry. Data are presented as mean + SEM (n ≥ 7 mice/group; *P < .05).
Aggravated lung injury and coagulation in Sirt1 knock-out mice after PM2.5 exposure. (A) To generate inducible Sirt1 knock-out mice, floxed Sirt1 mice containing a loxP-flanked Sirt1 exon 4 were bred with UBC-Cre-ERT2 mice. Tamoxifen-inducible and Cre-mediated recombination resulted in deletion of Sirt1 exon 4. Inducible knock-out mice were given tamoxifen by intraperitoneal injection. Ten days after tamoxifen treatment, spleen, lung, and liver were collected. Tissue homogenates were subjected to immunoblotting to confirm Sirt1 deletion. (B) Sirt1−/− and Sirt1+/+ littermate mice were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were perfused with PBS via the right ventricle to remove blood from the vascular bed of the lung. Lung images show that PM2.5 exposure led to blood clot formation (arrows). Images are representative of 6 mice in each experimental group. (C) Histologic examination by H&E staining shows increased leukocyte infiltration and blood clot formation in the lungs of Sirt1−/− mice after PM2.5 exposure. Images are representative of 4 mice in each experimental group. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, BAL fluids were collected. (D) BAL albumin levels were assessed by ELISA. (E) Total cells and neutrophils in the BAL fluids were counted by flow cytometry. Data are presented as mean + SEM (n ≥ 7 mice/group; *P < .05).
Exacerbated lung injury and coagulation in Sirt1 knock-out mice after PM2.5 exposure
We first examined the dose response of PM2.5 exposure on lung coagulation. Twenty-four hours after PM exposure, the lungs of heparinized mice were perfused with PBS to remove blood. Lung tissue fibrin levels were assessed by immunoblotting. PBS-exposed mice showed little fibrin production. In contrast, PM2.5-exposed mice showed lung fibrin formation in a dose-dependent manner (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). PM2.5 usually concentrates in areas close to roadways, power plants, and places with active wood burning.6 The doses used in our study have been reported to be comparable with the amount of respiratory exposure found in those neighborhoods).12,43 To explore the role of Sirt1 in PM-induced lung coagulation, inflammation and vascular dysfunction, Sirt1−/− mice and Sirt1+/+ littermates were exposed to PBS or 100 μg PM2.5 by intranasal instillation as previously described.7,12 Blood clots were observed on the lung surface of both Sirt1+/+ and Sirt1−/− mice after PM2.5 exposure, which could not be removed by PBS perfusion from the right ventricle. The clot formation was more severe in the lungs of Sirt1−/− mice, indicating increased lung coagulation in Sirt1−/− mice after PM2.5 exposure (Figure 1B). H&E staining showed increased leukocyte infiltration and blood vessel clot formation in the lungs of Sirt1−/− mice (Figure 1C). Furthermore, PM2.5 exposure led to lung vascular leakage as indicated by high BAL albumin levels, which were further elevated in Sirt1−/− mice. Total cell and neutrophil counts in BAL fluids were also markedly increased in Sirt1−/− mice after PM2.5 exposure (Figure 1D).
Sirt1 modulates NF-κB acetylation/activation and lung inflammation after PM2.5 exposure
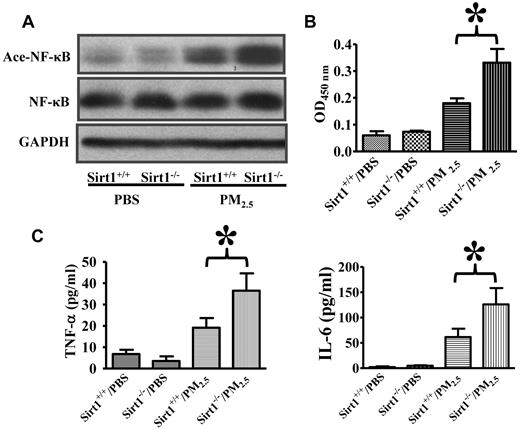
Inflammation is known to promote coagulation responses.44 NF-κB activation has been shown to up-regulate the expression of a wide variety of inflammatory mediators.45,46 It has been reported that Sirt1 inhibits NF-κB activity by deacetylation.27-30 We therefore decided to examine whether Sirt1 may modulate NF-κB acetylation and activation after PM2.5 exposure. Lung NF-κB acetylation was examined by immunoblotting using an antibody specific for acetylated NF-κB (Acetyl–NF-κB/p65 Lys310),27-30 and nuclear NF-κB DNA binding activity in the lung tissues was measured. Results showed that NF-κB acetylation and activation were induced in the lungs of both Sirt1+/+ and Sirt1−/− mice after PM2.5 exposure, with the levels of acetylation and activation being considerably higher in Sirt1−/− mice than in Sirt1+/+ littermates (Figure 2A-B). The levels of pro-inflammatory/prothrombotic cytokines, TNF-α and IL-6, were substantially higher in the BAL fluids of Sirt1−/− mice than those in Sirt1+/+ littermates after PM2.5 (Figure 2C), which might have contributed to the aggravated lung inflammation and coagulation in Sirt1−/− mice.
Sirt1 regulates NF-κB acetylation/activation and lung inflammation after PM2.5 exposure. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. (A) Six hours later, lung homogenates prepared were subjected to immunoblotting to examine NF-κB acetylation (Ace-NF-κB). Blots are representative of 5 mice in each experimental group. (B) NF-κB DNA binding activity was measured by ELISA. Data are presented as mean + SEM (n ≥ 7 mice/group; *P < .05). (C) Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, BAL TNF-α and IL-6 levels were assessed by ELISA. Data are presented as mean + SEM (n = 7 mice/group; *P < .05).
Sirt1 regulates NF-κB acetylation/activation and lung inflammation after PM2.5 exposure. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. (A) Six hours later, lung homogenates prepared were subjected to immunoblotting to examine NF-κB acetylation (Ace-NF-κB). Blots are representative of 5 mice in each experimental group. (B) NF-κB DNA binding activity was measured by ELISA. Data are presented as mean + SEM (n ≥ 7 mice/group; *P < .05). (C) Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, BAL TNF-α and IL-6 levels were assessed by ELISA. Data are presented as mean + SEM (n = 7 mice/group; *P < .05).
Sirt1 controls fibrin formation in the lung after PM2.5 exposure
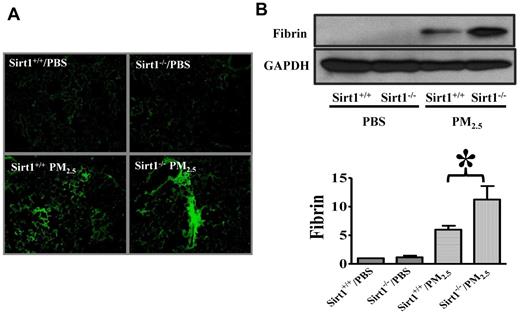
Thrombin converts fibrinogen to fibrin, which then forms blood clots.17 To confirm the role of Sirt1 in lung coagulation, we assessed fibrin production in the lung by immunofluorescence assay. Sirt1 knock-out mice exhibited more fibrin deposition in lung tissues than did their Sirt1+/+ littermates after PM2.5 exposure (Figure 3A). Immunoblotting also showed that PM2.5-induced fibrin production in the lungs of Sirt1−/− mice was markedly higher than that in Sirt1+/+ littermates, indicating that lung coagulation was indeed aggravated in Sirt1−/− mice (Figure 3B).
Sirt1 modulates fibrin production in the lung after PM2.5 exposure. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. (A) Fibrin immunofluorescent staining of lung cryosections of PM2.5 24 hour treated lung tissues. Images are representative of 6 independent experiments. (B) Lung fibrin generation was examined by immunoblotting. Blots and normalized bar graphs show fibrin production in the lung. Data are presented as mean + SEM (n = 6 mice/group; *P < .05).
Sirt1 modulates fibrin production in the lung after PM2.5 exposure. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. (A) Fibrin immunofluorescent staining of lung cryosections of PM2.5 24 hour treated lung tissues. Images are representative of 6 independent experiments. (B) Lung fibrin generation was examined by immunoblotting. Blots and normalized bar graphs show fibrin production in the lung. Data are presented as mean + SEM (n = 6 mice/group; *P < .05).
Sirt1 regulation of TFPI and PAI-1 in the lung after PM2.5 exposure
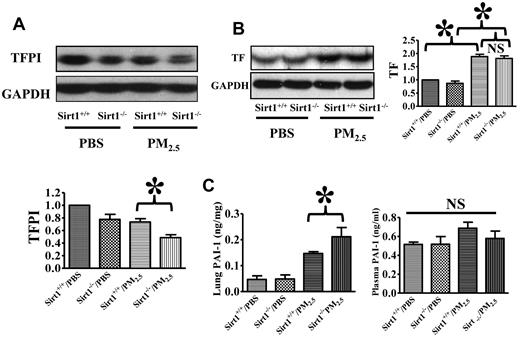
To investigate Sirt1 regulation of coagulation and fibrinolysis pathways in the lung after PM2.5 exposure, we examined TF, TFPI, and PAI-1 expression. Results showed that lung TFPI levels were decreased after PM2.5 exposure. Sirt1 deletion resulted in further reduction of TFPI level, which might have contributed to enhanced fibrin deposition in Sirt1−/− mice (Figure 4A). TF levels were increased in the lung tissues after PM2.5 exposure, whereas there was no significant difference in PM-induced TF expression between Sirt1−/− and Sirt1+/+ littermates (Figure 4B). PAI-1levels in the lung were also increased after PM2.5 exposure, and was higher in the lungs of Sirt1−/− mice than in Sirt1+/+ littermate. Interestingly, plasma PAI-1 assay revealed only a minor change after PM2.5 exposure, and there was no significant difference between Sirt1−/− and Sirt1+/+ littermates (Figure 4C). These results suggest that Sirt1 may function as a modulator for both coagulation and fibrinolysis.
Sirt1 regulation of TFPI, PAI-1 and TF expression in the lung. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. TFPI (A) and TF (B) expression was examined by immunoblotting. The blots and normalized bar graphs show TFPI and TF expression. Data are presented as mean + SEM (n ≥ 10 mice/group; *P < .05). (C) Lung and plasma PAI-1 levels were assessed by ELISA. Data are presented as mean + SEM (n = 8 mice/group; *P < .05).
Sirt1 regulation of TFPI, PAI-1 and TF expression in the lung. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. TFPI (A) and TF (B) expression was examined by immunoblotting. The blots and normalized bar graphs show TFPI and TF expression. Data are presented as mean + SEM (n ≥ 10 mice/group; *P < .05). (C) Lung and plasma PAI-1 levels were assessed by ELISA. Data are presented as mean + SEM (n = 8 mice/group; *P < .05).
Sirt1 expression in lung endothelial cells
To assess the contribution of endothelial cells in Sirt1 regulation of PM-induced lung coagulation, we examined the expression of Sirt1 in mouse lungs. Lung cryosections of WT mice were stained for Sirt1 and CD31 (a marker for endothelial cells).47 Immunofluorescence microscopy showed that Sirt1 was widely expressed in the lung including endothelial cells (Figure 5A).
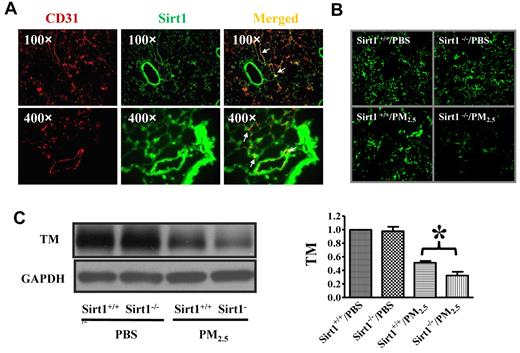
Sirt1 is expressed in lung endothelial cells and Sirt1 deletion increases PM2.5-induced TM down-regulation in the lung. (A) Immunofluorescence images showing Sirt1 expression (green) in lung tissues of WT C57BL/6 mice. No staining was detected using control normal IgG (data not shown). CD31 (red) was used as a specific marker for endothelial cells. Merged image indicates that Sirt1 is expressed in lung endothelial cells. Images are representative of 6 independent experiments. (B) Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Immunofluorescence microscopy revealed decreased TM expression in the lung after PM2.5 exposure. Images are representative of 6 independent experiments. (C) Lung homogenates were subjected to immunoblotting to examine TM expression. Blots and normalized bar graphs show TM expression in the lungs of Sirt1+/+ and Sirt1−/− littermates. Data are presented as mean + SEM (n ≥ 6 mice/group; *P < .05).
Sirt1 is expressed in lung endothelial cells and Sirt1 deletion increases PM2.5-induced TM down-regulation in the lung. (A) Immunofluorescence images showing Sirt1 expression (green) in lung tissues of WT C57BL/6 mice. No staining was detected using control normal IgG (data not shown). CD31 (red) was used as a specific marker for endothelial cells. Merged image indicates that Sirt1 is expressed in lung endothelial cells. Images are representative of 6 independent experiments. (B) Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Immunofluorescence microscopy revealed decreased TM expression in the lung after PM2.5 exposure. Images are representative of 6 independent experiments. (C) Lung homogenates were subjected to immunoblotting to examine TM expression. Blots and normalized bar graphs show TM expression in the lungs of Sirt1+/+ and Sirt1−/− littermates. Data are presented as mean + SEM (n ≥ 6 mice/group; *P < .05).
Decreased TM expression in Sirt1 knock-out mice after PM2.5 exposure
To explore the mechanisms underlying Sirt1 control of PM2.5-induced lung coagulation, we examined lung TM expression after PM2.5 exposure. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 by intranasal instillation. TM expression was found to be significantly decreased in the lungs of both Sirt1+/+ and Sirt1−/− littermates after PM2.5 exposure, and the level of TM was much lower in Sirt1−/− mice than in Sirt1+/+ littermates (Figure 5B-C), indicating that Sirt1 regulates the TM/PC anticoagulation system in the lung vasculature.
Sirt1 overexpression inhibits TM down-regulation after PM2.5 exposure
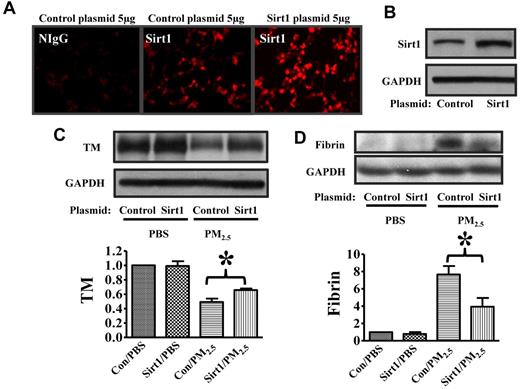
To further assess the protective effect of Sirt1 against PM2.5-induced lung coagulation, we used a PEI-plasmid in vivo transfection method to increase Sirt1 expression. Sirt1 gene delivery increased Sirt1 protein expression in the lung tissues, with high Sirt1 expression at 48 hours (Figure 6A-B) and 72 hours (data not shown) after transfection with Sirt1 plasmid. Importantly, Sirt1 overexpression inhibited PM2.5-induced TM down-regulation and fibrin deposition in the lung of WT mice (Figure 6C-D), which provided support for the protective role of Sirt1 against lung coagulation.
Sirt1 overexpression inhibits PM2.5-induced TM down-regulation and fibrin formation. WT C57BL/6 mice were given 5 μg of pAdTrack-CMV (control) or pAdTrack-Sirt1 (Sirt1) by tail vein injection as described in “In vivo gene delivery.” Forty-eight hours later, lungs were collected. (A) Lung cryosections were incubated with nonimmune IgG (NIgG) or Sirt1 antibody (Sirt1) for immunofluorescence study. (B) Lung homogenates were subjected to immunoblotting to examine Sirt1 expression (n = 4 mice/group). Forty-eight hours after Sirt1 gene delivery, mice were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Lung homogenates prepared were subjected to immunoblotting. Blots and normalized bar graphs show TM expression (C) and fibrin production (D) in the lungs of Sirt1+/+ and Sirt1−/− littermates. Data are presented as mean + SEM (n ≥ 6 mice/group; *P < .05).
Sirt1 overexpression inhibits PM2.5-induced TM down-regulation and fibrin formation. WT C57BL/6 mice were given 5 μg of pAdTrack-CMV (control) or pAdTrack-Sirt1 (Sirt1) by tail vein injection as described in “In vivo gene delivery.” Forty-eight hours later, lungs were collected. (A) Lung cryosections were incubated with nonimmune IgG (NIgG) or Sirt1 antibody (Sirt1) for immunofluorescence study. (B) Lung homogenates were subjected to immunoblotting to examine Sirt1 expression (n = 4 mice/group). Forty-eight hours after Sirt1 gene delivery, mice were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Lung homogenates prepared were subjected to immunoblotting. Blots and normalized bar graphs show TM expression (C) and fibrin production (D) in the lungs of Sirt1+/+ and Sirt1−/− littermates. Data are presented as mean + SEM (n ≥ 6 mice/group; *P < .05).
Sirt1 regulation of KLF2 expression in the lung after PM2.5 exposure
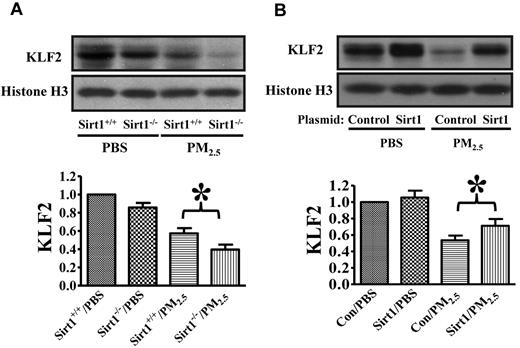
TM expression in endothelial cells is controlled by Kruppel-like factor 2 (KLF2) at transcriptional level.48 To explore the mechanisms of Sirt1 regulation of TM expression in the lung vasculature, we examined lung KLF2 expression after PM2.5 exposure. Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 by intranasal instillation. Afterward, lung nuclear protein was extracted as previously described.39 Nuclear KLF2 expression was found to be decreased in the lungs of both Sirt1+/+ and Sirt1−/− littermates after PM2.5 exposure, with the level being much lower in Sirt1−/− lungs than in Sirt1+/+ lungs (Figure 7A). In a separate study, Sirt1 overexpression was found to inhibit PM2.5-induced KLF2 down-regulation in the lungs of WT mice (Figure 7B), which indicates that Sirt1 could regulate lung TM expression by controlling KLF2 expression.
Sirt1 regulates KLF2 expression in the lung after PM2.5 exposure. (A) Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Nuclear proteins were extracted from the lung samples and subjected to immunoblotting to examine lung KLF2 expression. Data are presented as mean + SEM (n ≥ 7 mice/group). (B) WT C57BL/6 mice were given 5 μg of pAdTrack-CMV (control) or pAdTrack-Sirt1 (Sirt1) by tail vein injection as described “In vivo gene delivery.” Forty-eight hours after Sirt1 gene delivery, mice were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Nuclear proteins were extracted from the lung samples and subjected to immunoblotting to examine lung KLF2 expression. The blots and normalized bar graphs show KLF2 expression. Data are presented as mean + SEM (n ≥ 7 mice/group; *P < .05).
Sirt1 regulates KLF2 expression in the lung after PM2.5 exposure. (A) Sirt1+/+ and Sirt1−/− littermates were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Nuclear proteins were extracted from the lung samples and subjected to immunoblotting to examine lung KLF2 expression. Data are presented as mean + SEM (n ≥ 7 mice/group). (B) WT C57BL/6 mice were given 5 μg of pAdTrack-CMV (control) or pAdTrack-Sirt1 (Sirt1) by tail vein injection as described “In vivo gene delivery.” Forty-eight hours after Sirt1 gene delivery, mice were exposed to PBS or PM2.5 (100 μg) by intranasal instillation. Twenty-four hours later, lungs were collected. Nuclear proteins were extracted from the lung samples and subjected to immunoblotting to examine lung KLF2 expression. The blots and normalized bar graphs show KLF2 expression. Data are presented as mean + SEM (n ≥ 7 mice/group; *P < .05).
Discussion
PM exposure is known to be associated with increased risk of cardiovascular diseases.6 Altered coagulation responses and increased thrombosis have been suggested to be major mediators of PM-induced cardiopulmonary dysfunction.1-5 Pulmonary exposure to PM may cause both local and systemic inflammation, which could also contribute to increased thrombotic events.1-5 However, molecular mechanisms underlying these pathologic changes remain unclear.
The inflammation and coagulation cascades are intertwined events.31,32,44 TNF-α has been reported to down-regulate TM and KLF2 expression by activating NF-κB.49 An IL-6–mediated pathway has been demonstrated to play a critical role in PM-induced thrombotic events.2 Our data showed that the levels of pro-inflammatory/prothrombotic TNF-α and IL-6 were markedly increased in the lungs of Sirt1−/− mice after PM2.5 exposure, suggesting that Sirt1 may help maintain a quiescent environment.
NF-κB activation could be a potential link in PM-induced inflammation and coagulation responses. NF-κB is a master regulator of multiple inflammatory pathways.45,46 NF-κB also physically interacts with p300, a transcriptional coactivator required for many transcription factors including those controlling TM expression.31,32,49,50 NF-κB has been reported to down-regulate TM expression indirectly by competing for the limited pool of p300 in the nucleus.31,32,49,50 Acetylation has recently been demonstrated as a key mechanism for NF-κB activation.27,29,30 Our data showed that Sirt1 may act as a suppressor of NF-κB acetylation/activation in the mouse lung after PM2.5 exposure. Sirt1 knock-out mice were highly susceptible to PM2.5-induced lung coagulation and inflammation. These results reveal a new pathway that controls the initiation and amplification of NF-κB–mediated lung dysfunction after PM2.5 exposure.
TFPI inhibits TF-mediated coagulation cascades, and acts as a primary inhibitor of clot formation.15 It has been demonstrated that short-term PM exposure significantly decreased plasma TFPI level.51 Decreased circulating TFPI levels have been linked to venous thrombosis52 ; however, lung TFPI expression and regulation by Sirt1 after PM exposure have not been investigated. In this study, we demonstrated that lung TFPI expression was decreased after PM exposure, and Sirt1 deletion resulted in further reduction of TFPI levels in the lung, suggesting that Sirt1 could control coagulation responses by regulating TFPI expression.
PAI-1 plays an important role in fibrinolytic process by inhibiting the conversion of plasminogen to plasmin.53 PAI-1 is also a marker of endothelial cells dysfunction.53 Elevated PAI-1 promotes formation of endothelial microparticles and accelerates thrombin generation.54 Several studies have demonstrated an association between PM exposure and plasma PAI-1 levels in humans and in mice.51,55 In this study, we detected only a slight increase of circulating PAI-1 but a 3-fold increase of the lung PAI-1 level after PM2.5 exposure, indicating that the dose of PM2.5 exposure (100 μg/mouse) used in our studies is not sufficient to trigger systemic coagulation responses, but potent enough to induce localized lung pathologic changes. Although there was no significant difference in plasma PAI-1 levels between Sirt1−/− and Sirt1+/+ littermates, lung PAI-1 level was higher in Sirt1−/− mice after PM2.5 treatment, indicating that Sirt1 deletion may lead to abnormal lung PAI-1 expression. Therefore, Sirt1 may also act as a modulator of fibrinolytic responses in the lung.
The studies using Sirt1−/− mice suggest that Sirt1 may play a protective role in maintaining TM expression in lung endothelial cells after PM2.5 exposure. We also used a gene delivery method to overexpress Sirt1 in the lung to potentiate Sirt1 function. Sirt1 gene delivery was found to inhibit PM2.5-induced TM down-regulation in the lung. Fibrin production after PM2.5 exposure was also inhibited after Sirt1 gene delivery, which confirmed the role of Sirt1 in protection against PM2.5-induced lung coagulation. KLF2 is known to be a major regulator of TM expression.48 Our results showed that PM exposure led to the reduction of KLF2 expression in the lung. KLF2 expression was more profoundly suppressed in the lungs of Sirt1−/− mice than in Sirt1+/+ littermates. Interestingly, Sirt1 overexpression prevented PM2.5-induced KLF2 reduction. Our data therefore suggest that Sirt1 is a novel regulator of KLF2 expression, and that Sirt1 could regulate TM expression by modulating KLF2 expression in the lung. The mechanisms of Sirt1 regulation of KLF2 expression, nevertheless, remain to be determined. A recent study showed that Sirt1 activation could up-regulate KLF expression in endothelial cells via a myocyte enhancing factor 2 (MEF2)–dependent signaling pathway.56 Inhibition of MEF2 by NF-kB activation has also been shown to down-regulate KLF2 expression by inhibiting KLF2 promoter activity.49 Our data demonstrated that PM2.5 exposure led to NF-κB activation in mouse lungs, which was associated with decreased KLF2 expression.
The incidence of thrombosis increased sharply among the elderly,57 and the aged population has been reported to be highly susceptible to PM-induced cardiovascular diseases and mortality.6,33 Interestingly, Sirt1 expression is down-regulated in multiple organs during aging.24 In Sirt1flox/flox/Cre-ERT mice generated in the current study, Cre-ERT fusion protein expression is controlled by a human ubiquitin C (UBC) promoter, which drives tamoxifen-inducible and Cre-mediated Sirt1 deletion ubiquitously. The inducible Sirt1−/− mice used in our studies may mimic some of the responses to PM2.5 exposure in aging.21-26 Our study suggests that decreased Sirt1 expression in the elderly could be a potential risk factor for PM-induced coagulation and inflammation.
In conclusion, our studies demonstrate that Sirt1 modulates thrombomodulin, TFPI, PAI-1, and KLF2 expression, modulates NF-κB activation, and regulates inflammation/coagulation responses in the lung after PM exposure, which indicates that Sirt1 may control multiple proinflammatory and procoagulation pathways. Sirt1 functions as a suppressor of coagulation/inflammation axis. Our studies also indicate that Sirt1 is a potential therapeutic target for treating PM-induced cardiopulmonary dysfunction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs G. R. Scott Budinger, Asrar B. Malik, and Steven Idell for helpful discussion.
This work was supported by the American Heart Association National Scientist Development Award (J.F.), the Texas Allergy, Indoor Environmental, and Energy (TxAIRE) Institute Fund (J.F.), and the Parker B. Francis Pulmonary Research Award (J.F.).
Authorship
Contribution: Z.W. and J.F. designed research, performed research, analyzed data, and wrote the paper; and M.-C.L. and M.L. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jian Fu, Texas Lung Injury Institute, Center for Biomedical Research, University of Texas Health Science Center at Tyler, Tyler, Texas 75708; e-mail: jian.fu@uthct.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal