Abstract
To clarify which is preferable, a related donor with an HLA-1 Ag mismatch at the HLA-A, HLA-B, or HLA-DR loci in the graft-versus-host (GVH) direction (RD/1AG-MM-GVH) or an HLA 8/8-allele (HLA-A, HLA-B, HLA-C, and HLA-DRB1)–matched unrelated donor (8/8-MUD), we evaluated 779 patients with acute leukemia, chronic myelogenous leukemia, or myelodysplastic syndrome who received a T cell–replete graft from an RD/1AG-MM-GVH or 8/8-MUD. The use of an RD/1AG-MM-GVH donor was significantly associated with a higher overall mortality rate than the use of an 8/8-MUD in a multivariate analysis (hazard ratio, 1.49; P < .001), and this impact was statistically significant only in patients with standard-risk diseases (P = .001). Among patients with standard-risk diseases who received transplantation from an RD/1AG-MM-GVH donor, the presence of an HLA-B Ag mismatch was significantly associated with a lower overall survival rate than an HLA-DR Ag mismatch because of an increased risk of treatment-related mortality. The HLA-C Ag mismatch or multiple allelic mismatches were frequently observed in the HLA-B Ag-mismatched group, and were possibly associated with the poor outcome. In conclusion, an 8/8-MUD should be prioritized over an RD/1AG-MM-GVH donor during donor selection. In particular, an HLA-B Ag mismatch in the GVH direction has an adverse effect on overall survival and treatment-related mortality in patients with standard-risk diseases.
Introduction
An HLA-matched unrelated donor (MUD) is considered to be an alternative donor in hematopoietic stem cell transplantation (SCT) for patients who lack an HLA-identical sibling. However, it is difficult to find an MUD for patients with rare HLA haplotypes. SCT from a related donor with 1 Ag mismatch at HLA-A, HLA-B, or HLA-DR loci in the graft-versus-host (GVH) direction results in a higher but acceptable incidence of acute GVHD and outcomes comparable to that of SCT from a matched related donor (MRD) in patients with high-risk diseases because it reduces the risk of relapse via a graft-versus-leukemia (GVL) effect.1-3 In previous studies, HLA mismatches in the host-versus-graft (HVG) direction were associated with higher graft failure and lower overall survival (OS).1,2,4 However, strategies to reduce the risk of graft failure might have been improved by the use of conditioning regimens that strongly suppress recipient immune system.5 Therefore, in current clinical practice in Japan, SCT from a related donor with 1 Ag mismatch in the GVH direction and accepting multiple Ag mismatches in the HVG direction without specific stem cell manipulation is being performed,1,2 although such an approach has not yet been evaluated in a large cohort.
Our previous study showed that SCT from an HLA-1 Ag-mismatched donor in the GVH or HVG direction is comparable to that from an HLA-A, HLA-B, or HLA-DR Ag-MUD.1 However, this study is relatively old (1991-2000) and may not reflect current practice. Furthermore, the analysis was mainly performed based on serological matching, because information on HLA allele matching in unrelated transplantation was insufficient at that time. The importance of allele matching at the HLA-A, HLA-B, and HLA-DRB1 loci in unrelated donor transplantation has been established previously.6-8 In addition, the importance of allele matching at the HLA-C locus has been highlighted in several recent studies of unrelated transplantation, although HLA-C matching is, in general, still not considered in related transplantation.9-12 Therefore, we conducted a nationwide retrospective study to compare the clinical outcomes of transplantation from a related donor with an HLA-1 Ag mismatch at the HLA-A, HLA-B, or HLA-DR loci in the GVH direction (RD/1AG-MM-GVH) with an HLA 8/8-allele (HLA-A, HLA-B, HLA-C, and HLA-DRB1)–MUD (8/8-MUD).
Methods
Data collection
Data for patients 16-70 years of age with acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndrome (MDS), or chronic myelogenous leukemia (CML) who received a first allogeneic transplantation from a related donor or HLA-6/6–Ag-MUD between January 1, 2001 and December 31, 2008 were obtained from the Transplant Registry Unified Management Program,13 which includes data from the Japan Society for Hematopoietic Cell Transplantation and the Japan Marrow Donor Program. Our analysis included 344 patients who received a graft from an RD/1AG-MM-GVH donor and 453 patients who received a graft from an 8/8-MUD. The following patients were excluded: 11 patients who lacked data on survival status, survival date, sex of recipient and donor, stem cell source, GVHD prophylaxis, or performance status; 2 patients who received both BM and peripheral blood in related transplantation; and 5 patients who received stem cells manipulated by ex vivo T-cell depletion or CD34 selection. Finally, 327 patients who received a graft from an RD/1AG-MM-GVH donor and 452 patients who received a graft from an 8/8-MUD fulfilled the criteria. The data on 2318 patients who received transplantation from an MRD were also collected on the basis of similar inclusion and exclusion criteria to compare the OS rate. The study was approved by the data management committees of Transplant Registry Unified Management Program and by the institutional review board of Saitama Medical Center (Jichi Medical University, Saitama, Japan), where this study was organized.
Histocompatibility
Histocompatibility data for serological and genomic typing for the HLA-A, HLA-B, HLA-C, and HLA-DR loci were obtained from reports obtained from the institution at which the transplantation was performed. To reflect current practice in Japan, HLA matching in RD/1AG-MM-GVH donors was assessed by serological data for HLA-A, HLA-B, and HLA-DR loci, whereas that in 8/8-MUD was assessed by genomic data for HLA-A, HLA-B, HLA-C, and HLA-DR loci. When the recipient's Ags or alleles were not shared by the donor, this was considered an HLA mismatch in the GVH direction; when the donor's Ags or alleles were not shared by the recipient, this was considered a mismatch in the HVG direction. SCT from a related donor with 1 Ag mismatch in the GVH direction has been performed by accepting multiple Ag mismatches in the HVG direction,1,2 and therefore was included in this study.
End points and statistical analyses
The primary end point of the study was to compare OS rates between the RD/1AG-MM-GVH and 8/8-MUD groups. For exploratory purposes, OS, treatment-related mortality (TRM), relapse, acute and chronic GVHD, and cumulative incidences of neutrophil engraftment were analyzed in a subset of cohorts. The physicians who performed transplantation at each center diagnosed and graded acute and chronic GVHD according to standard criteria.14,15 The incidence of chronic GVHD was evaluated in patients who survived for at least 100 days. Neutrophil recovery was considered to have occurred when the absolute neutrophil count exceeded 0.5 × 109/L for 3 consecutive days after transplantation.
Descriptive statistics were used to summarize variables related to patient characteristics. Comparisons between groups were performed with the χ2 statistic or extended Fisher exact test as appropriate for categorical variables and the Mann-Whitney U test or the Kruskal-Wallis test as appropriate for continuous variables. The probability of OS was estimated according to the Kaplan-Meier method, and the groups were compared with the log-rank test. The probabilities of TRM, relapse, acute and chronic GVHD, and neutrophil engraftment were estimated on the basis of cumulative incidence curves to accommodate the following competing events16 : death for relapse, relapse for TRM, death without GVHD for acute and chronic GVHD, and death without engraftment for neutrophil engraftment; the groups were compared with a Gray test.17 Cox proportional-hazards regression was used to evaluate variables that may affect OS, whereas the Fine and Gray proportional-hazard model was used to evaluate variables that may affect TRM, relapse, acute and chronic GVHD, and neutrophil engraftment.18 For patients for whom conditioning intensity (myeloablative or reduced-intensity) was not reported, we reclassified the conditioning regimen as either myeloablative or reduced-intensity according to the National Marrow Donor Program/Center for International Blood and Marrow Transplant Research operational definitions.19 To be consistent with our previous study, acute leukemia in the first or second remission, CML in the first or second chronic phase, and MDS without leukemic transformation were defined as standard-risk diseases, and others were defined as high-risk diseases.1 The following variables were considered: the recipient's age group (≤ 50 years or > 50 years at transplantation), recipient's sex, presence of female (donor) to male (recipient) sex mismatch, performance status (0-1 or 2-4), disease (AML, ALL, CML, or MDS), disease status before transplantation (standard- or high-risk), type of conditioning regimen (myeloablative or reduced-intensity), type of GVHD prophylaxis (cyclosporine-based, tacrolimus-based, or other), use of antithymocyte globulin or alemtuzumab, and the time from diagnosis to transplantation (< 6 months or ≥ 6 months). In addition, a variable of graft source (BM or peripheral blood) was also considered in the analysis specific to related donors. Factors with P < .10 in the univariate analysis were used in the first multivariate model without donor type and deleted in a stepwise manner from the model by backward selection. We added donor type to the final model. All tests were 2-sided, and P < .05 was considered to indicate statistical significance. All statistical analyses were performed with STATA Version 11 software (StataCorp) and R Version 2.12.0 software (The R Foundation for Statistical Computing).
Results
Patient characteristics
Compared with recipients of an 8/8-MUD, recipients of an RD/1AG-MM-GVH were more likely to be younger, to be male receiving a transplantation from a female donor, to have a shorter duration from diagnosis to transplantation, to have a high-risk disease, to receive cyclosporine for GVHD prophylaxis, to receive antithymocyte globulin or alemtuzumab, and to have a longer follow-up period (Table 1). Approximately half of the recipients in the RD/1AG-MM-GVH group received peripheral blood stem cells, whereas during this period in Japan, the source of transplantation from an MUD was restricted to BM. In the RD/1AG-MM-GVH group, the number of Ag mismatches in the HVG direction was 0 in 11%, 1 in 67%, 2 in 20%, and 3 in 2%. HLA-A, HLA-B, and HLA-DRB1 allelic information in both recipients and donors was available in 148 of 327 transplantations from an RD/1AG-MM-GVH donor and information on HLA-C Ag mismatch in either the GVH or HVG direction was available in 123 of 327.
Patient characteristics
| Variable . | RD/1AG-MM-GVH (n = 327) . | 8/8 MUD (n = 452) . | P . |
|---|---|---|---|
| Median age at transplantation, y (range) | 45 (16-69) | 48 (16-68) | .043 |
| Recipient sex, n (%) | |||
| Male | 184 (56%) | 267 (59%) | .434 |
| Female | 143 (44%) | 185 (41%) | |
| Sex combination of donors and recipients, n (%) | |||
| Female to male | 91 (28%) | 73 (16%) | < .001 |
| Other combinations | 236 (72%) | 379 (84%) | |
| Performance status, n (%) | |||
| 0/1 | 298 (91%) | 415 (92%) | .736 |
| 2/3/4 | 29 (9%) | 37 (8%) | |
| Disease, n (%) | |||
| AML | 167 (51%) | 249 (55%) | .512 |
| ALL | 90 (28%) | 107 (24%) | |
| CML | 19 (6%) | 21 (5%) | |
| MDS | 51 (16%) | 75 (17%) | |
| Duration from diagnosis to transplantation, n (%) | |||
| < 6 mo | 124 (38%) | 102 (23%) | < .001 |
| ≥ 6 mo | 191 (58%) | 350 (77%) | |
| Unknown | 12 (4%) | 0 (0%) | |
| Disease risk, n (%) | |||
| Standard | 175 (54%) | 317 (70%) | < .001 |
| High | 133 (41%) | 129 (29%) | |
| Unknown | 19 (6%) | 6 (1%) | |
| Source of stem cells, n (%) | |||
| BM | 142 (43%) | 452 (100%) | < .001 |
| Peripheral blood | 185 (57%) | ||
| HLA compatibility in the HVG direction, n (%)* | |||
| Matched | 36 (11%) | 452 (100%) | < .001 |
| 1-antigen mismatch | 218 (67%) | ||
| 2-antigen mismatch | 65 (20%) | ||
| 3-antigen mismatch | 8 (2%) | ||
| HLA compatibility in the GVH direction, n (%)* | |||
| Matched | 0 (0%) | 452 (100%) | < .001 |
| 1-allele mismatch | 111 (34%) | ||
| 2-allele mismatch | 36 (11%) | ||
| 3-allele mismatch | 1 (0%) | ||
| Uncertain/missing | 179 (55%) | ||
| Conditioning regimen, n (%) | |||
| Myeloablative | 243 (74%) | 338 (75%) | .883 |
| Reduced-intensity | 84 (26%) | 114 (25%) | |
| GVHD prophylaxis, n (%) | |||
| Cyclosporine-based | 113 (35%) | 108 (24%) | 0.004 |
| Tacrolimus-based | 209 (64%) | 338 (75%) | |
| Others | 5 (2%) | 6 (1%) | |
| Use of ATG/alemtuzumab, n (%) | |||
| Yes | 33 (10%) | 13 (3%) | < .001 |
| No | 294 (90%) | 439 (97%) | |
| Median follow-up of survivors, mo (range) | 36.2 (3.0-95.7) | 13.5 (1.7-62.8) | < .001 |
| Variable . | RD/1AG-MM-GVH (n = 327) . | 8/8 MUD (n = 452) . | P . |
|---|---|---|---|
| Median age at transplantation, y (range) | 45 (16-69) | 48 (16-68) | .043 |
| Recipient sex, n (%) | |||
| Male | 184 (56%) | 267 (59%) | .434 |
| Female | 143 (44%) | 185 (41%) | |
| Sex combination of donors and recipients, n (%) | |||
| Female to male | 91 (28%) | 73 (16%) | < .001 |
| Other combinations | 236 (72%) | 379 (84%) | |
| Performance status, n (%) | |||
| 0/1 | 298 (91%) | 415 (92%) | .736 |
| 2/3/4 | 29 (9%) | 37 (8%) | |
| Disease, n (%) | |||
| AML | 167 (51%) | 249 (55%) | .512 |
| ALL | 90 (28%) | 107 (24%) | |
| CML | 19 (6%) | 21 (5%) | |
| MDS | 51 (16%) | 75 (17%) | |
| Duration from diagnosis to transplantation, n (%) | |||
| < 6 mo | 124 (38%) | 102 (23%) | < .001 |
| ≥ 6 mo | 191 (58%) | 350 (77%) | |
| Unknown | 12 (4%) | 0 (0%) | |
| Disease risk, n (%) | |||
| Standard | 175 (54%) | 317 (70%) | < .001 |
| High | 133 (41%) | 129 (29%) | |
| Unknown | 19 (6%) | 6 (1%) | |
| Source of stem cells, n (%) | |||
| BM | 142 (43%) | 452 (100%) | < .001 |
| Peripheral blood | 185 (57%) | ||
| HLA compatibility in the HVG direction, n (%)* | |||
| Matched | 36 (11%) | 452 (100%) | < .001 |
| 1-antigen mismatch | 218 (67%) | ||
| 2-antigen mismatch | 65 (20%) | ||
| 3-antigen mismatch | 8 (2%) | ||
| HLA compatibility in the GVH direction, n (%)* | |||
| Matched | 0 (0%) | 452 (100%) | < .001 |
| 1-allele mismatch | 111 (34%) | ||
| 2-allele mismatch | 36 (11%) | ||
| 3-allele mismatch | 1 (0%) | ||
| Uncertain/missing | 179 (55%) | ||
| Conditioning regimen, n (%) | |||
| Myeloablative | 243 (74%) | 338 (75%) | .883 |
| Reduced-intensity | 84 (26%) | 114 (25%) | |
| GVHD prophylaxis, n (%) | |||
| Cyclosporine-based | 113 (35%) | 108 (24%) | 0.004 |
| Tacrolimus-based | 209 (64%) | 338 (75%) | |
| Others | 5 (2%) | 6 (1%) | |
| Use of ATG/alemtuzumab, n (%) | |||
| Yes | 33 (10%) | 13 (3%) | < .001 |
| No | 294 (90%) | 439 (97%) | |
| Median follow-up of survivors, mo (range) | 36.2 (3.0-95.7) | 13.5 (1.7-62.8) | < .001 |
HLA compatibility was defined according to the HLA-A, HLA-B, and HLA-DR loci.
OS
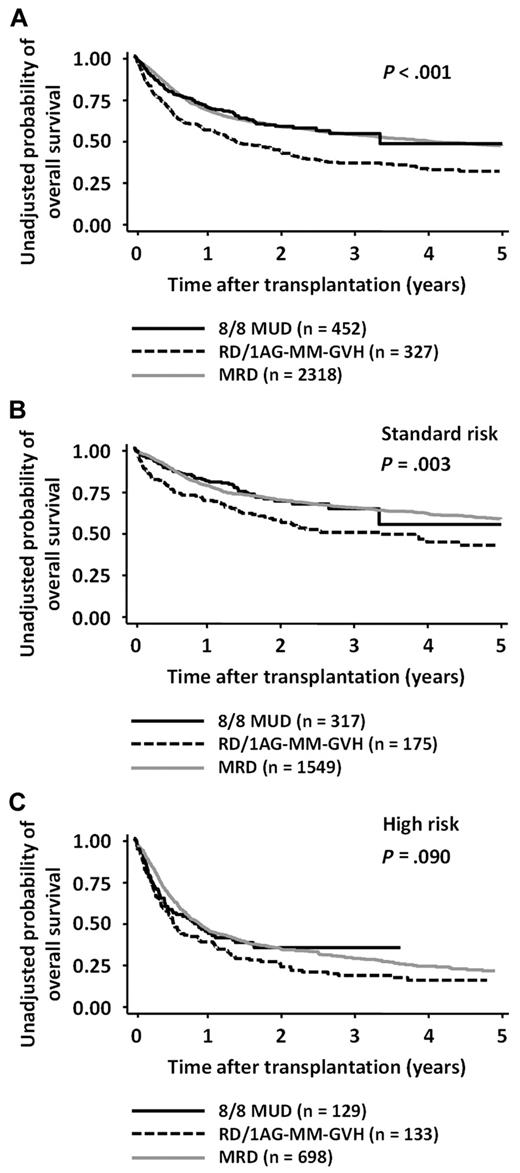
The 2-year OS rates in the 8/8-MUD and RD/1AG-MM-GVH groups were 0.59 (95% confidence interval [CI], 0.53-0.64) and 0.44 (95% CI, 0.38-0.49), respectively (log-rank test; P < .001; Figure 1A). Multivariate analysis revealed that, compared with the use of an 8/8-MUD, the use of an RD/1AG-MM-GVH was a significant adverse factor for OS (hazard ratio [HR], 1.49; 95% CI, 1.19-1.86; P < .001; Table 2). Age > 50 years, performance status ≥ 2, and high-risk disease were also found to be significant adverse factors, whereas other variables, such as the time from diagnosis to transplantation, were not.
OS according to donor type and risk of disease. OS after transplantation from an RD/1AG-MM-GVH donor, an 8/8-MUD, and HLA-MRD in patients with both-risk (A), standard-risk (B), or high-risk diseases (C). Survival rates in the 8/8-MUD and RD/1AG-MM-GVH groups were compared with the log-rank test.
OS according to donor type and risk of disease. OS after transplantation from an RD/1AG-MM-GVH donor, an 8/8-MUD, and HLA-MRD in patients with both-risk (A), standard-risk (B), or high-risk diseases (C). Survival rates in the 8/8-MUD and RD/1AG-MM-GVH groups were compared with the log-rank test.
Multivariate analysis of OS
| Variable . | Total (n = 779) . | Standard-risk (n = 492) . | High-risk (n = 262) . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Donor type | ||||||
| 8/8 MUD | 1.00 | 1.00 | 1.00 | |||
| RD/1AG-MM-GVH | 1.49 (1.19-1.86) | < .001 | 1.72 (1.24-2.39) | .001 | 1.30 (0.96-1.76) | .095 |
| Age, y | ||||||
| ≤ 50 | 1.00 | 1.00 | ||||
| > 50 | 1.44 (1.16-1.79) | .001 | 1.55 (1.13-2.15) | .007 | ||
| Performance status | ||||||
| 0/1 | 1.00 | 1.00 | ||||
| 2/3/4 | 1.79 (1.30-2.48) | < .001 | 1.76 (1.24-2.52) | .002 | ||
| Disease risk | ||||||
| Standard | 1.00 | |||||
| High | 2.41 (1.92-3.03) | < .001 | ||||
| Unknown | 1.38 (0.82-2.33) | .227 | ||||
| Variable . | Total (n = 779) . | Standard-risk (n = 492) . | High-risk (n = 262) . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Donor type | ||||||
| 8/8 MUD | 1.00 | 1.00 | 1.00 | |||
| RD/1AG-MM-GVH | 1.49 (1.19-1.86) | < .001 | 1.72 (1.24-2.39) | .001 | 1.30 (0.96-1.76) | .095 |
| Age, y | ||||||
| ≤ 50 | 1.00 | 1.00 | ||||
| > 50 | 1.44 (1.16-1.79) | .001 | 1.55 (1.13-2.15) | .007 | ||
| Performance status | ||||||
| 0/1 | 1.00 | 1.00 | ||||
| 2/3/4 | 1.79 (1.30-2.48) | < .001 | 1.76 (1.24-2.52) | .002 | ||
| Disease risk | ||||||
| Standard | 1.00 | |||||
| High | 2.41 (1.92-3.03) | < .001 | ||||
| Unknown | 1.38 (0.82-2.33) | .227 | ||||
Only variables that remained after backward selection in the multivariate analysis are shown.
Because our previous study showed that the impact of an HLA-1 Ag mismatch in a related transplantation on OS differed according to whether patients had standard-risk or high-risk diseases,1 the survival rates were compared separately in each disease-risk group. The OS rates of patients with standard-risk diseases in the 8/8-MUD group were significantly higher than those in the RD/1AG-MM-GVH group (P = .003), whereas there was no significant difference in high-risk patients (P = .090; Figure 1B-C). Although the interaction between the donor type and disease risk did not reach statistical significance (P = .140), multivariate analyses in each disease-risk group showed that the adverse impact of the use of an RD/1AG-MM-GVH donor was significant in standard-risk patients (HR, 1.72; 95% CI, 1.24-2.39; P = .001), but not in high-risk patients (Table 2).
To visually compare MRDs and other stem-cell sources, the OS rate for MRDs was layered on those for MUDs and RD/1AG-MM-GVHs (Figure 1). The OS curve of transplantation from an MRD was superimposed on that from an MUD in both standard- and high-risk patients (MRD vs MUD: standard-risk group, P = .895, and high-risk group, P = .581). Multivariate analysis confirmed that OS in the MRD group was comparable to the MUD group (MRD vs MUD: standard-risk group, HR, 1.02; 95% CI, 0.79-1.32; P = .878; high-risk group, HR, 0.98; 95% CI, 0.76-1.26; P = .865).
Effect of HLA mismatches on OS
To identify factors that may contribute to the inferior OS in standard-risk patients in the RD/1AG-MM-GVH group compared with those in the 8/8-MUD group, we evaluated the impact of each HLA-A, HLA-B, or HLA-DR Ag mismatch in the GVH direction and the number of Ag mismatches in the HVG direction on OS rates in the RD/1AG-MM-GVH group.
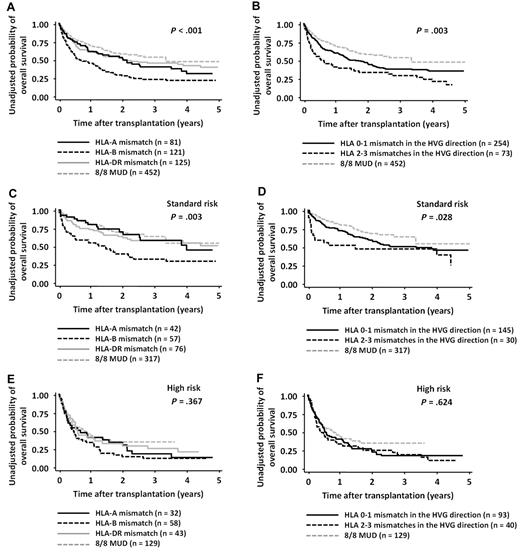
In the RD/1AG-MM-GVH group, the OS rate for patients who received a transplantation from a related donor with an HLA-B Ag mismatch in the GVH direction and that from a donor with 2 or 3 Ag mismatches in the HVG direction were significantly lower than those in other groups (log-rank test for HLA-A Ag mismatch vs HLA-B Ag mismatch vs HLA-DR Ag mismatch in the GVH direction, P < .001, and 0-1 mismatches vs 2-3 mismatches in the HVG direction, P = .003; Figure 2). However, multivariate analysis revealed that only the presence of an HLA-B Ag mismatch in the GVH direction (HR, 1.57; 95% CI, 1.13-2.18; P = .007) was significantly associated with a lower OS (Table 3).
OS in patients with both-risk, standard-risk, or high-risk diseases according to the locus of HLA mismatch in the GVH direction and the number of mismatches in the HVG direction. Survival rates in patients with HLA-A, HLA-B, and HLA-DR Ag mismatches in the GVH direction were compared with the log-rank test (A,C,E). Survival rates in patients with 0-1 and 2-3 mismatches in the HVG direction were compared with the log-rank test (B,D,F). Survival rates of the 8/8-MUD group are shown for visual comparison.
OS in patients with both-risk, standard-risk, or high-risk diseases according to the locus of HLA mismatch in the GVH direction and the number of mismatches in the HVG direction. Survival rates in patients with HLA-A, HLA-B, and HLA-DR Ag mismatches in the GVH direction were compared with the log-rank test (A,C,E). Survival rates in patients with 0-1 and 2-3 mismatches in the HVG direction were compared with the log-rank test (B,D,F). Survival rates of the 8/8-MUD group are shown for visual comparison.
Multivariate analysis of OS in patients receiving transplantation from a related donor with a 1-antigen mismatch in the GVH direction
| Variable . | Total (n = 327) . | Standard-risk (n = 175) . | High-risk (n = 133) . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| HLA mismatch in the GVH direction | ||||||
| HLA-DR mismatch | 1.00 | 1.00 | 1.00 | |||
| HLA-A mismatch | 1.07 (0.73-1.56) | .737 | 0.98 (0.54-1.81) | .966 | 1.11 (0.65-1.89) | .701 |
| HLA-B mismatch | 1.57 (1.13-2.18) | .007 | 1.86 (1.14-3.01) | .012 | 1.36 (0.86-2.17) | .193 |
| HLA mismatch in the HVG direction | ||||||
| 0-1 mismatches | 1.00 | 1.00 | 1.00 | |||
| 2-3 mismatches | 1.27 (0.91-1.76) | .154 | 1.67 (0.98-2.85) | .061 | 1.06 (0.69-1.61) | .799 |
| Age, y | ||||||
| ≤ 50 | 1.00 | 1.00 | ||||
| > 50 | 1.52 (1.14-2.03) | .004 | 1.87 (1.21-2.91) | .005 | ||
| Disease risk | ||||||
| Standard | 1.00 | |||||
| High | 2.06 (1.53-2.78) | < .001 | ||||
| Unknown | 1.00 (0.53-1.89) | .989 | ||||
| Variable . | Total (n = 327) . | Standard-risk (n = 175) . | High-risk (n = 133) . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| HLA mismatch in the GVH direction | ||||||
| HLA-DR mismatch | 1.00 | 1.00 | 1.00 | |||
| HLA-A mismatch | 1.07 (0.73-1.56) | .737 | 0.98 (0.54-1.81) | .966 | 1.11 (0.65-1.89) | .701 |
| HLA-B mismatch | 1.57 (1.13-2.18) | .007 | 1.86 (1.14-3.01) | .012 | 1.36 (0.86-2.17) | .193 |
| HLA mismatch in the HVG direction | ||||||
| 0-1 mismatches | 1.00 | 1.00 | 1.00 | |||
| 2-3 mismatches | 1.27 (0.91-1.76) | .154 | 1.67 (0.98-2.85) | .061 | 1.06 (0.69-1.61) | .799 |
| Age, y | ||||||
| ≤ 50 | 1.00 | 1.00 | ||||
| > 50 | 1.52 (1.14-2.03) | .004 | 1.87 (1.21-2.91) | .005 | ||
| Disease risk | ||||||
| Standard | 1.00 | |||||
| High | 2.06 (1.53-2.78) | < .001 | ||||
| Unknown | 1.00 (0.53-1.89) | .989 | ||||
Only variables that remained after backward selection in the multivariate analysis are shown.
The OS rates were also compared separately in the standard-risk and high-risk disease groups (Figure 2). Although the interaction between the presence of an HLA-B Ag mismatch and disease risk did not reach statistical difference (P = .232), the adverse impact of an HLA-B Ag mismatch in the GVH direction was observed in the standard-risk group (HR, 1.86 95% CI, 1.14-3.01; P = .012), but not in the high-risk group (Table 3). Conversely, the survival curve for the HLA-A Ag or HLA-DR Ag-mismatched group was almost superimposed on that for 8/8-MUDs (Figure 2; standard-risk group: for the HLA-A Ag-mismatched group vs the 8/8-MUD group, HR, 1.26; 95% CI, 0.73-2.19; P = .411; for the HLA-DR Ag-mismatched group vs the 8/8-MUD group, HR, 1.37; 95% CI, 0.89-2.11; P = .154; high-risk group: for the HLA-A Ag-mismatched group vs the 8/8-MUD group, HR, 1.26; 95% CI, 0.80-2.00; P = .320; and for the HLA-DR Ag-mismatched group vs the 8/8-MUD group, HR, 1.03; 95% CI, 0.67-1.59]; P = .880). The impact of 2 or 3 Ag mismatches in the HVG direction was not significant in either the standard-risk or high-risk group (Table 3).
Effect of an HLA-B mismatch on TRM, relapse, GVHD, and neutrophil engraftment in patients with standard-risk diseases
Our findings showed that an HLA-B Ag mismatch in the GVH direction strongly contributed to the low survival rate in standard-risk patients, which can explain the inferior survival rates in the RD/1AG-MM-GVH group compared with the 8/8-MUD group. Therefore, we evaluated the impact of an HLA-B Ag mismatch in the GVH direction on other outcomes in patients with standard-risk diseases in the RD/1AG-MM-GVH group.
First, we compared the characteristics of patients with standard-risk diseases who received transplantation from a related donor with an HLA-A, HLA-B, and HLA-DR Ag mismatch in the GVH direction (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Two or 3 Ag mismatches in the HVG direction were observed more frequently in the HLA-B Ag-mismatched group (28%) than in the HLA-A Ag-mismatched group (2%) or the HLA-DR Ag-mismatched group (17%). Although there was no information available on allelic mismatch or HLA-C Ag mismatch in more than half of the patients, an HLA-C Ag mismatch in either the GVH or HVG direction was observed more frequently in the HLA-B Ag-mismatched group (61% among the available data) than in the HLA-A Ag-mismatched group (25%) or the HLA-DR Ag-mismatched group (17%).
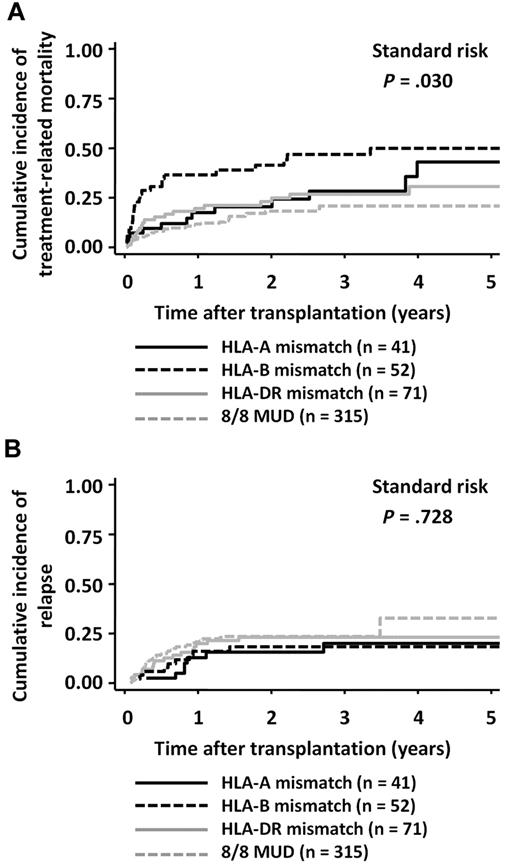
The incidence of TRM was higher in the HLA-B Ag-mismatched group (3-year mortality rate: HR, 0.47; 95% CI, 0.32-0.60) than in the HLA-A Ag-mismatched group (HR, 0.28; 95% CI, 0.14-0.44) or the HLA-DR Ag-mismatched group (HR, 0.27; 95% CI, 0.17-0.38; Figure 3A; log-rank test, P = .030). The presence of an HLA-B Ag mismatch in the GVH direction was an independent significant adverse factor that affected TRM in the RD/1AG-MM-GVH group (Table 4). Conversely, the incidence of relapse did not significantly differ among the 3 groups (Figure 3B and Table 4).
Cumulative incidence according to the locus of HLA mismatch in the GVH direction in patients with standard-risk diseases. Cumulative incidences in the related transplantation groups were compared with the Gray test. (A) TRM. (B) Relapse.
Cumulative incidence according to the locus of HLA mismatch in the GVH direction in patients with standard-risk diseases. Cumulative incidences in the related transplantation groups were compared with the Gray test. (A) TRM. (B) Relapse.
Multivariate analysis of TRM and relapse in patients with standard-risk diseases receiving transplantations from a related donor with a 1-antigen mismatch in the GVH direction
| Variable . | TRM (n = 164) . | Relapse (n = 164) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| HLA mismatch in the GVH direction | ||||
| HLA-DR mismatch | 1.00 | 1.00 | ||
| HLA-A mismatch | 1.22 (0.59-2.52) | .587 | 0.70 (0.29-1.67) | .418 |
| HLA-B mismatch | 2.00 (1.09-3.65) | .025 | 0.80 (0.34-1.87) | .605 |
| HLA mismatch in the HVG direction | ||||
| 0-1 mismatches | 1.00 | 1.00 | ||
| 2-3 mismatches | 2.21 (1.14-4.28) | .019 | 0.67 (0.23-1.98) | .467 |
| Age, y | ||||
| ≤ 50 | 1.00 | |||
| > 50 | 2.08 (1.18-3.65) | .011 | ||
| Duration from diagnosis to transplantation | ||||
| < 6 mo | 1.00 | |||
| ≥ 6 mo | 2.40 (1.19-4.82) | .014 | ||
| Unknown | 2.23 (0.77-6.48) | .140 | ||
| Variable . | TRM (n = 164) . | Relapse (n = 164) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| HLA mismatch in the GVH direction | ||||
| HLA-DR mismatch | 1.00 | 1.00 | ||
| HLA-A mismatch | 1.22 (0.59-2.52) | .587 | 0.70 (0.29-1.67) | .418 |
| HLA-B mismatch | 2.00 (1.09-3.65) | .025 | 0.80 (0.34-1.87) | .605 |
| HLA mismatch in the HVG direction | ||||
| 0-1 mismatches | 1.00 | 1.00 | ||
| 2-3 mismatches | 2.21 (1.14-4.28) | .019 | 0.67 (0.23-1.98) | .467 |
| Age, y | ||||
| ≤ 50 | 1.00 | |||
| > 50 | 2.08 (1.18-3.65) | .011 | ||
| Duration from diagnosis to transplantation | ||||
| < 6 mo | 1.00 | |||
| ≥ 6 mo | 2.40 (1.19-4.82) | .014 | ||
| Unknown | 2.23 (0.77-6.48) | .140 | ||
Only variables that remained after backward selection in the multivariate analysis are shown.
The incidence of grade 2-4 acute GVHD in the HLA-B Ag-mismatched group was higher than that in the HLA-A Ag-mismatched group, but comparable to that in the HLA-DR Ag-mismatched group (supplemental Figure 1 and supplemental Table 2). There was no significant difference in the incidence of grade 3-4 acute GVHD among the 3 groups. Regarding neutrophil engraftment, multivariate analysis showed that an HLA-B Ag mismatch was significantly associated with inferior neutrophil engraftment and 2 or 3 Ag mismatches in the HVG direction were associated with inferior neutrophil engraftment, with marginal significance (supplemental Table 2).
Discussion
In this nationwide retrospective study, we found that the survival rate of the RD/1AG-MM-GVH group was significantly inferior to that of the 8/8-MUD group, and this significant difference was observed only in patients with standard-risk diseases, although the interaction between donor type and disease risk did not reach statistical significance. We reported previously that transplantation from a related donor with 1 Ag mismatch in the GVH or HVG direction gave a clinical outcome comparable to that of transplantation from a 6/6-Ag-MUD in patients with either standard-risk or high-risk diseases.1 However, because HLA matching at the allelic level in unrelated transplantation significantly reduces the risk of GVHD, in the present study, the survival curve of transplantation from an 8/8-MUD was substantially improved, and could be superimposed on a curve corresponding to that from an MRD. Consistent with our findings, several studies have shown that the clinical outcomes of transplantation from an 8/8-10/10 MUD are comparable to those from an MRD.20,21 The significant difference in survival rates between transplantation from an RD/1AG-MM-GVH donor and an 8/8-MUD disappeared in patients with high-risk diseases, probably because the adverse impact of acute GVHD on survival might be offset by the potential GVL effect in transplantation from an RD/1AG-MM-GVH donor.1,2,22
We evaluated factors that may contribute to the inferior OS in patients with standard-risk diseases in the RD/1AG-MM-GVH group and found that, compared with the presence of an HLA-DR Ag mismatch, the presence of an HLA-B Ag mismatch in the GVH direction was significantly associated with lower OS and higher TRM. Conversely, the rates of OS and TRM in the HLA-A Ag- or HLA-DR Ag-mismatched group were superimposed on those in the MUD group. However, HLA-A, HLA-B, and HLA-DR Ag mismatches had similar effects on the incidence of severe acute GVHD; consequently, the causal relationship between an HLA-B Ag mismatch in the GVH direction and higher TRM remains unknown. In contrast to our findings, Valcarcel et al reported that there was no significant difference in OS between the use of 1-Ag–mismatched related donors (n = 89) and 8/8-MUDs (n = 700) in transplantation for AML and ALL during the first or second complete remission.23 This difference from our results can be partly explained by the fact that the MUD group in their study included a significantly smaller number of ALL patients with low-risk cytogenetics. In addition, in our study, genetic homogeneity in the Japanese population might affect the lower incidence of severe acute GVHD in MUD transplantation because of the less frequent mismatches in minor histocompatibility Ags.24,25
The frequency of an HLA-C Ag mismatch was substantially higher in the HLA-B Ag-mismatched group than in the HLA-A or HLA-DR Ag-mismatched groups. This finding may represent linkage disequilibrium between the HLA-B and HLA-C genes, which are located at a very close physical proximity within the major histocompatibility complex.26,27 Therefore, the impact of HLA-B-Ag might be affected by the co-presence of HLA-C Ag mismatch. We could not evaluate the impact of HLA-C Ag mismatch on OS rates because of the limited information on HLA-C Ag mismatch; therefore, an analysis with larger cohorts with complete HLA-C Ag information will be needed to evaluate the impact of HLA-C and/or HLA-B mismatch in transplantation from an RD/1AG-MM-GVH donor. Accordingly, we could not evaluate the impact of the KIR ligand mismatch. Although the impact of KIR ligand mismatch is still controversial, several studies analyzing T cell–replete transplantation showed that KIR ligand mismatch is associated with lower OS.12,28,29 The analysis of KIR matching would be helpful in elucidating the mechanism underlying the adverse effect of HLA-B mismatch in T cell–replete transplantation from an RD/1AG-MM-GVH donor.
Whether the presence of allelic mismatches in addition to the 1-Ag mismatch (2 or more allelic mismatches in total) affects transplantation outcome is also an important clinical question in transplantation from an RD/1AG-MM-GVH donor. A high frequency of 2-allele mismatches in the GVH direction was seen in the HLA-B Ag-mismatched group, suggesting a possible association between 2-allele mismatches and low OS. However, we did not observe a significant effect of the number of allelic mismatches on OS after transplantation from an RD/1AG-MM-GVH donor, possibly because of the small sample size.
Our study has several limitations. First, because several months are required to arrange unrelated transplantations, patients at low risk for relapse may more often be selected for these procedures. To minimize this bias, we included the duration from diagnosis to transplantation in the multivariate analysis; however, this variable did not have a significant effect in the multivariate analysis. Second, heterogeneous backgrounds may have resulted in a bias. In particular, the stem-cell source in unrelated transplantation was exclusively BM. However, the analysis of OS in the subgroup of patients who received a BM graft from an RD/1AG-MM-GVH donor or an 8/8-MUD showed similar results. Third, because we have incomplete Ag and allele information on the HLA-C and -DQB1 loci, we may have underestimated the degree of mismatching in transplantation from an RD/1AG-MM-GVH donor. Fourth, the difference in the impact of donor type between standard- and high-risk diseases should be cautiously interpreted, because the interaction between the donor type and disease risk did not reach statistical significance. This may be partly because of the lower statistical power to detect the interaction than the main effect.
In conclusion, our findings suggest that an 8/8-MUD, if available, should be prioritized over an RD/1AG-MM-GVH donor for patients without an MRD if an immediate transplantation is not necessary. In particular, the presence of an HLA-B Ag mismatch in the GVH direction has an adverse effect on OS because of treatment-related complications. This may be because of the high frequencies of additional mismatches of HLA-C Ag or allele in the HLA-B Ag-mismatched group. To elucidate the mechanism of the adverse outcomes in RD/1AG-MM-GVH donors with an HLA-B Ag mismatch, HLA Ag/allele matching including HLA-C should be performed in transplantations from an RD/1AG-MM-GVH donor.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to all of the physicians and data managers at the centers who contributed valuable data on transplantation to the Japan Society for Hematopoietic Cell Transplantation and the Japan Marrow Donor Program. They also thank all of the members of the data management committees of the Japan Society for Hematopoietic Cell Transplantation and the Japan Marrow Donor Program for their management of data.
J.K. is a Research Fellow of the Japan Society for the Promotion of Science. This work was supported in part by a grant-in-aid for Japan Society for the Promotion of Science (to J.K.).
Authorship
Contribution: Y.K. designed the research and organized the project; J.K., H. Saji, and Y.K. reviewed and analyzed the data and wrote the manuscript; J.K. and Y.K. performed the statistical analysis; H. Sakamaki, J.T., R.S., and Y.A. collected data from Japan Society for Hematopoietic Cell Transplantation; K.K. and Y.M. collected data from Japan Marrow Donor Program; and all authors interpreted the data and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshinobu Kanda, MD, Division of Hematology, Saitama Medical Center, Jichi Medical University, 1-847 Amanuma Town, Omiya Ward, Saitama City, Saitama, Japan 330-8503; e-mail: ycanda-tky@umin.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal