Abstract
The targeting of BCR-ABL, a hybrid oncogenic tyrosine (Y) kinase, does not eradicate chronic myeloid leukemia (CML)–initiating cells. Activation of β-catenin was linked to CML leukemogenesis and drug resistance through its BCR-ABL–dependent Y phosphorylation and impaired binding to GSK3β (glycogen synthase kinase 3β). Herein, we show that GSK3β is constitutively Y216 phospho-activated and predominantly relocated to the cytoplasm in primary CML stem/progenitor cells compared with its balanced active/inactive levels and cytosolic/nuclear distribution in normal cells. Under cytokine support, persistent GSK3β activity and its altered subcellular localization were correlated with BCR-ABL–dependent and –independent activation of MAPK and p60-SRC/GSK3β complex formation. Specifically, GSK3β activity and nuclear import were increased by imatinib mesylate (IM), a selective ABL inhibitor, but prevented by dasatinib that targets both BCR-ABL– and cytokine-dependent MAPK/p60-SRC activity. SB216763, a specific GSK3 inhibitor, promoted an almost complete suppression of primary CML stem/progenitor cells when combined with IM, but not dasatinib, while sparing bcr-abl–negative cells. Our data indicate that GSK3 inhibition acts to prime a pro-differentiative/apoptotic transcription program in the nucleus of IM-treated CML cells by affecting the β-catenin, cyclinD1, C-EBPα, ATF5, mTOR, and p27 levels. In conclusion, our data gain new insight in CML biology, indicating that GSK3 inhibitors may be of therapeutic value in selectively targeting leukemia-initiating cells in combination with IM but not dasatinib.
Introduction
Normal hematopoietic stem cells (HSCs) exit quiescence to maintain a balanced number of self-renewing cells or to generate a lineage-differentiated progeny that compensates for the loss of mature blood cells.1 Glycogen synthase kinase-3β (GSK3β) has attracted attention because of its role in preserving a quiescent HSC pool despite its negative effects on self-renewal (through inhibitory phosphorylation of Hedgehog, Notch, or WNT-related signaling effectors).2-6 This has prompted efforts to develop GSK3 inhibitors for augmenting overall hematopoietic reconstitution in vivo.7,8 Moreover, emerging evidence points to GSK3β as a therapeutic target in acute human leukemias (mixed-lineage leukemias) and multiple myelomas, indicating a hitherto unknown multifaceted role of GSK3β in normal and malignant hematopoiesis.9-12
Chronic myeloid leukemia (CML) is a myeloproliferative disorder that begins as an indolent chronic phase (CP) when an HSC expresses the causative protein tyrosine kinase BCR-ABL encoded by the Philadelphia chromosome (Ph).13,14 CML-initiating cells express BCR-ABL at the highest levels compared with their differentiated progeny, but they fail to expand at the same rate as downstream leukemic progenitors. This explains the long latency (5-7 years) required for the aberrant outgrowth of Ph+ granulocytes as a clinical hallmark of CML patients at diagnosis.
Previous studies have demonstrated that primitive Ph+ HSCs tend to remain in a deeply quiescent state despite prolonged cytokine stimulation in vitro, whereas Ph+ downstream progenitors show an increased proliferation and sensitivity to cytokine-induced maturation.15,16 The molecular mechanisms controlling the heterogeneity of primary CML stem/progenitor cells with respect to their cycling control remain elusive, but an intrinsic defect in executing symmetrical (self-renewing) cell divisions in long-term cell-initiating cultures (LTC-ICs) ex vivo has been described previously.17 CML progresses from the CP toward an acute blast crisis (BC) in which cells of the bulk tumor, referred as Ph+ committed granulocyte/macrophage precursors, may emerge as a second CML stem pool.18 The ability of Ph+ granulocyte/macrophage precursors to self-renew has been linked to WNT/β-catenin activation as result of GSK3β missplicing or a BCR-ABL–mediated phosphorylation of β-catenin that impairs its binding to intact Axin/GSK3β–destruction complexes.18-20
In the present study, we extend these data showing that GSK3β activity and its nuclear import/export are altered in freshly isolated CML stem/progenitor cells compared with their normal counterparts. From a biologic and therapeutic perspective, unraveling signaling differences in HSC-restricted pathways could be critical to devising strategies aimed at CML eradication. Indeed, imatinib mesylate (IM), a selective ABL inhibitor used as a first-line therapy for CML patients at diagnosis, minimally affects the survival of Ph+ stem/progenitor cells, and the disease usually relapses if therapy is discontinued or stopped in patients achieving remission.21-24
The results of this study validate the combined inhibition of BCR-ABL and GSK3 as a promising approach with which to selectively target CML-initiating versus bcr-abl–negative cells and illustrate why this strategy could be more promising using IM rather than dasatinib.
Methods
Inhibitors
IM (Novartis), dasatinib (Bristol-Myers Squibb), SB216763 and LY294002 (both from Sigma-Aldrich), or UO216 (Calbiochem) were dissolved in DMSO (Sigma-Aldrich).
Selection of CD34+ progenitors
Blood samples from healthy donors and CML patients in CP who had not received prior IM treatment or in myeloid BC with at least 70% of Ph+ blasts were purified by Ficoll (Amersham) density gradient centrifugation and enriched for CD34+ cells by immunomagnetic selection (CliniMACS, Miltenyi Biotec). CD34+CD38− cells were enriched using biotinylated anti-CD38 mAb and anti-biotin MACSiBeads (Miltenyi Biotec). Cell purity was confirmed by staining with anti-CD34–PE and anti-CD38–FITC (both from BD Biosciences) and by flow cytometric analysis (FACSCalibur; BD Biosciences). This study was approved by the Institutional Review Boards at University of Salento (Lecce, Italy) and met all requirements of the Declaration of Helsinki.
Cell cultures
CD34+ cells were cultured in IMDM (Sigma-Aldrich) supplemented with a serum substitute (BIT; StemCell Technologies), l-glutamine (2mM), penicillin (105 units/mL), streptomycin (100 mg/L, all from Gibco-BRL) and 5 growth factors (StemCell Technologies) used at low (1 ng/mL of SCF and Flt-3 ligand plus 0.2 ng/mL of IL-3, IL-6, and GM-CSF) or high (100 ng/mL of SCF and Flt-3 ligand plus 20 ng/mL of IL-3, IL-6, and G-CSF) 5 growth factor (5GF) concentrations, except where indicated (−5GF).
Functional studies
Viable cells were counted in a hemocytometer with Trypan blue dye solution, and differentiation was monitored by May-Grunwald-Giemsa staining (Sigma-Aldrich).
Proliferation assays
Cell growth was quantified with an MTT assay (Sigma-Aldrich) or a 5- and 6-CFSE assay (Molecular Probes).
Cell-cycle analysis
Cells were fixed with ethanol, resuspended in PBS (Sigma-Aldrich) containing 0.1 mg/mL of RNase A (Boehringer Mannheim) and 100 μg/mL of propidium iodide (Sigma-Aldrich). Cell-cycle analysis was assessed using flow cytometry and CellQuest Version 3.3 software (BD Biosciences).
Apoptosis assay
CFSE-stained cells were labeled with annexin V–PE (BD Pharmingen) and analyzed using flow cytometry.
Limiting-dilution analysis of LTC-ICs
After culture for 96 hours in the presence of high 5GF concentrations and different drug treatments, normal or CML cells were washed to remove the drug(s) and seeded by limiting dilutions (1000, 300, 100, 33, or 11 cells/well) on irradiated (8000 cGy) M2-10B4 fibroblast feeders subcultured in 96-well plates containing long-term BM culture medium (StemCell Technologies).22 The high 5GF content used during the drug preincubation period allowed us to test the impact of each treatment directly on primitive cells remaining capable of colony formation in the absence of effects on differentiating cells generated during the clonogenic progenitor assays. Cultures were maintained for 6 weeks with weekly half-medium changes. At the end of this culture period, half of the medium was removed and wells were overlaid with MethoCult medium (StemCell Technologies). Individual wells were then scored as negative or positive for the presence of colony-forming cell (CFC) colonies after 2 weeks. The frequency of LTC-ICs was calculated with L-Calc software (StemCell Technologies) as the reciprocal of the concentration of a test cell suspension that gave 37% negative wells.
CFC assays
After culture for 96 hours in the presence of high 5GF concentrations and different drug treatments, normal or CML CD34+ cells were washed to remove the drug(s), set up in MethoCult medium, and the total numbers of CFC colonies determined after 2 weeks.
FISH
FISH was performed using the LS1 dual-labeled bcr-abl DNA probe according to the manufacturer's instructions (Vysis).22
Immunofluorescence GSK3β staining
Cells were resuspended using the FIX and PERM kit (Caltag Laboratories) before incubation with an anti–phospho-GSK3β (phospho-tyrosine-216 [pY216]) Ab (BD Transduction Laboratories), followed by incubation with an Alexa Fluor 488–conjugated goat anti–mouse IgG-FITC as a fluorescent secondary Ab (Molecular Probes) and flow cytometry analysis. To assess GSK3β localization, cells were fixed in 4% paraformaldehyde, permeabilized in PBS/0.3% BSA/0.5% Triton X-100, and blocked with PBS/3%BSA/1% horse serum (Invitrogen) before staining with an anti-GSK3β (27C10) Alexa Fluor 488–conjugated Ab (Cell Signaling Technology). After washing, cells were mounted in VECTASHIELD medium (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole, and observed using a Leica confocal scanning system mounted to a Leica TCS SP5 microscope equipped with a 60× oil-immersion objective and a spatial resolution of approximately 200 nm in the X-Y axis and 100 nm in the Z-axis.
Visualization of MDC-labeled vacuoles
Monodansylcadaverine (MDC; Sigma-Aldrich) was used as a marker for late autophagic vacuoles but not endosomes.25 Cells were incubated with 0.05mM MDC in PBS at 37°C for 10 minutes, washed, and analyzed with a fluorescence microscope (Eclipse TE 300; Nikon) equipped with a filter system (excitation filter, 380 nm; emission filter, 525 nm) and a 60× oil-immersion objective. Images were captured with a CCD camera and Image Pro-Plus Version 6.0 software (MediaCybernetics) and imported into Adobe Photoshop.
Immunoprecipitation and Western blotting analysis
Cells (3 × 105 cells/sample) were subjected to immunoprecipitation/blotting analyses as described previously.20 Primary Abs were as follows: total GSK3β, phospho-GSK3β pY216, and anti–β-catenin C-terminal (immunogenic epitope within the amino acids 560-781) were from BD Transduction Laboratories; phospho-GSK3β phospho-serine-9 (pSer9), total ABL (clone 8E9), phospho-ABL (pY245), and NFκB/p50 were from Millipore; total SRC, phospho-SRC family (pY416), anti–β-catenin N-terminal Ser33/37-phosphorylated serine/threonine β-catenin (pS/T β-Cat), anti–β-catenin N-terminal Ser33/37/Thr41-nonphosphorylated (clone 8E4; non-pS/T β-Cat), phospho-AKT (pSer473, clone 587F11), total ATF5, total mTOR (clone 7C10), phospho-mTOR (pSer2481/2448), and phospho-C/EBPα (pThr222/226) were from Cell Signaling Technology or Upstate Biotechnology; and β-actin (AC-15), phospho-MAPK (pSer42/44, sc-7383), and p27/Kip1 (c-19) were from Santa Cruz Biotechnology. HRP-conjugated secondary Abs were from Jackson ImmunoResearch Laboratories. Protein bands were detected using the Superfemto kit (Pierce), and quantified by Image-Quant TL v2003 software (Amersham Pharmacia Biotech).
Kinase assay
GSK3β was immunoprecipitated by CML stem/progenitor cells (5 × 105 cells/sample) using an anti–total GSK3β mAb (BD) and then subjected to an in vitro kinase assay using recombinant human β-catenin protein as a substrate. The phosphorylated fraction of β-catenin was detected by autoradiography.
Statistical analysis
Data obtained from multiple independent experiments are reported as the means ± SEM. Significance levels were determined with the Student t test.
Results
Constitutive GSK3β activation in primary CML stem/progenitor cells
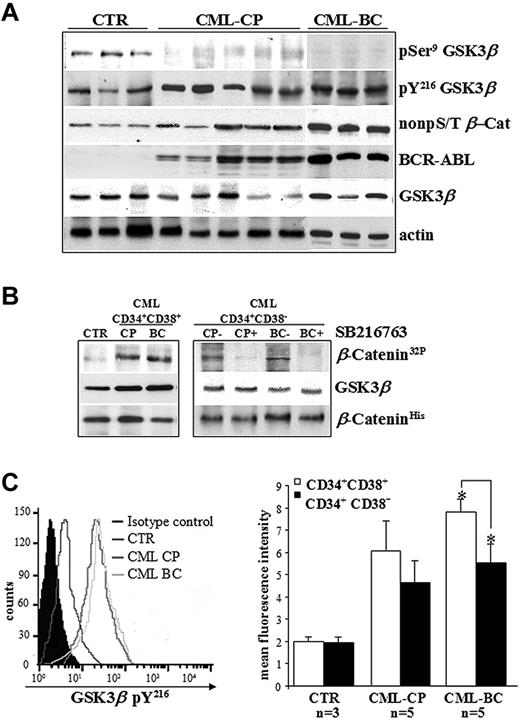
The phospho-GSK3β status was assessed in CD34+ cells freshly isolated from healthy donors (controls) and CML patients in CP or BC, and cultured in the presence of 5GF at low concentrations (culture conditions that maintained normal LTC-ICs for up to 10 days).26,27 Clinical features of CML patients are reported in Table 1. The detection of balanced levels of inhibitory pSer9 and stimulatory pY216 of GSK3β is considered a hallmark of its transient kinase regulation in normal CD34+ cells and in a broad range of human cell types.2,6 Conversely, no detectable levels of GSK3β pSer9 in CML CD34+ cells indicate that the kinase can no longer be regulated physiologically without significant changes on its total expression levels (Figure 1A). An in vitro kinase assay confirmed that GSK3β immunoprecipitated from Ph+ progenitors and that the more primitive CD34+CD38− cells that contain the majority of stem cell activity (< 3% of total CD34+ cells) were active (Figure 1B). Similar GSK3β pY216 levels in CML CP and BC samples were observed, although there was a trend toward lower GSK3β pY216 levels in CD34+CD38− cells compared with CD34+CD38+ progenitors in CML BC (P = .003; Figure 1C). These data also confirmed the apparently contradictory coexistence of a BCR-ABL–stabilized pool of β-catenin (non-pS/T β-Cat) and active GSK3β pY216 in CML cells versus normal CD34+ cells, as described previously.20
Clinical data from CML patient samples
| Patient no. . | WBCs, 109/L . | BM metaphases . | LTC-ICs (Ph+/total) . | Cells used . | % CD34+ cells before enrichment . | % CD34+ cells after enrichment . |
|---|---|---|---|---|---|---|
| CP1 | 116 | 46XY, t(9;22), 27/27 | 7/8 | BM | 5.5 | 86 |
| CP2 | 172 | 46XX, t(9;22), 25/25 | 9/9 | BM | 12.4 | 99 |
| CP3 | 740 | 46XY, t(9;22), 25/25 | 35/35 | BM | 7.3 | 92 |
| CP4 | 560 | 46XY, t(9;22), 29/29 | 18/18 | BM | 5.2 | 79 |
| CP5 | 240 | 46XX, t(9;22), 25/25 | 4/4 | BM | 21.2 | 98 |
| CP6 | 125 | 46XY, t(9;22), 21/21 | 12/12 | Blood | 6.8 | 92 |
| CP7 | 275 | 46XY, t(9;22), 25/25 | 8/8 | BM | 26.7 | 97 |
| CP8 | 145 | 46XX, t(9;22), 22/22 | 16/16 | BM | 5.3 | 96 |
| CP9 | 242 | 46XX, t(9;22), 30/30 | 10/10 | Blood | 7.8 | 95 |
| CP10 | 223 | 46XX, t(9;22), 28/28 | 10/12 | BM | 14.7 | 92 |
| CP11 | 354 | 46XX, t(9;22), 32/32 | 18/18 | BM | 22.5 | 98 |
| CP12 | 144 | 46XY, t(9;22), 44/45 | 22/22 | BM | 25.8 | 96 |
| CP13 | 645 | 46XX, t(9;22), 25/25 | 10/10 | BM | 15.6 | 91 |
| CP14 | 531 | 46XX, t(9;22), 30/30 | 13/13 | BM | 21.3 | 92 |
| CP15 | 232 | 46XX, t(9;22), 23/23 | 10/10 | BM | 10.9 | 95 |
| CP16* | 573 | 46XX, t(9;22), 21/26 | 30/35 | BM | 22.3 | 99 |
| CP17* | 640 | 46XX, t(9;22), 22/24 | 35/42 | BM | 27.5 | 99 |
| CP18* | 442 | 46XX, t(9;22), 25/28 | 21/25 | BM | 26.5 | 99 |
| BC1 | 675 | 46XY, t(9;22), 42/42 | 29/29 | BM | 15.8 | 91 |
| BC2 | 580 | 46XY, t(9;22), 35/35 | 32/32 | Blood | 13.3 | 95 |
| BC3 | 575 | 46XY, t(9;22), 22/22 | 15/15 | BM | 9.4 | 92 |
| BC4 | 450 | 46XY, t(9;22), 35/35 | 25/25 | BM | 23.2 | 88 |
| BC5 | 425 | 46XY, t(9;22), 30/30 | 35/35 | BM | 28.5 | 96 |
| Patient no. . | WBCs, 109/L . | BM metaphases . | LTC-ICs (Ph+/total) . | Cells used . | % CD34+ cells before enrichment . | % CD34+ cells after enrichment . |
|---|---|---|---|---|---|---|
| CP1 | 116 | 46XY, t(9;22), 27/27 | 7/8 | BM | 5.5 | 86 |
| CP2 | 172 | 46XX, t(9;22), 25/25 | 9/9 | BM | 12.4 | 99 |
| CP3 | 740 | 46XY, t(9;22), 25/25 | 35/35 | BM | 7.3 | 92 |
| CP4 | 560 | 46XY, t(9;22), 29/29 | 18/18 | BM | 5.2 | 79 |
| CP5 | 240 | 46XX, t(9;22), 25/25 | 4/4 | BM | 21.2 | 98 |
| CP6 | 125 | 46XY, t(9;22), 21/21 | 12/12 | Blood | 6.8 | 92 |
| CP7 | 275 | 46XY, t(9;22), 25/25 | 8/8 | BM | 26.7 | 97 |
| CP8 | 145 | 46XX, t(9;22), 22/22 | 16/16 | BM | 5.3 | 96 |
| CP9 | 242 | 46XX, t(9;22), 30/30 | 10/10 | Blood | 7.8 | 95 |
| CP10 | 223 | 46XX, t(9;22), 28/28 | 10/12 | BM | 14.7 | 92 |
| CP11 | 354 | 46XX, t(9;22), 32/32 | 18/18 | BM | 22.5 | 98 |
| CP12 | 144 | 46XY, t(9;22), 44/45 | 22/22 | BM | 25.8 | 96 |
| CP13 | 645 | 46XX, t(9;22), 25/25 | 10/10 | BM | 15.6 | 91 |
| CP14 | 531 | 46XX, t(9;22), 30/30 | 13/13 | BM | 21.3 | 92 |
| CP15 | 232 | 46XX, t(9;22), 23/23 | 10/10 | BM | 10.9 | 95 |
| CP16* | 573 | 46XX, t(9;22), 21/26 | 30/35 | BM | 22.3 | 99 |
| CP17* | 640 | 46XX, t(9;22), 22/24 | 35/42 | BM | 27.5 | 99 |
| CP18* | 442 | 46XX, t(9;22), 25/28 | 21/25 | BM | 26.5 | 99 |
| BC1 | 675 | 46XY, t(9;22), 42/42 | 29/29 | BM | 15.8 | 91 |
| BC2 | 580 | 46XY, t(9;22), 35/35 | 32/32 | Blood | 13.3 | 95 |
| BC3 | 575 | 46XY, t(9;22), 22/22 | 15/15 | BM | 9.4 | 92 |
| BC4 | 450 | 46XY, t(9;22), 35/35 | 25/25 | BM | 23.2 | 88 |
| BC5 | 425 | 46XY, t(9;22), 30/30 | 35/35 | BM | 28.5 | 96 |
Numbers after the karyotype indicate the numbers of metaphases with that karyotype of the total number analyzed. All CML patients in CP were collected at diagnosis before therapy. BC samples with at least 70% of blasts were used. Percentages of CD34+ cells before and after enrichment were also indicative of the efficiency of cell selection.
CP indicates chronic phase; BC, blast crisis; WBC, white blood cell count at sampling; BM, bone marrow; LTC-IC, long-term-culture initiating-cells; and Ph+, Philadelphia chromosome positive.
Selected CML CP patients from whom CD34+CD38− cells were isolated and analyzed by D-FISH after LTC-IC experiments.
Deregulated GSK3βactivation in CD34+ cells isolated from CML patients. (A) Primary CD34+ cells freshly isolated from healthy donors (CTR, n = 3) and CML patients in CP (n = 5) or BC (n = 3) were immunoblotted as indicated. Total levels of BCR-ABL and active β-catenin are also indicated. Total β-actin levels are reported as a loading control. (B) GSK3β was immunoprecipitated from CML progenitors (CD34+CD38+) and more primitive stem cells (CD34+CD38−) pooled from CML patients in CP (n = 5) or BC (n = 3) and incubated with (CP+ and BC+) or without (CP− and BC−) SB216763 5μM for 4 hours. Normal CD34+CD38− cells were pooled from healthy donors (CTR, n = 3). GSK3β activity was examined by an in vitro kinase assay using histidine-tagged recombinant β-catenin (β-CateninHis) as a substrate of GSK3β. Phosphorylated β-catenin was detected by autoradiography (β-Catenin32P). Levels of GSK3β and β-catenin were detected by Western blotting. (C) Representative histogram of intracellular pY216 levels of GSK3β (GSK3β pY216) in CD34+ cells isolated from healthy donors (CTR; n = 3) and CML patients in CP (n = 5) or BC (n = 5). Background signal was assessed in the same populations by staining with a matched-isotype control. Mean fluorescence intensities (MFI) ± SD relative to GSK3β pY216 signal in CD34+CD38+ progenitors and more primitive CD34+CD38− cells from healthy donors (CTR; n = 3) and CML patients in CP (n = 5) or BC (n = 5) are shown. Significant differences in paired t tests for CD34+CD38+ cells versus CD34+CD38− cells in CML BC patients are indicated (*P < .005).
Deregulated GSK3βactivation in CD34+ cells isolated from CML patients. (A) Primary CD34+ cells freshly isolated from healthy donors (CTR, n = 3) and CML patients in CP (n = 5) or BC (n = 3) were immunoblotted as indicated. Total levels of BCR-ABL and active β-catenin are also indicated. Total β-actin levels are reported as a loading control. (B) GSK3β was immunoprecipitated from CML progenitors (CD34+CD38+) and more primitive stem cells (CD34+CD38−) pooled from CML patients in CP (n = 5) or BC (n = 3) and incubated with (CP+ and BC+) or without (CP− and BC−) SB216763 5μM for 4 hours. Normal CD34+CD38− cells were pooled from healthy donors (CTR, n = 3). GSK3β activity was examined by an in vitro kinase assay using histidine-tagged recombinant β-catenin (β-CateninHis) as a substrate of GSK3β. Phosphorylated β-catenin was detected by autoradiography (β-Catenin32P). Levels of GSK3β and β-catenin were detected by Western blotting. (C) Representative histogram of intracellular pY216 levels of GSK3β (GSK3β pY216) in CD34+ cells isolated from healthy donors (CTR; n = 3) and CML patients in CP (n = 5) or BC (n = 5). Background signal was assessed in the same populations by staining with a matched-isotype control. Mean fluorescence intensities (MFI) ± SD relative to GSK3β pY216 signal in CD34+CD38+ progenitors and more primitive CD34+CD38− cells from healthy donors (CTR; n = 3) and CML patients in CP (n = 5) or BC (n = 5) are shown. Significant differences in paired t tests for CD34+CD38+ cells versus CD34+CD38− cells in CML BC patients are indicated (*P < .005).
BCR-ABL and exogenous cytokines sustain GSK3β pY216 levels in primary CML CP cells
We tested the active pools of GSK3β (pY216) and β-catenin (non-pS/T) in normal and CML cells exposed to high 5GF concentrations in the presence of IM, a specific ABL tyrosine kinase inhibitor, and SB216763, an ATP-competitive inhibitor of GSK3 (Figure 2A).
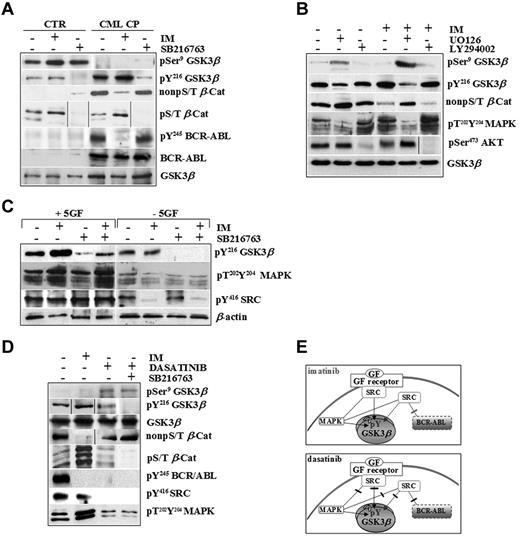
CML CP progenitors integrate signals by BCR-ABL and exogenous cytokines to sustain GSK3βpY216 activation. (A) Normal (CTR, n = 3) and CML CP (n = 5) CD34+ cells were exposed to IM (1μM) or SB216763 (5μM) for 4 hours in serum-free medium (SFM) containing 5GF concentrations. Representative immunoblots for total and pSer9 and pY216 of GSK3β, active β-catenin (non-pS/T β-Cat), phosphorylated β-catenin (non-pS/T β-Cat), and total and phosphorylated BCR-ABL (pY245 BCR-ABL) in one healthy and one CML CP patient sample are reported. (B) CML CP cells (n = 5) were cultured for 4 hours in the presence of 5GF, either without inhibitors (−) or in the presence of 1μM IM, 25μM UO126 (MAPK inhibitor), 30μM LY294002 (PI3K/AKT signaling inhibitor), or 5μM SB216763 or combinations as indicated. Representative immunoblots for total and pSer9 and pY216 levels of GSK3β, active β-catenin, dual phosphorylated MAPKs (pT202Y204MAPKs), and pSer473AKT are shown. (C) CML CP cells (n = 5) were exposed to IM (1μM), SB216763 (5μM), or the combination for 4 hours in the absence of serum and cytokines (−5GF) or in the presence of high 5GF-concentrations (+5GF), and then analyzed with the indicated Abs. Representative immunoblots for one CML CP sample are shown. (D) CML CP progenitors were treated with IM (1μM), dasatinib (0.15μM) or SB216763 (5μM) for 4 hours in SFM with 5GF. Representative immunoblots are indicated. (E). BCR-ABL or GF receptors both signal through MAPK and SRC kinases. SRC signaling activated by exogenous cytokines is not affected by IM. Conversely, both BCR-ABL– and GF-dependent activation of SRC and MAPKs is inhibited by dasatinib.
CML CP progenitors integrate signals by BCR-ABL and exogenous cytokines to sustain GSK3βpY216 activation. (A) Normal (CTR, n = 3) and CML CP (n = 5) CD34+ cells were exposed to IM (1μM) or SB216763 (5μM) for 4 hours in serum-free medium (SFM) containing 5GF concentrations. Representative immunoblots for total and pSer9 and pY216 of GSK3β, active β-catenin (non-pS/T β-Cat), phosphorylated β-catenin (non-pS/T β-Cat), and total and phosphorylated BCR-ABL (pY245 BCR-ABL) in one healthy and one CML CP patient sample are reported. (B) CML CP cells (n = 5) were cultured for 4 hours in the presence of 5GF, either without inhibitors (−) or in the presence of 1μM IM, 25μM UO126 (MAPK inhibitor), 30μM LY294002 (PI3K/AKT signaling inhibitor), or 5μM SB216763 or combinations as indicated. Representative immunoblots for total and pSer9 and pY216 levels of GSK3β, active β-catenin, dual phosphorylated MAPKs (pT202Y204MAPKs), and pSer473AKT are shown. (C) CML CP cells (n = 5) were exposed to IM (1μM), SB216763 (5μM), or the combination for 4 hours in the absence of serum and cytokines (−5GF) or in the presence of high 5GF-concentrations (+5GF), and then analyzed with the indicated Abs. Representative immunoblots for one CML CP sample are shown. (D) CML CP progenitors were treated with IM (1μM), dasatinib (0.15μM) or SB216763 (5μM) for 4 hours in SFM with 5GF. Representative immunoblots are indicated. (E). BCR-ABL or GF receptors both signal through MAPK and SRC kinases. SRC signaling activated by exogenous cytokines is not affected by IM. Conversely, both BCR-ABL– and GF-dependent activation of SRC and MAPKs is inhibited by dasatinib.
IM increased GSK3β pY216 levels in CML cells dose dependently without affecting normal cells (supplemental Figures 1 and 2A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A CML-restricted pool of active β-catenin (non-pS/T) was reduced by IM and increased by SB216763, indicating that the rate of stabilized β-catenin was still exogenously inducible by GSK3β-inactivation in primary CML CP cells. Accordingly, SB216763 also decreased a pool of β-catenin that can be marked for degradation via GSK3-dependent phosphorylation of its S/T-residues (pS/T β-Cat) in normal and CML cells without affecting total GSK3β levels or BCR-ABL activity.
We attempted to determine the upstream mechanisms controlling GSK3β pY216 using LY294002 or UO126 as inhibitors of 2 major pathways involved in BCR-ABL–positive leukemogenesis (PI3K/AKT and Ras/MAPK/MEK).28,29 Targeting of PI3K/AKT signaling by LY294002 did not affect GSK3β pY216 in CML CP progenitors (Figure 2B), as was also observed after incubation with WNT3a, a negative regulator of GSK3β (through its pSer9) in control cells (supplemental Figure 3). In contrast, targeting of MAPK activity by UO126 reduced GSK3β pY216, indicating that MAPKs are upstream positive regulators of GSK3β in CML CP progenitors (Figure 2B). Interestingly, IM-increased GSK3β pY216 was correlated with a compensatory response of CML cells to signals that can be elicited by multiple GF receptors converging on up-regulated p60-SRC and MAPK phospho-levels (Figure 2B-C). As reported previously,30 we confirmed that GSK3β is associated with a cluster of signaling proteins, including JAK2 and p60-SRC, in primary CML CP progenitors, providing the first evidence that IM treatment increases GSK3β binding to active p60-SRC under cytokine-enriched culture conditions (supplemental Figure 4).
The use of dasatinib, a dual SRC/ABL inhibitor approved for clinical use in CML patients who fail IM therapy,31-33 clarified the relative contribution of BCR-ABL and exogenous cytokines on GSK3β activity. Whereas IM inhibited BCR-ABL and its dependent signaling, both BCR-ABL–dependent and –independent (ie, GF mediated) p60-SRC/MAPK activation were targeted by dasatinib (Figure 2D-E). This was consistent with the finding that GSK3β pY216 levels were increased by IM but reduced by dasatinib (Figure 2D). The increased degradation of a phospho-S/T β-catenin (hence its reduced non-pS/T–stabilized levels) in IM-treated CML cells was much less apparent after dasatinib treatment, even when dasatinib was used in combination with SB216763 (Figure 2D).
These results indicate that CML progenitors finely integrate BCR-ABL and cytokine-activated signaling pathways that converge on GSK3β and β-catenin through active MAPK and p60-SRC/GSK3β complex formation. It seems conceivable that BCR-ABL expression, rather than its tyrosine kinase activity (which is selectively inhibited by IM), may be sufficient to sustain active GSK3β pY216 levels through a GF-dependent mechanism (which is annulled by dasatinib in addition to its anti–ABL kinase activity).
Mislocalization of GSK3β in CML CP stem/progenitor cells
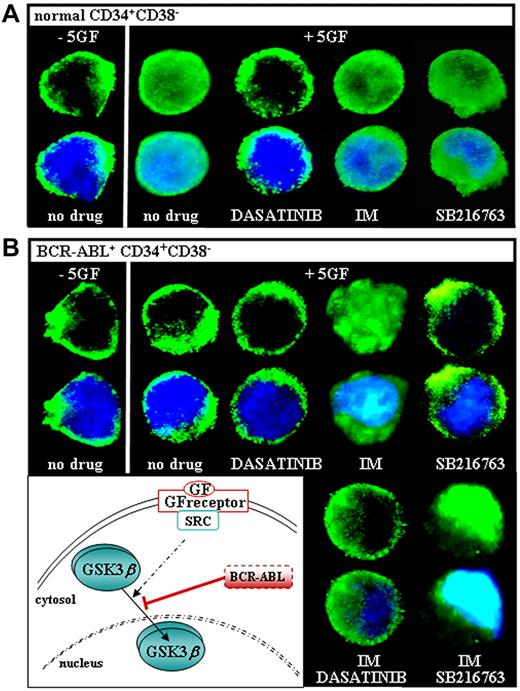
We examined the intracellular distribution of GSK3β and observed a rapid shuttling of the enzyme from the cytoplasm to the nucleus of primitive CD34+CD38− cells sorted from 3 healthy donors and then cultured in the absence or presence of 5GF (−5GF or +5GF, respectively; Figure 3A). This diffuse pattern did not change in 5GF-stimulated CD34+CD38− cells treated with SB216763 or IM, whereas it was inhibited by dasatinib. Regardless of GF stimulation, CD34+CD38− cells isolated from 10 CML CP patients showed a cytosolic retention of GSK3β that was unaffected by SB216763, dasatinib alone, or dasatinib combined with IM (Figure 3B). Nuclear GSK3β levels were markedly increased in CML CD34+CD38− cells treated with IM alone or in combination with SB216763. GSK3β mislocalization was confirmed in either primitive/quiescent cells (CD34+CD38−) or committed/cycling progenitors (CD34+CD38+) isolated from the majority of CML CP patients (9/10) and scored for BCR-ABL by double-fusion–FISH (D-FISH; data not shown).
GSK3βlocalization in CML CP progenitors in response to GF stimulation and IM. Freshly isolated normal (A) and CML (B) CD34+CD38− cells were cultured in the absence of serum and cytokines (−5GF) for 6 hours, and then exposed for 2 hours to a 5 GF cocktail (+5GF) in the absence (no drug) or presence of dasatinib (0.15μM) and/or IM (1μM), SB216763 (5μM) alone, or the combination of IM and SB216763. Each confocal image is representative of 3 immunofluorescence analyses of GSK3β using an anti-GSK3β Alexa Fluor 488–conjugated Ab (top panels). Overlap of GSK3β fluorescence signal and 4′,6-diamidino-2-phenylindole staining (bottom panels) is also shown (magnification, 60×). The depicted model illustrates that the shuttling of GSK3β in and out of the nucleus of normal cells is promoted by a GF-receptor engagement of SRC tyrosine kinases, because it is selectively inhibited by dasatinib but not imatinib. GSK3β is mislocalized to the cytoplasm of GF-stimulated CML cells and relocated to the nucleus after IM treatment, indicating a BCR-ABL kinase–dependent regulation.
GSK3βlocalization in CML CP progenitors in response to GF stimulation and IM. Freshly isolated normal (A) and CML (B) CD34+CD38− cells were cultured in the absence of serum and cytokines (−5GF) for 6 hours, and then exposed for 2 hours to a 5 GF cocktail (+5GF) in the absence (no drug) or presence of dasatinib (0.15μM) and/or IM (1μM), SB216763 (5μM) alone, or the combination of IM and SB216763. Each confocal image is representative of 3 immunofluorescence analyses of GSK3β using an anti-GSK3β Alexa Fluor 488–conjugated Ab (top panels). Overlap of GSK3β fluorescence signal and 4′,6-diamidino-2-phenylindole staining (bottom panels) is also shown (magnification, 60×). The depicted model illustrates that the shuttling of GSK3β in and out of the nucleus of normal cells is promoted by a GF-receptor engagement of SRC tyrosine kinases, because it is selectively inhibited by dasatinib but not imatinib. GSK3β is mislocalized to the cytoplasm of GF-stimulated CML cells and relocated to the nucleus after IM treatment, indicating a BCR-ABL kinase–dependent regulation.
Treatment with IM and SB216763 selectively targets Ph+ LTC-ICs, preserving normal stem cells
Previous studies demonstrated that targeting of GSK3β by SB216763 preserves normal HSCs, inhibiting leukemic cell growth in vivo.7-10 The survival of CML and normal stem/progenitor cells is not affected by IM or dasatinib.21-24 These considerations prompted us to investigate whether the combined use of SB216763 with IM or dasatinib may selectively target primitive CML HSCs while sparing normal stem cells.
Normal and CML CD34+ cells were treated with the highest clinically achievable doses of IM (1μM), dasatinib (0.15μM), and SB216763 (5μM) alone or in combination with IM or dasatinib in the presence of high 5GF, and then analyzed in LTC-IC assays in the absence of further drug exposure (Figure 4A).
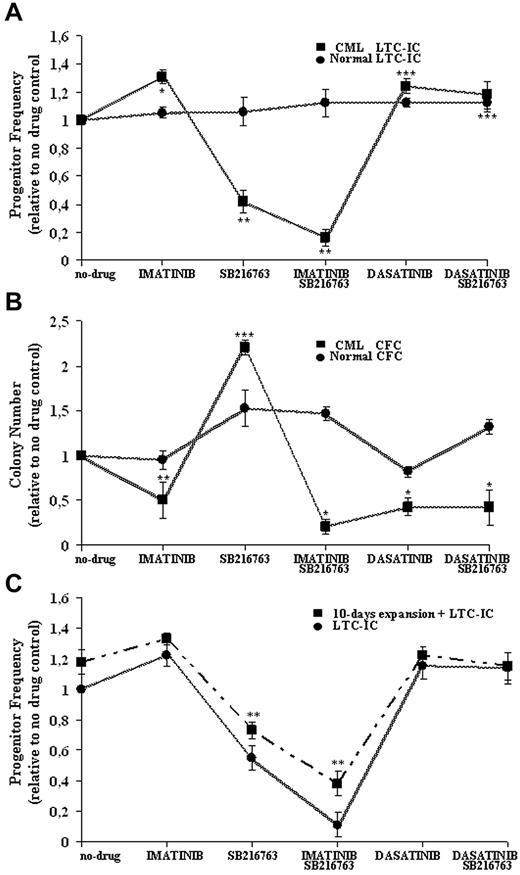
Effective and selective targeting of CML CP stem/progenitor cells by combined treatment with IM and SB216763. Normal and CML CD34+ cells were cultured for 96 hours in SFM supplemented with 5GF in the absence (−) or presence (+) of IM (1μM), dasatinib (0.15μM), and SB216763 (5μM) alone or in combination with IM or dasatinib. Cells were then assayed for primitive (A) or committed (B) progenitors in the absence of further drug exposure. The indicated mean ± SD graphed for CML and normal cells is based on replicate experiments (CML, n = 5; normal, n = 3). LTC-IC frequency was calculated in limiting-dilutions assays and normalized to the frequency of no-drug samples for each drug treatment. LTC-IC frequency (mean ± SD) of untreated CD34+ cells was 22 ± 13/1000 input cells for CML samples and 9 ± 5/1000 cells for normal cells. CFC frequency was plotted for each drug treatment and normalized to the colony number obtained in no-drug samples. CFC frequency (mean ± SD) of untreated cells was 345 ± 56/1000 input cells for CML samples and 122 ± 30/1000 cells for normal cells. Drug treatments at which CML progenitor frequency was significantly different from no-drug controls are indicated as follows: ***P < .001; **P < .01; and *P < .05. (C) CML CD34+CD38− cells (n = 3) were treated as indicated and then directly assayed as LTC-ICs or expanded for 10 days in stroma-free liquid-suspension cultures enriched with high 5GF concentrations before being analyzed in LTC-IC assays. LTC-IC frequency was calculated in limiting-dilution assays and normalized to the frequency of no-drug samples for each drug treatment. LTC-IC frequency (mean ± SD) of untreated CD34+CD38− cells was 11 ± 2/1000 input cells for CML samples before GF-mediated liquid enrichment and 21 ± 2/1000 cells afterward. **P < .01.
Effective and selective targeting of CML CP stem/progenitor cells by combined treatment with IM and SB216763. Normal and CML CD34+ cells were cultured for 96 hours in SFM supplemented with 5GF in the absence (−) or presence (+) of IM (1μM), dasatinib (0.15μM), and SB216763 (5μM) alone or in combination with IM or dasatinib. Cells were then assayed for primitive (A) or committed (B) progenitors in the absence of further drug exposure. The indicated mean ± SD graphed for CML and normal cells is based on replicate experiments (CML, n = 5; normal, n = 3). LTC-IC frequency was calculated in limiting-dilutions assays and normalized to the frequency of no-drug samples for each drug treatment. LTC-IC frequency (mean ± SD) of untreated CD34+ cells was 22 ± 13/1000 input cells for CML samples and 9 ± 5/1000 cells for normal cells. CFC frequency was plotted for each drug treatment and normalized to the colony number obtained in no-drug samples. CFC frequency (mean ± SD) of untreated cells was 345 ± 56/1000 input cells for CML samples and 122 ± 30/1000 cells for normal cells. Drug treatments at which CML progenitor frequency was significantly different from no-drug controls are indicated as follows: ***P < .001; **P < .01; and *P < .05. (C) CML CD34+CD38− cells (n = 3) were treated as indicated and then directly assayed as LTC-ICs or expanded for 10 days in stroma-free liquid-suspension cultures enriched with high 5GF concentrations before being analyzed in LTC-IC assays. LTC-IC frequency was calculated in limiting-dilution assays and normalized to the frequency of no-drug samples for each drug treatment. LTC-IC frequency (mean ± SD) of untreated CD34+CD38− cells was 11 ± 2/1000 input cells for CML samples before GF-mediated liquid enrichment and 21 ± 2/1000 cells afterward. **P < .01.
Compared with no-drug controls, CML LTC-IC recoveries were increased by IM and dasatinib (131% ± 12% and 125% ± 11%, respectively) that exerted only a minor anti-proliferative effect on normal progenitors (105% ± 4% and 112% ± 3% respectively). Treatment with SB216763 alone significantly reduced CML LTC-ICs (42% ± 8%) without affecting normal LTC-ICs (106% ± 10%).
For normal LTC-ICs, no adverse effects were also observed after treatment with SB216763 in combination with IM or dasatinib (112% ± 10% and 109% ± 6%, respectively). The addition of SB216763 resulted in a more effective targeting of CML LTC-ICs (16% ± 6%) compared with IM alone, without statistically significant effects in the setting of dasatinib treatment (112% ± 6%).
The effects of each drug treatment on normal and CML cycling progenitors, which were assayed directly as CFCs under cytokine-supported cultures, are shown in Figure 4B. The numbers of CML CFCs were significantly decreased by IM and dasatinib (51% ± 20% and 43% ± 10%, respectively), whereas normal CFCs showed minor suppression (95% ± 2% and 82% ± 6%, respectively). Treatment with SB216763 alone significantly increased normal or CML CFCs (220% ± 8% and 153% ± 20%, respectively). Similarly to CML LTC-ICs, the addition of SB216763 to IM led to an increased inhibition of CML CFCs (21% ± 8%) compared with IM alone, whereas the drug had no effect in combination with dasatinib (42% ± 20%).
Experiments were then performed to further test the degree of Ph+ versus Ph− selectivity for treatment with IM and SB216763. CD34+CD38− cells were isolated from selected cryopreserved samples of CML CP patients who had been shown previously to have higher Ph− stem/progenitor cell reserves (Table 1), and treated for 96 hours with IM, dasatinib, SB216763 alone, or SB216763 in combination with IM or dasatinib in the presence of high 5GF concentrations. Cells were harvested after each indicated treatment, washed to remove drug(s), and used for establishing by limiting dilutions in a series of LTC-IC assay cultures. In parallel, stroma-free suspension cultures were maintained for 10-days in the presence of high 5GF concentrations to ensure that all cells had divided at least once before performing the LTC-IC experiments.
As shown in Figure 4C, treatment with SB216763 alone or in combination with IM significantly reduced the frequency of CML LTC-ICs, preserving the long-term activity of residual stem cells that were expanded previously by 10 days of cytokine-enriched liquid culture in the absence of further drug exposure (Figure 4C). The Ph status of such GF-enriched LTC-ICs generated for each drug treatment was evaluated by D-FISH and compared with that of cells at baseline (Table 2). All CML cells were less than 90% Ph+ at baseline. After GF-mediated liquid enrichment, more than 95% of LTC-IC colonies derived by samples treated with SB216763 and IM were Ph−, indicating that residual normal stem cells survived exposure to the drugs.
D-FISH results for LTC-IC experiments with CD34+CD38− cells isolated from CML CP patients
| Patient no. . | Ph+/total cells (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Baseline . | No-Drug . | Imatinib . | SB216763 . | Imatinib SB216763 . | Dasatinib . | Dasatinib SB216763 . | |
| CP16 | 223/263 (85) | 93/112 (83) | 86/121 (71) | 32/118 (27) | 2/88 (2) | 86/94 (92) | 105/121 (87) |
| CP17 | 273/314 (87) | 82/126 (65) | 128/156 (82) | 34/106 (32) | 3/96 (4) | 129/148 (87) | 120/136 (88) |
| CP 18 | 242/276 (88) | 103/138 (75) | 119/140 (85) | 21/84 (25) | 2/42 (4) | 132/174 (76) | 77/107 (72) |
| Patient no. . | Ph+/total cells (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Baseline . | No-Drug . | Imatinib . | SB216763 . | Imatinib SB216763 . | Dasatinib . | Dasatinib SB216763 . | |
| CP16 | 223/263 (85) | 93/112 (83) | 86/121 (71) | 32/118 (27) | 2/88 (2) | 86/94 (92) | 105/121 (87) |
| CP17 | 273/314 (87) | 82/126 (65) | 128/156 (82) | 34/106 (32) | 3/96 (4) | 129/148 (87) | 120/136 (88) |
| CP 18 | 242/276 (88) | 103/138 (75) | 119/140 (85) | 21/84 (25) | 2/42 (4) | 132/174 (76) | 77/107 (72) |
Colonies were harvested after LTC-IC experiments combined with prior cell expansion under cytokine-enriched liquid suspension cultures pooled for each drug treatment condition and then FISH was performed on the cells from these colonies and cells at baseline.
In summary, these data indicate that combination treatment with IM and SB216763 selectively targets primitive Ph+ cells while preserving functional bcr-abl–negative LTC-ICs.
Combined treatment with IM and SB216763 enforces CML CD34+ stem/progenitor cell cycling, differentiation, and apoptosis
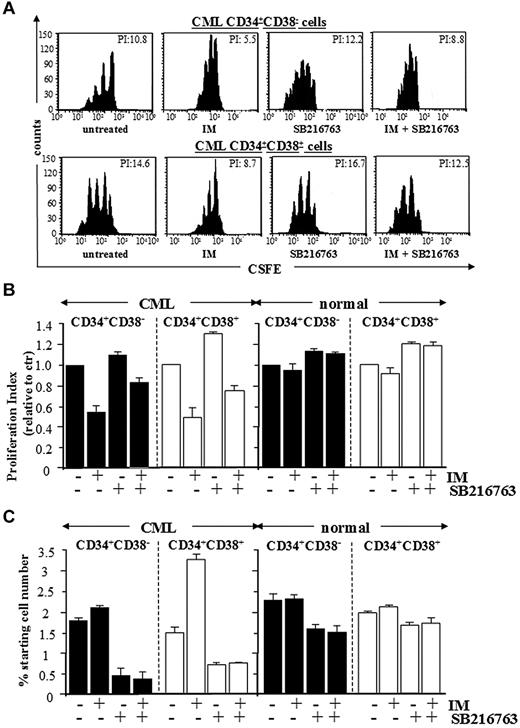
To investigate the growth-inhibitory effects of IM and SB216763, primitive CD34+CD38− cells and committed CD34+CD38+ progenitors from healthy or CML CP patients were labeled with CFSE and cultured for 96 hours with IM, SB216763, or the combination in serum-free medium containing high 5GF, referred as culture conditions that support the long-term expansion of normal LTC-ICs for up to 10 days.26,27 Subsequently, CFSE+ cells were labeled with annexin-PE and analyzed for apoptosis and proliferation (reduction of CFSE fluorescence in successive cell generations). Representative dot plots are shown in Figure 5A and Figure 6A.
Combined treatment with IM and SB216763 is cytotoxic by inducing apoptosis in CML CP stem/progenitor cells. CML and normal CD34+CD38− primitive and CD34+CD38+ committed progenitors were labeled with CFSE and cultured for 96 hours with IM (1μM), SB216763 (5μM), or the combination. (A) Representative data for apoptosis of CML CD34+CD38− primitive and CD34+CD38+ committed progenitors. (B-C) Compiled data (expressed as percentages of cells positively labeled by annexin V–PE) for apoptosis of total and undivided CML and normal CD34+ cell subsets (n = 5), respectively.
Combined treatment with IM and SB216763 is cytotoxic by inducing apoptosis in CML CP stem/progenitor cells. CML and normal CD34+CD38− primitive and CD34+CD38+ committed progenitors were labeled with CFSE and cultured for 96 hours with IM (1μM), SB216763 (5μM), or the combination. (A) Representative data for apoptosis of CML CD34+CD38− primitive and CD34+CD38+ committed progenitors. (B-C) Compiled data (expressed as percentages of cells positively labeled by annexin V–PE) for apoptosis of total and undivided CML and normal CD34+ cell subsets (n = 5), respectively.
Proliferation-induced decline of CML primitive progenitors in response to SB216763 alone or in combination with IM. CFSE-labeling assays were used to measure the effects of IM (1μM), SB216763 (5μM), or the combination on cell division of CML and normal CD34+CD38− primitive and CD34+CD38+ committed progenitors after 96 hours of culture for each treatment. (A) Representative CFSE plots for CML CD34+CD38− primitive and CD34+CD38+ committed progenitors treated as indicated. The calculated proliferation index for each dot plot is indicated. (B) Compiled data for proliferation (expressed relative to proliferation of untreated cells) of inhibitor-treated CML (n = 5) and normal (n = 3) CD34+ cell subsets, respectively. The mean ± SD values of replicate experiments are shown (n = 3, CML primitive progenitors; n = 5, CML committed progenitors; n = 3, normal primitive and committed progenitors). (C) The percentage of viable, nondividing CML primitive progenitors retaining maximal CFSE fluorescence after 96 hours of culture for each indicated treatment.
Proliferation-induced decline of CML primitive progenitors in response to SB216763 alone or in combination with IM. CFSE-labeling assays were used to measure the effects of IM (1μM), SB216763 (5μM), or the combination on cell division of CML and normal CD34+CD38− primitive and CD34+CD38+ committed progenitors after 96 hours of culture for each treatment. (A) Representative CFSE plots for CML CD34+CD38− primitive and CD34+CD38+ committed progenitors treated as indicated. The calculated proliferation index for each dot plot is indicated. (B) Compiled data for proliferation (expressed relative to proliferation of untreated cells) of inhibitor-treated CML (n = 5) and normal (n = 3) CD34+ cell subsets, respectively. The mean ± SD values of replicate experiments are shown (n = 3, CML primitive progenitors; n = 5, CML committed progenitors; n = 3, normal primitive and committed progenitors). (C) The percentage of viable, nondividing CML primitive progenitors retaining maximal CFSE fluorescence after 96 hours of culture for each indicated treatment.
Whereas the indicated treatments did not affect the survival of normal stem/progenitor cells, apoptosis of CML stem and progenitor cells was induced by combining IM and SB216763, but not by using IM or SB216763 as single agents (Figure 5B). Cell death was restricted to either divided or undivided CML CD34+ cell subsets (with CFSE fluorescence reduced or equivalent to relative parental generations; Figure 5A,C). The net effect of IM, SB216763, or the combination was then assessed on undivided and total normal and CML cells, correlating the proliferation index of total CFSE-labeled cells (expressed as the sum of the cells in all generations divided by number of parental cells present at the start of the experiments) with the absolute numbers of viable cells retaining maximal CFSE fluorescence (CFSEmax) after each treatment (Figure 6B-C). Primitive CD34+CD38− cells and committed CD34+CD38+ progenitors from CML CP patients were more proliferative compared with the corresponding normal populations, as indicated by reduced numbers of quiescent CFSEmax progenitors. Treatment with IM and SB216763 reversed the anti-proliferative arrest elicited by IM by decreasing the number of undivided CFSEmax cells. Particularly, the proliferation index for CML CD34+CD38− and CD34+CD38+ cells treated with IM and SB216763 was no longer higher than that of untreated cells, but the fractions of quiescent CFSEmax cells were more efficiently depleted. These effects were correlated with an enforced G1-to-S cycling of primary CML CP CD34+ cells that showed morphological features distinctive of early myeloid commitment and apoptosis (supplemental Figure 5).
Overall, these data demonstrate that SB216763 primarily induces a quiescence-to-cycling switch in primary CML CP cells, leading to an increase in sensitivity to the killing effects of IM while sparing normal stem/progenitor cells.
Molecular changes induced by SB216763 alone and in combination with IM or dasatinib in primary CML stem/progenitor cells
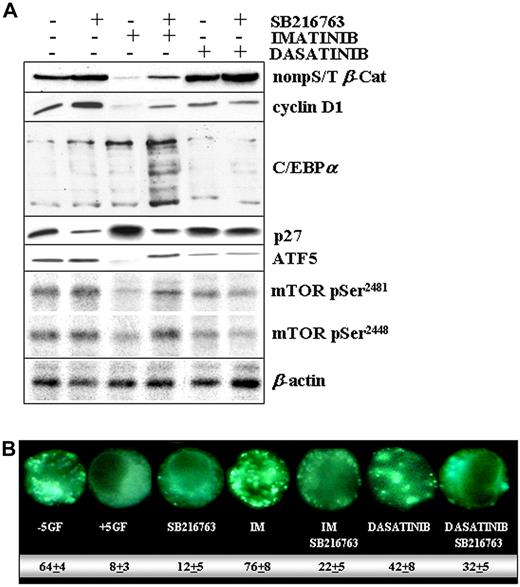
Combination treatment with IM and SB216763 resulted in much higher levels of β-catenin, cyclin D1, C/EBPα, ATF5, mTOR, and lower amounts of p27 compared with IM alone (Figure 7A). Except for ATF5 and mTOR, which remained unchanged, the turnover of β-catenin, cyclin D1, C/EBPα, and p27 was already altered by SB216763 alone, suggesting a GSK3-dependent regulation.2 Consistently, the delayed rate of β-catenin degradation and of p27 accumulation observed in the setting of dasatinib treatment may be related, at least in part, to the drug-mediated inhibition of GSK3β activity (Figure 2D).
Molecular changes in CML CP progenitors treated with IM and SB216763. (A) CML CP (n = 5) CD34+ cells were exposed to IM (1μM), dasatinib (0.15μM), or SB216763 (5μM) alone or in combination with IM or dasatinib for 24 hours in SFM containing high 5GF concentrations. Representative immunoblots for active β-catenin, total cyclinD1, C/EBPα, p27, ATF5, and phospho-mTOR (pSer2481/2448) in a representative CML CP patient sample are reported. Total β-actin levels are reported as a loading control. (B) CML CD34+ cells were treated with the indicated drug(s) and then analyzed for the presence of MDC-positive autophagosomes, distinct dot-like structures trapped in acidic, membrane-rich organelles distributed in the cytoplasm or localizing in the perinuclear regions of CML cells (magnification, 60×). The numbers indicate the percentage of autophagy induction for each drug treatment condition.
Molecular changes in CML CP progenitors treated with IM and SB216763. (A) CML CP (n = 5) CD34+ cells were exposed to IM (1μM), dasatinib (0.15μM), or SB216763 (5μM) alone or in combination with IM or dasatinib for 24 hours in SFM containing high 5GF concentrations. Representative immunoblots for active β-catenin, total cyclinD1, C/EBPα, p27, ATF5, and phospho-mTOR (pSer2481/2448) in a representative CML CP patient sample are reported. Total β-actin levels are reported as a loading control. (B) CML CD34+ cells were treated with the indicated drug(s) and then analyzed for the presence of MDC-positive autophagosomes, distinct dot-like structures trapped in acidic, membrane-rich organelles distributed in the cytoplasm or localizing in the perinuclear regions of CML cells (magnification, 60×). The numbers indicate the percentage of autophagy induction for each drug treatment condition.
A simple interpretation of these results is that inhibition of GSK3β (SB216763 or dasatinib dependent), but also changes on its compartmentalization (IM and dasatinib dependent, Figure 3B), may work in concert to modulate the net GSK3β signaling through some substrates but not others. Our initial observations indicated that targeting of a nucleus-localized pool of GSK3β (with SB216763 plus IM) may be required to stabilize ATF5, which in turn has been reported to promote the transcription of mTOR.34 This effect may account for a SB216763-mediated reduction of autophagy (Figure 7B), a state of metabolic inertia that is prevented by mTOR and critical to avoiding IM-mediated apoptosis.34,35
Discussion
Mechanisms underlying GSK3β activation in normal and malignant HSCs remain enigmatic despite the notion that GSK3β undergoes a transient, cytokine-dependent regulation in a broad range of cell types.2,6 The detection of pY216 is considered to be a hallmark of GSK3β kinase activation in living cells, because Y216 is located in the “activation loop” of the enzyme, acting as an autophosphorylation site or as a substrate for Pyk2, MEK1, and SRC-family tyrosine kinases (SFKs).36-40 In the present study, we have shown that freshly isolated CML stem/progenitor cells express a constitutively pY216 pool of GSK3β that can be reduced using SB216763, an ATP-competitive inhibitor of GSK3.
Consistent with GSK3β inhibition, SB216763 decreased the pool of β-catenin that is marked for degradation through GSK3-dependent phosphorylation on specific N-terminal S/T residues. Reciprocally, the rate of β-catenin protein stabilization (assayed through its non-pS/T pool) in primary CML CP cells remained inducible by SB216763. It is therefore consistent that GSK3β missplicing or increasing levels of BCR-ABL can progressively promote β-catenin activation during CML progression, as reported previously.18-20
IM, which was used herein as a specific BCR-ABL inhibitor, prevented the accumulation of β-catenin, causing a dose-dependent increase of GSK3β pY216 levels in cytokine-stimulated CML cells, indicating that GSK3β may function independently of β-catenin. In human mixed-lineage leukemias, GSK3 inactivation by SB216763 was correlated with increased levels of β-catenin, but tumor suppression in vivo through the inhibition of transcription complexes involving HOX, MEIS1, and CREB.9-11 In multiple myeloma, GSK3-mediated phosphorylation was reported to couple 2 apparently opposing cellular processes, as ubiquitination/degradation and transcriptional activation, promoting MAF-transforming activity.12 Therefore, which factors ultimately determine whether GSK3β may function as a tumor promoter or suppressor remains a challenge for future investigations.
An interesting finding of the present study was that GSK3β pY216 levels were up-regulated as part of an IM-mediated compensatory survival response of CML CP progenitors to exogenously added cytokines that rescue p60-SRC and MAPK phospho-activation beyond effective targeting of BCR-ABL.24,29,33 Accordingly, dasatinib used as a dual ABL and SFK antagonist delayed the turnover of β-catenin by inhibiting GSK3βby targeting BCR-ABL and cytokine-activated p60-SRC and MAPK. To our knowledge, these data represent the first evidence that active GSK3β may be differentially regulated by IM and dasatinib in primary CML CP progenitors that are rescued by cytokine support.24,29,33
GSK3β has been found to be associated with a cluster of signaling proteins, including JAK2 and p60-SRC kinases.30 We herein extend these data by showing that IM increased the binding of GSK3β to phospho-p60SRC (pY416) and β-catenin (thereby restoring its GSK3-mediated protein turnover), but not to JAK2. These observations, coupled with the inhibitory effect of U0126 on GSK3β, identify SFK and MAPK as upstream positive regulators of GSK3β activity in cytokine-stimulated CML CP progenitors. Moreover, a BCR-ABL–mediated signaling attenuation of AKT41 that is known to phosphorylate GSK3β at S9 may also contribute to the defective inactivation of GSK3β reported in primary CML versus normal CD34+ cells.
In addition to constitutive activity, our data show that GSK3β is located primarily in the cytoplasm and its nuclear levels are reduced in cytokine-stimulated CML stem/progenitor cells. In contrast, a diffuse pattern of GSK3β in normal HSCs is indicative of its physiologic shuttling in/out of the nucleus. Whereas the GSK3β import relies on a bipartite nuclear localization signal, GSK3β has no definable nuclear export signals, indicating that chaperone proteins (eg, FRAT or Tau) may be involved.2 How and whether these chaperone proteins rely on kinases or phosphatases that may act selectively in a cell compartment remain unclear. Our data indicate that the shuttling of GSK3β is independent of its kinase activity in normal and CML cells, as has also been reported in embryonic stem cells.42 However, in a broad range of cell types, nuclear GSK3β pY216 levels markedly increase during the S-phase of the cell cycle or in response to apoptotic stimuli, but it remains to be determined whether this represents translocation of GSK3β or modification of the enzyme already in the nucleus.43,44 In the present study, we show that nuclear import of GSK3β is prevented through a BCR-ABL kinase–dependent manner, because a nucleus-relocated pool of GSK3β (that is expected to be highly active) was detected in CML cells exposed to IM under cytokine support. Under such culture conditions, dasatinib fails to promote nuclear translocation of GSK3β in both normal and CML cells. From a biologic and therapeutic prospective, these data suggest that the net biologic outcome of GSK3β signaling may be reorientated through some substrates, but not others, in CML cells treated with dasatinib or IM. By evaluating potential cytosolic and nuclear targets of GSK3β, we describe changes in β-catenin, cyclin D1, C/EBPα, ATF5, mTOR, and p27 levels that may account for the most effective suppression of CML LTC-ICs by combining SB216763 with IM, but not dasatinib.
Mechanistically, a reasonable explanation for our results is that targeting of GSK3β in the setting of IM treatment may prime a differentiative/apoptotic-transcription program in CML cells, which is consistent with GSK3β nuclear relocation, higher levels of C/EBPα, ATF5, mTOR, and lower amounts of p27 compared with IM alone. In particular, SB216763/IM-mediated increases in ATF5 and mTOR protein levels are consistent with a significative reduction of autophagy, a state of metabolic inertia that has been involved in IM resistance.34,35 Conversely, targeting of cytosolic GSK3β may be ineffective in rescuing the cytostatic effect of dasatinib through sustained levels of β-catenin and p27. SB216763 alone increases CML CD34+CD38− cell cycling and an early myeloid commitment, which is consistent with higher levels of β-catenin, cyclin D1, C/EBPα, and CD38 and reduced amounts of p27. These data are suggestive of a GSK3β-mediated stabilization of p27 that is known to be predominantly relocated to the cytoplasm in primary CML cells via a BCR-ABL kinase–dependent mechanism.45 One can speculate that a restored, nucleus-localized pool of p27 may be reduced by adding SB216763 to IM but remain substantially unaffected by dasatinib alone or in combination with SB216763, which agrees with cytosolic retention of GSK3β and the lack of cooperation between GSK3β inhibition and dasatinib.
Previous studies have shown that GSK3 is critical for maintaining stem-cell characteristics, but whether GSK3 inactivation may be sufficient per se for expanding normal HSCs in vivo remains elusive.3,7,8,42 Pharmacologic targeting or genetic ablation of GSK3 has been reported to promote short-term neutrophil and megakaryocyte recoveries in BM-transplanted mice, while preserving or depleting a stem cell pool.3,7,8 Lithium, another GSK3 inhibitor, has already been tested in human clinical trials to enhance hematopoietic recovery after myelosuppressive chemotherapy, but this approach has not seen wide use because of limited success in restoring hematopoiesis in patients with reduced numbers of primitive HSCs.2 Accordingly, the results of the present study indicate that GSK3β inactivation promotes the expansion of normal and CML progenitors (as determined by increased CFC units) that are unable to regenerate the stem pool. Indeed, the long-term maintenance of normal and bcr-abl–negative stem cells is not affected by SB216763, whereas a smaller proportion of CML CD34+CD38− cells may retain the ability to execute self-renewing divisions, thus explaining the reduced Ph+ LTC-IC frequency. It seems reasonable that SB216763 mediates a “differentiative priming” that sensitizes CML stem cells by increasing apoptosis of undivided (CFSEmax) CD34+CD38− cells under IM treatment and cytokine support.
The role of GSK3β in glycogen metabolism suggests other interesting correlations. Quiescent CML cells have an increased BCR-ABL–mediated glucose uptake/metabolism that reduces in cis their proliferation through an HIF-1α–induced metabolic reprogramming.46 Whereas IM does not modify the glycemic profile of CML cells in vitro, and an altered glycemic control is a first sign of IM resistance in patients, fasting glucose improvement can be observed under dasatinib treatment in CML patients resistant to IM.47,48 Therefore, persistent GSK3β activity in CML primitive cells would be expected to impair storage mechanisms of glucose-derived carbon (through a phosphorylation-dependent inactivation of the glycogen synthase), thereby enforcing a metabolic compensatory response that would be expected to promote IM resistance.
In conclusion, the results of the present study show that targeting of GSK3β may be a fruitful approach to increasing the apoptotic effects of IM on CML stem cells without compromising bcr-abl–negative LTC-ICs or the short-term expansion of normal committed progenitors. This strategy may offer therapeutic value as an alternative to the use of cytokines to stimulate myelopoiesis or to resolve IM-mediated neutropenia in CML patients or during mobilization regimens of autologous peripheral blood stem cells in CML patients in complete cytogenetic response on IM therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Connie Eaves (Terry Fox Laboratory, Vancouver, BC), Dr Tessa Holyoake (University of Glasgow, Glasgow, United Kingdom), Drs Antonio Danieli and Raffaele Acierno (University of Salento, Lecce, Italy), and Dr Franco Sanapo (Azienda Sanitaria Locale Leece [ASLLE], Lecce, Italy) for insightful comments and discussion. This work is in memory of Pietro Coluccia.
This work was supported by the Italian Association for Cancer Research, Milan, Italy (research grants 1243 and 6189 to A.M.L.C.).
Authorship
Contribution: A.M.L.C. designed the research and wrote the manuscript; A.M.L.C., G.R., C.T., I.P., S.D.L., E.d.L., S.D.M., and L.D. performed the experiments and analyzed the data; C.G.P., N.D.R., and M.M. enrolled CML patients and supervised the project; and all authors commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Addolorata Maria Luce Coluccia, Hematology Unit and Clinical Proteomics Laboratory, Polo Oncologico Giovanni Paolo II, Ospedale Vito Fazzi ASL (LE), University of Salento, via Monteroni I-73100, Lecce, Italy; e-mail: malu.coluccia@unisalento.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal