Abstract
Leukemia cells from patients with chronic lymphocytic leukemia (CLL) express a highly restricted immunoglobulin heavy variable chain (IGHV) repertoire, suggesting that a limited set of antigens reacts with leukemic cells. Here, we evaluated the reactivity of a panel of different CLL recombinant antibodies (rAbs) encoded by the most commonly expressed IGHV genes with a panel of selected viral and bacterial pathogens. Six different CLL rAbs encoded by IGHV1-69 or IGHV3-21, but not a CLL rAb encoded by IGHV4-39 genes, reacted with a single protein of human cytomegalovirus (CMV). The CMV protein was identified as the large structural phosphoprotein pUL32. In contrast, none of the CLL rAbs bound to any other structure of CMV, adenovirus serotype 2, Salmonella enterica serovar Typhimurium, or of cells used for propagation of these microorganisms. Monoclonal antibodies or humanized rAbs of irrelevant specificity to pUL32 did not react with any of the proteins present in the different lysates. Still, rAbs encoded by a germ line IGHV1-69 51p1 allele from CMV-seropositive and -negative adults also reacted with pUL32. The observed reactivity of multiple different CLL rAbs and natural antibodies from CMV-seronegative adults with pUL32 is consistent with the properties of a superantigen.
Introduction
Chronic lymphocytic leukemia (CLL) is a low-grade malignancy of mature B cells that express low levels of functional surface membrane immunoglobulin (Ig), the B-cell antigen receptor (BCR).1 The Ig repertoire of healthy B cells is vast and in a constant state of flux. Diversity is generated in particular by recombination of germ line segments and, after ligation of antigen by a BCR with adequate affinity, somatic hypermutation. This process of Ig maturation is initiated on first encounter with a specific antigen, and subsequent or prolonged antigen exposure leads to generation of antibodies of even higher affinity to the specific antigen.2
In contrast, the Ig repertoire expressed by leukemia B cells from unrelated CLL patients is highly restricted and skewed, and it is not representative of the Ig repertoire expressed by naive B cells.3-5 Approximately 50% of CLL B cells express Ig heavy chains encoded by unmutated Ig heavy chain variable (IGHV) region genes, and of these more than 20% express closely homologous, if not identical, stereotyped heavy chain third complementarity determining regions (HCDR3).5-7 IGHV1-69 genes belong to the most frequently used IGHV genes and are mostly unmutated.6 Greater than 70% of IGHV1-69 cases use a 51p1-like allele and these 51p1-encoded Ig heavy chains are clearly distinct from those found in the normal adult blood B-cell repertoire.8,9 The highly restricted Ig repertoire expressed by CLL B cells is the basis of the widely accepted notion that a limited set of antigens may react with the Ig of the leukemic cells and that this interaction is relevant for the homeostasis of leukemic cells.
For CLL B cells, the functional consequences of Ig-mediated ligation of antigens are poorly defined, primarily because of the lack of knowledge of the relevant antigens. Therefore, a search for relevant antigens has been conducted in recent years. Candidate antigens should be distributed widely and provide chronic or repetitive stimuli for B cells as found in selected infections or autoimmune diseases.10,11 CLL recombinant antibodies (rAbs) that were derived from Ig expressed by leukemic cells were generated by several research groups to screen for reactivity with antigen panels that represent antibody targets in autoimmune diseases for reactivity. This limited set of CLL rAbs were found to react with multiple different antigens, including IgG, cardiolipin, single-stranded DNA, actin, thyroglobulin,12 nonmuscle myosin heavy chain IIA,13 and molecular motifs on oxidized low-density lipoprotein and apoptotic cells.14 Binding reactivity of several Abs with multiple antigens suggests the presence of a common antigenic determinant on the proteins screened. Alternatively, reactivity of multiple CLL rAbs with very different stereotyped Ab motifs could indicate the involvement of a superantigen that has antigen-receptor–mediated interactions with a large proportion of the leukemic cell pool. However, the identity and specificity of these antigen-antibody interactions that presumably drive the evolution of CLL is still undefined.
Infectious pathogens may provide chronic or repetitive stimuli for B cells and therefore represent potential targets for CLL BCRs.1 Human cytomegalovirus (CMV) in particular has been linked to CLL by several lines of evidence. Our group previously demonstrated that the amino acid sequence of the κ light chain variable region from a human CMV-specific IgG Ab shares extensive homology with the deduced amino acid sequences of an Ig commonly expressed on leukemic cells.15,16 Recently, we found a higher herpesvirus-specific seropositivity in selected CLL cohorts compared with the general population.17 Persistence of CMV and EBV in a subset of CLL patients expressing IGHV4-34 has been demonstrated previously.18 Furthermore, immune senescence is characterized in CMV-seropositive individuals by expansion of CD8+ T cells, of which a substantial portion is directed against CMV,19,20 and this expansion of CMV-specific CD8+ T cells is even more pronounced in CLL patients than in age-matched healthy adults.21 This suggests that an antigenic determinant of CMV could be involved as a specific antigen or superantigen in BCR-mediated stimulation of CLL cell.
To investigate the hypothesis that the Ig of CLL B cells react with an antigenic determinant of an infectious pathogen, we evaluated the reactivity of a panel of different CLL rAbs with a panel of selected viral and bacterial pathogens. These infectious pathogens chosen each have worldwide distribution, and humans are frequently exposed to these antigens by reactivation from latency with periods of viremia or extensive cross-reactivity with closely related pathogens.
Methods
Viral and bacterial antigen preparations
The Towne strain of CMV was obtained from the American Type Tissue Collection (ATCC; VR-977) and propagated with the use of human foreskin fibroblasts (HFFs), as described previously.22 HFFs were obtained after informed consent from the Medical Center of the University of California, San Diego and were cultured in minimal essential medium with Earle salts supplemented with 10% heat-inactivated fetal bovine serum, 1.5 μg/mL amphotericin B, 2mM l-glutamine, 200 U/mL penicillin, and 200 μg/mL streptomycin. All experiments were performed under G0 synchronization conditions.23 Cells were trypsinized 3 days after the monolayer became confluent and were replated at a lower density to induce progression into the cell cycle. At the time of replating, cells either were infected with CMV at a multiplicity of infection of 5 or were mock-infected with tissue culture supernatants as described previously.23 Cells were harvested at the indicated times postinfection (p.i.) and processed as described for each experiment. All experiments were performed at least twice.
Lysates of adenovirus serotype 2 (Ad2) and A549 culture cells at 72 hours p.i. were kindly provided by Lieve Naesens (Katholieke Universiteit Leuven, Leuven, Belgium). Single-colony isolates of the wild-type Salmonella enterica serovar Typhimurium 14028s were grown in Luria broth containing tryptone, yeast extract, and sodium chloride, and then they were incubated at 37°C and harvested at 24 hours p.i.
For Western blot analysis, virus-infected cells were lysed in Laemmli reducing sample buffer (50mM Tris, pH 6.8, 2% sodium dodecyl sulfate [SDS], 10% glycerol, and 5% β-mercaptoethanol) supplemented with a protease inhibitor cocktail (Roche) and phosphatase inhibitors (25mM sodium fluoride, 1mM sodium orthovanadate, and 5mM β-glycerophosphate). The lysate was sonicated and heated at 95°C for 5 minutes.
Recombinant expression of virus proteins
The expression vector for the open reading frame encoding the product of UL32 (pUL32) of CMV strain AD169 was kindly provided by Dr V. Sanchez (Texas A&M Health Science Center, College Station, TX). Orientation and sequence were verified by nucleotide sequencing (Eton Bioscience). The vector was further modified by addition of a Kozak consensus sequence for improved protein expression. Human 293FT cells were used for transient expression of pUL32 after transfection using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. Human 293FT cells were purchased from Invitrogen and grown in Dulbecco minimal essential medium with 10% heat-inactivated fetal bovine serum, 2mM l-glutamine, 200 U/mL penicillin, 200 μg/mL streptomycin, 1× DMEM nonessential amino acids, and 0.5 mg/mL G418 (Mediatech Cellgro). All cell culture media, serum, and supplements, except where noted, were from Invitrogen. Cells were maintained at 37°C and 7% CO2. As a negative control, 293FT cells were transfected with the pCDNA vector only. At 48 hours after transfection, cell lysates for use in Western blotting were prepared in reducing SDS sample buffer and solubilized at 42°C for 10 minutes. For use in enzyme immunoassay (EIA), untransfected or transfected cells were resuspended in 0.1M carbonate buffer (pH 9.6) and sonicated, and the then the sonicate was clarified by low-speed centrifugation. Sonicates were subsequently enriched for high-molecular-weight proteins by 10-fold dilution in coating buffer followed by concentration using Amicon Ultra-15 100-kDa molecular weight cut-off centrifugal filter units (Millipore).
Antibodies
The CMV-specific mAbs specific for pUL32 and pUL86 were kindly provided by Dr W. Britt (University of Alabama at Birmingham, Birmingham, AL). Sera from healthy, adult volunteers (n = 8) were screened for CMV-specific IgG Abs with the use of a commercially available EIA (medac). Sera positive for CMV-specific IgG Abs (n = 4) were used as positive controls, and sera negative for CMV-specific IgG Abs (n = 4) were used as negative controls in the different CMV-specific immunoassays. Human immune sera reactive against human herpesvirus-6 were obtained from Virion/Serion, a mAb to the human herpesvirus 6 glycoprotein 60/110 (Santa Cruz Biotechnology), and sera reactive against adenoviruses were from Panbio Diagnostics. Serum from 1 of the 8 healthy volunteers who was immunized against Salmonella infection 6 months before blood collection was used as positive control in the Salmonella Typhimurium-specific immunoassay. Human sera that are nonreactive in the Salmonella. Typhimurium-specific immunoassay were not available because of the extensive cross-reactivity among antibodies specific for the different Enterobacteriacea.24
Recombinant CLL Abs were expressed in vectors as described previously.25 In brief, PCR-amplified Ig heavy and light chain variable region products were digested with the appropriate restriction enzymes and ligated into a human IgG1, IgK, or IgL expression vector. 293A human embryonic kidney fibroblasts were cultured in DMEM supplemented with 10% ultra-low IgG FBS (Invitrogen) and cotransfected by calcium phosphate precipitation with equimolar amounts of Ig heavy and light chain plasmid DNA. Twelve hours after transfection, cells were washed with serum-free DMEM and thereafter cultured in DMEM supplemented with 1% Nutridoma SP (Roche Diagnostics). Supernatants were collected after 8 days of culture, and cell debris was removed by centrifugation at 800g for 10 minutes. Antibodies were purified using protein G Sepharose (GE Healthcare) and eluted in 0.1M glycine (pH 2.6) and neutralized with 1M Tris-HCl (pH 8.8). The integrity of all CLL rAbs was confirmed by SDS-PAGE analysis. After electrophoresis, protein bands were detected with use of Silver Stain Plus according to the manufacturer's instructions (Bio-Rad Laboratories). For use as a negative control in these studies, the heavy and light chain variable regions of 4A5, a high-affinity mouse IgG2b mAb specific for a human oncofetal antigen, were coexpressed using the human IgG1 and IgK expression vectors. The purified rAb retains binding specificity for its target antigen, as assessed flow cytometry, but it does not react with cellular components of the cells lysates used in these studies (data not shown).
Healthy individuals expressing IGHV1-69 receptors on their B cells were identified with use of the 51p1-specific mAb G6.8 Blood was collected with informed consent in accordance with the Declaration of Helsinki from 3 normal volunteers with known CMV serostatus. Approval was granted by the institutional review board of the University of California, San Diego. Leukocytes were isolated from these samples with the use of BD Vacutainer CPT tubes (BD Biosciences) according to the manufacturer's instructions. Secreted 51p1-encoded Abs were isolated from total serum of a CMV-seropositive individual and a CMV-seronegative individual positive for expression of IGHV1-69, respectively, by chromatography using the 51p1-specific G6 mAb15 coupled to CNBr-activated Sepharose (GE Healthcare). In addition, 51p1-encoded Abs were further purified using protein G-Sepharose (GE Healthcare) by elution with 0.1M glycine (pH 2.6), neutralized with 1M Tris-HCl (pH 8.8), and then transferred to PBS at physiologic pH with use of PD MiniTrap G-25 columns (GE Healthcare). Integrity of the purified 51p1-encoded Abs from healthy individuals was confirmed by SDS-PAGE and silver staining, as described above.
Western blot analysis
Equal amounts of lysate normalized by protein concentration were loaded onto SDS polyacrylamide gels unless otherwise stated. After electrophoresis, the proteins were transferred to nitrocellulose membranes with the iBlot Dry Blotting System (Invitrogen) and visualized by staining with Ponceau S (Sigma-Aldrich) to confirm efficient and homogenous transfer of protein. Western blot analysis was performed using the Cassette Miniblot System (Immunetics) with 28 channels, which allowed for blotting in parallel multiple different Abs against several different protein preparations on a single membrane without the need for further manipulation such as cutting of the membrane. Positive control and recombinant CLL mAbs were used at 5 to 10 μg/mL and incubated overnight at 4°C. Primary Ab were detected with horseradish peroxidase–conjugated anti–human IgG (Southern Biotechnology Associates). The blots were prepared for enhanced chemiluminescence using SuperSignal West Pico and West Femto chemiluminescent detection methods (Thermo Scientific) and subsequent autoradiography.
pUL32-specific EIA
Microtiter plates (Corning) were coated with 25 μL of a 10 μg/mL solution of clarified sonicates from either untransfected 293FT cells or cells transfected with vectors expressing either CMV pUL32 or HSV-2 pUL29,26 as described above. Carbonate buffer (0.1M) at pH 9.6 was used for dilution of the protein sonicates. Recombinant pUL32 and pUL29 protein were purified and concentrated before coating using an Amicon Ultra-15 Centrifugal Filter Unit (Millipore). After 12 hours incubation at 4°C, plates were washed 5 times with 10mM PBS containing 0.05% Tween 20 and then blocked with 150 μL of StartingBlock blocking buffer according to the manufacturer's instructions (Thermo Scientific). Plates were incubated overnight at 4°C with 25 μL of Ab preparation diluted in blocking buffer. After washing 5 times, plates were incubated with anti–human, horseradish peroxidase–labeled secondary Ab for 1 hour at room temperature, washed again 5 times, and then incubated with TMB peroxidase substrate (Kirkegaard and Perry Laboratories) for 10 minutes. The reaction was stopped with 1M phosphoric acid and the optical density (OD) measured at a wavelength of 450 nm. Background signal was defined as mean OD without primary Ab + 2 SD.
Statistical analysis
Comparison of 2 groups was carried out using the paired sample t test or 1-way ANOVA for normally distributed quantitative parameters and the Fisher exact test for categorical data. The OD values measured by EIA were related to antibody dilutions by a 4-parameter logistic model. Model fit was better than 99% in all cases. OD values measured when probing the different protein lysates from each experiment were modeled as a parallel line assay with a shift parameter. The shift parameter was tested for significance by Walsh tests. A P value less than .05 is considered statistically significant.
Results
Reactivity of CLL rAbs with bacterial and viral lysates
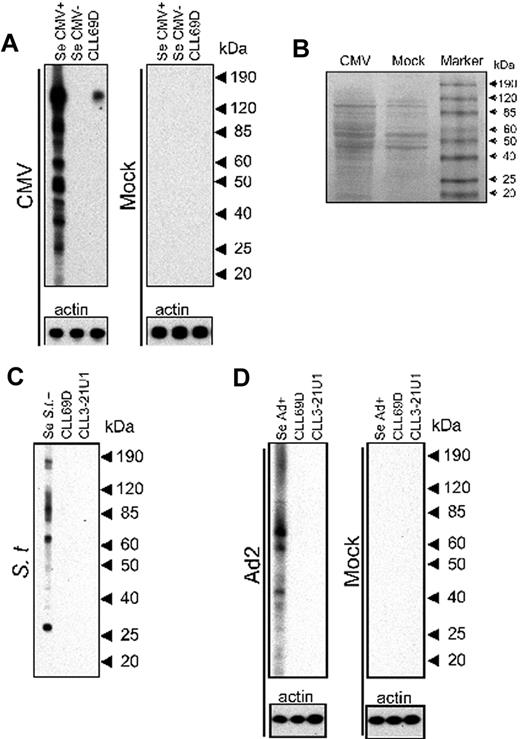
Screening of CMV protein lysate for reactivity with CLL69D, a stereotypic Ig receptor encoded by an unmutated IGHV1-69 gene and κ light chain (Table 1), showed a single, strong signal of approximately 150 kDa (Figure 1A). In addition, multiple bands were identified when the same CMV lysate was probed in a separate channel with reference sera available from healthy, adult CMV-seropositive volunteers (Figure 1, representative experiment with 1 immune serum). In contrast, immunoblots of protein lysate from mock-infected HFFs run in parallel and probed with the same rAb CLL69D did not result in a detectable signal, and no specific signal was detected in either CMV or mock-infected cells using reference sera from a healthy CMV-seronegative donor. Visualization of the proteins present on the membranes loaded with CMV- or mock-infected cell lysates demonstrated the presence of multiple bands with distinct staining patterns (Figure 1B), indicating the presence of adequate protein loaded in both lanes, and also confirmed by the signal detected on probing with an antibody to β-actin (Figure 1A).
Characteristics of CLL rAbs used in present investigation
| Recombinant IgG . | IGHV gene . | IGHD gene . | IGHJ gene . | % germ line identity . | HCDR3 sequence . | IGKV/IGLV gene . | % germ line identity . | LCDR3 sequence . |
|---|---|---|---|---|---|---|---|---|
| CLL69A | IGHV1-69 | D3-16 | JH3 | 100 | GGIYDYVWGSYRPNDAFDI | IGKV3-20 | 100 | QQYGSSPGT |
| CLL69B | IGHV1-69 | D2-02 | JH6 | 100 | MRPDIVVVPAAISYYYGMDV | IGKV1-39 | 100 | QQSYSTPRT |
| CLL69D | IGHV1-69 | D2-02 | JH6 | 100 | GGDIVVVPAAMSYYYYGMDV | IGKV3-11 | 100 | QQRSNT |
| CLL3-21U1 | IGHV3-21 | JH6 | 99 | DQNGMDV | IGLV3-21 | 99.3 | QVWDAGSDHPWV | |
| CLL3-21U2 | IGHV3-21 | D6-13 | JH4 | 100 | DPSFYSSSWTLFDY | IGKV3-20 | 100 | QQYGSSPLT |
| CLL3-21M1 | IGHV3-21 | JH6 | 95.4 | DGNGMDV | IGLV3-21 | 97 | QVWDSGSDHPWV | |
| CLL4-39A | IGHV4-39 | D2-02 | JH6 | 100 | HRLGYCSSTSCYYYYYGMDV | IGLV1-40 | 99.7 | QSYDSSLSVV |
| GW21 | IGHV1-69 | D5-24 | JH4 | 99.7 | DGEMATDDHFDY | IGLV1-40 | 99.7 | QSYDSSLSVV |
| MU08 | IGHV1-69 | D3-10 | JH5 | 100 | AGYGSGTYWFDP | IGLV1-40 | 99.7 | QSYDSSLSVV |
| Recombinant IgG . | IGHV gene . | IGHD gene . | IGHJ gene . | % germ line identity . | HCDR3 sequence . | IGKV/IGLV gene . | % germ line identity . | LCDR3 sequence . |
|---|---|---|---|---|---|---|---|---|
| CLL69A | IGHV1-69 | D3-16 | JH3 | 100 | GGIYDYVWGSYRPNDAFDI | IGKV3-20 | 100 | QQYGSSPGT |
| CLL69B | IGHV1-69 | D2-02 | JH6 | 100 | MRPDIVVVPAAISYYYGMDV | IGKV1-39 | 100 | QQSYSTPRT |
| CLL69D | IGHV1-69 | D2-02 | JH6 | 100 | GGDIVVVPAAMSYYYYGMDV | IGKV3-11 | 100 | QQRSNT |
| CLL3-21U1 | IGHV3-21 | JH6 | 99 | DQNGMDV | IGLV3-21 | 99.3 | QVWDAGSDHPWV | |
| CLL3-21U2 | IGHV3-21 | D6-13 | JH4 | 100 | DPSFYSSSWTLFDY | IGKV3-20 | 100 | QQYGSSPLT |
| CLL3-21M1 | IGHV3-21 | JH6 | 95.4 | DGNGMDV | IGLV3-21 | 97 | QVWDSGSDHPWV | |
| CLL4-39A | IGHV4-39 | D2-02 | JH6 | 100 | HRLGYCSSTSCYYYYYGMDV | IGLV1-40 | 99.7 | QSYDSSLSVV |
| GW21 | IGHV1-69 | D5-24 | JH4 | 99.7 | DGEMATDDHFDY | IGLV1-40 | 99.7 | QSYDSSLSVV |
| MU08 | IGHV1-69 | D3-10 | JH5 | 100 | AGYGSGTYWFDP | IGLV1-40 | 99.7 | QSYDSSLSVV |
Screening of different bacterial and viral lysates for reactivity with CLL rAbs by immunoblot. (A) CMV- and mock-infected cells were both harvested at 96 hours p.i. for Western blot analysis with the CLL rAbs CLL69D. Human sera (Se CMV+ and Se CMV−) served as positive and negative controls, respectively. All bacterial and viral lysates were used at the same total protein concentration, and all experiments with virus- and mock-infected cell lysates were done in parallel on the same blot, respectively. (B) Visualization of protein bands on the nitrocellulose membranes after electrophoresis and protein transfer of CMV- and mock-infected cell lysates, respectively, with the use of Ponceau S staining solution. (C) Salmonella Typhimurium (St) was harvested after overnight incubation in Luria broth for Western blot analysis with the CLL rAbs CLL69D and CLL3-21U1. Immune serum (Se St+) served as positive control. (D) Ad2 or mock-infected A549 (Mock) cells were harvested at 48 hours p.i. for Western blot analysis with the CLL rAbs CLL69D and CLL3-21U1. Immune serum (Se Ad+) served as positive control. Experiments with mock- and adenovirus-infected cells were done in parallel on the same blot, respectively. Human sera without reactivity to any of the proteins present in the Salmonella Typhimurium or Ad2 lysates were not available, and all CMV reference sera used showed reactivity with several of the proteins (data not shown) because of extensive cross-reactivity between different serotypes. Actin served as loading control. The culture medium used for propagation of Salmonella Typhimurium was protein- and eukaryotic cell-free; therefore, probing with an actin-specific Ab was not done.
Screening of different bacterial and viral lysates for reactivity with CLL rAbs by immunoblot. (A) CMV- and mock-infected cells were both harvested at 96 hours p.i. for Western blot analysis with the CLL rAbs CLL69D. Human sera (Se CMV+ and Se CMV−) served as positive and negative controls, respectively. All bacterial and viral lysates were used at the same total protein concentration, and all experiments with virus- and mock-infected cell lysates were done in parallel on the same blot, respectively. (B) Visualization of protein bands on the nitrocellulose membranes after electrophoresis and protein transfer of CMV- and mock-infected cell lysates, respectively, with the use of Ponceau S staining solution. (C) Salmonella Typhimurium (St) was harvested after overnight incubation in Luria broth for Western blot analysis with the CLL rAbs CLL69D and CLL3-21U1. Immune serum (Se St+) served as positive control. (D) Ad2 or mock-infected A549 (Mock) cells were harvested at 48 hours p.i. for Western blot analysis with the CLL rAbs CLL69D and CLL3-21U1. Immune serum (Se Ad+) served as positive control. Experiments with mock- and adenovirus-infected cells were done in parallel on the same blot, respectively. Human sera without reactivity to any of the proteins present in the Salmonella Typhimurium or Ad2 lysates were not available, and all CMV reference sera used showed reactivity with several of the proteins (data not shown) because of extensive cross-reactivity between different serotypes. Actin served as loading control. The culture medium used for propagation of Salmonella Typhimurium was protein- and eukaryotic cell-free; therefore, probing with an actin-specific Ab was not done.
To evaluate whether CLL69D also reacts with other viral or bacterial pathogens to which humans are frequently and repeatedly exposed, we screened protein lysates from Salmonella Typhimurium and Ad2 for reactivity with rAb CLL69D. These pathogens were specifically selected because virtually every adult has serum antibodies against adenoviruses or Salmonella spp because of extensive reactivity of Abs across adenovirus subtypes and all Enterobacteriaceae, respectively.24,27 Immunoblots results show that neither the bacterial and viral lysates of Salmonella Typhimurium or Ad2, respectively, nor of lysate from mock-infected A549 cells reacted with CLL69D (Figure 1C-D). Additional probing of Salmonella Typhimurium or Ad2 lysates with a CLL rAb with a different stereotyped Ab motif (CLL3-21U1, Table 1) was also negative. In contrast, multiple bands were identified on both the bacterial and viral cell culture lysates when probed with the reference sera available from healthy, adult volunteers (Figure 1, representative experiments with 1 serum each).
Identification of the CMV protein bound by CLL rAb
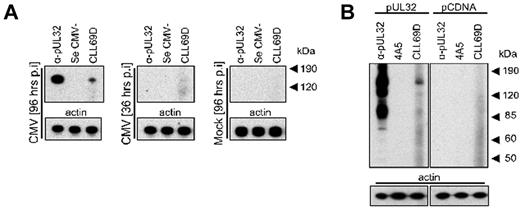
To further investigate and characterize the binding of the CLL69D rAb to a putative CMV protein, several additional observations were made that helped define the potential CMV candidate proteins bound by the CLL rAb. First, as noted previously, the band detected from CMV cell lysate corresponds in molecular size to a protein of ∼ 150 kDa. Second, each of the sera available from healthy CMV-seropositive donors (n = 4), but none of the CMV-seronegative sera (n = 4), reacted with this ∼ 150-kDa protein (data not shown). Third, multiple CMV proteins of other sizes also were detected using the CMV immune serum, but unlike the reactivity with the 150-kDa protein, the patterns of reactivity of these other CMV proteins were variable (data not shown). Finally, a time course experiment showed that the ∼ 150-kDa CMV protein bound by the CLL rAb is expressed later than 36 hours after CMV infection of permissive cells (Figure 2A). CMV-infected cells were harvested at 36 and 96 hours p.i. for Western blot analysis with the CLL rAbs CLL69D. We observed a signal at 150 kDa for CLL69D at 96 hours p.i. but not at 36 hours p.i., or with mock-infected cells at 96 hours p.i. CMV-seronegative serum (Se CMV−) was negative for all 3 conditions, and a signal was detected with mAb against pUL32 only at 96 hours p.i. The CMV large structural phosphoprotein pUL32 is a CMV protein of 150 kDa, highly immunogenic in the human host,28,29 and is expressed at late times during the CMV infection of permissive cells, that is, not before 48 hours p.i.30
Identification of the CMV protein reactive with the CLL rAb CLL69D. (A) Time course experiment for presence of the 150-kDa CMV protein detected by CLL69D at early and late times during CMV infection of permissive cells. CMV-infected cells were harvested at 36 and 96 hours p.i. for Western blot analysis with the CLL rAbs CLL69D. A monoclonal Ab specific for pUL32 (α-pUL32) and CMV-negative serum (Se CMV−) served as positive and negative controls, respectively. (B) The CMV large structural phosphoprotein pUL32 (pp150) was generated recombinantly for Western blot analysis with the CLL rAb CLL69D. A monoclonal Ab specific for pUL32 (α-pUL32) and a humanized mouse monoclonal Ab of irrelevant specificity (4A5) served as positive and negative controls, respectively. Actin served as a loading control.
Identification of the CMV protein reactive with the CLL rAb CLL69D. (A) Time course experiment for presence of the 150-kDa CMV protein detected by CLL69D at early and late times during CMV infection of permissive cells. CMV-infected cells were harvested at 36 and 96 hours p.i. for Western blot analysis with the CLL rAbs CLL69D. A monoclonal Ab specific for pUL32 (α-pUL32) and CMV-negative serum (Se CMV−) served as positive and negative controls, respectively. (B) The CMV large structural phosphoprotein pUL32 (pp150) was generated recombinantly for Western blot analysis with the CLL rAb CLL69D. A monoclonal Ab specific for pUL32 (α-pUL32) and a humanized mouse monoclonal Ab of irrelevant specificity (4A5) served as positive and negative controls, respectively. Actin served as a loading control.
To test whether the ∼ 150-kDa CMV protein bound by CLL69D is actually pUL32, recombinant pUL32 was generated in an eukaryotic expression system. 293FT cells were transfected with a plasmid vector encoding pUL32, and cell lysates were prepared after 48 hours for Western blotting. Probing pUL32 cell lysate with CLL69D or a mAb specific for pUL32 resulted in a signal at the expected molecular size but not when probing recombinant pUL32 with a chimeric mouse rAb of irrelevant specificity (4A5) or when testing pCDNA-transfected cell lysate (Figure 2B).
Reactivity of a panel of CLL rAbs with pUL32
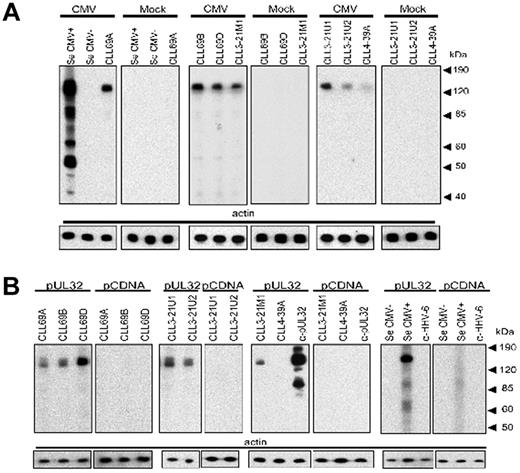
To determine whether binding to pUL32 is limited to rAb CLL69D or is a property of other CLL antibodies that express Ig heavy chains with highly related HCDR3, we screened additional CLL rAbs for reactivity with CMV cell lysate by Western blot. Our results demonstrate that all other CLL rAbs with Ig heavy chains encoded by IGHV1-69 detected an ∼ 150-kDa band in CMV-infected, but not mock-infected, cells regardless of the conserved HCDR3 or paired light chain expression. In addition, 3 different CLL rAb that express IgH encoded by IGHV3-21 and κ or λ light chains also detect an ∼ 150-kDa band, regardless of percent identity to germ line, or HCDR3 sequence (Figure 3A; Table 1). The signal obtained when probing with a CLL rAb encoded by the IGHV4-39 gene was close to the detection limit of the assay and did not increase with increasing rAb concentration (data not shown). Probing pUL32–transfected cell lysate with the same CLL rAbs by Western blot also resulted in a signal with each of the same rAbs, at the predicted molecular size for pUL32 as indicated by the mAb to pUL32 (Figure 3B). Probing mock-infected or pCDNA-transfected cell lysate with the different CLL rAbs gave uniformly negative results. Similarly, screening of the different CLL rAbs for reactivity with Salmonella Typhimurium or adenovirus lysate by Western blot gave also negative signals (data not shown). Reactivity of the different CLL rAbs observed with CMV lysate was similar as with pUL32 lysate, except for CLL rAb CLL4-39A that reacted weakly with CMV lysate but not with pUL32 lysate. Blotting sera from CMV-seropositive individuals against recombinant pUL32 also gave a strong signal, whereas sera from CMV-seronegative individuals gave a uniformly negative result in the pUL32 Western blot (Figure 3B).
Reactivity of a panel of CLL rAbs with different motifs and light chains to pUL32. Blotting of different stereotypic CLL rAbs with IGHV1-69 (CLL69A, CLL69B, and CLL69D), IGHV3-21 (CLL3-21M1, CLL3-21U1, and CLL3-21U2), and IGHV4-39 (CLL4-39A) gene against lysates of CMV- and mock-infected cells (A) and lysates of pUL32- and pCDNA-transfected cells (B). A mAb specific for pUL32 (α-UL32), serum from a CMV-seronegative individual, and a CMV-seropositive individual (Se CMV− and CMV+, respectively), and a mAb specific for the human herpesvirus-6 glycoprotein 60/110 (α–HHV-6) were used as controls. Concentrations of CLL rAbs ranged between 5 and 7.5 μg/mL. Actin served as a control for protein loading.
Reactivity of a panel of CLL rAbs with different motifs and light chains to pUL32. Blotting of different stereotypic CLL rAbs with IGHV1-69 (CLL69A, CLL69B, and CLL69D), IGHV3-21 (CLL3-21M1, CLL3-21U1, and CLL3-21U2), and IGHV4-39 (CLL4-39A) gene against lysates of CMV- and mock-infected cells (A) and lysates of pUL32- and pCDNA-transfected cells (B). A mAb specific for pUL32 (α-UL32), serum from a CMV-seronegative individual, and a CMV-seropositive individual (Se CMV− and CMV+, respectively), and a mAb specific for the human herpesvirus-6 glycoprotein 60/110 (α–HHV-6) were used as controls. Concentrations of CLL rAbs ranged between 5 and 7.5 μg/mL. Actin served as a control for protein loading.
Reactivity of CLL rAbs with pUL32 under native conditions
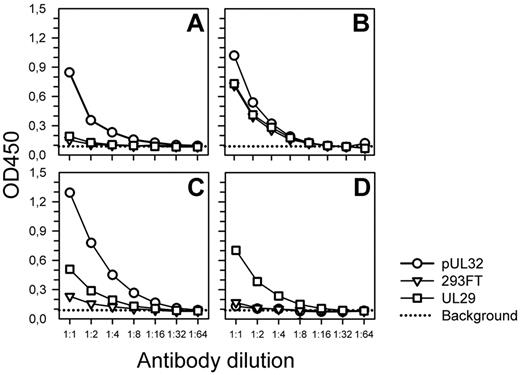
To validate findings obtained by Western blotting under denaturing conditions, CLL rAbs also were evaluated for reactivity with pUL32 by EIA under native conditions (Figure 4). Signals obtained when testing CLL rAbs CLL69B and CLL69D in 2-fold dilutions for reactivity with pUL32 cell sonicate decreased with decreasing Ab concentration and were clearly distinct from background signals obtained with untransfected 293FT or HSV-2 pUL29-transfected cell sonicates, respectively (Figure 4A-B). The relative difference in signals obtained when testing immune sera from CMV- or HSV-2–seropositive healthy individuals against pUL32, pUL29, or 293FT cell sonicates, respectively, were even more pronounced than those obtained when testing CLL rAb CLL69B (Figure 4B-C). The shift in OD values from assays on cell lysates of transfected versus mock-transfected cells was statistically significant for all experiments (P < .001, Walsh tests). The shift is expressed as a 1:13.0, 1:1.6, 1:12.6, or 1:8.9 dilution for representative experiments 4A through D, respectively.
Reactivity of CLL rAbs with the native pUL32. Recombinant pUL32, pUL29, and untransfected 293FT cell sonicates were probed in parallel with 2-fold serial dilutions of CLL rAb CLL69B (stock solution, 10 μg/mL; A), CLL rAb CLL69D (stock solution, 10 μg/mL; B), CMV-specific immune serum (Se CMV+; C), and HSV-2–specific immune serum (Se HSV+; stock dilution of serum samples, 1:1500; D). Cell sonicates were harvested at 48 hours after transfection for EIA analysis with equal amounts of total protein used. Ab preparations were used at 1 to 10 μg/mL and were titrated to avoid saturation of binding sites. All experiments were done in duplicate and each Ab was tested against the 3 different protein preparations in parallel on the same EIA plate.
Reactivity of CLL rAbs with the native pUL32. Recombinant pUL32, pUL29, and untransfected 293FT cell sonicates were probed in parallel with 2-fold serial dilutions of CLL rAb CLL69B (stock solution, 10 μg/mL; A), CLL rAb CLL69D (stock solution, 10 μg/mL; B), CMV-specific immune serum (Se CMV+; C), and HSV-2–specific immune serum (Se HSV+; stock dilution of serum samples, 1:1500; D). Cell sonicates were harvested at 48 hours after transfection for EIA analysis with equal amounts of total protein used. Ab preparations were used at 1 to 10 μg/mL and were titrated to avoid saturation of binding sites. All experiments were done in duplicate and each Ab was tested against the 3 different protein preparations in parallel on the same EIA plate.
Secreted 51p1 Abs isolated from healthy adults
The unmutated IGHV1-69 heavy chains of the rAb in this study are each encoded by a germ line IGHV1-69 51p1 allele. To evaluate whether IGHV1-69 or 51p1-encoded Ig expressed in healthy adults also may react with pUL32, we isolated and purified serum Abs with 51p1-encoded Ig heavy chains from 1 CMV-seropositive and 2 CMV-seronegative adults using of the anti-idiotypic Ab G6, an antibody specific for IGHV regions encoded by unmutated 51p1 alleles of IGHV1-69.8 The 51p1-encoded Ab from the CMV-seropositive adult reacted with pUL32 at the detection limit of the Western blot assay (Figure 5A), whereas the 51p1-encoded Ab from the 2 CMV-seronegative adults did not react detectably with pUL32. These signals did not change when the Ab concentrations were increased 2-fold (data not shown). Overall patterns of reactivity observed for the different 51p1-encoded Abs from healthy adults and CLL rAbs by Western blot analysis was similar to that observed by EIA (Figure 5B). Probing with any of the CLL rAbs, except CLL4-39A, gave significantly stronger signals when tested against native pUL32 than against 293FT or pUL29 cell sonicate indicative for a higher reactivity to pUL32 than to any other protein present in the cell sonicates. Regarding the 51p1 Abs from healthy adults, EIA signals were discernible between pUL32 and 293FT or pUL29 cell sonicate only when probing with those from the CMV-seropositive adults but not with those from the CMV-seronegative adults (P = .018, ANOVA). EIA signals obtained when probing the different protein lysates with a monoclonal Ab specific for a human herpesvirus-6 protein were similar to background signal, suggesting that none of the proteins present in the different lysates binds IgG molecules nonspecifically.
Reactivity of secreted 51p1 Abs isolated from blood of healthy individuals with CMV cell lysate. (A) CMV- and mock-infected cells were harvested at 96 hours p.i. for Western blot analysis with total immune serum and secreted 51p1 Abs isolated and purified from a healthy CMV-seropositive (Se and 51p1 CMV+, respectively) and 2 CMV-seronegative (51p1 CMV−) individuals, respectively. Final Ab concentration used for the 51p1 Ab preparations was 5 μg/mL, respectively. Actin served as a loading control. (B) EIA analysis of the reactivity of secreted 51p1 Abs (51p1 CMV+ and CMV−) to pUL32, pUL29, and untransfected 293FT cell sonicates in comparison that of the different CLL rAbs, and monoclonal Ab of irrelevant specificity (a-HHV-6). Recombinant pUL32, pUL29, and untransfected 293FT cells were harvested at 48 hours after transfection for EIA analysis with equal amounts of total protein used. All rAbs and mAbs were tested at the same final concentration (10 μg/mL). Immune sera against CMV (Se CMV+) and HSV-2 (Se HSV-2+) served as positive controls and were tested at the same serum dilutions (1:1500) against the different protein preparations. All experiments were done in duplicate and each Ab was tested against the 3 different protein preparations in parallel on the same EIA plate. Mean OD readings for the respective Ab preparations tested to each cell sonicate were compared by the use of 1-way analysis of variance, and a statistically significant difference in signal between groups (P < .05) is denoted by an asterisk.
Reactivity of secreted 51p1 Abs isolated from blood of healthy individuals with CMV cell lysate. (A) CMV- and mock-infected cells were harvested at 96 hours p.i. for Western blot analysis with total immune serum and secreted 51p1 Abs isolated and purified from a healthy CMV-seropositive (Se and 51p1 CMV+, respectively) and 2 CMV-seronegative (51p1 CMV−) individuals, respectively. Final Ab concentration used for the 51p1 Ab preparations was 5 μg/mL, respectively. Actin served as a loading control. (B) EIA analysis of the reactivity of secreted 51p1 Abs (51p1 CMV+ and CMV−) to pUL32, pUL29, and untransfected 293FT cell sonicates in comparison that of the different CLL rAbs, and monoclonal Ab of irrelevant specificity (a-HHV-6). Recombinant pUL32, pUL29, and untransfected 293FT cells were harvested at 48 hours after transfection for EIA analysis with equal amounts of total protein used. All rAbs and mAbs were tested at the same final concentration (10 μg/mL). Immune sera against CMV (Se CMV+) and HSV-2 (Se HSV-2+) served as positive controls and were tested at the same serum dilutions (1:1500) against the different protein preparations. All experiments were done in duplicate and each Ab was tested against the 3 different protein preparations in parallel on the same EIA plate. Mean OD readings for the respective Ab preparations tested to each cell sonicate were compared by the use of 1-way analysis of variance, and a statistically significant difference in signal between groups (P < .05) is denoted by an asterisk.
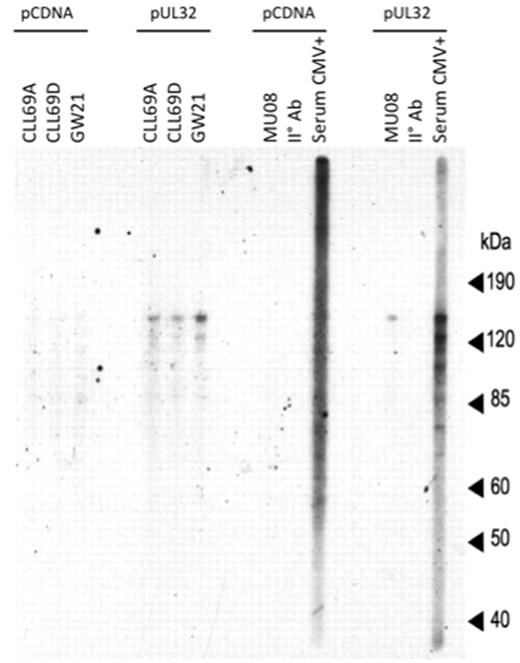
To further determine whether binding to pUL32 is specific to rAb encoded by the 51p1-encoded heavy chains or a property of all 51p-encoded heavy chains, we further isolated, expressed in vitro, purified, and analyzed rAb with 51p1-encoded Ig heavy chains isolated from B cells from CMV-seropositive or CMV-seronegative adults tested previously. MU08 and GW21 rAbs were isolated from CMV-seropositive and CMV-seronegative individuals, respectively. Each rAbs differs from CLL69A, CLL69B, and CLL69D only in the HCDR3 because all are encoded by an unmutated 51p1-allele (Table 1). MU08 and GW21 rAbs, CLL69A, CLL69D, and CMV-seropositive serum were tested against protein lysates from pUL32 transfected cells or 293FT cells transfected with control pCDNA vector (Figure 6). Each rAbs showed a detectable signal of ∼ 150 kDa for pUL32 transfected cells but not pCDNA-transfected 293FT cells. These results indicate that 51p1-encoded Abs are able to bind to pUL32 regardless of whether they are isolated from CMV-seropositive or CMV-seronegative individuals.
Reactivity of 51p1-encoded Abs isolated from blood of a healthy CMV-seropositive and CMV-seronegative individual with pUL32. Recombinant CMV protein pUL32 and control cell lysate from 293FT cells transfected with pCDNA were size separated on SDS-PAGE and transferred to nitrocellulose membrane. The membrane was probed with CLL 51p1 rAbs and 51p1 rAbs isolated and purified from a healthy CMV-seronegative and CMV-seropositive individual. Secondary Ab alone and CMV-seropositive serum was used as negative and positive control, respectively. All rAbs were tested at the same final concentration (5 μg/mL).
Reactivity of 51p1-encoded Abs isolated from blood of a healthy CMV-seropositive and CMV-seronegative individual with pUL32. Recombinant CMV protein pUL32 and control cell lysate from 293FT cells transfected with pCDNA were size separated on SDS-PAGE and transferred to nitrocellulose membrane. The membrane was probed with CLL 51p1 rAbs and 51p1 rAbs isolated and purified from a healthy CMV-seronegative and CMV-seropositive individual. Secondary Ab alone and CMV-seropositive serum was used as negative and positive control, respectively. All rAbs were tested at the same final concentration (5 μg/mL).
Discussion
Here, we found that several recombinant Abs with different Ab motifs react with a single CMV protein. In contrast to previous studies that reported a polyspecificity of CLL Igs,14,31 none of the CLL rAb tested reacted with any of the other bacterial, viral, or cellular structures presently evaluated. Identification of this specific interaction between a viral structure and Ig expressed by leukemic cells may have far reaching virological, immunologic, and hematologic implications, but foremost it opens up new avenues for studying functional consequences of ligation of Ig expressed on leukemic cells to a specific antigen.
The observed reactivity of CLL rAbs with CMV is not very surprising when considering the extraordinary characteristics of this herpesvirus. Infection with CMV is common and found worldwide: ∼ 60% of the general population has serologic evidence for CMV infection.32 After primary infection, CMV persists in monocytes and macrophages and in endothelial cells, with frequent reactivations from latency associated with periods of viremia and strong stimulation of humoral and cellular immunity.33-35 CMV has multiple mechanisms available for modulating the host's immune system, thereby allowing the virus to establish latency in cells of the immune system (reviewed in Hengel et al36 ).
CMV infection and CLL also share remarkable characteristics. Rates of CMV seroprevalence, frequency, and magnitude of CMV reactivation,19 and risk for CLL increase in parallel with increasing age.37,38 The seroprevalence of CMV may be significantly higher in selected CLL cohorts than in age-matched healthy adults.17 Furthermore, immune senescence is characterized in CMV-seropositive individuals by expansion of CMV-specific CD8+ T cells, particularly in CLL patients.21
The reactivity of CLL rAbs to pUL32 and not of any other of the numerous, highly immunogenic CMV proteins, however, is surprising.30,39 The CMV basic phosphoprotein pUL32 (pp150 or BPP) is the major and second most abundant tegument phosphoprotein,40,41 is essential for viral replication by retaining nucleocapsid organization through secondary envelopment at the assembly compartment,42-44 and is highly immunogenic in the human host.28,29 pUL32 is the polypeptide most frequently reactive in immunoblotting analyses with antisera compared with other viral proteins.30 Still, it may be only speculated at this point why the CLL rAbs reacted with pUL32 and not any of the other immunogenic CMV proteins.
The observed reactivity of multiple CLL rAbs with different stereotyped Ab motifs and some 51p1-Abs and -rAbs from healthy adults with this single CMV protein is consistent with the characteristics of a superantigen. Superantigens are microbial proteins that are oligo- or multivalent; have broad reactivity with a large proportion of the B-cell pool; and induce proliferation, activation, migration, and deletion of B cells.45 Several superantigens have been identified so far, including proteins of Staphylococcus aureus, Peptostreptococcus magnus, HIV-1, and Plasmodium falciparum.45 The mitogenic and immunomodulatory properties of superantigens for B cells include antiapoptotic effects, up-regulation of activation markers on B cells, and supraclonal deletion.45 Nevertheless, only 6 of 7 CLL rAbs reacted with pUL32, and several rAbs, mAbs, and serum Abs did not react detectably with pUL32. Furthermore, the capacity of a superantigen to interact with the BCR is inhibited by immunoglobulins that bind and neutralize the superantigen.45 We found in concordance with others that a significant proportion of the humoral immune response in CMV-seropositive adults and CLL patients is directed against pUL32.30
Therefore, alternative explanations for the reactivity of multiple, different CLL rAbs with a single protein also have to be considered. Reactivity of Abs with very different Ab motifs with a single viral protein is commonly observed such as in the human humoral immune response to influenza virus infection.46 pUL32 is one of the largest proteins expressed by CMV, and multiple epitopes were found when studying reactivity of human immune sera with pUL32.30,47,48 The reactivity of CLL rAbs with very different antibody motifs with pUL32 may point to the presence of multiple CLL rAb binding sites on this single CMV protein.
It also may be speculated that stimulation of naive B cells by a specific antigen may be an initiating event for CLL. As many as 20% of CLL patients have malignant cells that express 51p1,8,9 an allele of IGHV1-69 that frequently is expressed by leukemia cells with little or no somatic mutation and also seems to be overrepresented in the fetal and adult primary B-cell repertoire.49 The 51p1-encoded IGHV expressed on the majority of normal B cells, however, are distinct from those expressed on leukemic cells.8 and a single amino-acid substitution within the HCDR3 was shown to dramatically alter the specificity of this Ab.50 In contrast, a subset of healthy B cells express 51p1-Abs that are very similar to those found on leukemic cells.8,51 Conserved patterns in the 51p1-encoded BCR of healthy B cells suggest a restricted sequence repertoire shaped by evolution to recognize common pathogens. Conceivably, a subset of such normal B cells may be selected to undergo transformation by virtue of their expression of surface Ig with some distinctive binding activity for pUL32.
Still, a considerable fraction of patients who lacked serological evidence for past infection with CMV had CLL cells that were not uniquely associated with any of the IGHV subgroups.17 However, it should be noted that CMV genome have been detected in clinical samples of CMV-seronegative, healthy blood donors.52 Larsson et al even detected the CMV genome in 55% of peripheral leukocytes of CMV-seronegative blood donors.53 The presence of the CMV genome in samples of CMV-seronegative individuals, however, could not be confirmed by other large studies.54 Consequently, it is possible that only a fraction of CMV-seronegative patients who have CLL cells with stereotyped receptors had prior infection with CMV.
Cross-reactivity or molecular mimicry of stereotyped receptors with other self- or environmental antigens also has to be considered, especially in CMV-seronegative patients. Chu et al reported previously binding activity of 1 of the CLL rAbs also presently evaluated (CLL69A) with nonmuscle myosin heavy chain IIA (MYHIIA).13 MYHIIA has a molecular size of 225 kDa and is highly abundant in eukaryotic cells such as the HFFs and 293 cells, cell types that are used for propagation of CMV and expression of recombinant proteins.55 The consistently negative results obtained when probing lysates of HFFs or 293FT cells with CLL69A suggests that this rAb has stronger binding for pUL32 than with other mammalian cell proteins. Nevertheless, stereotyped receptors were reported to preferentially recognize modified MYHIIA in apoptotic cells, especially when complexed with other putative targets of stereotyped receptors.12,13 Because we did not evaluate in parallel the binding activity our rAbs for pUL32 and MYHIIA in apoptotic blebs, we can only speculate that there may be a structural relationship between pUL32 with other self-antigens that may account for the reactivity of the rAbs from CMV-seronegative patients.
In conclusion, we found that multiple CLL rAbs with different Ab motifs have higher reactivity with the highly immunogenic CMV structural phosphoprotein pUL32 than with other viral, bacterial, or cellular proteins. Nevertheless, some secreted 51p1 Abs and all 51p1 rAbs available from healthy adults also reacted with pUL32, consistent with the properties of a superantigen. Identification of an antigen that reacts specifically with CLL rAbs, however, provides the basis for future evaluation of functional consequences of ligation of Ig expressed on CLL cells with a specific antigen. Our findings have the potential for opening completely new avenues to the study of the disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for the excellent technical assistance of Monica Cook and Trang Le.
This study was funded in part by National Institutes of Health grants 5 P01-CA081534 for the CLL Research Consortium and R37-CA049870 (T.J.K.); a training grant from the European Society of Clinical Microbiology and Infectious Diseases and Austrian Science Fund grant J2750-B11 (C.S.); and a grant from the Austrian Society of Hematology and Oncology, the Initiative for Cancer Research of the Medical University of Vienna.
National Institutes of Health
Authorship
Contribution: C.S., U.J., and T.J.K. conceived, planned, and prepared the study; C.S. wrote the manuscript; G.F.W., E.M.G., and T.J.K. were responsible for generation of the recombinant antibodies; C.S. and D.G. were responsible for the bacteriologic investigation; C.S. and G.F.W. were responsible for the immunologic evaluation of Ab reactivity; C.S., C.S.M., R.S., K.V., and D.S. were responsible for the virologic investigation; and C.S., C.S.M., and D.S. generated the recombinant viral proteins.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas J. Kipps, Moores University of California–San Diego Cancer Center, 3855 Health Sciences Dr, 0820, La Jolla, CA 92093-0820; e-mail: tkipps@ucsd.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal